Covoiturage.univ-mrs.fr
8722 • The Journal of Neuroscience, September 24, 2003 • 23(25):8722– 8732
Brain-Derived Neurotrophic Factor Modulation of
GABAergic Synapses by Postsynaptic Regulation of
Rinda A. Wardle1,2
and Mu-ming Poo1
1Division of Neurobiology, Department of Molecular and Cell Biology, University of California, Berkeley, California 94720-3200, and 2Division of Biology,
University of California at San Diego, La Jolla, California 92093-0357
Brain-derived neurotrophic factor (BDNF) potentiates excitatory synapses in a variety of systems by promoting presynaptic transmitter
release. The existing evidence indicates that BDNF attenuates inhibitory transmission, but reports differ considerably in their character-
ization of the effect and proposed mechanisms. We examined the effects of exogenously applied BDNF on EPSCs and IPSCs recorded from
functionally identified neurons in dissociated rat hippocampal cultures. When recording from glutamatergic neurons, we found that
BDNF exerted differential effects at excitatory versus inhibitory synapses: increasing amplitude of EPSCs but slightly decreasing that of
IPSCs. Furthermore, when recording from GABAergic neurons, we found that BDNF increased the IPSC amplitude. That these differential
BDNF effects reflect distinct presynaptic and postsynaptic mechanisms was suggested by the BDNF-induced changes in miniature EPSCs
and IPSCs. An increased mini-frequency was found at all synapses, indicating elevated presynaptic transmitter secretion; a change in the
amplitude of mini-IPSCs was found at GABAergic cells, suggesting postsynaptic modulation of GABA responses. Selective postsynaptic
mechanisms were further examined by comparing the effect of BDNF on GABA-induced currents recorded from glutamatergic versus
GABAergic cells. For GABAergic but not glutamatergic postsynaptic cells, BDNF induced a shift in the reversal potential (EIPSC) toward
more positive levels, hence reducing the inhibitory action of IPSCs. This BDNF-induced effect correlates with the existing level of
furosemide-sensitive K⫹
–Cl⫺
transport activity in the postsynaptic cell. Thus, BDNF may decrease the efficacy of inhibitory transmis-
sion by acute postsynaptic downregulation of Cl⫺
transport, in addition to its well known presynaptic effect.
Key words: BDNF; inhibitory synapses; GABAergic transmission; chloride transporter; synaptic plasticity; hippocampal cultures
lation have been most extensively studied at excitatory synapses.
Neurotrophins are crucial for the survival and differentiation of
With a few exceptions (Levine et al., 1995; Kovalchuk et al.,
neurons (Levi-Montalcini, 1987; Lewin and Barde, 1996) and are
2002), neurotrophin effects have been attributed to increased
known to modulate a variety of synapses (McAllister et al., 1999;
presynaptic transmitter release. They have also been associated
Poo, 2001). Neurotrophins serve long-term trophic functions by
with activation of Trk receptor tyrosine kinase (Lohof et al.,
regulating expression of synaptic proteins (Wang et al., 1995;
1993), PI-3 (phosphoinositide-3) kinase (Yang et al., 2001) and
Narisawa-Saito et al., 1999; Loeb et al., 2002) and maturation of
MAP (mitogen-activated protein) kinase (Jovanovic et al., 1996),
synaptic properties (Wang et al., 1995; Liou et al., 1997). They
and elevation of cytoplasmic Ca 2⫹ (Zhang and Poo, 2002). The
also acutely modulate the efficacy of basal synaptic transmission
downstream actions of these cytoplasmic signals may increase
at developing neuromuscular junctions in cell cultures (Lohof et
phosphorylation of synaptic vesicle-associated proteins (Jo-
al., 1993) and at central excitatory synapses in hippocampal and
vanovic et al., 2000), causing enhanced transmitter loading, ves-
cortical cultures (Lessmann et al., 1994; Levine et al., 1995; Li et
icle mobilization, or secretion at excitatory synapses. Interest-
al., 1998), acute slices (Kang and Schuman, 1995; Akaneya et al.,
ingly, the existing evidence indicates that neurotrophins reduce
1997), and
in vivo (Messaoudi et al., 1998). In addition, brain-
the efficacy of inhibitory transmission (Kim et al., 1994; Tanaka
derived neurotrophic factor (BDNF), a member of the neurotro-
et al., 1997; Frerking et al., 1998; Brunig et al., 2001, Rivera et al.,
phin family, plays an important role in activity-induced long-
2002). Together, these reports suggest that BDNF-induced acute
term potentiation (LTP) in the hippocampus (Korte et al., 1995;
synaptic modulation is specific to presynaptic neuron type. In
Figurov et al., 1996; Patterson et al., 1996).
addition, the effect of BDNF at excitatory synapses is specific to
The mechanisms for neurotrophin-induced synaptic modu-
postsynaptic cell type. In hippocampal cultures, BDNF induceselevated glutamate release at synapses with glutamatergic (E) butnot GABAergic (I) postsynaptic cells (Schinder et al., 2000).
Received Jan. 13, 2003; revised July 31, 2003; accepted Aug. 12, 2003.
Analogously, LTP can be induced in hippocampal cultures
This work was supported by National Institutes of Health Grant NS37831.
(Bi and Poo, 1998) and slices (McMahon and Kauer, 1997;
Correspondence should be addressed to M-m. Poo at the above address. E-mail:
[email protected].
Copyright 2003 Society for Neuroscience 0270-6474/03/238722-11$15.00/0
Maccaferri et al., 1998) with glutamatergic but not GABAergic
Wardle and Poo • Postsynaptic BDNF Modulation of GABAergic Synapses
J. Neurosci., September 24, 2003 • 23(25):8722– 8732
• 8723
postsynaptic cells. It is unknown whether the modulatory
aptic cell type could be identified. For most recordings, neurons were
effect of BDNF on GABAergic synapses is also specific to the
voltage clamped (
V ) at ⫺70 mV, resulting in inward currents for both
postsynaptic cell type.
EPSCs and IPSCs. For perforated-patch recordings of EPSCs and IPSCs,
In the present study, we demonstrated that BDNF exerts op-
pipettes were tip filled with internal solution and then backfilled with
posite modulatory effects on glutamatergic and GABAergic syn-
internal solution containing gramicidin D (25 g/ml; Sigma) or ampho-
apses in hippocampal cultures. In addition, we found that the
tericin B (200 g/ml; Calbiochem, San Diego, CA). The internal solution
effect of BDNF on GABAergic synapses depends on the postsyn-
for gramicidin D perforated-patch recordings contained the following
aptic cell type. Although BDNF modulates presynaptic transmit-
(in mM): 150 KCl and 10 HEPES. The internal solution for amphotericin
ter release at GABAergic synapses on both glutamatergic and
B perforated-patch recordings contained the following (in mM): 154K-gluconate, 9 NaCl, 1 MgCl , 10 HEPES, and 0.2 EGTA. All perforated-
GABAergic postsynaptic cells, BDNF acts postsynaptically to re-
patch recordings of EPSCs were made with amphotericin B. Perforated-
duce inhibition only at GABAergic synapses on GABAergic
patch recordings of IPSCs were made with either gramicidin D or am-
postsynaptic cells. This action is mediated by a BDNF-induced
photericin B. Gramicidin D forms pores permeable to monovalent
shift in the reversal potential (
EIPSC) for Cl⫺ currents toward
cations and small uncharged molecules but not to Cl ⫺, permitting reli-
more positive levels, an effect that correlates with a rapid down-
able recordings of GABAergic currents (Kyrozis and Reichling, 1995;
regulation of K ⫹–Cl ⫺ cotransporter activity. These findings
Owens et al., 1996). However, because of the difficulty in maintaining
demonstrate a novel mechanism for BDNF modulation of
stable access resistance during long-term recordings with gramicidin D,
GABAergic synapses.
we performed additional experiments using amphotericin B as the per-forating agent. Amphotericin B forms pores that are partially permeable
Materials and Methods
to Cl ⫺, which can cause an initial perturbation of [Cl ⫺] . However, we
Culture preparation. Low-density cultures of dissociated hippocampal
found that a new steady-state [Cl ⫺] was rapidly established after perfo-
neurons were prepared from embryonic day 18 (E18) to E20 rat embryos
ration. Therefore, we started experiments only after recording 10 –20
as described previously (Bi and Poo, 1998). Hippocampi were
min of control period, during which the IPSC amplitude and
E
trypsinized for 20 min at 37°C and then gently triturated. Dissociated
mained constant, indicating that a new steady-state [Cl ⫺] had been
neurons were plated at 25,000 –100,000 cells/ml on poly-L-lysine-coated
established. For breakthrough whole-cell recordings of EPSCs and IP-
glass coverslips in 35 mm Petri dishes. The plating medium used was
SCs, pipettes were backfilled with internal solution containing the fol-
DMEM (BioWhittaker, Walkersville, MD) supplemented with 10% fetal
lowing (in mM): 135 K-gluconate, 15 KCl, 5 NaCl, 0.5 EGTA, 10 HEPES,
calf serum (Hyclone, Logan, UT), 10% Ham's F-12 with glutamine (Bio-
and 2 Mg-ATP. No difference was observed between experiments per-
Whittaker) and 50 U/ml penicillin–streptomycin (Sigma, St. Louis,
formed with amphotericin B or gramicidin D perforated-patch or break-
MO). The culture medium was supplemented with 20 mM KCl 24 hr after
through whole-cell recording. The synaptic reversal potential of IPSCs
plating. Both glia and neurons are present under these conditions. Neu-
was determined by varying the
V of the postsynaptic cell in 5–10 mV
rons were recorded after 10 –14 d in culture.
