Powerpoint プレゼンテーション




Regional Workshop on IP management around WIPO Re: Search and WIPO GREEN
Practices of IP applied to economic and social development: Experience from Japan Industry
March 3, 2016, Manila
Takeda Pharmaceutical Company Limited Head of Operations (IP), Intellectual Property
Introduction of Takeda
Pharma Industry and Patent Unmet Medical Needs High Risk and Huge R&D cost on Drug Discovery Unique Nature of Pharma Patent
Japan's capability to generate New Drug Generation
Patent Drives Innovation Secure Investment to Develop New Drug Facilitate Flow of New Technology Takeda's case
New Paradigm in Drug Generation Unmet Medical Needs Open Innovation in Japan for Drug Discovery Open Innovation to Improve Global Health
• Background • GHIT • WIPO Re:Search

1. Introduction of Takeda
Christophe Weber,
Business Overview
Sales (2014): 1,777.8 bn
R&D (2014 ) : 382. 1 bn(21.5% of Sales)
Presence in over 70 countries
Employee: 31,328 (as of 2015 March)
2. Pharma Industry and Patent
■Unmet Medical Needs
Strive to develop and
provide new therapies
Chlamydial infection
Irregular heartbeat
Bronchial asthma
to Patients
Allergic rhinitis
Herpesvirus infection
Anxiety neurosis
Myocardial infarction
Benign prostatic hypertrophy
Parkinson's disease
Atopic dermatitis
Chronic rheumatoid arthritis
Osteoporosis Nephrosis syndrome
Autonomic disturbance
Cerebral infarction
Hemorrhage (including subarachnoid hemorrhage)
Chronic hepatitis C
Chronic glomerulonephritis
Diabetic(al) neuropathy
Urinary incontinence/pollakiuria
Degenerative arthritis
Chronic hepatitis B
Diabetic(al) retinopathy
Diabetic(al) nephropathy
Alzheimer's Disease
Multiple sclerosis
Chronic renal failure
Senile dementia Lung cancer Liver cancer
Neuromuscular disturbance and myopathy
Satisfaction with treatment
Source: questionnaire survey by mail
Unmet medical needs
Survey period: October 15 to December 22, 1999 Target: medical doctors (128 respondents)
Source : The Japan Health Sciences Foundation: "Report on Key Domestic Technologies 2000 – Outlook of medical needs in 2010 –"
Takeda Key Products and Pipeline (as of Aug, 2015)
■High Risk and Huge R&D cost on Drug Discovery
2–3 years 3–5 years 3–7 years 1–2 years
for preclinical for clinical
Number of compounds 652,336 203 75 26 21
1:3,213 1:8,698 1:25,090 1:31,064
success rate • Extremely low success rate • R & D costs : several 10 billions over100 billions yen (JPMA)
1,300 millions $ (PhRMA)
2005 to 2009 Survey by JPMA
Lowering of PTS for Drug Development
2005-2009 : 1/31,064 2004-2008 : 1/25,482 2003-2007 : 1/21,677 1999-2003 : 1/12,324 1989-1993 : 1/ 3,700
■Unique nature of pharma patent
Patent for Automobiles, IT
Patent for Pharma Products
Substance Patent
a)Hundreds, thousands patents for one
a) One basic patent for one product
b) Value of individual patent is high
b)Value of individual patent is not so high
c) Not rare to give up product
c)Unlikely that a third party patent
development due to a third party patent
constitutes an absolute bar for product
development and launch d)International Standardization
3. Japan's Capability to Generate New Drug
<Japan-originated new medicines marketed in more than 20 countries by 1999>
Before introduction of product patent system
1960s ; 2 products
1970s ; 4 products
Introduction of product patent system ;
After introduction of product patent system
1980s ; 18 products
1990s ; 14 products
