New drugs for rheumatoid arthritis
The new england journal
of medicine
Alastair J.J. Wood, M.D.,
Editor
New Drugs for Rheumatoid Arthritis
Nancy J. Olsen, M.D., and C. Michael Stein, M.B., Ch.B.
heumatoid arthritis affects approximately 1 percent of the
From the Divisions of Rheumatology(N.J.O., C.M.S.) and Clinical Pharmacology
U.S. population and can cause irreversible joint deformities and functional im-
(C.M.S.), Departments of Medicine (N.J.O.,
pairment. The cause of this autoimmune disease remains obscure, but greater C.M.S.), Pharmacology (C.M.S.), and Mi-
understanding of the underlying mechanisms has facilitated the development of new crobiology and Immunology (N.J.O.),
Vanderbilt University School of Medicine,
drugs and revolutionized treatment.
Specific CD4+ T cells are involved in the induction of the immune response in rheu-
matoid arthritis, most likely as a response to an unknown exogenous or endogenous N Engl J Med 2004;350:2167-79.
Copyright 2004 Massachusetts Medical Society.
antigen. Consequently, recruited monocytes, macrophages, and fibroblasts producecytokines such as tumor necrosis factor
a (TNF-
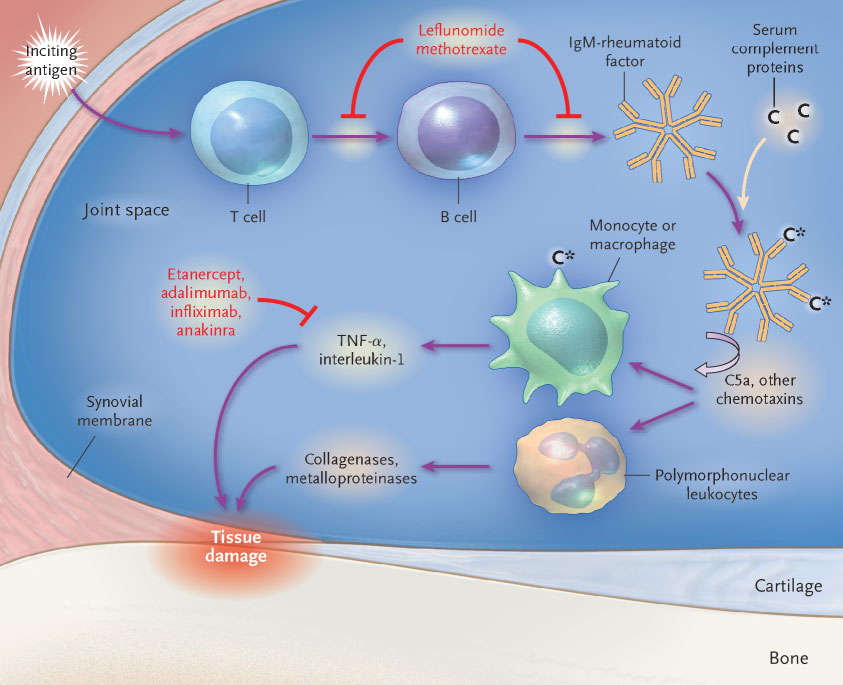
a) and interleukin-1 within the synovialcavity. These cytokines are central to a damaging cascade, ultimately triggering the pro-duction of matrix metalloproteinases and osteoclasts, which results in irreversible dam-age to soft tissues and bones. The occurrence of B-lymphocyte dysregulation is suggest-ed by the association of erosive disease with the presence of rheumatoid factor, whichmediates further damage through complement fixation (Fig. 1).2 Several new drugs havebecome available for the treatment of rheumatoid arthritis (Table 1), and in this article,we review their properties.
Both the short-term efficacy and the toxic effects of new drugs for rheumatoid arthritisare usually evaluated in clinical trials of 6 to 12 months' duration. Improvement is mostoften defined by an outcome measure of the American College of Rheumatology (ACR)called the ACR 20.3 The ACR 20 is defined as a reduction by 20 percent or more in thenumber of tender and swollen joints plus similar improvement in at least three of thefollowing five measures: pain, global assessments by the patient and the physician,self-assessed physical disability, and levels of acute-phase reactant. Two other outcomemeasures that are deemed to be more clinically relevant, the ACR 50 (improvement of50 percent or more) and the ACR 70 (improvement of 70 percent or more), are also oftenreported.4,5 All the drugs we discuss below appear to be more effective than placeboand to slow the progression of disease as measured radiologically.6-9 These medicationsare thus classified as disease-modifying antirheumatic drugs.