increments from ⫺80 to ⫺50 mV and measuring the resulting IPSC
Electrophysiology. Double whole-cell perforated-patch or break-
amplitude. A best-fit line for the current–voltage (
I–V) relationship was
through recordings were simultaneously made from pairs of reciprocally
calculated using a linear regression, and the interpolated intercept of this
connected neurons in culture as described previously (Bi and Poo, 1998).
line with the abscissa was taken as the reversal potential. The slope of the
Recording pipettes were prepared from glass microcapillaries (VWR Sci-
same line was taken as the respective slope conductance.
entific, Brisbane, CA) with a resistance of 2– 4 M⍀. Internal solutions
Recordings of transmitter-induced currents. After the cell type was de-
differed in various experiments and are described in detail below. Allexperiments were performed at room temperature in an external bath
termined using synaptically evoked responses, glutamate or GABA was
solution containing the following (in m
focally applied at the soma of the identified cell, and transmitter-induced
M): 150 NaCl, 3 KCl, 3 CaCl , 2
MgCl , 10 HEPES, and 5 glucose, pH 7.4 (310 mOsm). Neurons were
currents were recorded by puffing transmitter pulses (100 mM, 1 msec,
visualized by phase-contrast optics (Nikon Diaphot; Nikon, Tokyo, Ja-
10 –20 psi) through a micropipette (2 M⍀; VWR Scientific) at a 30 sec
pan). Recordings were performed with two patch-clamp amplifiers
interval, using an electrically gated Picospritzer (General Valve, Fairfield,
(Axopatch 200B; Axon Insturments, Foster City, CA). Signals were fil-
NJ). Glutamate (100 mM) was puffed at a 90 sec interval to reduce poten-
tered at 5 Hz using amplifier circuitry. Data was acquired and analyzed
tial toxic effects on the cells. All transmitter-induced currents were re-
using Axoscope 8.0 (Axon Instruments). Series resistance was assessed at
corded using the perforated-patch method. Either gramicidin D or am-
5 min intervals and compensated at 75% throughout the experiment.
photericin B was used as the perforating agent for GABA-induced
Experiments were rejected if changes in series resistance exceeded 10%.
current recordings, with similar results. All glutamate-induced current
Unless otherwise noted, average values are expressed as mean ⫾ SEM,
recordings were performed with amphotericin B perforation.
and statistical analyses were performed using one-tailed Student's
t tests,
Recordings of miniature EPSCs and IPSCs. After the cell type had been
either paired or unpaired as noted. BDNF (PeproTech, Rocky Hill; NJ)
identified using the criteria described above, the bath solution was re-
was added directly to the recording bath for a final concentration of 100
placed with one that contained tetrodotoxin (TTX) (1 M), 0.05% BSA,
ng/ml in experiments that did not require perfusion of the culture. When
and either bicuculline (15 M) or CNQX (10 M) to record miniature
cells were being continuously perfused, the bath solution was recycled
EPSCs (mEPSCs) or mIPSCs, respectively. Recordings of mEPSCs were
once BDNF had been added for a final concentration of 100 ng/ml. We
made using the amphotericin B perforated-patch method described
found no difference in results for these two procedures of BDNF appli-
above. Because the amplitudes of mIPSCs with
V of ⫺ 70 mV were too
cation. All recordings of currents induced by focally applied transmitter
small to be consistently detected, mIPSCs were recorded as outward
were made with constant perfusion to avoid desensitization of transmit-
currents at
V of 0 mV. To stabilize the cells while voltage clamped at 0
ter receptors and toxicity to the cell.
Recordings of EPSCs and IPSCs. To assess synaptic connectivity, the
mV, mIPSCs were recorded in breakthrough mode using whole-cell in-
presynaptic neuron was stimulated at a low frequency (0.03– 0.05 Hz)
ternal solution (described above) that replaced K ⫹-gluconate with Cs ⫹-
with a 1 msec step depolarization from ⫺70 to ⫹30 mV in voltage-clamp
gluconate to block K ⫹ channels. A few whole-cell recordings of mIPSCs
mode. We identified EPSCs and IPSCs by their characteristic decay times
were made with
V of ⫺70 mV by increasing the [Cl ⫺] (155 m
(⬃10 and 40 msec, respectively), reversal potentials (⫺5 to 5 mV and
to a level approaching 0 mV, thus allowing us to record
⫺70 to ⫺40 mV, respectively), and, in some cases, their sensitivity to
amplified inward mIPSCs at
V of ⫺70 mV. This recording technique
6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10 M) or bicuculline
allowed us to investigate potential presynaptic BDNF effects on mIPSC
methiodide (15 M; Research Biochemicals, Natick, MA), respectively.
frequency recorded from I3 I synapses, which were masked when re-
Experiments were performed only if both the presynaptic and postsyn-
cording at 0 mV because of the postsynaptic BDNF effects on mIPSC
8724 • J. Neurosci., September 24, 2003 • 23(25):8722– 8732
Wardle and Poo • Postsynaptic BDNF Modulation of GABAergic Synapses
amplitude. However, this recording techniquedid not allow us to observe postsynapticchanges because the elevated [Cl ⫺] (155 m
in the whole-cell pipette solution rendered anyBDNF-induced postsynaptic changes in [Cl ⫺]itoo small in percentage to cause a noticeablechange in E
(average BDNF-induced
changes in [Cl ⫺] were found to be 4 –5 m
experiments using either gramicidin or ampho-tericin perforated-patch recording, as esti-mated by Nernst's equation and known valuesfor [Cl ⫺]
and BDNF-induced changes in
Immunocytochemistry. Hippocampal cul-
tures prepared as described above were washedwith PBS two to three times before and aftereach of the following steps. Cultures were fixedwith 4% paraformaldehyde for 20 min, perme-abilized with 0.2% Triton X-100 for 20 min,incubated for 90 min with rabbit anti-KCC2antibody (Upstate Biotechnology, Lake Placid,NY) diluted (1:200) in 2% PBS–BSA, incubatedfor 60 min with Alexa Fluor 568 goat anti-rabbitsecondary antibody (Molecular Probes, Eu-gene, OR) diluted (1:1000) in 2% PBS–BSA,and mounted with Prolong Antifade (Molecu-lar Probes). Images of fluorescent neurons wereacquired using a Leica (Nussloch, Germany)confocal imaging system (TCS SP) equippedwith a krypton gas ion laser and a Leica invertedmicroscope (DM IRBE) fitted with a Leica 40⫻objective (PL Apo; 1.25– 0.75 oil immersion).
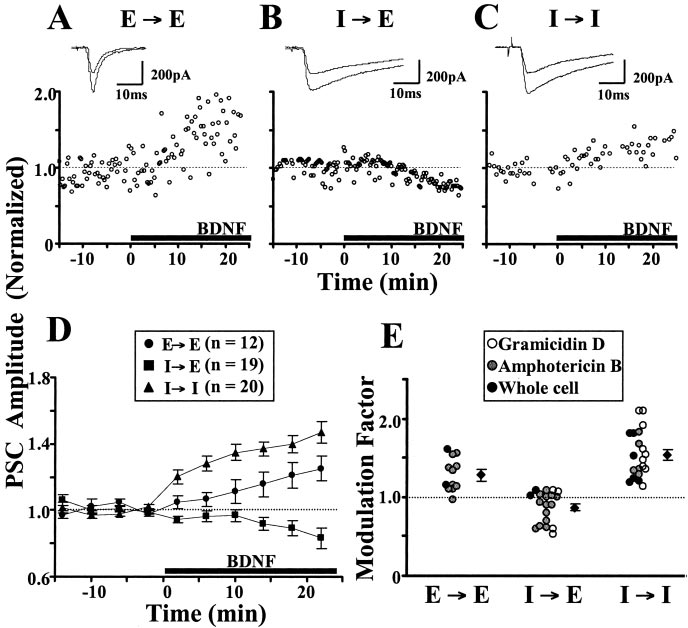
Figure 1. BDNF effect on evoked PSCs. A–C, Example recordings of PSCs. The presynaptic cells were stimulated at 0.05 Hz, and
each data point shows the amplitude of a PSC normalized against the average amplitude (dotted line) during the last 10 min ofcontrol period (t ⫽ ⫺10 to 0 min). At t ⫽ 0, BDNF (100 ng/ml) was added (black line). V
The effect of BDNF on GABAergic
h of ⫺70 mV. Insets, Sample traces of
synapses depends on the postsynaptic
using amphotericin B perforated whole-cell recording. B, Example of IPSCs in a glutamatergic neuron (I3E), using amphotericin
cell type
B perforated recording. C, Example of IPSCs in a GABAergic neuron (I3I), using gramicidin D perforated recording. D, Summary
Synaptic modulation by BDNF was exam-
of all experiments on BDNF effects at E3E, I3E, and I3I. Events averaged over 4 min bins and normalized to the last 10 min
ined at both glutamatergic and GABAergic
of the control period. Data points represent mean ⫾ SEM. E, Scatter plot showing the extent of BDNF-induced modulation of
synapses in cultures of dissociated rat
individual synapses. Modulation factor is defined as the ratio between the mean PSC amplitude observed 15–30 min after the
hippocampal neurons. Simultaneous re-
onset of BDNF application and the mean PSC amplitude during the control period. The average factor was 1.29 ⫾ 0.07 at E3E
cordings of two reciprocally connected
(n ⫽ 12; p ⬍ 0.0001; paired t test), 0.87 ⫾ 0.05 at I3E (n ⫽ 19; p ⬍ 0.0001), and 1.54 ⫾ 0.07 at I3I (n ⫽ 20; p ⬍ 0.0001).
neurons were made using whole-cell re-
For inhibitory synapses, there was no significant difference between recordings made with gramicidin D (white circles) or am-photericin B (gray circles) ( p ⫽ 0.08 and p ⫽ 0.22, I3I and I3E, respectively; unpaired t test).
cording methods. Because the BDNF ef-fect on glutamatergic synapses has been
observed in glutamatergic versus GABAergic neurons (I3 E vs
shown to be target-cell specific (Schinder et al., 2000), we re-corded from neuron pairs only when both presynaptic and
I3 I). Neurons were voltage clamped at ⫺70 mV, at which most
postsynaptic cells could be unequivocally identified as either glu-
IPSCs were inward currents with distinctively longer decay times
tamatergic (E) or GABAergic (I). The cell type of the neuron was
than EPSCs. After a 10 –20 min control period of stable EPSC or
determined by sequentially recording synaptic currents evoked
IPSC recording, BDNF (100 ng/ml) was applied to the culture by
by stimulation of each neuron in the pair. EPSCs and IPSCs were
either direct bath addition or constant perfusion. In agreement with
identified by their characteristic time course, reversal potential,
previous findings (Lessmann et al., 1994; Levine et al., 1995; Li et al.,
and, in some cases, their sensitivity to CNQX and bicuculline,
1998; Schinder et al., 2000), the amplitude of EPSCs recorded at
which block AMPA and GABA
E3E synapses increased within the first 10–15 min and reached a
A receptors, respectively (see Ma-
terials and Methods).
final level of 129 ⫾ 7.3% (n ⫽ 12) (Fig. 1A,D,E) of the control level.
