Pmed.1000191 1.8
The APPLe Study: A Randomized, Community-Based,Placebo-Controlled Trial of Azithromycin for thePrevention of Preterm Birth, with Meta-Analysis
Nynke R. van den Broek1, Sarah A. White2, Mark Goodall2, Chikondi Ntonya2, Edith Kayira2,
George Kafulafula3{, James P. Neilson4*
1 Liverpool School of Tropical Medicine, Liverpool, United Kingdom, 2 Malawi-Liverpool-Wellcome Trust Clinical Research Programme, Blantyre, Malawi, 3 Department of
Obstetrics & Gynaecology, College of Medicine, University of Malawi, Blantyre, Malawi, 4 School of Reproductive & Developmental Medicine, University of Liverpool,
Liverpool, United Kingdom
Background: Premature birth is the major cause of perinatal mortality and morbidity in both high- and low-incomecountries. The causes of preterm labour are multiple but infection is important. We have previously described an unusuallyhigh incidence of preterm birth (20%) in an ultrasound-dated, rural, pregnant population in Southern Malawi with highburdens of infective morbidity. We have now studied the impact of routine prophylaxis with azithromycin as directlyobserved, single-dose therapy at two gestational windows to try to decrease the incidence of preterm birth.
Methods and Findings: We randomized 2,297 pregnant women attending three rural and one peri-urban health centres inSouthern Malawi to a placebo-controlled trial of oral azithromycin (1 g) given at 16–24 and 28–32 wk gestation. Gestationalage was determined by ultrasound before 24 wk. Women and their infants were followed up until 6 wk post delivery. Theprimary outcome was incidence of preterm delivery, defined as ,37 wk. Secondary outcomes were mean gestational age atdelivery, perinatal mortality, birthweight, maternal malaria, and anaemia. Analysis was by intention to treat. There were nosignificant differences in outcome between the azithromycin group (n = 1,096) and the placebo group (n = 1,087) in respectof preterm birth (16.8% versus 17.4%), odds ratio (OR) 0.96, 95% confidence interval (0.76–1.21); mean gestational age atdelivery (38.5 versus 38.4 weeks), mean difference 0.16 (20.08 to 0.40); mean birthweight (3.03 versus 2.99 kg), meandifference 0.04 (20.005 to 0.08); perinatal deaths (4.3% versus 5.0%), OR 0.85 (0.53–1.38); or maternal malarial parasitaemia(11.5% versus 10.1%), OR 1.11 (0.84–1.49) and anaemia (44.1% versus 41.3%) at 28–32 weeks, OR 1.07 (0.88–1.30). Meta-analysis of the primary outcome results with seven other studies of routine antibiotic prophylaxis in pregnancy (.6,200pregnancies) shows no effect on preterm birth (relative risk 1.02, 95% confidence interval 0.86–1.22).
Conclusions: This study provides no support for the use of antibiotics as routine prophylaxis to prevent preterm birth inhigh risk populations; prevention of preterm birth requires alternative strategies.
Trial registration: Current Controlled Trials
Please see later in the article for the Editors' Summary.
Citation: van den Broek NR, White SA, Goodall M, Ntonya C, Kayira E, et al. (2009) The APPLe Study: A Randomized, Community-Based, Placebo-Controlled Trial ofAzithromycin for the Prevention of Preterm Birth, with Meta-Analysis. PLoS Med 6(12): e1000191. doi:10.1371/journal.pmed.1000191
Academic Editor: Gordon C. Smith, Cambridge University, United Kingdom
Received July 23, 2009; Accepted October 23, 2009; Published December 1, 2009
Copyright:
ß 2009 van den Broek et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permitsunrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: The study was funded by the Wellcome Trust (project grant 065810/Z/01/Z). Drug and placebo were supplied free of charge by Pfizer. The funders hadno role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests: The authors have declared that no competing interests exist.
Abbreviations: CI, confidence interval; OR, odds ratio.
* E-mail:
[email protected]
PLoS Medicine www.plosmedicine.org
December 2009 Volume 6 Issue 12 e1000191
Azithromycin to Prevent Preterm Birth
women; of six randomized trials, four reported preterm deliveryrates (1,310 women) [24]. Pooled results from these diverse
Of the 4 million neonatal deaths each year, 99% occur in low-
populations did not show a statistically significant reduction in the
income countries and 28% are attributable to preterm birth [1].
incidence of preterm delivery with prophylactic antibiotics (relative
Preterm delivery is one of the nine main causes of death in
risk 0.88, 95% CI 0.71–1.08). but the wide CIs were compatible
children below the age of 5 y [2]. Reducing the incidence of
with a clinically important reduction in preterm birth.
prematurity is important if Millennium Development Goal 4 for
Our aims were 2-fold. First, to investigate whether antibiotic
child survival (MDG-4) is to be achieved [2,3] and important to
prophylaxis would be of future practical benefit in the studied
reduce health service costs [4].
