Ajmsc.info
African Journal of Medical Sciencesfo
Citation: Balsam A. Gagran and Nossiba O. Ali. Prevalence of Extended Spectrum Beta-
Lactamase Producing
Escherichia coli in Hospital Acquired Urinary Tract Patients.
African
Journal of Medical Sciences, 2016, 1(3) ajmsc.info
Prevalence of Extended Spectrum Beta-Lactamase Producing
Escherichia coli in Hospital Acquired Urinary Tract Patients
Balsam A. Gagran and Nossiba O. Ali
Department of Medical Microbiology, Faculty of Medical Laboratory Sciences,
Al Neelain University, Khartoum, Sudan
Abstract
Background: Urinary tract infection is one of the most prevalent infections worldwide.
Extended spectrum beta lactamase (ESBL) is a beta lactamase enzyme capable of hydrolyzing
third generation cephalosporins, and is inhibited by beta lactamase inhibitor.
Objective: To estimate the prevalence of extended spectrum beta-lactamase producing
Escherichia coli (
E. coli) in hospital acquired urinary tract infections.
Materials and methods: A total of 50
Escherichia coli isolates were collected from the
microbiology laboratories of Khartoum and Omdurman Teaching Hospitals (Sudan). The isolates
were obtained originally from urine cultures of patients with urinary tract infections. To obtain
pure culture, isolates were sub-cultured on nutrient agar. Identification of the isolates was
confirmed by conventional microbiological techniques.
E. coli isolates were tested for their
in
vitro multidrug antimicrobial resistance against the third generation cephalosporins using the
Kirby-Bauer disk diffusion method. The antibiotics used were amoxyclav, cefepime,
ceftazidime, cefuroxime, and cephalothin. Double disk synergy test was performed to detect the
extended spectrum beta-lactamase producing
Escherichia coli. Results: The antibiogram of ESBL producing
E. coli strains showed resistance to cefepime
(42/84%), amoxyclav (35/70%), cefuroxime (35/70%), cephalothin (44/88%), and ceftazidime
(20/40%). From the 50
E. coli strains investigated, six strains (12%) were found producing
ESBL with the synergy double disc diffusion test. The non-ESBL producers were 44 (88%)
strains. The ESBL's producer strains were highly resistant (88%) to cephalothin and least
resistant (40%) to ceftazidime.
Conclusion: E .coli strains are the commonest aetiological agents of hospital acquired urinary
tract infection. Ceftazidime, amoxyclav, cefuroxime, and cephalothin have a significant
resistance against
E. coli ESBL producing strains.
Key words: Extended spectrum beta-lactamase,
Escherichia coli, Urinary tract infections.
Gagran, et al, 2016: Vol 1(3)
African Journal of Medical Sciencesfo
ESBL-producing strains of
E. coli were first noted in 2003 when South East and West Midlands regions of England reported to the Health Protection Agency about the appearance of infections with highly cephalosporin-resistant strains of
E. coli, some of w
hich were thought to have arisen in the community. The resistance of these bacteria is due to the fact that they have acquired genes that enable them to produce a particular class of extended-spectrum β-lactamase enzymes (ESBLs) called CTX-M that attack and destroy the β-lactam antibiotics (penicillins and cephalosporins), thereby making themselves resistant to their action. Most CTX-M-producing
E. coli are exceptionally resistant to multiple antibiotics including ampicillin and the cephalosporins1.
They are often resistant to other antibiotics such as quinolones and trimethoprim, which are some of the most important and widely used classes of antibiotics. As a result, there are limited options for oral treatment of these infections. Urinary tract infections (UTIs) and bacteraemia caused by
E. coli can be life-threatening, that is why the emergence of the ESBL-producing strains is a serious concern
. Urinary tract infections are among the most prevalent infectious diseases, with a substantial financial burden on society.
Microorganisms can reach the urinary tract by haematogenous or lymphatic spread, but there is abundant clinical and experimental evidence to show that the ascent of microorganisms from the urethra is the most common pathway that leads to a UTI, especially organisms of enteric origin (e.g.
E. coli and other Enterobacteriaceae). This provides a logical explanation for the greater frequency of UTIs in women than in men, and for the increased risk of infection following bladder catheterization or instrumentation. A single insertion of a catheter into the urinary bladder in ambulatory patients results in urinary infection in 1-2% of cases. Indwelling catheters with open-drainage systems result in bacteriuria in almost 100 within 3-4 days2.
Urinary tract infections typically occur when bacteria enter the urinary tract through the urethra. The bacteria then multiply in the bladder and can result in an infection. The bacteria that usually cause an infection come from the intestinal tract and live on the skin near the rectum or in the vagina in women. The most common organism is
E. coli but other bacteria can also cause infections. Hospital units surfaces and environment may become contaminated by bacterial pathogens especially ESBL. Microorganisms are considered major health proplem in hospital worldwide. The use of disinfectants is essential in infection control in hospital and health care centers cephalosporin can be used for of treatment of ESBL bacteria unfortunately some strain can mutate and resist cephalosporin therefore they become difficult to diagnose and treat. The threat of ESBL has been the topic of intensive research and discussion. Although ESBL remains extremely rare there is widespread concern that ESBL poses by far the greatest risk to patients, given the virulence of the organism3.