<World top 50; 10 Japan-originated new medicines in 2008>
Crestor (Shionogi, antilipidemic agent)
4.1 billion dollars
Actos (Takeda, Type II diabetes)
4.1 billion dollars
Blopress (Takeda, hypotensive agent)
3.8 billion dollars
Aricept (Eisai, anti-Alzheimer agent)
3.4 billion dollars
Abilify (Otsuka, antipsychotic agent)
3.3 billion dollars
Takepron (Takeda, antiulcer agent)
3.2 billion dollars
Cravit (Daiichi-Sankyo, antibiotic agent)
2.9 billion dollars
Pariet (Eisai, antiulcer agent)
2.7 billion dollars
Harnal (Astellas, prostatic hypertrophy)
2.7 billion dollars
Olmetec (Daiichi-Sankyo, hypotensive agent) 2.3 billion dollars
Source: Uto Brain
New Drug Generation in World
Japan is rated as the third capable country to generate new drugs in the world
Germany: 4
Others: 3
Denmark: 5
France : 5
Origin: The JPMA's Office of Pharmaceutical Industry Research Pharmaprojects, @2013 IMS Health. IMS World Review
4. Patent Drives Innovation
■Secure investment to develop new drug
Sales Revenue(B)-R&D Investment (A)⇒ Investment for new drug
Sales Revenue(B)
Exclusive period
R&D Investment (A)
■Patent facilitates flow of new technology
☆ Innovation by Disclosure of Technology
☆Innovation by Technology Transfers
■Takeda's cases
1. Leuprorelin Product Case
2. Lansoprazole Product Case
3. Collaboration Research Cases
Overview of Leuprorelin Product Case
Leuprorelin Acetate
Product Options Daily injection
1-month Depot(Administration: daily to 1 month)
1-month Depot(improved)(Elimination of BSE risk)
3-month Depot(Administration: 1 month to 3 months)
6-month Depot(Administration: 3 months to 6 months)
6-month Depot(improved)(Decrease of administration volume by
higher drug content)
70-80 countries including Asian countries such as Philippines,
Malaysia, Singapore, Indonesia, Taiwan, Thai, China, Korea, etc.
1M, 3M, 6M Depot is sustained-release microsphere production
consisting of Leuprorelin Acetate and Biodegradable Polymer such as PLGA, PLA. Those Depots can suppress the initial excess release and achieve a stable release speed over a long period of time.
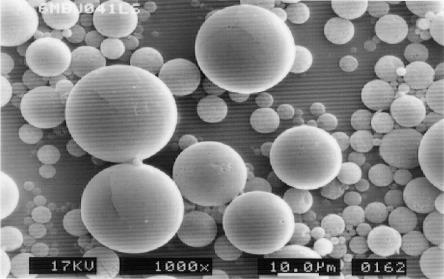
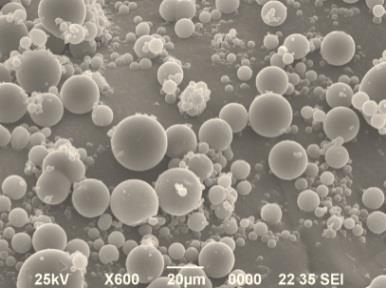

Characteristic of Leuprorelin Products
All Leuprorellin
The products need to be injected.
substance patent
ss expired before
neli Japanese Patent
Patient Fr
Philippines Patent
Contributions to Health Care Professionals sing Leuprorelin
DPS (dual-chamber prefilled syringe) was applied for workload reduction of health care professionals.
Philippines Patent
DPS with safe device
(needle stick protection )
Simple, convenient,
efficient and safe!!!
Complex procedures
and some potential risk
Overview of Lansoprazole Product Case
Stomach and intestinal ulcers Erosive esophagitis(damage to the esophagus from stomach acid) Other conditions involving excessive stomach acid such as Zollinger-Ellison syndrome
Capsules 15mg and 30 mg 1992
Oral disintegrating (OD) tablets, 2002
Pack products for a helicobacter pylori-eradicating, 2002
Downsizing 30mg capsules, 2004
Intravenous 30mg 2006
Pack products for a helicobacter pylori-eradicating, 2010 Combination tablets 2014
about 890 countries including Asian countries such as Philippines,
Malaysia, Singapore, Taiwan, Thailamd, China, Korea, etc.
Stabilized gastro-resistant capsules can protect against degradation in the
stomach, improve bioavailability and acquire better absorption properties.
OD tablets can improve adherence.