population in Malawi. Second, to test the intervention in the
The incidence of preterm birth (before 37 completed wk of
population with the highest reported incidence of preterm birth—
pregnancy) is between 5% and 10% in most industrialised countries
as this could have generalizable importance to other high risk
[5]. A recently reported rise in preterm birth among primigravid
women in Denmark from 3.8% to 5.7% [6] caused sufficient concernto merit an accompanying editorial [7]. The incidence of preterm
birth is higher in the United States—rising from 10.7% in 1992 to12.3% in 2003 [8]. Estimates in low-income countries are difficult
Participants and Setting
because of common uncertainties about gestational age. However, we
Women were recruited from three rural and one peri-urban
have previously reported much higher rates of 24% (95% confidence
antenatal clinic in Southern Malawi. Eligibility criteria were:
interval [CI] 21%–28%) and 20% (95% CI 17%–24%) in rural,
gestational age less than 24 wk as determined by ultrasound
community-based, ultrasound-dated studies in Malawi of, respective-
(biparietal diameter measurement), intention to remain in the
ly, anaemic [9] and unselected [10] pregnant women. We are not
study area for the duration of the pregnancy, and signed informed
aware of any other similar, rural studies from sub-Saharan Africa,
consent. Biparietal diameter measurement [25] was performed by
although an urban study in Mozambique (using ultrasound) reported
specially trained midwives and used to calculate gestational age
an incidence of 15% [11].
(Concept 200l Dynamic Imaging). All women with confirmed
The causes of preterm labour are multiple, and the processes
gestational age ,24 completed wk at this first visit were invited to
that ultimately lead to preterm birth may start many weeks before
participate in the trial.
labour starts [12,13]. There is compelling evidence for the
Recruited women were randomly allocated to either 1 g
etiological importance of infection, mainly ascending genital tract
azithromycin or placebo given at both 16–24 and 28–32 wk
infection, and principally in association with earlier rather than
gestational windows. Antenatal care was provided to all women
later preterm birth [14,15]. There is considerable evidence to
according to the usual schedule (planned 4-weekly visits until 32 wk;
suggest that intrauterine infection may occur quite early in
2-weekly thereafter). At the booking visit, all women were screened
pregnancy but remain undetected for months [14]. For example,
for malaria (thick film), anaemia (Hb ,11 g/dl by battery operated
women with high levels of C-reactive protein in early pregnancy
HemoCue device), and syphilis (VDRL). Haemoglobin and syphilis
have a much higher risk of spontaneous preterm birth (odds ratio
results were available on the same day; those found positive for
[OR] 4.64, 95% CI 0.94–22.96) [16]. Thus, antibiotic prophylaxis
syphilis were treated on the same day with intramuscular benzyl
to treat clinically unsuspected infection during pregnancy could,
penicillin (1 g). All women received iron tablets daily (60 mg
potentially, avoid later preterm births.
elemental iron as ferrous sulphate) with 0.25 mg folic acid, and
Our studied pregnant populations in Malawi carry high burdens
antimalarial prophylaxis (two doses of Fansidar: 500 mg sulpha-
of infective morbidity, including HIV (seropositivity 30%) [17],
doxine with 25 mg pyrimethamine). All azithromycin (or placebo)
malaria (33%) [9], syphilis (10% positive Treponema pallidum
and Fansidar tablets were taken under supervision at the clinic.
haemagglutination [TPHA]), and other sexually transmitted
Women who failed to attend for their 28–32 week visit were
infections, e.g., trichomoniasis 26%, candidiasis 37% (unpublished
followed up, where possible, in the community.
data). Anaemia is also common (haemoglobin ,11 g/dl 72%) [18]
Women were asked to report when they had delivered and to
and attributable not only to nutritional deficiencies but also to
return for routine visits at 1 and 6 wk postnatally; women who
chronic inflammation. [19]
withdrew from the study were followed up in an effort to obtain their
We hypothesised that routine antibiotic prophylaxis would
delivery date and the survival status of the woman and her neonate.
decrease the incidence of preterm labour and birth, and conducteda placebo-controlled randomised trial of single-dose azithromycin
1 g orally at two time windows of pregnancy: 16–24 and 28–32 wk
At booking and throughout antenatal care all women were
(Text S2). Azithromycin was chosen because of its broad spectrum
encouraged to consider voluntary counselling and testing for HIV
of antibacterial activity including effectiveness against Ureaplasma
status, which was available in the clinic, as were antiretroviral
urealyticum (implicated as an important cause of preterm labour), its
drugs to prevent maternal to child transmission. We did not seek to
efficacy against sexually transmitted infections including syphilis
collect prospective data about the HIV status of women. Our
and chlamydia, its antimalarial effects (malaria is also a cause of
objective was to determine whether routine prophylactic treatment
prematurity), its safety profile in pregnancy [20], and the
with an antibiotic in a population with a known high prevalence of
convenience of a single oral dose with few side-effects. A recently
infection and preterm labour would reduce the incidence of
reported randomized trial showed that prophylactic azithromycin
preterm labour (primary outcome). Secondary outcomes were
reduces the risk of miscarriage after amniocentesis [21].
mean gestational age at delivery, perinatal mortality, birthweight,
We also hypothesised that routine azithromycin would decrease
and maternal malarial status and anaemia at 28–32 wk.
the incidence of malarial parasitaemia, because of its antimalarial
Preterm birth was defined as gestational age at delivery of at
properties [22,23], and anaemia, because of the association of
least 24 wk and less than 37 wk. Perinatal mortality included
anaemia with chronic inflammation in this population [19].
stillbirths and deaths within the first week of life.