The immunomodulatory drug leflunomide, an isoxazole derivative, is a competitive in-hibitor of dihydroorotate dehydrogenase, the rate-limiting intracellular enzyme re-quired for the de novo synthesis of pyrimidines.10 Resting lymphocytes can derive pyri-midines from salvage pathways, but activated lymphocytes are dependent on the de novosynthesis of pyrimidines. Therefore, blockade of the pyrimidine-synthesis pathway hasantiproliferative effects. In vitro, relatively high concentrations of leflunomide (5¡10 M)
n engl j med 350;21
The New England Journal of Medicine
Downloaded from nejm.org on February 3, 2011. For personal use only. No other uses without permission.
Copyright 2004 Massachusetts Medical Society. All rights reserved.
The new england journal of medicine
Figure 1. Inflammation in the Rheumatoid Joint.
The identity of the inciting antigen is not known, but it most likely drives lymphocyte proliferation, which contributes to the production of the rheumatoid-factor autoantibody. The fixation of complement amplifies the destructive cascade, attracting additional inflammatory cells and resulting in the production of cytokines and enzymes. These, in turn, medi-ate tissue damage, including cartilage loss and bone erosion. Likely sites of action of the major drugs described in this article are shown. C denotes serum complement protein, and C* activated serum complement protein.
modulate nuclear factor-kB,11 tyrosine kinases in drug in the circulation. This metabolite is highlythe signaling pathway made up of Janus kinases protein-bound and has a long half-life of 15 to 18and signal transducers and activators of transcrip- days.15,16 Therefore, without a loading dose, it maytion (the JAK–STAT pathway) and growth-factor take as long as two months to achieve steady-statereceptors,12,13 interleukin-6, matrix metallopro- concentrations. In addition, the active metaboliteteinases, and prostaglandin E .14 Because of the undergoes extensive enterohepatic recirculation,
theoretically additive effects of leflunomide and and it may take up to two years for the amount ofmethotrexate in inhibiting pyrimidine synthesis and drug in plasma to decrease to an undetectable level.
purine synthesis, respectively, these drugs have been Renal excretion appears to be limited, and the doseproposed as potentially complementary therapies.10 generally does not have to be reduced because of de-
creased renal function, although caution is advised
because information is limited.
Leflunomide is a prodrug that, after oral adminis-
tration, undergoes rapid chemical conversion to its interactions with other drugs
primary active metabolite, A77 1726, which ac- Leflunomide inhibits cytochrome P-450 2C9
counts for more than 95 percent of the levels of the (CYP2C9) in vitro and, according to a case report,
The New England Journal of Medicine
Downloaded from nejm.org on February 3, 2011. For personal use only. No other uses without permission.
Copyright 2004 Massachusetts Medical Society. All rights reserved.
Table 1. New Drugs for the Treatment of Rheumatoid Arthritis.
Usual Dose
Inhibits pyrimidine
Loading dose of 100 mg daily for 3 days, then
Binds TNF-a and TNF-b
25 mg twice/wk or 50 mg once/wk
Human anti–TNF-a
40 mg every second wk
Chimeric anti–TNF-a
3 mg/kg of body weight at 0, 2, and 6 wk, then
For incomplete response, maintenance dose
may be gradually increased to a maximum of 10 mg/kg
may increase the anticoagulant activity of warfarin, period. During 104,000 patient-years of exposure,a substrate of this enzyme.18 However, there have liver-function abnormalities were identified in 296been no specific studies assessing the potential for patients. Fifteen of these patients died from eitherinteractions between leflunomide and warfarin or liver failure or concomitant illness; hepatic dysfunc-other CYP2C9-substrate drugs. Rifampin raises the tion was considered to be possibly related to lefluno-concentration of the active metabolite of lefluno- mide treatment in 10 of these 15 patients.24 Moremide by 40 percent, and the dose may have to be ad- recently, the Arthritis Advisory Committee of thejusted. Leflunomide reduces serum uric acid con- Food and Drug Administration (FDA) reviewed thecentrations in treated patients19 by increasing renal clinical trials and postmarketing studies.25,26 Ele-urate excretion, perhaps through effects on the vated transaminase concentrations affected 2 per-proximal renal tubule.20
cent to 4 percent of patients, although the incidenceof hepatocellular necrosis was far lower, at approx-
imately 0.02 percent to 0.04 percent. The risk of
Large placebo-controlled studies comparing leflu- severe liver injury was deemed to be small and, giv-nomide with sulfasalazine19 or methotrexate21,22 en the benefits of treatment, acceptable. On the ba-suggest that the drugs have similar efficacy (Table sis of the surveillance data, patients with preexist-2). As compared with placebo, leflunomide slowed ing liver abnormalities or a history of heavy alcoholprogression, as measured radiographically, over a intake or hepatitis virus infections should not beperiod of 6 to 12 months.19,21 After two years, more treated with leflunomide.