Lansoprazole capsules and granules
15mg 30mg
(#3) (#1)
downsized 30mg
capsules
Easy for patients
to take because
of adherence
30mg 30mg
(#3) (#1)
Japanese Patent Philippines Patent
Lansoprazole oral disintegrating (OD) tablets and microgranules
Enteric-coated microgranules
Lansoprazole layer
Intermediate layer
1st enteric layer
2nd enteric layer
3rd enteric layer
Inactive granules
Over coating layer
Enteric-coated microgranules comprised of seven layers
Comparison of granules of between capsules and OD tablets
Philippines Patent
number of particles
(30mg products)
Easy for patients to
take without water
because of adherence
Collaboration Research Cases
The following technologies produced with the third party are
being used for Leuprorelin Product.
Formulation Technology
Collaboration Research
Biodegradable polymers
Japanese Company A
Dual chamber pre-filled
Japanese Company B
5. New Paradigm in Drug Development ■Unmet Medical Needs
Strive to develop and
provide new therapies
Chlamydial infection
Irregular heartbeat
Bronchial asthma
to Patients
Allergic rhinitis
Herpesvirus infection
Anxiety neurosis
Myocardial infarction
Benign prostatic hypertrophy
Parkinson's disease
Atopic dermatitis
Chronic rheumatoid arthritis
Osteoporosis Nephrosis syndrome
Autonomic disturbance
Cerebral infarction
Hemorrhage (including subarachnoid hemorrhage)
Chronic hepatitis C
Chronic glomerulonephritis
Diabetic(al) neuropathy
Urinary incontinence/pollakiuria
Degenerative arthritis
Chronic hepatitis B
Diabetic(al) retinopathy
Diabetic(al) nephropathy
Alzheimer's Disease
Multiple sclerosis
Chronic renal failure
Senile dementia Lung cancer Liver cancer
Neuromuscular disturbance and myopathy
Satisfaction with treatment
Source: questionnaire survey by mail
Unmet medical needs
Survey period: October 15 to December 22, 1999 Target: medical doctors (128 respondents)
Source : The Japan Health Sciences Foundation: "Report on Key Domestic Technologies 2000 – Outlook of medical needs in 2010 –"
Authentic Drug Discovery Model
Pharmaceutical Innovation 2012年5月 JPMA
Up-to-date model (diversified / Complicated )
Many areas are connected by advanced networks
Pharmaceutical Innovation 2012年5月 JPMA
New drug seeds from academia or bio-venture (1998-2007)
US:60% from Academia or biotechs Open innovation looks the mainstream.
Japan: Less than 20% from academia or biotechs
Large
Pharma
Robert Kneller , Nature Reviews Drug Discovery 9, 867-882, 2010
More drugs from academia / biotech company
Facilitate networking
・Pursue originality by conjugating various and creative research activities ・Needs to create new mechanism where academia and pharma companies get together more easily and spontaneously
More Openness and Accessibility
(data, information, strategy, etc.)
Academia, Biotechs
Shift into More "Open Innovation"
Traditional Model
Diversified, Complicated
・Large molecule, Molecularly
targeted drug, biologics,
・Lifestyle Disease
regenerative medicine
Open Innovation for
Closed - all things
innovative therapy /
done in house –
Patent Strategy to
Open Innovation for
protect in-house R&D
Global Health by
Innovative R&D model
Unmet medical needs in
Neglected tropical diseases and
More exposure to cutting -
communicable diseases
edge technologies.
■Open Innovation in Japan
Academia - Pharma
• Kyoto Univ. - Takeda :T-CIRA
Pharma – Pharma
• Daiichi-Sankyo – Astellas: Co-use of Compound Library
Public offer by Pharma
• Shionogi : FINDS
• Daiichi-Sankyo: TaNeDS • Astellas: a-cube • Takeda: Cockpi-T, RINGO-T
Academia – Academia
• Tokyo Univ.: Drug Discovery Open Innovation Center • Kyoto Univ. Center for Innovation in Immunoregulative Technologies
and Therapeutics
Government Driven
• Drug Discovery Supporting Network
Highlights of the Research Collaboration
1. Center for iPS Cell Research and Application (CiRA) at Kyoto
University and Takeda Pharmaceutical Company Ltd. (Takeda) start a ten-year-long research collaboration (T-CiRA Joint Program).