At the time of planning our study, a Cochrane systematic review
We documented outcomes including date, type and place of
had been published on routine antibiotic administration to pregnant
delivery, type of assistance, and condition of mother and baby. For
PLoS Medicine www.plosmedicine.org
December 2009 Volume 6 Issue 12 e1000191
Azithromycin to Prevent Preterm Birth
babies born in a hospital or health centre, birthweight was recorded.
tailed test was planned for the primary outcome since an increase
Babies were also weighed at postnatal visits at weeks 1 and 6.
in the incidence of preterm delivery would be of no more interestthan equivalence [26,27]. Two-tailed tests were planned for
secondary outcomes, to ensure that an impact in either direction
Ethical approval was obtained from the College of Medicine
could be identified and reported. After agreeing to the analysis
Research Ethics Committee (COMREC), Malawi, and permission
plan, a single interim analysis was performed using a significance
to work at the Health Centres was obtained from the Ministry of
level of 0.001 to avoid inflation of the final false positive error rate.
Health in Malawi. The study was designed to have 90% power todetect a reduction in the incidence of preterm birth from 20% [10]
to 15%, using a one-tailed test of significance at the 5% level. This
The randomization schedule was prepared by a statistician not
required 987 women per arm. To account for an anticipated 15%
involved in the trial analysis using a random generation procedure
dropout rate the total number recruited was to be 2,300. A one-
with variable block size to assign both treatments equally within
Figure 1. Trial profile.
doi:10.1371/journal.pmed.1000191.g001
PLoS Medicine www.plosmedicine.org
December 2009 Volume 6 Issue 12 e1000191
Azithromycin to Prevent Preterm Birth
each block of consecutive numbers. The azithromycin and placebo
errors observed when opening envelopes; (iii) five women were
treatments allocated were provided as identical capsules (Pfizer)
recruited with gestational age .24 wk during the first 5 wk of
and packed in pairs of sealed envelopes for each individual study
recruitment (their gestational ages were all less than 25 wk by
number, according to the randomization schedule, by staff who
ultrasound scan) and five women were recruited at ,6 wk. The
were not involved in the conduct of the trial. The randomization
second dose was received by 1,048 (91%) of women assigned to
schedule was placed in sealed envelopes and not disclosed to
azithromycin and 1,056 (92%) of women assigned to placebo. 131
anyone involved in the trial; it was only provided to the trial
women received their second dose either before week 28 or after
statistician for the interim and final analyses.
week 32; 14 (20) assigned to azithromycin (placebo) were early by
Numbers were assigned sequentially, by the study midwives,
up to 12 (30) d and 51 (46) were late by up to 20 (31) d. Two
stratified by the two midwife teams, each serving two health
women (both randomised to placebo) received azithromycin in
centres, at the time of enrolment to the study. Both participants
error (wrong envelope opened) at the second dose. The women for
and study midwives were blinded to the study assignment. At no
whom these doses were intended did not receive a second dose.
time during the study was there cause to unblind the treatment
Baseline characteristics were similar for the two treatment
allocation for any participant.
groups (Table 1).
The overall incidence of preterm birth was 17.1% and there was
little difference between the treatment groups. The OR for
In accordance with the analysis plan, logistic regression was
preterm birth for women given azithromycin was 0.96 (one-sided
used to estimate the effect of azithromycin on the incidence of
95% upper confidence limit: 1.21). Likewise, no statistically
preterm labour, prevalence of malaria parasitaemia at the 28–32-
significant difference was found between the treatment arms for
wk visit, and perinatal mortality. Analysis of covariance was used
any of the secondary outcomes (Table 2). Although not
to estimate the effect of azithromycin on gestational age at delivery
prespecified as an outcome, there was also no statistically
and on birth-weight. Variables included in these analyses as
significant difference (Fisher's exact, p = 0.38) between the
potentially influencing outcomes were: health centre, gravidity,
treatment arms in the incidence of early preterm birth
body mass index (BMI), previous preterm delivery, anaemia,
(,34 wk): azithromycin (4.6%), placebo (5.4%).