In the first set of experiments, we examined the effects of
In contrast, the amplitude of IPSCs recorded from I3E synapses
bath-applied BDNF on the efficacy of both glutamatergic and
showed a slight reduction over the same time course (87 ⫾ 4.5% of
GABAergic synapses. Recordings were made with gramicidin D
the control; n ⫽ 19) (Fig. 1B,D,E), suggesting a differential BDNF
or amphotericin B perforated patch or with breakthrough whole
effect that depends on the presynaptic cell type. Surprisingly, the
cell. Similar results were obtained with all three methods as long
amplitude of IPSCs recorded from I3I synapses showed a marked
as a stable control recording was achieved before the application
increase in response to BDNF (154 ⫾ 6.7% of the control; n ⫽ 20),
of BDNF (see Materials and Methods). Presynaptic specificity of
starting within 5–10 min after the addition of BDNF (Fig. 1C–E),
the action of BDNF was determined by comparing the effects of
indicating a postsynaptic cell-type specificity for BDNF modulation
BDNF on EPSCs and IPSCs observed only in glutamatergic neu-
of IPSCs. This increase in the amplitude of inward (depolarizing)
rons (E3 E vs I3 E). Postsynaptic specificity of BDNF actions
IPSCs represents a reduction of inhibition at these synapses. To-
was determined by comparing the effects of BDNF on IPSCs
gether, these experiments indicate that the modulation of synaptic
Wardle and Poo • Postsynaptic BDNF Modulation of GABAergic Synapses
J. Neurosci., September 24, 2003 • 23(25):8722– 8732 • 8725
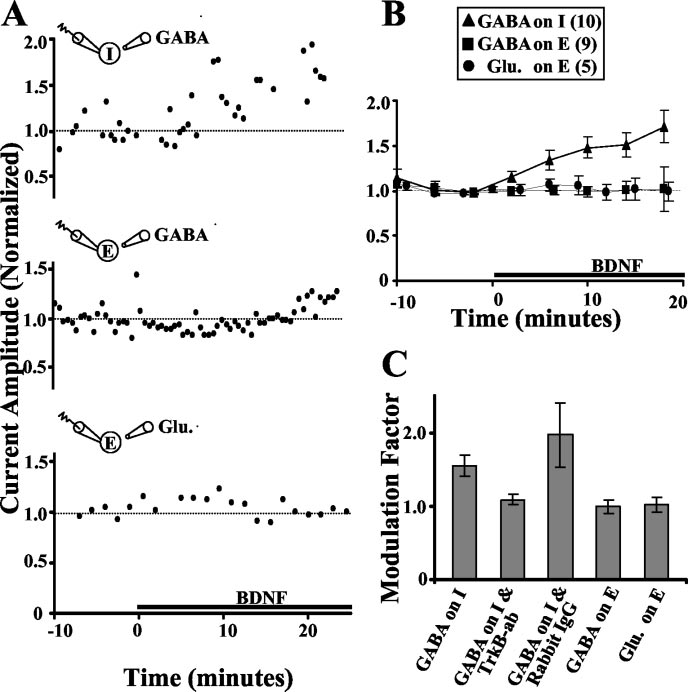
the amplitude of IGABA after removingBDNF by perfusion with fresh culture me-dium. In three of four cases, we found thatthe elevated IGABA persisted for as long as astable recording was made (5–15 min afterwashout). In contrast, BDNF had no effecton IGABA or glutamate-induced currents(Iglu) recorded from glutamatergic cells(Fig. 2 A, B). This demonstrates a selectivesusceptibility of GABAergic neurons toBDNF-dependent modulation of IGABA,consistent with a potential postsynapticBDNF effect at I3 I synapses. The lack ofany BDNF-induced change in Iglu at gluta-matergic neurons is consistent with a pre-synaptic mechanism for BDNF action onEPSCs at E3 E synapses, whereas the lackof change of IGABA in glutamatergic neu-rons confirms the postsynaptic specificityof the BDNF effect on IPSCs.
BDNF acts postsynaptically through
TrkB receptors
To determine whether the observed BDNF
effect on IGABA recorded from GABAergic
cells was attributable to activation of TrkB
receptors, we examined the effect of BDNF
on cells that were preincubated with an an-
tibody raised against the extracellular do-
main of the TrkB receptor (amino acids
160 –320; rabbit polyclonal). We found
that preincubation with TrkB antibody for
Figure 2. The effects of BDNF on transmitter-induced currents. A, Example of IGABA recorded from GABAergic (top) and gluta-
15–20 min before recording blocked
recorded using amphotericin B. BDNF was added at t ⫽ 0. B, Summary of the normalized amplitude of I
BDNF modulation of I
GABA and Iglu at various
GABA amplitudes re-
times before and after BDNF treatment (black bar). At GABAergic cells, BDNF caused an increase in I
corded from GABAergic cells (Fig. 2C).
GABA amplitude (164 ⫾ 13%
of control), but at glutamatergic cells, BDNF caused no significant change in IGABA or Iglu amplitude (94 ⫾ 10 and 92 ⫾ 6%,
Preincubation with rabbit IgG had no ef-
respectively). No significant difference was found between IGABA recorded with gramicidin D and amphotericin B, and the data
fect on the BDNF-induced elevation of the
were pooled. C, Summary of the average BDNF effect on transmitter-induced currents. Modulation factor defined as the ratio
IGABA amplitude (Fig. 2C). The tyrosine ki-
between the mean transmitter-induced current amplitude 10 –20 min after BDNF application and that during the last 10 min of
nase inhibitor K252a is commonly used to
the control period. BDNF caused an increase in IGABA recorded from GABAergic cells (1.54 ⫾ 0.15; p ⬍ 0.001; n ⫽ 10), but this
demonstrate TrkB receptor involvement
effect was blocked in cells that had been preincubated with a TrkB antibody (20 g/ml; 1.09 ⫾ 0.08; n ⫽ 5; p ⬎ 0.1).
in an observed BDNF effect. We found
Preincubation with rabbit IgG (50 g/ml) did not block the BDNF effect (1.96 ⫾ 0.43; n ⫽ 4; p ⬍ 0.05). Glu, Glutamate; ab,
that, in the presence of K252a, BDNF
caused a marked decrease in IGABA ampli-tude (data not shown), indicating that ty-
transmission by BDNF is unique to each of four different synaptic
rosine kinase activity may be involved in the BDNF effect. How-
configurations, suggesting both presynaptic and postsynaptic cell-
ever, K252a is a general tyrosine kinase inhibitor, and both the
type specificity of BDNF action.
K ⫹–Cl ⫺ cotransporter 2 (KCC2) and GABAA receptors areknown to be regulated by tyrosine kinase activity (Dunne et al.,
BDNF effects on GABA-induced currents
1998; Kelsch et al., 2001; Brandon et al., 2002). Thus, the above
To determine whether the postsynaptic cell-type specificity of the
K252a effect may result from multiple actions on other tyrosine
effect of BDNF on IPSCs reflects a postsynaptic mechanism, we
kinases besides TrkB.
examined the effect of bath-applied BDNF on membrane cur-rents induced by focally applied transmitter. Pairs of neurons
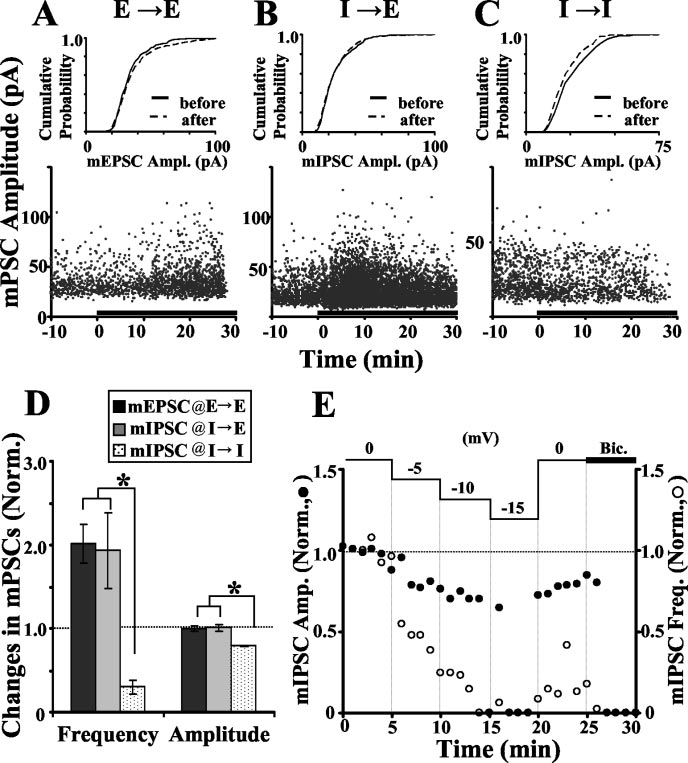
BDNF effects on mIPSCs also indicate
were patched and identified. A GABA- or glutamate-containing
micropipette was positioned near the surface of the soma, the
To further study the loci of BDNF-induced synaptic modulation,
transmitter was pressure ejected with a 1 msec pulse at regular
mIPSCs and mEPSCs were analyzed before and after BDNF treat-
intervals (see Materials and Methods), and membrane currents
ment. Functional identification of the postsynaptic cell type as
were recorded (at Vh of ⫺70 mV) before and after application of
glutamatergic or GABAergic was made before selective recording
BDNF. We found that BDNF treatment caused an increase in the
of mIPSCs or mEPSCs, using action potential blocker TTX, to-
amplitude of inward GABA-induced currents (IGABA) recorded
gether with CNQX or bicuculline, respectively. At E3 E syn-
from GABAergic cells, with a time course and extent of increase
apses, the frequency of mEPSCs increased markedly after the
similar to that observed for IPSCs at I3 I synapses (Fig. 2 A, B).