than 80 percent of the patients who received meth-
Combination therapy with leflunomide and
otrexate or leflunomide in a blinded, randomized, methotrexate may be associated with a higher riskcontrolled trial (the Utilization of Leflunomide in of hepatotoxic effects than treatment with lefluno-the Treatment of Rheumatoid Arthritis [ULTRA] tri- mide alone. In one trial including 30 patients, 3 pa-al) had no new erosions.21
tients (10 percent) were withdrawn because of ele-vated levels of liver enzymes.27 In a larger study, 41
of 130 patients who received leflunomide with
The most important serious reactions to lefluno- methotrexate (31.5 percent) had an elevation of themide are hepatic. In clinical trials, approximately alanine aminotransferase level to more than 1.25 percent of patients had elevated transaminase lev- times the upper limit of normal, but only 2.3 percentels23 that were generally less than twice the upper were withdrawn from the study because of abnor-limit of normal and were reversible after the discon- mal results on liver-function tests.28 However, se-tinuation of treatment with the drug. In May 2001, vere hepatotoxic effects have been reported with thisthe manufacturer issued a letter to clinicians detail- combination in a case report.29ing reports of hepatotoxicity in the postmarketing
Weight loss was reported in the earliest trials of
n engl j med 350;21
The New England Journal of Medicine
Downloaded from nejm.org on February 3, 2011. For personal use only. No other uses without permission.
Copyright 2004 Massachusetts Medical Society. All rights reserved.
The new england journal of medicine
Table 2. Rates of Response to Leflunomide as Compared with Other Disease-Modifying Agents and Placebo.*
percentage of patients
* The American College of Rheumatology (ACR) response is defined as an improvement of at least 20 percent (ACR 20),
an improvement of at least 50 percent (ACR 50), and an improvement of at least 70 percent (ACR 70) in the number of tender and swollen joints plus similar improvement in at least three of five measures specified by the ACR (see text for details). Data for 6 months are from Smolen et al.,19 and data for 12 months are from Strand et al.22 The rates of re-sponse to all drugs were significantly higher than those in the corresponding placebo group. NS denotes not stated.
leflunomide, and loss of 20 percent of body weight clinical use in rheumatoid arthritis
has been observed in clinical practice, although the Methotrexate remains the most commonly used dis-
mechanisms are unknown.30 However, weight loss ease-modifying antirheumatic drug,36 but lefluno-
seldom necessitates the discontinuation of therapy. mide is a useful alternative in the face of intolerance
Although diarrhea was reported in 32 percent of the to methotrexate. As mentioned above, given its po-
patients who were treated with leflunomide in the tential for hepatotoxic effects, leflunomide is con-
ULTRA trial,21 weight loss can occur in the absence traindicated in patients with any type of liver impair-
of gastrointestinal symptoms.
ment, and regular monitoring is required (Table 3).
Hypertension of unclear cause was reported in A loading dose of 100 mg daily for three days can be
18 percent of the patients in the same trial and was used to achieve therapeutic concentrations quicklya new finding in 5 percent.21 Elevations in blood but may cause gastrointestinal intolerance. In clin-pressure can occur within the first two months of ical practice, the loading dose is often omitted or re-treatment; therefore, regular monitoring of blood duced. The usual daily dose (20 mg) is tolerated wellpressure is advisable.31 Reversible alopecia occurred by most patients. If minor adverse effects such as di-in approximately 10.5 percent of patients in the arrhea or abdominal cramps occur, a dose of 10 mgULTRA trial,21 and pancytopenia,32,33 peripheral can be effective and may be better tolerated.37neuropathy,24 and interstitial pneumonitis34 have
The combination of leflunomide and metho-
also been reported with this agent.
trexate appears to be more effective than metho-trexate and placebo, resulting in ACR 20 response
rates of 46.2 and 19.5 percent, respectively. The
Preclinical studies indicated that leflunomide causes primary drawback of this combination is its poten-fetal death or is teratogenic35; thus, women of child- tial for hepatotoxic effects28,29; thus, patients whobearing potential must have a negative pregnancy are treated with both agents should be monitoredtest before beginning treatment and must use reli- closely.
able contraception. Because of the drug's long half-life, discontinuing treatment before conception is
inadequate. The manufacturer suggests an elimina-
tion protocol of oral cholestyramine, 8 g three timesdaily for 11 days, and verification that the plasma TNF-a, an inflammatory cytokine that is released bylevel of the drug is below 0.02 mg per liter on two activated monocytes, macrophages, and T lympho-separate tests performed at least 2 weeks apart. This cytes, promotes inflammatory responses that areprotocol should also be followed by men who wish important in the pathogenesis of rheumatoid ar-to father a child.35 There are no reported effects of thritis.1,38,39 TNF-a binds to two receptors, the typedecreased fertility in patients who have taken 1 TNF receptor (p55) and the type 2 TNF receptorleflunomide.
(p75), that are expressed on many types of cells.40,41
The New England Journal of Medicine
Downloaded from nejm.org on February 3, 2011. For personal use only. No other uses without permission.
Copyright 2004 Massachusetts Medical Society. All rights reserved.