malaria, and syphilis status at the week 16–24 visit. Gestational age
Meta-analysis of the results of eight trials of routine antibiotic
at delivery and multiplicity of pregnancy was also included in the
prophylaxis, including APPLe, using a random effects model,
analysis of birth-weight. Gestational age at delivery was also
showed the relative risk of preterm birth (,37 wk) with routine
included (as linear and quadratic functions) in the analysis of
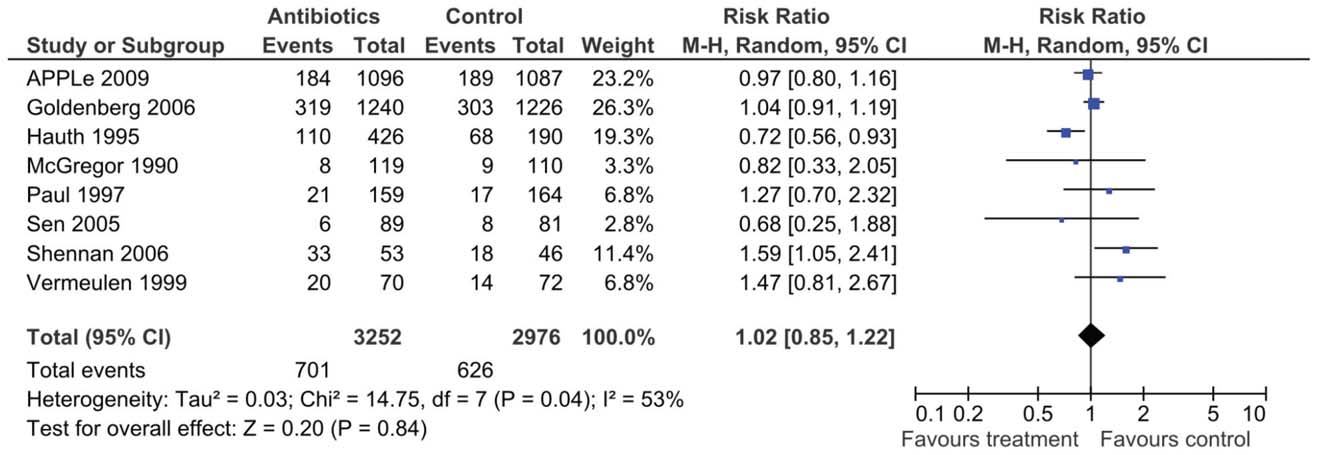
prophylactic antibiotics to be 1.02 (95% CI 0.86–1.22) (Figure 2).
perinatal mortality. All analyses were performed, using Statasoftware versions 9 or 10, on an intention-to-treat basis using all
available data; for all secondary outcomes two tailed tests wereperformed using the 5% significance level.
The overall incidence of preterm birth in our trial was 17.1%,
An interim report, including analyses of safety and efficacy data
which is higher than the figure reported in other populations, and
for the 1,151 women with an estimated date of delivery prior to 8
which is not dissimilar to the findings of our previous, smaller
February 2005 was prepared for the data and safety monitoring
study (incidence 20%; 95% CI 17%–24%) that formed the basis
board in June 2005.
for the sample size calculation [10]. The incidence of preterm
A limited meta-analysis was planned to include the results of this
birth was the same for the two groups and our trial provided no
study together with the results of other randomized trials of routine
support for our hypothesis that this regimen of prophylactic
antibiotic prophylaxis during pregnancy. These were identifiedusing a comprehensive search of the Cochrane Pregnancy andChildbirth Database of Clinical Trials (details of search strategy not
Table 1. Baseline comparability of randomised groups by
included). Only the primary outcome of the APPLe (Azithromycin
treatment group.
for the Prevention of Preterm Labor) study (delivery ,37 wk) was tobe meta-analysed (Review Manager 5; Cochrane Collaboration). Arandom effects model was to be used if there was significant
Statistic/Category Treatment Group
heterogeneity. There were no plans for subgroup or sensitivity
Azithromycin Placebo
Gestational age at
Over a period of 19 mo (February 2004 to September 2005)
11,713 women were seen for their first antenatal care visit in one
of the four antenatal clinics. Of these 2,297 met the inclusion
criteria and consented to enter the trial. Of the 9,416 women not
recruited approximately 85% were more than 24 wk pregnant atthis visit and 15% were either intending to move out of the area or
Weight for height
did not want to join the study. The last follow-up visit was on 24
A trial profile is presented in Figure 1. The primary outcome
Haemoglobin (g/dl)
(whether delivery was preterm or not) was known for 2,183(95.0%) women; 1,744 (75.9%) were followed up until 6 wk post
Positive malaria slide
partum. The following protocol deviations occurred (Text S1): (i)
sd, standard deviation; VDRL, venereal disease research laboratory; + ve,
study numbers were assigned out of sequence on six occasions; (ii)
three numbers were not assigned because of study drug shortage
PLoS Medicine www.plosmedicine.org
December 2009 Volume 6 Issue 12 e1000191
Azithromycin to Prevent Preterm Birth
Table 2. Summary and comparison of outcomes by treatment group.