BDNF treatment (202 ⫾ 24% of the control), whereas the
The persistence of the BDNF effect was examined by monitoring
mEPSC amplitude distribution remained unchanged (Fig.
8726 • J. Neurosci., September 24, 2003 • 23(25):8722– 8732
Wardle and Poo • Postsynaptic BDNF Modulation of GABAergic Synapses
3 A, D). These results are consistent withprevious reports (Lessmann et al., 1994; Liet al., 1998; Schinder et al., 2000).
The amplitude of inward mIPSCs re-
corded with Vh of ⫺70 mV was too smallto be consistently detected above the noiselevel because the Vh was only slightly morenegative than the EIPSC for most cells. Re-cording with much more negative Vh in-creases the observed size of inward cur-rents but is damaging to the cell.
Therefore, mIPSCs were recorded at Vh of0 mV as amplified outward currents (seeMaterials and Methods). We found thatBDNF caused a rapid increase in themIPSC frequency at I3 E synapses (193 ⫾45% of controls), without significantchange in the distribution of mIPSC am-plitudes (Fig. 3 B, D). This is in sharp con-trast to the BDNF-induced reduction ofIPSC amplitude at I3 E synapses, becausean increased mini-frequency usually sug-gests an increased release probability. Asdiscussed later, however, the disparatechanges in spontaneous and evoked re-lease are not unprecedented.
When recording outward mIPSCs at
I3 I synapses (Vh of 0 mV), we found thatBDNF caused a reduction of the mIPSCamplitude (79 ⫾ 1% of controls) and amarked decrease in the mIPSC frequency(30 ⫾ 9% of controls) (Fig. 3C,D). Thechange in mIPSC amplitude is consistentwith a postsynaptic effect. This BDNF-induced reduction of outward mIPSC cur-
Figure 3. BDNF effect on miniature postsynaptic currents. A, Example of mEPSCs at an E3E synapse. Bottom, Scatter plot of
rents at Vh of 0 mV is reconciled with the
mEPSC amplitudes recorded before and after adding BDNF (black bar) in the presence of TTX and bicuculline. Top, Amplitude
previous finding of increased inward IP-
distribution for mEPSCs before (last 10 min of control; black line) and 15–25 min after (dashed line) BDNF treatment. No signifi-
SCs and IGABA at ⫺70 mV if both resulted
cant difference was found between distributions ( p ⫽ 0.13; Kolmogorov–Smirnov test). Recording made with amphotericin B
from a BDNF-induced shift in the reversal
(Vh of ⫺70 mV). B, Example of mIPSCs at an I3E synapse in the presence of TTX and CNQX, using breakthrough whole-cell
potential of Cl ⫺ currents toward more
recording at Vh of 0 mV (see Materials and Methods). Amplitude distribution shows no significant difference in mIPSC amplitudes
positive levels. The reduction of mIPSC
frequency could in principle result from
similar to that described in B. Amplitude distributions show a significant difference before and after BDNF treatment ( p ⫽0.008;
either a reduction in the release probability
Kolmogorov–Smirnov test). D, Summary of BDNF-induced changes in mPSC amplitude and frequency at E3E (n ⫽ 8), I3E
(n ⫽ 7), and I3I (n ⫽ 4) synapses. Each bar represents the mean amplitude or frequency 15–30 min after the onset of BDNF
or, indirectly, the reduction in mIPSC am-
treatment, normalized to the mean amplitude or frequency during the control period. Significant differences are indicated by
plitudes. Because of the skewed amplitude
asterisks (frequency, p ⬍ 0.01; amplitude, p ⬍ 0.001; ANOVA; Scheffe post hoc test). E, Recording of mIPSCs while changing V
distribution of mIPSCs toward smaller
from 0 to ⫺15 mV in 5 mV steps. Amplitude and frequency averaged over 1 min bins and normalized to the first 5 min of the
events, reduction in mIPSC amplitudes
recording (t ⫽ 0–5 min). At t ⫽ 25 min, bicuculline (Bic) was added to the recording solution.
would result in a disproportionately largedecrease in the perceived frequency as a
frequency (data not shown; see Materials and Methods), sug-
result of a loss of events with amplitudes below the detection
gesting that BDNF similarly elevates spontaneous presynaptic
threshold. To further test the latter possibility, mIPSC were re-
transmitter release at all three synapse types.
corded at I3 E or I3 I synapses at different clamping voltagesranging from 0 to ⫺15 mV in 5 mV decrements. We found thatthe decrease in mIPSC amplitude as the membrane potential
Differential BDNF effects on IPSCs at I3 I and I3 E synapses
approached the reversal potential was accompanied by a progres-
Changes in the amplitude of IPSCs or GABA-induced membrane
sive decrease in the observed mIPSC frequency (Fig. 3E), in a
currents may result from a change in membrane conductance or
manner that is quantitatively consistent with the idea that the
in the driving force for Cl ⫺ ions. Changes in membrane conduc-
observed decrease in mIPSC frequency and amplitude reflects a
tance could reflect presynaptic or postsynaptic modifications,
change in amplitude alone. The extent of the observed decrease
e.g., changes in transmitter release or in response properties of
in mIPSC frequency attributable to amplitude change could
GABAA receptors, respectively. Changes in the driving force,
also have masked the presynaptic BDNF effects. In two cases in
however, could result directly from changes in the postsynaptic
which we were able to record mIPSCs from GABAergic cells at
Cl ⫺ concentration ([Cl ⫺]i), altering the reversal potential for
⫺70 mV, we indeed observed a robust increase in mIPSC
IPSCs (EIPSC). To determine the mechanism underlying the

Wardle and Poo • Postsynaptic BDNF Modulation of GABAergic Synapses
J. Neurosci., September 24, 2003 • 23(25):8722– 8732 • 8727
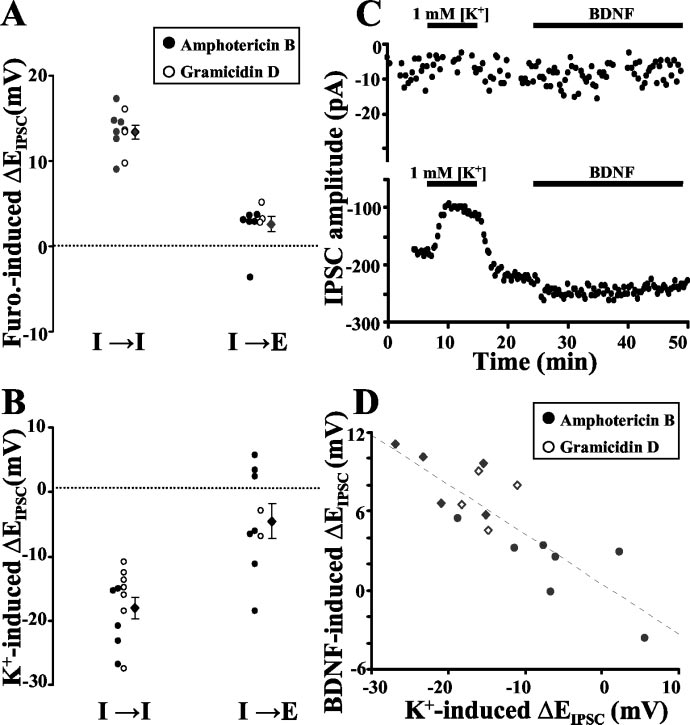
synapses (Fig. 4 B–D). We also noted that,although the EIPSC recorded showed alarge cell-to-cell variation, BDNF consis-tently induced a similar degree of changein EIPSC at I3 I synapses (Fig. 4C). Thus,these postsynaptic GABAergic neuronsappear to be more susceptible to [Cl ⫺]imodulation by BDNF than the glutama-tergic neurons. We found an overall de-crease in the slope conductance inducedby BDNF at both synapse types, with aslightly larger decrease observed at I3 Ethan I3 I synapses, although the differ-ence was not statistically significant (Fig.
4 D). These findings also account for theapparent difference in the BDNF effects onIPSCs versus mIPSCs at I3 I synapses de-scribed above. Shifts in EIPSC toward morepositive values would increase the ampli-tude of inward IPSCs (recorded at ⫺70mV) (Fig. 1C–E) and decrease the ampli-tude of outward mIPSCs (recorded at 0mV) (Fig. 3C,D).