The biologic activity of TNF-a can be attenuated bysoluble TNF receptors.42
Table 3. Monitoring Recommendations.*
Patients with rheumatoid arthritis have high
concentrations of TNF-a in the synovial fluid.43,44
Obtain a complete blood count and alanine aminotransfer-
TNF-a is localized to the junction of the inflamma-
ase measurements at baseline and then monthly until
tory pannus and healthy cartilage,44 and high sy-
stable. Usual clinical practice is to repeat these tests
novial fluid TNF-a concentrations are associated
at intervals of 2 to 3 mo.
with the erosion of bone.45 Studies in animals have
Be clinically alert for tuberculosis, histoplasmosis, and other
provided key evidence that antagonizing TNF-a is
a viable therapeutic strategy. Inflammatory arthritis
Same as for etanercept.
developed in transgenic mice that expressed human
Same as for etanercept.
TNF-a,46 and in another animal model, treatment
Obtain a complete blood count at baseline, monthly for
with anti-TNF antibodies ameliorated arthritis.47
3 mo, and every 3 mo thereafter.
Subsequent proof-of-concept studies in patientswith rheumatoid arthritis indicated that blocking * Patients who are to receive etanercept, adalimumab, or infliximab should be
screened for previous exposure to tuberculosis before treatment is started.
TNF improved symptoms.48,49 Currently, three
Patients who are also receiving another disease-modifying antirheumatic
TNF-blocking drugs — etanercept, infliximab, and
drug such as methotrexate require additional monitoring as appropriate
adalimumab — are available for clinical use. Their
for that drug.
usual doses and pharmacokinetic characteristics arelisted in Table 1.
Methotrexate has been considered the standard
e t a n e r c e p t
against which newer disease-modifying antirheu-
Etanercept, a soluble TNF-receptor fusion protein, matic drugs should be evaluated.59 For example, ais composed of two dimers, each with an extracel- double-blind, randomized study in 632 patientslular, ligand-binding portion of the higher-affinity with early rheumatoid arthritis compared 10 or 25type 2 TNF receptor (p75) linked to the Fc portion mg of etanercept twice weekly with methotrexateof human IgG1. This fusion protein binds to both in a dose escalated over the course of eight weeks toTNF-a and TNF-b, thereby preventing each from a final weekly dose of 20 mg.9 Patients who receivedinteracting with its respective receptors.
the higher dose of etanercept had a more rapid re-sponse within the first 2 weeks, but after 12 months,
the ACR 20 response rates were similar — 72 per-
After subcutaneous administration, etanercept is cent in the 25-mg etanercept group and 65 percentabsorbed slowly, with concentrations peaking at ap- in the methotrexate group (P=0.16).9 The averageproximately 50 hours. Its half-life is generally four increases in radiologic score with 25 mg of etaner-days (Table 1).50,51 In healthy volunteers, systemic cept and methotrexate — 1.00 and 1.59 units, re-exposure to the drug, measured as the area under spectively — were not significantly different (P=the concentration–time curve, varied by a factor of 0.11).9 Another study indicated that patients witheight.50 A regimen of 50 mg once weekly appears to an inadequate response to methotrexate receivebe as effective as a regimen of 25 mg twice weekly.52 benefit when etanercept is added to their regimen
rather than placebo (Table 4).55
Efficacy in Rheumatoid Arthritis
After dose-finding studies,53 a 10-mg dose of infliximab
etanercept, a 25-mg dose of etanercept, and place- Infliximab, first approved for the treatment of
bo were compared in 234 patients in a six-month Crohn's disease, is a chimeric IgG1 anti–TNF-a
randomized study.54 Both doses of etanercept ap- antibody containing the antigen-binding region of
peared to be effective, resulting in ACR 20 response the mouse antibody and the constant region of the
rates of 51 percent and 59 percent, respectively, as human antibody. It binds to soluble and mem-
compared with 11 percent in the placebo group. The brane-bound TNF-a with high affinity, impairing
25-mg dose resulted in a more rapid response and the binding of TNF-a to its receptor. Infliximab
more frequent ACR 50 responses (40 percent) than also kills cells that express TNF-a through anti-
the 10-mg dose (24 percent) or placebo (5 percent) body-dependent and complement-dependent cy-
(Table 4).54
n engl j med 350;21
The New England Journal of Medicine
Downloaded from nejm.org on February 3, 2011. For personal use only. No other uses without permission.
Copyright 2004 Massachusetts Medical Society. All rights reserved.