Mean Difference or ORa
Number (%) who had preterm birth
184/1,096 (16.8%)
189/1,087 (17.4%)
Mean gestational age (wk) at delivery
Mean birthweight (kg)
n (%) at 2nd dose with malaria parasitaemia
117/1,014 (11.5%)
103/1,017 (10.1%)
n (%) at 2nd dose with anaemiae
445/1,010 (44.1%)
418/1,017 (41.3%)
n (%) of perinatal deaths
Thirteen maternal deaths were reported; three occurred during pregnancy (one in the azithromycin group) and ten within 6 wk of delivery (seven in the azithromycingroup). Adverse events were reported for three other women (vomiting after taking medication), of whom two were in the azithromycin group. The event rates forthese deaths and adverse events were too low for statistical comparisons to be appropriate.
aDerived from multivariable analyses using women with available data.
bOR.
cOne-sided 95% CI as specified in the analysis plan.
dMean difference.
eThis analysis was not specified in the analysis plan.
*p-Values for univariable analyses are given in parentheses.
doi:10.1371/journal.pmed.1000191.t002
azithromycin would reduce the incidence of preterm birth and
either small-for-gestational age at term or preterm. We are
improve outcome.
currently studying the mortality and morbidity and developmental
Some researchers use early preterm birth (e.g., 34 wk) as their
outcome of these babies, with known gestational age at birth.
main outcome measure as neonatal mortality is higher after early
It has been convincingly argued that the results of clinical trials
preterm than late preterm birth. We chose, as the primary
should be discussed against the background of the totality of
outcome, overall preterm birth (,37 wk) because our previous
evidence from other similar studies [29,30]. Since the publication
studies had shown high rates of perinatal mortality (160/1,000)
of the Cochrane review [24] that incorporated data from four
associated with late preterm birth (33–36 wk) in this population
studies [31–34], results from an additional four trials of routine
[10]. In addition, morbidity is greater after late preterm than term
antibiotic prophylaxis with preterm birth as an outcome have
birth, even in high income communities [28]. Azithromycin was,
become available [35–37], including APPLe (Table 3). The largest
in any case, not shown in the current study to be effective in
trials, by far, are APPLe and HPTN 024. HPTN 024 was, like
preventing early, as well as overall, preterm birth.
APPLe, performed in central Africa but relied, unlike APPLe, on
As far as we are aware, our studied population of unselected
menstrual dates and clinical examination rather than ultrasound for
pregnant women in a rural population in sub-Saharan Africa is
gestational age assessment [37,38]. The eight trials took place in
unique in having had the gestational ages of their pregnancies
diverse settings (high and low income), with different types of
confirmed by ultrasound. Gestational dating by clinical examina-
participants (e.g., unselected women, women at high risk of preterm
tion in later pregnancy or by the date of the last menstrual period
birth by past histories, women who were predominantly HIV
is unreliable. Many studies in low-income countries have therefore
positive), differing timings of treatment, and different antibiotic
used ‘‘low birthweight'' (,2.5 kg) as a surrogate for preterm
regimens. As well as clinical heterogeneity, there was statistical
birth—but it is a poor surrogate as low birthweight babies may be
heterogeneity on analysis of the pooled data (I2, 51%) from, overall,
Figure 2. Random effects meta-analysis of trials of routine antibiotic prophylaxis in pregnancy that report preterm birth ,37 wk asoutcome.
doi:10.1371/journal.pmed.1000191.g002
PLoS Medicine www.plosmedicine.org
December 2009 Volume 6 Issue 12 e1000191
Azithromycin to Prevent Preterm Birth
Table 3. Randomised trials of antibiotic prophylaxis in pregnancy.
McGregor 1990 [31]
235 unselected women
Erythromycin versus placebo
624 women at high risk of preterm birth
Metronidazole + erythromycin versusplacebo
Vermeulen 1995 [33]
168 women with history of preterm birth
Vaginal clindamycin versus placebo
437 unselected women
Erythromycin versus placebo
224 unselected ‘‘urban poor''
Metronidaxzole + cephalexin versus notreatment
Shennan 2006 [36]
100 high risk women with +ve fetal fibronectin
Metronidazole versus placebo
Goldenberg 2006 [37,38]
2,098 HIV+ and 335 HIV2 women
Metronidazole + erythromycin versus
2,297 unselected women
16–24 and 28–32
Azithromycin versus placebo
6,228 pregnancies. Meta-analysis, using a random effects model
not be treated with antibiotics unless for specific infections and
showed the relative risk of preterm birth (,37 wk) with routine
with good evidence of likely benefit.
prophylactic antibiotics to be 1.02 (95% CI 0.86–1.22).
It is important to try to reconcile this finding that routine
Supporting Information
antibiotic prophylaxis does not prevent preterm birth, with the
Trial protocol.
considerable observational data that associates infection with
Found at: doi:10.1371/journal.pmed.1000191.s001 (0.07 MB
preterm labour. It is possible that different antibiotics or different
antibiotic regimens with more intensive treatment schedules mightimpact on preterm birth rates. However, more complicated
CONSORT checklist.
antibiotic regimens would have less appeal in resource-poor settings.