A BDNF-induced modulation of EIPSC
at I3 I synapses raises the question ofwhether endogenous levels of BDNF areinvolved in maintaining or regulatingEIPSC. To test this, we recorded EIPSC fromI3 I synapses before and after acutelyblocking endogenous BDNF activity usinga TrkB receptor antibody specific to theextracellular domain of the receptor andshown previously to functionally blockBDNF activation (Fig. 2C). Acutely block-ing endogenous BDNF activity caused nosignificant change in EIPSC but caused a de-crease in conductance (Fig. 4 D). This sug-gests that acute change in the basal level ofendogenous BDNF activity is not suffi-cient to modify EIPSC, although it may besufficient to modify synaptic conductance.
Figure 4. Differential BDNF effects on IPSCs at I3I and I3E. A, B, Example of IPSCs at I3I (A1; gramicidin D) and I3E (B1;
This finding does not rule out potential
effects of higher levels of endogenously se-
creted BDNF on EIPSC under some physi-
linear fit of data before (black line, black circles) and after (dashed line, white circles) BDNF treatment, its abscissa-intercept
ological conditions, e.g., high-frequency
determines the EIPSC, and its slope is taken as the synaptic conductance. C, Summary plots showing EIPSC at I3I and I3E
neuronal firing (Balkowiec and Katz,
synapses before (⫺) and after (⫹) BDNF treatment for individual recordings performed with gramicidin D (gray triangles) or
amphotericin B (black triangles). There was no significant difference between data obtained by the two methods at either I3Ior
I3E synapses ( p ⫽ 0.27 and 0.42, respectively; unpaired t test). D, Summary plots of acute changes in EIPSC (in millivolts) and
decrease in synaptic conductance (percentage) after adding BDNF (100 ng/ml), at I3I (n ⫽ 21) and I3E (n ⫽ 8) synapses, or
BDNF-induced shift in EIPSC requires
removing endogenous BDNF activity by adding TrkB antibody (2 g/ml;TrkB-ab)atI3Isynapses(n⫽4).AtI3IversusI3E,
significant difference was found between the BDNF effects on EIPSC ( p ⬍ 0.0001; unpaired t test) but not between BDNF effects
on conductance ( p
i characteristic of mature
⫽ 0.12; t test). TrkB antibody had no significant acute effect on EIPSC at I3I ( p ⬎ 0.1; t test).
neurons is attributed primarily to KCC2
postsynaptic BDNF effect on IPSCs, the total membrane conduc-
K ⫹–Cl ⫺ cotransporter activity, which is
tance and E
known to be responsible for setting EIPSC (Thompson and Gah-
IPSC were measured before and after BDNF treatment.
During the control period and at 10 –20 min after BDNF treat-
wiler, 1989; Kaila, 1994) and for the developmental switch of
ment, recordings of IPSCs were made at different membrane
GABAergic transmission from excitation to inhibition (Owens et
potentials from ⫺80 to ⫺40 mV. The I–V relationship was used
al., 1996; Ehrlich et al., 1999; Rivera et al., 1999; Ganguly et al.,
to determine the slope conductance and E
2001). During development, increased expression and activation
IPSC for each cell. Mea-
surements were made for IPSCs recorded from both GABAergic
of KCC2, the K ⫹–Cl ⫺ cotransporter predominant in neurons
and glutamatergic cells. As shown in Figure 4, BDNF caused a
(Payne et al., 1996), lowers [Cl ⫺]i and drives EIPSC toward more
shift in E
negative levels, resulting in inhibitory actions by GABA. To test
IPSC toward more positive levels at I3 I synapses (Fig.
4 A, C,D) but had no consistent effect on EIPSC recorded at I3 E
whether KCC2 is involved in BDNF-induced modulation of IP-
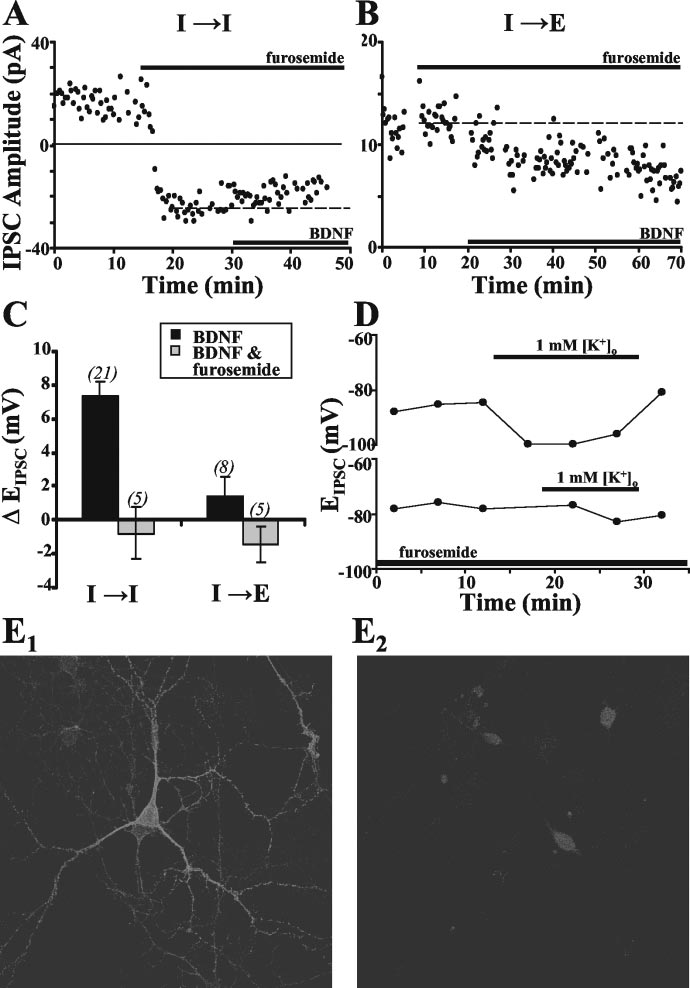
8728 • J. Neurosci., September 24, 2003 • 23(25):8722– 8732
Wardle and Poo • Postsynaptic BDNF Modulation of GABAergic Synapses
SCs, we treated hippocampal cultures withfurosemide (100 m), an antagonist ofK ⫹–Cl ⫺ cotransporters (Thompson andGahwiler, 1989; Payne et al., 1996; Payne,1997; Jarolimek et al., 1999), for 10 –15min before the addition of BDNF. Furo-semide treatment by itself caused a markedshift in EIPSC toward more positive levels atI3 I synapses, evidenced by a decrease inoutward IPSC amplitude and an increasein inward IPSCs (Fig. 5A). SubsequentBDNF treatment, however, resulted in noadditional alteration in IPSC amplitude orEIPSC at these synapses, suggesting thatfurosemide-sensitive K ⫹–Cl ⫺ cotrans-port is involved in the modulation of EIPSCby BDNF (Fig. 5 A, C). In contrast, furo-semide treatment by itself had no signifi-cant effect on EIPSC at I3 E synapses (Fig.
5B), and the effect of subsequent BDNFtreatment on IPSCs was not altered by thepresence of furosemide (Fig. 5 B, C). Thesefindings suggest that the postsynapticmodulatory action of BDNF at I3 I syn-apses is mediated through downregulationof furosemide-sensitive K ⫹–Cl ⫺ cotrans-port. On the basis of changes in EIPSC, weestimated that furosemide and BDNF in-creased [Cl ⫺]i to 169 ⫾ 6.8 and 144 ⫾5.6% of the control value, respectively.
These values are substantially lower thanthe variation of [Cl ⫺]i found in these neu-rons [up to fivefold difference between thelowest and highest [Cl ⫺]i as estimatedfrom the range of EIPSC (Fig. 4C)], suggest-ing that the absence of a BDNF effect infurosemide-treated cells was unlikely to becaused by a ceiling effect for [Cl ⫺]ielevation.
Furosemide is also known to block
other cotransporters, including the Na ⫹–K ⫹–2Cl ⫺ cotransporter (NKCC) andKCC1. Therefore, to further determinewhether the effect on EIPSC by furosemidewas primarily attributable to a block of
Figure 5. Effect of furosemide on BDNF-induced modulation of EIPSC.A,B,ExamplerecordingofIPSCs(amphotericinB)atI3I
KCC2 activity, we tested the effect of furo-
and I3E synapses, showing the effects of furosemide (100 m) and subsequent BDNF treatment. The dashed line shows the
semide on EIPSC sensitivity to changes in
mean IPSC amplitude during the last 10 min before adding BDNF. C, Summary plots comparing BDNF-induced changes in EIPSC in
external K ⫹ ([K ⫹]
the absence (black) or presence (gray) of furosemide at I3I and I3E synapses. Data obtained using both amphotericin B and
o). Changes in [K ⫹]o
activate KCC2 but not NKCC or KCC1
gramicidin D showed no difference and were thus pooled. D, Two example recordings (gramicidin D) showing changes in EIPSC
(Payne, 1997; DeFazio et al., 2000).
attributable to [K ⫹]o-induced activation of KCC2 cotransport in the absence (top) and presence (bottom) of furosemide. Normal
Thompson and Gahwiler (1989) have
Specific staining for KCC2 in these neurons using a KCC2 antibody as the primary antibody with cell-to-cell variation in KCC2
shown that [K ⫹]o-induced changes in
staining intensity. E
2, Control staining in parallel cultures showing the level of nonspecific staining with the secondary antibody
do not result from other K ⫹-
alone. For the control image, fluorescence intensity gain was increased more than twofold compared with that for specific KCC2
mediated components because blocking
image. Nonspecific staining was restricted to the soma, showing no variation among neurons.
K ⫹ channels with intracellular Cs ⫹ doesnot block the [K ⫹]o-induced effect onEIPSC. Pairs of neurons, patched and iden-tified as described previously, were recorded from for a control
[K ⫹]o was returned to 3 mM. In the presence of furosemide,
period in normal [K ⫹]
reduction of [K ⫹]
o (3 mM). A low [K ⫹]o (1 mM) solution
o had no effect on [Cl ⫺]i (Fig. 5D, bottom).
was then perfused into the culture. We found that this change in
These results are consistent with a prevalent furosemide-sensitive
KCC2 cotransporter activity in these neurons. To further test for
o induced a significant decrease in [Cl ⫺]i, as revealed by
changes in IPSC amplitude and a shift in E
the presence of KCC2 in these neurons, immunocytochemical
IPSC toward a more
negative level (Fig. 5D, top). The effect was reversed after the
experiments were performed with a rabbit anti-KCC2 antibody.