Gagran, et al, 2016: Vol 1(3)
African Journal of Medical Sciencesfo
Materials and methods
This is a descriptive, cross sectional, analytical study conducted at Khartoum Teaching and Omdurman Teaching Hospitals (Sudan). Inclusion criteria were
E. coli isolates of urine samples of patients with urinary tract infection; and exclusion criteria were
E. coli isolates of urine samples of patients without urinary tract infection. Sampling was a non-probability sampling type, and the sample size was 50
E. coli isolates of urine samples. The study was conducted during the period from March to May 2014. Approval of the study was issued from Al Neelain University (Khartoum) and administration of the microbiology laboratories of Khartoum Teaching and Omdurman Teaching Hospitals (Sudan). Verbal consent was obtained from all patients enrolled in the study.
Escherichia coli isolates were collected from the microbiology laboratories of Khartoum and Omdurman Teaching Hospitals. The isolates were obtained originally from urine cultures of patients with urinary tract infections. The isolates were inoculated on CLED agar and incubated overnight aerobically at 37º C. To obtain pure culture, isolates were sub-cultured on nutrient agar. Full identification of the organism was determined by standard conventional bacteriological techniques. All
E. coli isolates were tested for their
in vitro multidrug antimicrobial resistance against the third generation cephalosporins using the Kirby-Bauer disk diffusion method. Preparation of Mac Farland Standard was performed to give a turbidity suspension containing approximately 1.5 ×108 bacterial cells per ml. The antibiotics used were amoxyclav (30 μg),
cefepime (30 μg), ceftazidime (30 μg), cefuroxime (30 μg), and cephalothin (30 μg). The choice of these antibiotics and the susceptibility results interpretation were made according to the guidance of the National Committee for Clinical Laboratory Standards Institute (2001). Resistant
E. coli isolates to at least one of the third generation cephalosporins were checked for ESBL production. Double disk synergy test was performed by inoculating
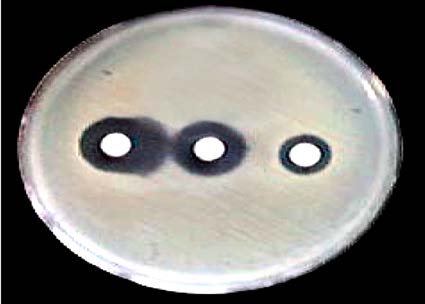
E. coli isolates on Muller-Hinton agar plates. Three discs were put on this medium: one disc containing ceftazidime (30 μg), the second disc containing cefotaxime (30 μg), and the third disc containing (20 µg amoxicillin+10 µg clavulanic acid). Each disc was placed 20 mm (center to center) away from the disc containing the cephalosporin antibiotic. Inoculated plates were incubated aerobically at 37°C for 18 - 24 h. Under sufficient illumination, the inhibition of growth around the discs was recorded. ESBL production was considered positive when the clavulanate mediated enhancement of the activity of an indicator drug produced a keyhole effect and regarded as a phenotypic confirmation of the presence of ESBL (Fig. 1).
50
E. coli strains
were isolated from urine samples of patients diagnosed clinically as cases of
UTIs. As illustrated in Fig. (2), the antibiogram of
E. coli strains showed resistance to cefepime
(42/84%), amoxyclav (35/70%), cefuroxime (35/70%), cephalothin (44/88%), and ceftazidime
(20/40%).
Gagran, et al, 2016: Vol 1(3)
African Journal of Medical Sciencesfo
Fig. (1): ESBL production by double disk diffusion method.
Ceftazidime (left) showing a keyhole effect with clavulanic acid (middle)
and cefotaxime (right) showing a negative result
Fig. (2): Resistance of E. coli strains
As shown in Table (I), from the 50 E. coli strains investigated, six strains (12%) were found producing ESBL with the synergy double disc diffusion test. The E. coli strains were highly resistant (88%) to cephalothin and least resistant (40%) to ceftazidime. The resistance ratio (84%) of cefepime against E. coli strains was not significant (p-value ˃ 0.05). However, the resistance ratios of the remaining antibiotics were significant (p-value ˂ 0.05).
Gagran, et al, 2016: Vol 1(3)
African Journal of Medical Sciencesfo
Table (I): Third generation antibiotics sensitivity
pattern against E. coli strains
Cefuroxime 35 / 70%
Cephalothin 44 / 88%
Discussion
Antimicrobial resistance is now recognized as an increasingly global problem, especially among
Gram-negative bacteria. Increasing resistance to broad spectrum cephalosporins amongst E. coli
predominantly due to the production of ESBLs were reported from different countries. Many
other reports from different countries and regions showed different prevalence rates of ESBLs
producing Entrobacteriaceae causing urinary tract infections. E. coli is the most common ESBL
positive organism, but all Enterobacteriaceae can harbor plasmid-mediated ESBL genes
(Bouchillon et al4). In this study the frequency rate of E. coli ESBLs strains were 6/12%.