Found at: doi:10.1371/journal.pmed.1000191.s002 (0.06 MB
Another explanation is that ascending intrauterine infection
may have been overemphasised as a primary cause of pretermbirth. If factors such as psychosocial stress or heavy work, for
example, are important in the premature triggering of the
The data monitoring panel was P.A. Williamson and M. Turner. The
placental corticotropin-releasing hormone (CRH) pathway that
HPTN024 trial team provided unpublished, pooled data on gestational age
ultimately leads to parturition [12], associated premature cervical
at delivery, for the meta-analysis. The late Tony Hart gave valuable advice
shortening and dilatation might permit secondary ascending
in the planning of the study.
bacterial invasion of the uterine cavity. This has been suggested
George Kafulafula died on 28 August 2009.
in the past [39] in the context of twin pregnancy in which pretermbirth is common, and early cervical dilatation does occur [40].
Author Contributions
Transvaginal ultrasound scanning has shown short cervices to be a
powerful predictor of preterm birth in singleton pregnancies [41].
At the time of planning of the trial, it was assumed that
antibiotic prophylaxis during pregnancy was unlikely to confer any
harm, whether or not it conferred any benefit. The publication of
the follow-up of the ORACLE trial has shown that this assumption
was wrong. This report showed that children of women treated
with antibiotics for preterm labour (not prophylactically) were
more likely to have neuro-developmental delay [42]. Our study
adds further weight to the conclusion that pregnant women should
1. Lawn JE, Cousens S, Zupan J for the Lancet Neonatal Survival Steering Team
8. Hoyert DL, Mathews TJ, Menacker F, Strobino DM, Guyer B (2006) Annual
(2005) 4 million neonatal deaths: When? Where? Why? Lancet 365: 891–900.
summary of vital statistics: 2004. Pediatrics 117: 168–183.
2. Jones GRW, Steketee RE, Black RE, Bhutta ZA, Morris SS and the Bellagio
9. van den Broek NR, White SA, Flowers C, Cook JD, Letsky EA, et al. (2006)
Child Survival Group (2003) How many child deaths can we prevent this year?
Randomised trial of vitamin A supplementation in pregnant women in rural
Lancet 362: 65–71.
Malawi found to be anaemic on screening by HemoCue. BJOG 113: 569–
3. Martines J, Paul VK, Bhutta ZA for the Lancet Neonatal Survival Steering
Team (2005) Neonatal survival: a call for action. Lancet 365: 1189–1197.
10. van den Broek NR, Ntonya C, Kayira E, White S, Neilson JP (2005) Preterm
4. Eichenwald EC, Stark AR. Management and outcomes of very low birth weight
birth in rural Malawi: high incidence in ultrasound-dated population. Hum
(2008) New Eng J Med 358: 1700–1711.
Reprod 20: 3235–3237.
5. Steer P (2005) The epidemiology of preterm labour. BJOG 112: s1–s3.
11. Challis K, Osman NB, Nystrom L, Nordahl G, Bergstrom S (2002) Symphysis-
6. Langhoff-Roos J, Kesmodel U, Jacobsson B, Rasmussen S, Vogel I (2006)
fundal height growth chart of an obstetric cohort of 817 Mozambican women
Spontaneous preterm delivery in primiparous women at low risk in Denmark:
with ultrasound-dated singleton pregnancies. Trop Med Int Health 7: 678–684.
population based study. BMJ 332: 937–939.
12. Smith R (2007) Parturition. New Eng J Med 356: 271–283.
7. Shennan AH, Bewley S (2006) Why should preterm births be rising? BMJ 332:
13. Simhan HN, Caritis SN (2007) Prevention of preterm delivery. New Eng J Med
357: 477–487.
PLoS Medicine www.plosmedicine.org
December 2009 Volume 6 Issue 12 e1000191
Azithromycin to Prevent Preterm Birth
14. Goldenberg RL, Hauth JC, Andrews WW (2000) Intrauterine infection and
31. McGregor JA, French JI, Richter R, Vuchetich M, Bachus V, et al. (1990)
preterm delivery. New Eng J Med 342: 1500–1507.
Cervicovaginal microflora and pregnancy outcome: results of a double-blind,
15. Goldenberg RL, Culhane JF, Iams JD, Romero R (2008) Epidemiology and
placebo-controlled trial of erythromycin treatment. Am J Obstet Gynecol 163:
causes of preterm birth. Lancet 371: 75–84.
16. Pitiphat W, Gillman WW, Joshipura KJ, Williams PL, Douglass CW, et al.
32. Hauth JC, Goldenberg LR, Andrews WW, DuBard MB, Copper RL (1995)
(2005) Plasma C-reactive protein in early pregnancy and preterm delivery.
Reduced incidence of preterm delivery with metronidazole and erythromycin in
Am J Epidemiol 162: 1108–1113.
women with bacterial vaginosis. N Eng J Med 333: 1732–1736.
17. van den Broek NR, White SA, Neilson JP (1998) The relationship between
33. Vermeulen GM, Bruinse HW (1999) Prophylactic administration of clindamycin
asymptomatic human immunodeficiency virus infection and the prevalence and
2% vaginal cream to reduce the incidence of spontaneous preterm birth in
severity of anaemia in pregnant Malawian women, Am J Trop Med Hyg 59:
women with an increased recurrence risk: a randomised placebo-controlled trial
double-blind trial. BJOG 106: 652–657.