The new england journal of medicine
Table 4. Rates of Response to TNF Antagonists Alone and in Combination with Methotrexate.*
No. of Patients
percentage of patients
Moreland et al.54
Weinblatt et al.55
Placebo plus methotrexate
Etanercept plus methotrexate
Placebo plus methotrexate
Infliximab plus methotrexate
Abbott Laboratories57
Weinblatt et al.58
Placebo plus methotrexate
Adalimumab plus methotrexate
* Infliximab is approved only for use in combination with methotrexate. The data presented are for the doses that are used
in clinical practice (infliximab, 3 mg per kilogram of body weight every 8 weeks; etanercept, 25 mg by subcutaneous in-jection twice a week; and adalimumab, 40 mg by subcutaneous injection every other week), as studied in double-blind, controlled studies lasting 24 to 30 weeks. The studies demonstrating efficacy for the various drugs had different designs; thus, the response rates cannot be compared directly. Response rates for all drugs at all levels of ACR response were sig-nificantly higher than those in the corresponding placebo group.
kilogram every 8 weeks) to 59 percent (with 10 mg
There are marked differences among patients in the per kilogram every 4 weeks).8 All regimens of in-pharmacokinetics of infliximab. In one study in- fliximab plus methotrexate were more effective thanvolving 428 subjects, trough concentrations eight methotrexate plus placebo in preventing progres-weeks after the intravenous administration of 3 mg sion as measured radiologically.8 The ACR 50 re-of infliximab per kilogram of body weight varied sponse rates in all the infliximab groups were simi-by a factor of more than 100.62 Pharmacokinetic lar after 24 weeks,56 but after 54 weeks, the responsemodeling indicated that shortening the interval be- rate in the group receiving 3 mg per kilogram everytween doses to six weeks would increase the trough 8 weeks (21 percent) was significantly lower thanlevels more effectively than increasing the dose by those in the two groups receiving 10 mg per kilo-100 mg.62
gram (39 percent among those given a dose every8 weeks and 38 percent among those given a dose
Efficacy in Rheumatoid Arthritis
every 4 weeks).8 As a result of these studies, a stan-
A single infusion of infliximab (1 or 10 mg per kilo- dard regimen has evolved (Table 1). Patients who do
gram) was reported to improve symptoms of rheu- not have an adequate response or who have an ini-
matoid arthritis rapidly, providing early evidence of tial response followed by a relapse may have a better
the effectiveness of TNF antagonism.63 Subsequent response either if the interval between infusions is
studies demonstrated that monotherapy with inflix- decreased to every four to six weeks or if the dose is
imab (at a dose of 3 or 10 mg per kilogram) was su- increased.62
perior to placebo,64 but the frequent development
of anti-infliximab antibodies led to its use in com- adalimumab
bination with methotrexate rather than as mono- Adalimumab is a recombinant human IgG1 mono-
therapy. Efficacy and the dose–response relation clonal antibody that binds to human TNF-a with
were defined in a study involving 428 patients who high affinity, both impairing cytokine binding to its
had active rheumatoid arthritis despite methotrex- receptors and lysing cells that express TNF-a on
ate therapy.8,56 Four regimens — infliximab at a their surface.
dose of 3 or 10 mg per kilogram every four or eight
weeks combined with methotrexate — were all sim- Clinical Pharmacology
ilarly and significantly more effective than metho- After subcutaneous administration, adalimumab is
trexate plus placebo. After 54 weeks, the ACR 20 re- absorbed slowly, with peak concentrations reached
sponse rate ranged from 42 percent (with 3 mg per after approximately 130 hours. There is substantial
The New England Journal of Medicine
Downloaded from nejm.org on February 3, 2011. For personal use only. No other uses without permission.
Copyright 2004 Massachusetts Medical Society. All rights reserved.
interpatient variability in disposition,65 but clear- older than among younger patients.83 The back-ance is similar in men and women and appears to be ground risk of serious infection is approximatelyunaffected by age or body weight.66 The addition of twice as high among patients with rheumatoid ar-methotrexate to the regimen reduced adalimumab thritis as among those without this condition84;clearance by 20 percent after a single dose and by therefore, it is difficult to interpret sporadic reports44 percent after multiple doses.57
of infection in patients receiving anti-TNF thera-py. However, the risk of opportunistic infections,
Efficacy in Rheumatoid Arthritis
including histoplasmosis and tuberculosis, is in-
In a randomized, double-blind study, the ACR 20 re- creased. Such observations are congruent with ani-sponse rate for 40 mg of adalimumab administered mal studies showing that TNF is important for gran-weekly was similar to the rate for the same dose ad- uloma formation85 and preventing the reactivationministered every other week (53 percent and 46 of latent tuberculosis.86percent, respectively), and both were significantly
Tuberculosis has been reported in association