Wardle and Poo • Postsynaptic BDNF Modulation of GABAergic Synapses
J. Neurosci., September 24, 2003 • 23(25):8722– 8732 • 8729
ferences in KCC2 activity and not otherfurosemide-sensitive
To determine whether the differences
in KCC2 activity alone can account for thedifferences in BDNF-induced modulationof IPSCs observed at different synapsetypes, neuron pairs were recorded contin-uously under the following conditions.
First, we recorded the shift in EIPSC in-duced by changing [K ⫹]o briefly from 3 to1 mM, to reveal KCC2 activity. After re-cording from the same cells for a secondcontrol period with 3 mM [K ⫹]o, wetreated the cells with BDNF and measuredchanges in EIPSC to determine the synapsesusceptibility to BDNF-induced modula-tion (Fig. 6C). We found that the degree ofEIPSC shift induced by low [K⫹]o, whichreflects the activity of KCC2, correlatedstrongly with the BDNF responsiveness foreach individual cell (Fig. 6 D), at eitherI3 I or I3 E synapses.
Discussion
In this study, we first show that, in hip-
pocampal cultures, BDNF has different
modulatory effects at glutamatergic and
GABAergic synapses: enhancing the am-
plitude of EPSCs at E3 E synapses but not
that of IPSCs at I3 E synapses. Addition-
ally, BDNF has differential effects on IP-
SCs at I3 E versus I3 I synapses. At I3 E
Figure 6. Changes in EIPSC induced by furosemide, [K⫹]o, and BDNF. A, B, Scatter plots showing the effect of furosemide (A)
synapses, we found that BDNF slightly at-
and changing [K ⫹]o (B) on EIPSC at I3I and I3E synapses recorded with gramicidin D (white circles) or amphotericin B (black
tenuated IPSC amplitude, apparently
circles). The change in EIPSC is defined as the difference between EIPSC measured during the control period and in the presence of
through a reduction of presynaptic evoked
furosemide ( A) or reduced [K ⫹]o (1 mM; B). C, Separate example recordings (amphotericin B) of IPSCs at two different synapses
GABA release. At I3 I synapses, however,
(top and bottom) showing responses to [K ⫹]o and BDNF. Control periods were recorded with normal solution (3 mM [K⫹]o, no
BDNF modified the IPSC amplitude
BDNF), and [K ⫹]o was temporarily changed to 1 mM during time indicated. BDNF was added after the return to 3 mM [K⫹]o. D,
through a shift in EIPSC toward more
Scatter plot showing that the magnitudes of ⌬EIPSC induced by the reduction of [K⫹]o (to 1 mM) and by BDNF (at normal [K⫹]o)
positive levels, brought about by down-
at individual synapses are inversely correlated (correlation coefficient, r ⫽ ⫺0.841; associated probability, p ⬍ 0.001; ordinary
least-squares regression). Recordings were made with amphotericin B (white symbols) and gramicidin D (black symbols) at both
regulating postsynaptic KCC2-mediated
I3I (diamonds) and I3E (circles) synapses.
K ⫹/Cl ⫺ cotransporter activity. Thestrong correlation between postsynapticKCC2 activity and susceptibility to
We found specific staining for KCC2 in these cultures, with a
BDNF-induced modulation of EIPSC fur-
large cell-to-cell variation in the staining intensity within the
ther suggests KCC2 regulation as a primary postsynaptic
same culture (Fig. 5E). This is consistent with a variable level of
mechanism underlying the target cell specificity of the effect
KCC2 expression among a heterogeneous population of neurons.
on GABAergic synapses by BDNF.
Differential BDNF effects on EPSCs and IPSCs
BDNF effects on IPSCs correlate with KCC2 activity
Our findings of the modulatory effects of BDNF at E3 E and
The above findings suggest that the effect on IPSCs by BDNF at
I3 E synapses are in general agreement with previous reports on
I3 I synapses involves modulation of KCC2 activity. To further
neurotrophin-induced acute synaptic modifications in dissoci-
test whether differences in the effects on IPSCs by BDNF at I3 I
ated cell cultures and slice preparations (Poo, 2001). Although
versus I3 E synapses result from differences in KCC2 activity, we
several reports have shown that BDNF does not acutely modify
compared the effects of either furosemide treatment or changing
basal glutamatergic synaptic transmission in slice preparations
[K ⫹]o on EIPSC at GABAergic and glutamatergic postsynaptic
(Figurov et al., 1996; Tanaka et al., 1997; Frerking et al., 1998), the
cells. Blocking K ⫹–Cl ⫺ cotransporter activity with furosemide
discrepancy may reflect differences in the experimental condi-
caused significant changes in EIPSC at GABAergic but not gluta-
tions or developmental stages of the preparations (Poo, 2001).
matergic cells (Fig. 6 A), suggesting that I3 I and I3 E synapses
For example, BDNF-induced effects on EPSCs were found to
differ by their postsynaptic K ⫹–Cl ⫺ cotransporter activity. Fur-
depend on synapse maturity, as gauged by the initial synaptic
thermore, activating KCC2 cotransporters by changing [K ⫹]o
strength and reliability (Berninger et al., 1999; Schinder et al.,
caused significant changes in EIPSC in GABAergic but not gluta-
2000). Previous reports on acute synaptic modulation by neuro-
matergic cells (Fig. 6 B), more specifically indicating cell-type dif-
trophins have, in general, examined either EPSCs or IPSCs but
8730 • J. Neurosci., September 24, 2003 • 23(25):8722– 8732
Wardle and Poo • Postsynaptic BDNF Modulation of GABAergic Synapses
not both in the same preparation. The present study demon-
transmitter release observed at E3 E, I3 E, and I3 I synapses,
strates that, for the same developmental stage and experimental
we also observed BDNF-induced changes in mini amplitude only
conditions, BDNF directly exerts differential synaptic effects by
at I3 I synapses, indicative of a postsynaptic BDNF effect at I3 I
potentiating E3 E and suppressing I3 E transmission.
synapses. Recordings of IGABA further confirmed the existence ofa postsynaptic BDNF effect independently functioning to selec-
Postsynaptic cell-type specificity
tively modulate IGABA at GABAergic cells. The existence of cell-
Synaptic modifications have been shown to be specific to the
type-specific modulation and both presynaptic and postsynaptic
postsynaptic cell type in a number of systems. In hippocampal
BDNF effects allows diverse physiological actions of BDNF on
culture and slice, activity-dependent LTP can be induced at E3 E
neural networks made of a heterogeneous population of neurons.
but not E3 I synapses (Bi and Poo, 1998; Maccaferri et al., 1998,respectively), apparently attributable to the lack of postsynaptic
BDNF regulates postsynaptic [Cl ⴚ]i at GABAergic synapses
Ca 2⫹/calmodulin kinase II in GABAergic cells (Liu and Jones,
At I3 I synapses, BDNF caused a shift in EIPSC toward more
1997; Sik et al., 1998). Similarly, acute BDNF application poten-
positive levels through postsynaptic regulation of a furosemide-
tiates presynaptic transmitter release at E3 E but not E3 I syn-
sensitive Cl ⫺ transporter activity. Previous studies have shown
apses (Schinder et al., 2000), perhaps attributable to a modifica-
that Cl ⫺ transport plays a critical role in the development of
tion of presynaptic terminal susceptibility to neurotrophins
inhibitory synapses (Owens et al., 1996; Ehrlich et al., 1999;
through retrograde signaling from the postsynaptic cell. We
Rivera et al., 1999; Ganguly et al., 2001) and in maintaining Cl ⫺
demonstrated that the effect on IPSCs by BDNF also depends on
homeostasis in mature neurons (Jarolimek et al., 1999). In many
postsynaptic cell type. The presynaptic and postsynaptic cell-type
developing nervous systems, age-specific expression of different
specificity of the effects of BDNF could play a critical role in
K ⫹–Cl ⫺ cotransporters renders GABAergic synaptic transmis-
maintaining coordinated modifications of excitatory and inhib-
sion initially depolarizing and later hyperpolarizing (Ben-Ari,
itory synaptic actions in heterogeneous neuronal populations
2002). Early in development, high NKCC expression levels ele-
during development and in mature nervous systems. This target-
vate [Cl ⫺]i (Fukuda et al., 1998; Kakazu et al., 1999). Later,
cell specificity may reflect differences in inhibitory synapse mat-
NKCC levels are reduced and KCC2 expression is upregulated,
uration at I3 I versus I3 E synapses, and maturation of inhibi-
lowering [Cl ⫺]i and causing a shift of EIPSC toward more negative
tory synapses has been shown to correlate with increased KCC2
levels (Ehrlich et al., 1999). After GABAergic transmission
expression and to depend on GABAergic activity (Ganguly et al.,
switches from depolarizing to hyperpolarizing, KCC2 normally
maintains low [Cl ⫺]i. However, studies on mature neurons havealso shown that the efficacy of GABAergic transmission may be
Presynaptic versus postsynaptic mechanisms
altered through modulation of K ⫹–Cl ⫺ cotransport and subse-
Previous reports have shown that BDNF effects on EPSCs result
quent changes in [Cl ⫺]i. Thompson and Gahwiler (1989)
primarily from increased presynaptic transmitter release (Lohof
showed that, in hippocampal slice cultures, repetitive low-
et al., 1993; Lessmann and Heumann, 1998; Li et al., 1998;
frequency stimulation, furosemide treatment, or changes in
Berninger et al., 1999). However, BDNF effects on IPSCs have
[K ⫹]o all caused a similar reduction in the efficacy of GABAergic
been attributed to both presynaptic and postsynaptic mecha-
synapses through changes in both conductance and driving force
nisms. Frerking et al. (1998) found that the reduction of inhibi-
for Cl ⫺. Later studies have shown that both furosemide treat-
tory transmission by BDNF was accompanied by changes in
ment and [K ⫹]o can affect KCC2 activity (Payne, 1997). Furo-
mean variance and paired-pulse depression, indications of pre-
semide is also known to act on other cotransporters, including
synaptic effects. However, Tanaka et al. (1997) showed that the
NKCC. However, it is unlikely that the furosemide effect ob-
effect on IPSCs by BDNF depends on postsynaptic tyrosine ki-
served in the present study results from a block of NKCC activity,
nase activity and Ca 2⫹ mobilization, and Brunig et al. (2001)
because blocking NKCC would result in a decreased [Cl ⫺]i, and
reported BDNF-induced downregulation of GABAA receptor
thus a shift of EIPSC toward more negative rather than positive
surface expression. Here we showed that BDNF modulation of
levels. Furthermore, the hippocampal cultures used for these ex-
IPSCs at I3 E synapses most likely results from presynaptic ac-
periments have already been shown to predominantly express
tions that reduce efficacy of GABA release, whereas at I3 I syn-
KCC2 (Ganguly et al., 2001). Rivera et al. (2002) have reported
apses, it is primarily attributable to a postsynaptic shift of EIPSC to
recently that, over the time course of hours, BDNF modulates
more positive levels. We found that BDNF increased frequency of
GABAergic synaptic transmission through a downregulation of
both mEPSCs and mIPSCs, indicating an increase in the presyn-
KCC2 mRNA and protein. The acute downregulation of K ⫹–Cl ⫺
aptic release probability. This is consistent with the BDNF-
cotransporter activity within 10 min after BDNF application that
induced enhancement of EPSCs at E3 E synapses but not reduc-
we observed appears to involve more rapid posttranslational reg-
tion of IPSCs at I3 E synapses, suggesting differential regulation
ulation, although a rapid BDNF-dependent modulation of pro-
of spontaneous and evoked GABA release by BDNF. Schinder
tein synthesis remains possible (Aakalu et al., 2001).