18. van den Broek NR, Rogerson S, Mhango CG, Bambala B, White SA, et al.
34. Paul VK, Singh M, Buckshee K (1998) Erythromycin treatment of pregnant
(2000) Anaemia in pregnancy in southern Malawi: prevalence and risk factors.
women to reduce the incidence of low birth weight and preterm deliveries.
BJOG 107: 445–451.
Int J Gynecol Obstet 62: 87–88.
19. van den Broek NR, Letsky EA (2000) Etiology of anemia in pregnancy in south
35. Sen A, Mahalanabis D, Mukhopadhyay S, Chakrabarty K, Singh AK, et al.
Malawi. Am J Clin Nutr 72(suppl): 247S–256S.
(2005) Routine use of antimicrobials by pregnant Indian women does not
20. Sarkar M, Woodland CC, Koren G, Einarson ARN (2006) Pregnancy outcome
improve birth outcome: a randomized controlled trial. J Health Popul Nutr 23:
following exposure to azithromycin. BMC Pregnancy and Childbirth 6: 18.
21. Giorlandino C, Cignini P, Cini M, Brizzi C, Carcioppolo O, et al. (2009)
Antibiotic prophylaxis before second-trimester genetic amniocentesis (APGA): a
36. Shennan A, Crawshaw S, Briley A, Hawken J, Seed P, et al. (2006) A
single-centre open randomized controlled trial. Prenat Diagn. DOI: 10.1002/
randomised controlled trial of metronidazole for the prevention of preterm birth
in women positive for cervicovaginal fetal fibronectin: the PREMET study.
22. Chico RM, Pittrof R, Greenwood B, Chandramohan D (2008) Azithromycin-
BJOG 113: 65–74.
chloroquine and the intermittent preventive treatment of malaria in pregnancy.
37. Taha TE, Brown ER, Hoffman IF, Fawzi W, Read JS, et al. (2006) A phase III
Malaria Journal 7: 255.
clinical trial of antibiotics to reduce chorioamnionitis-related perinatal HIV-1
23. Kalilani L, Mofolo I, Chaponda M, Rogerson SJ, Alker AP, et al. (2007) A
transmission. AIDS 20: 1313–1321.
randomized controlled trial of azithromycin or artesunate added to sulfadoxine-
38. Goldenberg RL, Mwatha A, Read JS, Adeniyi-Jones S, Sinkala M, et al. (2006)
pyrimethamine as treatment for malaria in pregnant women. PLoS ONE 2:
The HPTN 024 study: the efficacy of antibiotics to prevent chorioamnionitis and
preterm birth. Am J Obstet Gynecol 194: 650–661.
24. Thinkhamrop J, Hofmeyr GJ, Adetoro O, Lumbiganon P (2002) Prophylactic
39. Romero R, Shamma F, Jimenez C, Callahan R, Nores J, et al. (1990) Infection
antibiotic administration in pregnancy to prevent infectious morbidity and
and labor VI. Prevalence, microbiology, and clinical significance of intraamnio-
mortality. Cochrane Database Sys Rev CD 002250.
tic infection in twin gestations with preterm labor. Am J Obstet Gynecol 163:
25. Chitty LS, Altman DG, Henderson A, Campbell S (1994) Charts of fetal size: 2
Head measurements. BJOG 101: 35–43.
40. Neilson JP, Verkuul DAA, Crowther CA, Bannerman C (1988) Preterm labor in
26. Bland JM, Altman DG (1994) One and two sided tests of significance. BMJ 309:
twin pregnancies: prediction by cervical assessment. Obstet Gynecol 72:
27. Overall JE (1991) A comment concerning one-sided tests of significance in new
41. Honest H, Bachman LM, Coomarasamy A, Gupta JK, Kleijnen J, et al. (2003)
drug applications. J Biopharm Stat 1: 157–160.
Accuracy of cervical sonography in predicting preterm birth: a systematic
28. Saigal S, Doyle LW (2008) An overview of mortality and sequelae of preterm
review. Ultrasound Obstet Gynecol 22: 305–322.
birth from infancy to adulthood. Lancet 371: 261–269.
42. Kenyon S, Pike K, Jones DR, Brocklehurst P, Marlow N, et al. (2008) Childhood
29. Young C, Horton R (2005) Putting clinical trials into context. Lancet 366:
outcomes after prescription of antibiotics to pregnant women with spontaneous
preterm labour: 7-year follow-up of the ORACLE II trial. Lancet 372:
30. Clarke M, Hopewell S, Chalmers I (2007) Reports of clinical trials should begin
and end with up-to-date systematic reviews of other relevant evidence: a statusreport. J R Soc Med 100: 187–190.