higher than the rate with placebo (19 percent) (Ta- with all TNF antagonists. Data from the FDA Ad-ble 4).67,68 Adalimumab appears to have additive verse Event Reporting System, a passive surveil-effects when used with methotrexate. For example, lance system that has no reliable denominator andan ACR 20 response was achieved in a significantly may underestimate true incidence, indicated thatgreater proportion of patients who received metho- although a similar number of patients had been ex-trexate plus 20, 40, or 80 mg of adalimumab every posed, 70 cases of tuberculosis had been reportedtwo weeks than of those who received methotrexate after treatment with infliximab and 9 after etaner-plus placebo (48 percent, 67 percent, 66 percent, cept treatment.87 The rate of tuberculosis amongand 15 percent, respectively).58
patients with rheumatoid arthritis who had beentreated with infliximab was 24.4 cases per 100,000,as compared with the background rate of 6.2 cases
per 100,000 patients with this illness.87 In early
studies of adalimumab therapy, tuberculosis devel-
Initial studies reported substantial efficacy with al- oped in 8 of 542 patients.79 The introduction ofmost no serious adverse effects,9,53,56,58,69 and screening procedures and the use of lower doses ofpostmarketing data were also reassuring.70 How- adalimumab reduced the frequency to 5 of the nextever, wider use of TNF antagonists has resulted in 1900 patients.79reports — largely case reports or small series of pa-
Tuberculosis in patients who are receiving anti-
tients — that link TNF-antagonist use with a wide TNF therapy most often arises from the reactiva-
range of adverse events, including infections, can- tion of latent infection and usually occurs within
cer, vasculitis, lupus-like autoimmune disease, mul- the first two to five months of treatment.87 Extra-
tiple sclerosis–like demyelinating disorders, liver pulmonary and disseminated disease is common,
disease, hematologic abnormalities including aplas- and atypical clinical presentations may lead to de-
tic anemia and lymphoma, severe allergy, and asep- layed diagnosis and increased morbidity.87
tic meningitis.71-79 The relationships between anti-
TNF therapy and many of these adverse events are malignant disease
unknown. Much safety information regarding TNF Lymphoma has been reported in association with
antagonists is unpublished but may be found in the all three TNF antagonists,77 but whether or not
transcript of a meeting of the FDA Arthritis Adviso- there is a causal relationship is debated. The reason
ry Committee.79
for the uncertainty is that the incidence of lympho-ma is increased among patients with rheumatoid
i n f e c t i o n
arthritis and increases with the severity of the con-
Serious bacterial infections, tuberculosis, atypi- dition.88,89 Therefore, the increased incidence ofcal mycobacterial infection, aspergillosis, histo- lymphoma among patients who receive TNF an-plasmosis, coccidioidomycosis, listeriosis, Pneu- tagonists, which is estimated to be 2.3 to 6.4 timesmocystis carinii pneumonia, cryptococcal infections, that in the general population, could be ascribed tocytomegalovirus, and other infections have oc- either severe rheumatoid arthritis or its treatment.79curred,71,72,75,79-82 and such infections may be The transcripts of the FDA meeting in which all themore common among patients 65 years of age or studies were examined together indicate that lym-
n engl j med 350;21
The New England Journal of Medicine
Downloaded from nejm.org on February 3, 2011. For personal use only. No other uses without permission.
Copyright 2004 Massachusetts Medical Society. All rights reserved.
The new england journal of medicine
phoma developed in six patients who were receiv- with adalimumab plus methotrexate (in 4 per-
ing TNF antagonists during the controlled portion cent).58 However, drug-induced systemic lupus
of clinical trials, as compared with none of the con- erythematosus is rare78,92,93: the FDA Adverse Event
trol patients,79 indicating that a causal relationship Reporting System identified 16 cases after the use
is plausible. The type of lymphoma reported is sim- of etanercept.94
ilar to that occurring in the general population of pa-
tients with rheumatoid arthritis.77 Apart from lym- demyelinating syndromes
phoma, the incidence of cancer is not significantly Exacerbation of previously quiescent multiple scle-
altered.79
rosis and new-onset demyelinating neurologic dis-ease have been reported.76 The number of patients
affected is not known, but in the FDA Adverse Event
Minor redness and itching at the injection site, last- Reporting System, 18 cases were reported after
ing a few days, are common among patients who re- etanercept therapy and 2 after infliximab therapy.
ceive etanercept and adalimumab.54,68 Symptoms, The range of symptoms was broad and included
most often headache and nausea, occur in 20 per- paresthesia (in 65 percent of patients), optic neuri-
cent of patients during the infusion of infliximab tis (in 40 percent), and confusion (in 25 percent).76
and appear to be controllable with the use of anti- Although a causal relationship has not been estab-
histamines or by slowing the infusion rate.62 Symp- lished, the fact that another TNF antagonist, lener-
toms suggestive of an immediate hypersensitivity cept, worsened symptoms in patients with multiple
response, such as urticaria, occur in 2 percent of pa- sclerosis95 renders the association plausible.