et al. (2000) have shown previously in these cultures that
The postsynaptic specificity of the effect of BDNF could result
BDNF had no effect on evoked synaptic responses but caused
from differences in postsynaptic TrkB expression. Although pre-
an increase in mEPSC frequency at E3 I synapses. Interest-
vious studies have shown that TrkB expression levels in the hip-
ingly, in synaptotagmin-deficient neurons, increased mEPSC
pocampus remain constant from E17 through adulthood
frequency accompanied reduced evoked transmitter release
(Ivanova and Beyer, 2001), cell-to-cell variation in TrkB expres-
(Broadie et al., 1994). Opposite BDNF effects on spontaneous
sion could still arise from varied histories of activity and exposure
and evoked release at I3 E synapses may reflect presynaptic
to BDNF (Haapasalo et al., 2002). Nonetheless, differences in
regulation of synaptic vesicle proteins, resulting in release
TrkB expression patterns are unlikely to be the main cause for
modulation similar to that found in synatotagmin-deficient
specificity of BDNF at GABAergic synapses, because the magni-
tude of the effect of BDNF directly correlates with KCC2 activity
In addition to the BDNF-induced changes in presynaptic
levels, as revealed by [K ⫹]o-sensitive Cl⫺ transporter activity.
Wardle and Poo • Postsynaptic BDNF Modulation of GABAergic Synapses
J. Neurosci., September 24, 2003 • 23(25):8722– 8732 • 8731
Expression and activity of KCC2 varies with developmental stage
Frerking M, Malenka RC, Nicoll RA (1998) Brain-derived neurotrophic fac-
(Lu et al., 1999; DeFazio et al., 2000), neuronal type (Ueno et al.,
tor (BDNF) modulates inhibitory, but not excitatory, transmission in the
2002), and presence of GABAergic activity (Ganguly et al., 2001).
CA1 region of the hippocampus. J Neurophysiol 80:3383–3386.
Fukuda A, Muramatsu K, Okabe A, Shimano Y, Hida H, Fujimoto I, Nishino
The dependence of KCC2 activity on such diverse factors creates
H (1998) Changes in intracellular Ca 2⫹ induced by GABA receptor
a scenario in which the differential effects on GABAergic synapses
activation and reduction in Cl ⫺ gradient in neonatal rat neocortex. J Neu-
by BDNF are specific not only to KCC2 activity but also to the
rophysiol 79:439 – 446.
varied factors impinging on KCC2 expression and activity.
Ganguly K, Schinder AF, Wong ST, Poo M (2001) GABA itself promotes the
BDNF and other neurotrophins are involved in a variety of
developmental switch of neuronal GABAergic responses from excitation
critical nervous system functions, ranging from neuronal devel-
to inhibition. Cell 105:521–532.
opment and survival to learning and memory. In addition, they
Haapasalo A, Sipola I, Larsson K, Akerman KE, Stoilov P, Stamm S, Wong G,
Castren E (2002) Regulation of TRKB surface expression by brain-
have been linked to pathological conditions such as epilepsy (Es-
derived neurotrophic factor and truncated TRKB isoforms. J Biol Chem
clapez et al., 1997; Biagini et al., 2001; Binder et al., 2001; Scharf-
277:43160 – 43167.
man et al., 2002). Interestingly, previous studies have also shown
Ivanova T, Beyer C (2001) Pre- and postnatal expression of brain-derived
the importance of Cl ⫺ transport in epilepsy and other patholog-
neurotrophic factor mRNA/protein and tyrosine protein kinase receptor
ical conditions, including axonal injury (Nabekura et al., 2002).
B mRNA in the mouse hippocampus. Neurosci Lett 307:21–24.
Here, we report a link between neurotrophin activity and Cl ⫺
Jarolimek W, Lewen A, Misgeld U (1999) A furosemide-sensitive K ⫹–Cl ⫺
transport at GABAergic synapses that provides a promising lead
cotransporter counteracts intracellular Cl ⫺ accumulation and depletionin cultured rat midbrain neurons. J Neurosci 19:4695– 4704.
for additional investigation on the regulation of inhibitory mech-
Jovanovic JN, Benfenati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL, Green-
anisms involved in these pathological conditions.
gard P, Czernik AJ (1996) Neurotrophins stimulate phosphorylation of
synapsin I by MAP kinase and regulate synapsin I-actin interactions. ProcNatl Acad Sci USA 93:3679 –3683.
Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM (2001) Dynamic
Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS (2000) Syn-
visualization of local protein synthesis in hippocampal neurons. Neuron
apsins as mediators of BDNF-enhanced neurotransmitter release. Nat
30:489 –502.
Akaneya Y, Tsumoto T, Kinoshita S, Hatanaka H (1997) Brain-derived neu-
Kaila K (1994) Ionic basis of GABA receptor channel function in the ner-
rotrophic factor enhances long-term potentiation in rat visual cortex.
vous system. Prog Neurobiol 42:489 –537.
J Neurosci 17:6707– 6716.
Kakazu Y, Akaike N, Komiyama S, Nabekura J (1999) Regulation of intra-
Balkowiec A, Katz DM (2002) Cellular mechanisms regulating activity-
cellular chloride by cotransporters in developing lateral superior olive
dependent release of native brain-derived neurotrophic factor from hip-
neurons. J Neurosci 19:2843–2851.
pocampal neurons. J Neurosci 22:10399 –10407.
Kang H, Schuman EM (1995) Long-lasting neurotrophin-induced en-
Ben-Ari Y (2002) Excitatory actions of GABA during development: the na-
hancement of synaptic transmission in the adult hippocampus. Science
ture of the nurture. Nat Rev Neurosci 3:728 –739.
267:1658 –1662.
Berninger B, Schinder AF, Poo MM (1999) Synaptic reliability correlates
Kelsch W, Hormuzdi S, Straube E, Lewen A, Monyer H, Misgeld U (2001)
with reduced susceptibility to synaptic potentiation by brain-derived neu-
Insulin-like growth factor 1 and a cytosolic tyrosine kinase-activate chlo-
rotrophic factor. Learn Mem 6:232–242.
ride outward transport during maturation of hippocampal neurons.
Bi GQ, Poo MM (1998) Synaptic modifications in cultured hippocampal
J Neurosci 21:8339 – 8347.
neurons: dependence on spike timing, synaptic strength, and postsynap-
Kim HG, Wang T, Olafsson P, Lu B (1994) Neurotrophin 3 potentiates
tic cell type. J Neurosci 18:10464 –10472.
neuronal activity and inhibits gamma-aminobutyratergic synaptic trans-
Biagini G, Avoli M, Marcinkiewicz J, Marcinkiewicz M (2001) Brain-
derived neurotrophic factor superinduction parallels anti-epileptic–neu-
mission in cortical neurons. Proc Natl Acad Sci USA 91:12341–12345.
roprotective treatment in the pilocarpine epilepsy model. J Neurochem
Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T (1995) Hip-
76:1814 –1822.
pocampal long-term potentiation is impaired in mice lacking brain-
Binder DK, Croll SD, Gall CM, Scharfman HE (2001) BDNF and epilepsy:
derived neurotrophic factor. Proc Natl Acad Sci USA 92:8856 – 8860.
too much of a good thing? Trends Neurosci 24:47–53.
Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A (2002) Postsynaptic induc-
Brandon NJ, Jovanovic JN, Smart TG, Moss SJ (2002) Receptor for activated
tion of BDNF-mediated long-term potentiation. Science 295:1729 –1734.