2. iPS cell and its related technologies to pharmaceutical R&D activities,
such as drug discovery, cell therapy and drug safety.
3. Professor Shinya Yamanaka, the head of CiRA, directs the Program.
4. Takeda will provide:
Total research budget: Approximately 20 billion-yen per over ten years
Research facility: Dedicated space in Shonan Research Center (Fujisawa, Japan) of Takeda
iii. In-kind research support: 50 Takeda researchers, drug discovery
technologies and other R&D know-hows.
Unique T-CiRA academic/industry collaboration
1. Long term (10 years) commitment by both CiRA and Takeda to a
nationally important project.
2. The program is solely directed by Professor Yamanaka, the
discoverer of iPS cell.
3. "Open Innovation": Housed in the center of a drug company's
research headquarter.
4. A large scale collaboration: Over 100 researchers at one site.
5. Public Contribution: After filing patents, CiRA and Takeda encourage
publication of outcomes that will accelerate public research.
6. Internationality: The Program will recruit scientists from all over the
• Invite academia to present new assay
• Takeda will put its compound libraries
into screenings, using the assay system.
• Takeda reports back to the presenter the
findings gained by the screenings.
• The presenter can freely disclose and
utilize the outcome.
• The outcome may lead to breakthrough
to new drug discovery by academia, industries or Takeda.
• Encourage and invite junior researchers at universities, research
institutes and private industries to apply for sponsorship by Takeda.
• Innovative idea on drug target or research technology relating to
specific therapeutic areas and themes designated by Takeda
– Central Nervous System, Gastro Intestinal, Oncology, Immunology,
inflammatory, Microbiome
– 100,000 USD (at max.) funding – Relevant Takeda's technology and assets
• Resulting invention and patents
– Vested in Partner – Not enforced against Takeda's research activities – Takeda has first right to negotiate for exclusive license
■Open Innovation to Improve Global Health
Global Health and Open Innovation
• HIV/ AIDs, Tuberculosis, Malaria, NTDs (Neglected Tropical
Diseases) in developing countries ---> "Unmet Medical Needs"
• Public health care system, health insurance system,
distribution system, pricing system, healthcare professional, public education and new drug development.
• It is essential to realize Flexible Partnerships between public
sector and private sector as well as other forms of cooperation within the various stakeholders. >>> Product Partnership.
• Patent drives technology flow and open innovation, and brings
the life to the Technology
• Cases involving Japan
– GHIT – WIPO Re:Search
GHIT - The Global health Innovation Technology Fund
Why products not being provided?
GHIT Takeda's case: Lead Optimization for Aminopyrazole
• DNDi and Takeda Collaborate
• for the Lead Optimization for Aminopyrazole Series
for Visceral Leishmaniasis drugs
Takeda and DNDi collaboration
for Developing a Drug for Treating
Visceral Leishmaniasis
Efforts Funded by GHIT Fund
GHIT Collaboration by Multiple Pharmas
NTD Drug Discovery Booster Launched May 2015
Participants: DNDi, Eisai, Shionogi, Takeda, AstraZeneca
Target disease: Chagas Disease and Leishmaniasis, 450 million people are at risk of
contracting worldwide
Time frame: From 2015-2017 with the possibility of extension & expansion Speed up the process and cut the cost of finding new treatments for the target diseases DNDi will access millions of unique compounds, generated over many decades of research,
to screen for potential treatments or cures for the target diseases.
Companies will continually examine their libraries for better matches as the search is
The GHIT Fund supports EUR 640,000 (79.5 million JPY), and the involvement of the three
Japanese companies.
WIPO Re:Search - background
• Founded in 2011 by the World Intellectual Property Organization
(WIPO), BIO Ventures for Global Health (BVGH), and several leading pharmaceutical companies
• Global initiative established to encourage and facilitate the sharing of
intellectual property assets to advance drug, vaccine, and diagnostic
development for malaria, tuberculosis, and neglected tropical
diseases (NTDs)
• Consortium membership includes academic and non-profit research
institutions, governmental and non-governmental organizations, and biopharmaceutical companies
• Any resulting product would be made available royalty free in the least
developed countries and at a reasonable cost in other developing countries.