tients.56 Serious anaphylaxis is uncommon and oc-
curred in 2 of 500 patients with Crohn's disease who heart failure
were treated with infliximab.90
TNF-a levels are elevated in patients with heart fail-ure and associated with decreased cardiac contrac-
tility.96 Initial reports on anti-TNF therapy for heart
Antibodies to etanercept developed in 3 percent of failure were encouraging.97,98 However, subsequentpatients,9 but their clinical significance is unknown. studies of etanercept and infliximab in heart failureIn other reports, human antichimeric antibodies to were stopped early because of lack of evidence ofinfliximab developed in 53, 21, and 7 percent of pa- benefit and, in the case of infliximab, increasedtients who were receiving 1, 3, and 10 mg of inflix- mortality.79,99imab per kilogram, respectively.64 The frequencyof antichimeric antibodies was lower (8.5 percent)
among patients who were treated with infliximab
at a dose of 3 or 10 mg per kilogram plus concomi-tant methotrexate.62 These antibodies accelerated TNF antagonists appear to be among the most ef-the clearance of infliximab62 and were associated fective treatments available for rheumatoid arthritis.
with increased infusion reactions and shorter re- The response is generally rapid, often occurringsponses in patients with Crohn's disease.91 Anti- within a few weeks, although not all patients havebodies to adalimumab developed in 12 percent of a response. There is little information regardingpatients; the rate was reduced to 1 percent with con- head-to-head comparisons between various TNFcurrent methotrexate treatment.92 The ACR 20 re- antagonists or between TNF antagonists and othersponse rate was lower in patients treated with adal- disease-modifying antirheumatic drugs. There isimumab in whom antibodies developed.92
also a need for data that will show whether particu-
Antinuclear antibodies were detected in approx- lar adverse effects are class effects. Until convinc-
imately 60 percent of patients who were receiving ing data to the contrary are available, similar pre-infliximab and methotrexate, as compared with 26 cautions are appropriate with regard to all TNFpercent of those who were treated with methotrex- antagonists.
ate alone.8 Antibodies to double-stranded DNA
Anti-TNF therapy should not be started in pa-
developed after etanercept treatment (in 4 percent tients with active infection and should be discon-of patients),54 after treatment with infliximab plus tinued if a serious infection occurs. Chronic or re-methotrexate (in 10 percent),8 and after treatment current infection is a relative contraindication. All
The New England Journal of Medicine
Downloaded from nejm.org on February 3, 2011. For personal use only. No other uses without permission.
Copyright 2004 Massachusetts Medical Society. All rights reserved.
patients should be screened for latent tuberculosis pairment.106 Hemodialysis and peritoneal dialy-
before anti-TNF therapy is begun, and should be sis do not appear to remove substantial amounts of
treated before starting such therapy if they test pos- anakinra.106
itive.100 Physicians should be alert to the increased
risk of tuberculosis and other opportunistic infec- efficacy in rheumatoid arthritis
tions.
Anakinra, alone or in combination with metho-
TNF antagonists should also be avoided in pa- trexate, has been more effective than placebo in
tients with any demyelinating disorder or heart fail- randomized, controlled trials involving approxi-ure, and therapy should be discontinued if such an mately 900 patients with rheumatoid arthritis (Ta-illness develops. Rare cases of pancytopenia, aplas- ble 5).108,109 A long-term extension study docu-tic anemia, and liver failure have been reported,79 mented that responses seen in the first 24-weekbut no specific monitoring is recommended.
phase of the study were durable; after 48 weeks, 18percent of patients treated with anakinra had anACR 50 response, and 3 percent had an ACR 70 re-
sponse.110 Treatment with anakinra also signifi-
Interleukin-1, produced by monocytes, macrophag- cantly slows the rate of damage, as measured on ra-
es, and some specialized cells in the synovial lining, diography.7
has inflammatory effects that include the induction
of interleukin-6 and cyclooxygenase-2.101 The ac- adverse effects
tions of interleukin-1 are down-regulated by inter- The most common adverse event is dose-dependent
leukin-1–receptor antagonist, a natural inhibitor skin irritation at the injection site, occurring in 50
that competes for binding to interleukin-1 recep- to 80 percent of patients in trials. Most reactions
tors. In mice that are deficient in interleukin-1– appeared to be mild, and resolved within a few
receptor antagonist, a chronic inflammatory ar- weeks.105 A small number of patients reported
thritis develops, with features similar to those of more severe allergic-type reactions involving itch-
rheumatoid arthritis.102
ing, swelling, and pain.107
Anakinra is a recombinant form of human inter-
The risk of infection, primarily bacterial, appears
leukin-1–receptor antagonist that targets the type I to be increased. Serious infections occurred in 2.1
interleukin-1 receptor that is expressed in many tis- percent of patients receiving anakinra, as compared
sues. In patients with rheumatoid arthritis, levels with 0.4 percent of those receiving placebo, in one
of this receptor antagonist in the inflamed joint are study involving 1000 patients (P=0.07); no oppor-
lower than would be required for the inhibition of tunistic infections were observed.111
the amount of interleukin-1 present, and this im-
balance is thought to contribute to the persistence clinical use in rheumatoid arthritis
of joint inflammation.103,104
Anakinra may be useful in patients who have no re-sponse to or are unable to tolerate methotrexate,
leflunomide, or TNF antagonists. Anakinra therapy
Anakinra is identical to the nonglycosylated formof the endogenous protein except for the additionof one N-terminal methionine.105 Because it has a
Table 5. Rates of Response to 24 Weeks of Anakinra Therapy.*
short half-life (Table 1),106 daily administration is
more effective than injections given weekly or three
Combination Therapy with Methotrexate
times a week.107 Renal clearance eliminates 80 per-cent of infused anakinra in rats,104 and humans with
renal failure have markedly decreased clearance;
percentage of patients
clearance decreases by half in moderate renal dis-
ease, by two thirds in severe renal disease, and by 75percent in end-stage renal disease.106 In such pa-
tients, adjustment of the dose or frequency of injec-
tion may be indicated; computer simulation has * Data on monotherapy are from Bresnihan et al.,108 and data on combination
suggested that a dose of 100 mg every second day
therapy are from Cohen et al.109
may be appropriate in patients with severe renal im-
n engl j med 350;21
The New England Journal of Medicine
Downloaded from nejm.org on February 3, 2011. For personal use only. No other uses without permission.