PLoS Medicine www.plosmedicine.org
December 2009 Volume 6 Issue 12 e1000191
Azithromycin to Prevent Preterm Birth
Background. Most pregnancies last about 40 weeks. Labor
similar in the two groups of women. Finally, the researchers
that occurs before 37 weeks of gestation (the period during
did a meta-analysis (a statistical technique that combines the
which a baby develops in its mother) is defined as a preterm
results of several studies) of their study and seven published
birth. In industrialized countries, 5%–10% of all births are
studies of routine antibiotic prophylaxis in pregnancy, which
preterm. Figures for preterm births are harder to obtain for
indicated that the prophylactic use of antibiotics did not
low-income countries because of uncertainties about
alter the risk of preterm birth.
gestational dates but, in both rich and poor countries,preterm birth is a major cause of infant death and illness
What Do These Findings Mean? These findings provide
around the time of birth. Babies who are born prematurely
no support for the use of antibiotics as prophylaxis to
also often have long-term health problems and disabilities.
prevent preterm birth. The women included in this study had
There are many reasons why some babies are born
an unusually high incidence of preterm delivery and a high
prematurely. Structural problems such as a weak cervix
burden of infection so these findings may not be
(the neck of the womb, which dilates during labor to allow
generalizable. The results of the meta-analysis, however,
the baby to leave the mother's body) can result in a
also provide no support for prophylactic antibiotics. Given
premature delivery, as can pregnancy-induced diabetes,
that observational data have associated infection with
blood-clotting disorders, bacterial infections in the vagina
preterm labor, why are the results of the APPLe trial and
or the womb, and malaria. However, it is impossible to
the meta-analysis negative? One possibility is that different
predict which mothers will spontaneously deliver early.
antibiotics or dosing regimens might be more effective.
Another possibility is that infection might be a secondary
Why Was This Study Done? At present there is no
consequence of some other condition that causes preterm
effective way to prevent premature births. Because infection
birth rather than the primary cause of early delivery.
is often associated with preterm labor and can occur early in
Whatever the reason for the lack of effect of prophylactic
pregnancy but remain undetected, one way to reduce the
antibiotics, the researchers recommend that pregnant
incidence of preterm births may be to give pregnant women
women should not be given antibiotics prophylactically to
antibiotics even when they have no obvious infection
prevent preterm birth particularly since, in a recent study,
(prophylactic antibiotics). In this study, the researchers test
the babies of women given antibiotics to halt ongoing
this hypothesis by giving the antibiotic azithromycin to
preterm labor had an increased risk of developmental
pregnant women living in Southern Malawi in a randomized,
placebo-controlled trial. One baby in five is born before 37weeks gestation in Southern Malawi and the women living in
Additional Information. Please access these Web sites via
this part of sub-Saharan Africa have a high burden of
the online version of this summary at http://dx.doi.org/10.
infection. Azithromycin is a safe antibiotic that can treat
many of the bacterial infections that have been implicated inpreterm birth. It also has some antimalarial activity. In a
N The March of Dimes, a nonprofit organization for
pregnancy and baby health, provides information on
randomly assigned to receive a drug or identical-looking
(in English and Spanish)
‘‘dummy'' tablets (placebo).
N The Nemours Foundation, another nonprofit organization
for child health, also provides information on
What Did the Researchers Do and Find? The researchers
(in English and Spanish)
enrolled more than 2,000 pregnant women into the APPLe
N Tommy's is a nonprofit organization that funds research
study (Azithromycin for the Prevention of Preterm Labor)
and provides information on
and determined the gestational age of their unborn babies
using ultrasound. Half of the women were given an oral dose
N The US Centers for Disease Control and Prevention
of azithromycin at 16–24 weeks and at 28–32 weeks
provides information on (in
gestation. The remaining women were given a placebo at
English and Spanish)
similar times. The mothers and their babies were followed upuntil 6 weeks after delivery. There was no significant
N The US National Women's Health Information Center has
difference in the primary outcome of the study—the
detailed information about (in English and
incidence of delivery before 37 weeks gestation—between
the two groups of women. Secondary outcomes—including
N MedlinePlus provides links to other information on
mean gestational age at delivery, mean birth weight, and still
(in English and Spanish)
births and infant deaths within a week of birth—were also
PLoS Medicine www.plosmedicine.org
December 2009 Volume 6 Issue 12 e1000191
Source: http://www.docfleetwood.net/classfiles/sample_plos_paper_human_subjects.pdf
My Health, My Choice, My Child, My Life! Women demand the roll out of a comprehensive national action plan to end vertical transmission of HIV in India Globally, momentum has been built to reinvigorate efforts to reduce maternal and infant mortality and improve maternal health including for women living with HIV. Nationally, women and children have been the stated priority of the government HIV programme since the beginning. The Indian Constitution guarantees the right to equality for all women and the right to life and health of all. In order to succeed in meeting these goals, civil society, especially women and mothers living with HIV, must be engaged and listened to, as we know the ground realities in the communities we live and work in.
Breastfeeding is Best! It seems that every year in the summer just before remember being quite shocked to see an old picture of Canadians celebrate World Breastfeeding Week there one of my children bottle feeding when I ‘remembered' is media coverage of something that undermines him as exclusively breastfed. breastfeeding. This year we seem to have gotten an