Another study showed that ESBL production was detected in 42% of cases5. Other workers6
detected ESBL production in 48.3% of the strains studied by the double disc synergy test.
Furthermore, the frequency rate of ESBL production in some studies7 was 46%; however this
frequency rate was higher than that reported elsewhere 8 (9%).
In India Dharmishtha and his colleagues9 studied the antibiotic related resistance in UTI patients
and they found that the overall E.coli strains isolated were (70.96%) among the all Gram
negative bacilli of UTI patients. ESBL prevalence was 16.67% and 30% among community
acquired and hospital acquired E.coli respectively. Cephalosporin group of sensitivity was
widely varied among the patients studied and more resistance was found
in hospital acquired strains. Jalalpour10 studied in 2012 the antibiogram pattern in extended
spectrum beta lactamase enzyme producing gram negative bacilli in Iranian urinary tract
infection. She reported that the frequency of ESBLs in E. coli strains was 35.06%. According to
antibiogram result 59.2, 54.9, 30.3, 27.8, 19.5 and 16.7% of E. coli strains were resistant to co-
trimoxazole, nalidixic acid, ciprofloxacin, gentamicin, ceftazidime and nitrofurantoin
respectively. From this study, we recommend that screening for ESBL producing organisms
should be performed to assist in the judicious use of antibiotics in treatment of UTIs. Further
research work is also recommended to look for ESBL producing organisms other than E .coli
strains that may threaten the clinical setting.
Gagran, et al, 2016: Vol 1(3)
African Journal of Medical Sciencesfo
Conclusion: ESBL producing pathogens are an emerging threat of hospital settings; and E .coli strains are the commonest aetiological agents of hospital acquired urinary tract infection. Ceftazidime, amoxyclav, cefuroxime, and cephalothin have a significant resistance against E. coli ESBL producing strains.
References
1. Drieux L, Brossier, Sougakoff FW, Jarlier V. Phenotypic detection of extended spectrum β-
lactamase production in Enterobacteriaceae review and bench guide. Clinical Microbiology and
Infection. 2008; 14(1):90–103.
2. Doi Y, Adams J, O Keefe A, Quereshi Z, Ewan L, Paterson DL. Community-acquired extended-
spectrum b-lactamase producers, United States [letter] Emerg Infect Dis. htm;2007; 13/7/1121.
3. Mohanty S, Gaind R, Ranjan R, Deb M. Use of the cefepime-clavulanate ESBL test for
detection of extended-spectrum beta-lactamases in AmpC co-producing bacteria. J. Infect Dev.
2010; 4(1):24–29.
4-Bouchillon SK, Johnson BM, Hoban DJ, Johnson JL, Dowzicky MJ, Wu, DH. (2002).
Interscience Conference Antimicrob. Agents Chemother , 2002; 42: 27-30.
5. Naas T,Poirel L and Nordmann P. Minor extended-spectrum β-lactamases. Clin Microbiol
Infect 2008; 14 (Suppl. 1):42–52.
6. Pfaller MA, Segreti J. Overview of the epidemiological profile and laboratory detection of
extended spectrum β-lactamases. Clin Infect Dis. 2006; 42: S153–S163.
7. Karen B. and George A Jacoby. An updated functional classification of β-lactamases.
Antimicrob Agents Chemother. 2009; 10.1128/AAC.01009-09.
8. Thomas G Slama. Gram-negative antibiotic resistance: there is a price to pay. Critical Care,
2008; 12 (Suppl 4):S4.
9. Dharmishtha G, Gandhi Paragi J, Patel Kiran N. Study on antibiotic related resistance in UTI
patients: a comparison between community acquired and hospital acquired E. coli. National
Journal of Community Medicine. 2012; V. 3 (2).
10. Jalalpour, S. Antibiogram pattern in extended spectrum beta lactamase nano enzyme
producing gram negative bacilli in Iranian urinary tract infection. African Journal of Pharmacy
and Pharmacology, 2012; V. 6 (12), 899-903.
Gagran, et al, 2016: Vol 1(3)
Source: http://www.ajmsc.info/images/Vol1.3/Balsam%20Article.pdf
EI-01693; No of Pages 21 Available online at www.sciencedirect.com Environment International xx (2007) xxx – xxx Chemical contaminants in feedlot wastes: Concentrations, effects and attenuation S.J. Khan a,⁎, D.J. Roser a, C.M. Davies a, G.M. Peters a, R.M. Stuetz a, R. Tucker b, N.J. Ashbolt a a Centre for Water and Waste Technology, School of Civil and Environmental Engineering, University of New South Wales, NSW 2054, Australia
Making Education Easy Issue 97 – 2015 Welcome to issue 97 of GP Research Review. Italian research suggests that strict control of systolic blood pressure (SBP) may hasten cognitive decline among older adults with pre-existing dementia or mild cognitive impairment. Patients whose daytime SBP was lowered to ≤128 mmHg had greater