⇒ Takeda joined WIPO Re:Search in Sept. 2015
WIPO Re:Search - Structure
WIPO Re:Search – Advancing NTD Product R&D
• Dr. Jim McKerrow (University of California, San
Diego [UCSD], USA) & Eisai Co., Ltd. (Japan)
• Jim is assessing inhibitors of ergosterol synthesis as
potential leishmaniasis and Chagas disease drugs
• Eisai shared a novel squalene synthase inhibitor with
• Dr. Alister Craig (Liverpool School of Tropical Medicine) &
Eisai Co., Ltd. (Japan)
• Alister is searching for adjunct treatments for cerebral
malaria by targeting PAR1
• Eisai shared its PAR1 inhibitors with Alister to assess in his
in vitro cerebral malaria model
Marketed Drugs in Japan
( ):still under development or examination
In 2003 - Marketed drug among 99 (out of TOP103)
98 97(2) 97(2) 97(2) 94 70 63(23)
Improved Data Protection period in Japan 8 year period instead of 6 year one
In 2013 - Marketed drug among Top100
100 100 99(1) 100 95(2) 73(12) 93(3)
Origin:Ⓒ2015 IMS Health. World Review, LifeCycle、Pharmaprojects、Evaluatepharma
Existing Data
Any and all existing data and findings owned by a product development partner at the initiation of a project,
including but not limited to information, know-how or intellectual property, will remain that of the original holder.
The original holder may share, assign, or license their rights to a third party.
New Data
Ownership of any and all data and findings that is obtained or created through activities invested by the GHIT
will be discussed and negotiated between participants and/or product development.
Patent Applications
Any existing data owned by a product development partner and/or any new data obtained through activities
invested by the GHIT Fund may be disclosed by the GHIT Fund to a third party if such data is used in a patent
application for a product which was derived from the activities invested by the GHIT Fund; provided, however:
(1) that the disclosure of such data shall be limited to the proposed title of the invention, a draft of the abstract,
the international non-proprietary name (INN) where applicable, and an outline of the specifications of such
patent application; and (2) such third party shall take reasonable measures to keep confidential any such data
received from the GHIT Fund.
Principle of Product Access Policy
Licenses
When product development partners and/or participants are successfully granted a patent deriving from projects
invested by the GHIT Fund, product development partners and/or participants will grant royalty-free licenses to
users operating in Least Developed Countries (LDCs) as categorized by the United Nations classification and
Low-Income Countries (LICs) categorized by the World Bank classification. License-related matters concerning
middle income countries will be reviewed on an individual basis with the goal of ensuring reasonable royalty
licenses.
Pricing
In LDCs, LICs and middle income countries, product development partners and/or participants will set prices for
products on the basis of a no gains/no loss policy that can improve access to the product for patients and
citizens of LDCs, LICs and middle income countries
WIPO Re:Search – Sharing IP Assets
• Compounds & compound libraries
• HMG-CoA reductase inhibitors
• Technologies
• High-throughput screening platform
• Anti-dengue virus antibody
• Know-how and therapeutic area expertise
• Drug structure-activity relationship (SAR) advice
• Clinical/field samples
• Severe and asymptomatic malaria patient samples
• Investigator's brochure, clinical trials data summary
• Other support
• Vaccine thermostability formulation
Source: http://accessiblebookalliance.org/edocs/mdocs/aspac/en/wipo_ip_mnl_16/wipo_ip_mnl_16_t_1.pdf
DOSE-RESPONSE OF PORCINE OVARIAN GRANULOSA CELLS TO AMYGDALIN TREATMENT COMBINED WITH DEOXYNIVALENOL Marek Halenár*, Marína Medveďová, Nora Maruniaková, Dagmara Packová, Adriana Kolesárová Address(es): Ing. Marek Haklenár, Department of Animal Physiology, Faculty of Biotechnology and Food Sciences, Slovak University of Agriculture in Nitra, Tr. A. Hlinku 2, 949 76 Nitra, Slovak Republic. *Corresponding author: [email protected]
Facts and Myths about Valley Independent Pharmacies Today, 7 in 10 prescriptions filled in the United States are for generic drugs. This fact sheet explains how generic drugs are made and approved and debunks some common myths about these products. FACT: FDA requires generic drugs to have the same quality and performance as the brand name drugs.