C kinase-1 facilitates protein kinase C-dependent phosphorylation and
Kyrozis A, Reichling DB (1995) Perforated-patch recording with gramici-
functional modulation of GABA
receptors with the activation of
din avoids artifactual changes in intracellular chloride concentration.
G-protein-coupled receptors. J Neurosci 22:6353– 6361.
J Neurosci Methods 57:27–35.
Broadie K, Bellen HJ, DiAntonio A, Littleton JT, Schwarz TL (1994) Ab-
Lessmann V, Heumann R (1998) Modulation of unitary glutamatergic syn-
sence of synaptotagmin disrupts excitation-secretion coupling during
apses by neurotrophin-4/5 or brain-derived neurotrophic factor in hip-
synaptic transmission. Proc Natl Acad Sci USA 91:10727–10731.
pocampal microcultures: presynaptic enhancement depends on pre-
Brunig I, Penschuck S, Berninger B, Benson J, Fritschy JM (2001) BDNF
established paired-pulse facilitation. Neuroscience 86:399 – 413.
reduces miniature inhibitory postsynaptic currents by rapid downregula-
Lessmann V, Gottmann K, Heumann R (1994) BDNF and NT-4/5 enhance
tion of GABA(A) receptor surface expression. Eur J Neurosci
glutamatergic synaptic transmission in cultured hippocampal neurones.
13:1320 –1328.
DeFazio RA, Keros S, Quick MW, Hablitz JJ (2000) Potassium-coupled
Levi-Montalcini R (1987) The nerve growth factor 35 years later. Science
chloride cotransport controls intracellular chloride in rat neocortical py-
237:1154 –1162.
ramidal neurons. J Neurosci 20:8069 – 8076.
Levine ES, Dreyfus CF, Black IB, Plummer MR (1995) Brain-derived neu-
Dunne EL, Moss SJ, Smart TG (1998) Inhibition of GABA receptor func-
rotrophic factor rapidly enhances synaptic transmission in hippocampal
tion by tyrosine kinase inhibitors and their inactive analogues. Mol Cell
neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci
Neurosci 12:300 –310.
USA 92:8074 – 8077.
Ehrlich I, Lohrke S, Friauf E (1999) Shift from depolarizing to hyperpolar-
Lewin GR, Barde YA (1996) Physiology of the neurotrophins. Annu Rev
izing glycine action in rat auditory neurones is due to age-dependent Cl⫺
Neurosci 19:289 –317.
regulation. J Physiol (Lond) 520:121–137.
Li YX, Zhang Y, Lester HA, Schuman EM, Davidson N (1998) Enhancement
Esclapez M, Hirsch JC, Khazipov R, Ben-Ari Y, Bernard C (1997) Operative
of neurotransmitter release induced by brain-derived neurotrophic factor
GABAergic inhibition in hippocampal CA1 pyramidal neurons in exper-
in cultured hippocampal neurons. J Neurosci 18:10231–10240.
imental epilepsy. Proc Natl Acad Sci USA 94:12151–12156.
Liou JC, Yang RS, Fu WM (1997) Regulation of quantal secretion by neu-
Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B (1996) Regulation of
rotrophic factors at developing motoneurons in Xenopus cell cultures.
synaptic responses to high-frequency stimulation and LTP by neurotro-
J Physiol (Lond) 503:129 –139.
phins in the hippocampus. Nature 381:706 –709.
Liu X, Jones EG (1997) Alpha isoform of calcium-calmodulin dependent
8732 • J. Neurosci., September 24, 2003 • 23(25):8722– 8732
Wardle and Poo • Postsynaptic BDNF Modulation of GABAergic Synapses
protein kinase II (CAM II kinase-alpha) restricted to excitatory synapses
of a putative K-Cl cotransporter in rat brain. A neuronal-specific isoform.
in the CA1 region of rat hippocampus. NeuroReport 8:1475–1479.
J Biol Chem 271:16245–16252.
Loeb JA, Hmadcha A, Fischbach GD, Land SJ, Zakarian VL (2002) Neu-
Poo MM (2001) Neurotrophins as synaptic modulators. Nat Rev Neurosci
regulin expression at neuromuscular synapses is modulated by synaptic
activity and neurotrophic factors. J Neurosci 22:2206 –2214.
Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U,
Lohof AM, Ip NY, Poo MM (1993) Potentiation of developing neuromus-
Saarma M, Kaila K (1999) The K ⫹/Cl ⫺ co-transporter KCC2 renders
cular synapses by the neurotrophins NT-3 and BDNF. Nature
363:350 –353.
Lu J, Karadsheh M, Delpire E (1999) Developmental regulation of the
Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A,
neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat
Kokaia Z, Airaksinen MS, Voipio J, Kaila K, Saarma M (2002) BDNF-
brains. J Neurobiol 39:558 –568.
induced TrkB activation down-regulates the K ⫹-Cl ⫺ cotransporter
Maccaferri G, Toth K, McBain CJ (1998) Target-specific expression of pre-
KCC2 and impairs neuronal Cl ⫺ extrusion. J Cell Biol 159:747–752.
synaptic mossy fiber plasticity. Science 279:1368 –1370.
Scharfman HE, Goodman JH, Sollas AL, Croll SD (2002) Spontaneous lim-
McAllister AK, Katz LC, Lo DC (1999) Neurotrophins and synaptic plastic-
bic seizures after intrahippocampal infusion of brain-derived neurotro-
ity. Annu Rev Neurosci 22:295–318.
phic factor. Exp Neurol 174:201–214.
McMahon LL, Kauer JA (1997) Hippocampal interneurons express a novel
Schinder AF, Berninger B, Poo M (2000) Postsynaptic target specificity of
form of synaptic plasticity. Neuron 18:295–305.
neurotrophin-induced presynaptic potentiation. Neuron 25:151–163.
Messaoudi E, Bardsen K, Srebro B, Bramham CR (1998) Acute intrahip-
Sik A, Hajos N, Gulacsi A, Mody I, Freund TF (1998) The absence of a major
pocampal infusion of BDNF induces lasting potentiation of synaptic
Ca 2⫹ signaling pathway in GABAergic neurons of the hippocampus. Proc
transmission in the rat dentate gyrus. J Neurophysiol 79:496 – 499.
Natl Acad Sci USA 95:3245–3250.
Nabekura J, Ueno T, Okabe A, Furuta A, Iwaki T, Shimizu-Okabe C, Fukuda
Tanaka T, Saito H, Matsuki N (1997) Inhibition of GABA synaptic re-
A, Akaike N (2002) Reduction of KCC2 expression and GABA
sponses by brain-derived neurotrophic factor (BDNF) in rat hippocam-
receptor-mediated excitation after in vivo axonal injury. J Neurosci
pus. J Neurosci 17:2959 –2966.
22:4412– 4417.
Thompson SM, Gahwiler BH (1989) Activity-dependent disinhibition. II.
Narisawa-Saito M, Carnahan J, Araki K, Yamaguchi T, Nawa H (1999)
Effects of extracellular potassium, furosemide, and membrane potential
Brain-derived neurotrophic factor regulates the expression of AMPA re-
on ECl⫺ in hippocampal CA3 neurons. J Neurophysiol 61:512–523.
ceptor proteins in neocortical neurons. Neuroscience 88:1009 –1014.
Ueno T, Okabe A, Akaike N, Fukuda A, Nabekura J (2002) Diversity of
Owens DF, Boyce LH, Davis MB, Kriegstein AR (1996) Excitatory GABA
neuron-specific K ⫹-Cl ⫺ cotransporter expression and inhibitory
responses in embryonic and neonatal cortical slices demonstrated by
postsynaptic potential depression in rat motoneurons. J Biol Chem
gramicidin perforated-patch recordings and calcium imaging. J Neurosci
277:4945– 4950.
16:6414 – 6423.
Wang T, Xie K, Lu B (1995) Neurotrophins promote maturation of devel-
Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER (1996)
oping neuromuscular synapses. J Neurosci 15:4796 – 4805.
Recombinant BDNF rescues deficits in basal synaptic transmission and
Yang F, He X, Feng L, Mizuno K, Liu XW, Russell J, Xiong WC, Lu B (2001)
hippocampal LTP in BDNF knockout mice. Neuron 16:1137–1145.
PI-3 kinase and IP3 are both necessary and sufficient to mediate NT3-
Payne JA (1997) Functional characterization of the neuronal-specific K-Cl
induced synaptic potentiation. Nat Neurosci 4:19 –28.
cotransporter: implications for [K ⫹]
regulation. Am J Physiol
Zhang X, Poo MM (2002) Localized synaptic potentiation by BDNF re-
273:C1516 –C1525.
quires local protein synthesis in the developing axon. Neuron 36:675–
Payne JA, Stevenson TJ, Donaldson LF (1996) Molecular characterization
Source: http://covoiturage.univ-mrs.fr/upload/p239/Art_7_Poo_JN_2003.pdf
PSICOTERAPIAS Y/O PSICOFÁRMACOS 1 DR. HÉCTOR HUESO H.2 ResumenA pesar de los grandes avances en el terreno de la psicoterapia y las neurociencias, aún nos enfrentamos con muchas dificultades para aliviar el sufrimiento psíquico y existencial. El título deesta ponencia se refiere a las posibles acciones terapéuticas con que contamos: psicoterapias sinmedicación, psicoterapias combinadas con psicofármacos y psicofármacos sin psicoterapia. Se
repert med cir. 2 0 1 6;2 5(2):101–105 de Medicina y Cirugía Guía de práctica clínica Movimientos anormales y embarazo Eduardo Palacios a y Ángela Viviana Navas b,∗ a Servicio de Neurología, Hospital de San José, Sociedad de Cirugía de Bogotá, Fundación Universitaria de Ciencias de la Salud,Bogotá DC, Colombiab Servicio de Neurología, Fundación Universitaria de Ciencias de la Salud, Bogotá DC, Colombia