Copyright 2004 Massachusetts Medical Society. All rights reserved.
The new england journal of medicine
should not be started in patients with active infec- disease,115 uveitis,116 and vasculitis.117 However,tion and should be discontinued if a serious infec- much remains unknown. Hard data regarding thetion occurs. Caution should be exercised when con- comparative long-term efficacy and toxicity of thesesidering the use of anakinra in patients with chronic agents, as well as the variable rates of response, willor recurrent infection. Because reversible neutrope- be important for rational clinical use.
nia and thrombocytopenia have occurred in a small
Treatments for rheumatoid arthritis continue to
number of patients, monitoring the complete blood advance rapidly, and many new drugs are under in-count is recommended (Table 3).
vestigation; some have shown promise in clinical
The combination of anakinra and methotrexate trials that have been published. These include tac-
appears to be well tolerated.109 However, both drugs rolimus,118 rituximab,119 an interleukin-6 antago-can lower the white-cell count; thus, regular labora- nist,120 and a fusion protein — cytotoxic T-lympho-tory monitoring is required. Concomitant use of cyte–associated antigen 4-IgG1 (CTLA-4–Ig) —anakinra and a TNF antagonist may increase the that blocks T-cell costimulatory pathways.121 Oth-risk of infections and should be avoided.112
er drugs that are now at earlier stages of develop-ment include pegylated, soluble TNF receptor an-tagonists and agents that trap cytokines, block
interleukin-15, prevent the cleavage of human com-
plement component C5, or inhibit adhesion mole-
The drugs discussed above appear to be relatively cules.122 The introduction of additional effectivesafe and effective in the short-to-intermediate–term therapies for rheumatoid arthritis will improve thetreatment of rheumatoid arthritis. In addition, the outlook for patients, since even with the range ofrole of these agents in the treatment of illnesses oth- therapies currently available, some patients stiller than rheumatoid arthritis is evolving. Infliximab have poorly or incompletely controlled disease.
is an effective treatment for Crohn's disease,91 and
Supported by a grant (HS1-0384) from the Center for Education
TNF antagonists are finding a place in the treatment and Research on Therapeutics and grants (HL 65082 and HL 67964)
from the U.S. Public Health Service.
of many conditions, including psoriasis,113 anky-
Dr. Olsen reports having received an honorarium from Aventis
losing spondylitis,114 juvenile arthritis,113 Still's and research support from Bristol-Myers Squibb in the past two
r e f e r e n c e s
Choy EH, Panayi GS. Cytokine pathways
Arthritis Rheum 2000;43:495-505. [Erra-
12. Siemasko K, Chong AS, Jack HM, Gong
and joint inflammation in rheumatoid ar-
tum, Arthritis Rheum 2000;43:1345.]
H, Williams JW, Finnegan A. Inhibition of
thritis. N Engl J Med 2001;344:907-16.
Jiang Y, Genant HK, Watt I, et al. A mul-
JAK3 and STAT6 tyrosine phosphorylation
Bukhari M, Lunt M, Harrison BJ, Scott
ticenter, double-blind, dose-ranging, ran-
by the immunosuppressive drug lefluno-
DG, Symmons DP, Silman AJ. Rheumatoid
domized, placebo-controlled study of re-
mide leads to a block in IgG1 production.
factor is the major predictor of increasing
combinant human interleukin-1 receptor
J Immunol 1998;160:1581-8.
severity of radiographic erosions in rheuma-
antagonist in patients with rheumatoid ar-
13. Xu X, Shen J, Mall JW, et al. In vitro and
toid arthritis: results from the Norfolk Ar-
thritis: radiologic progression and correla-
in vivo antitumor activity of a novel immu-
thritis Register Study, a large inception co-
tion of Genant and Larsen scores. Arthritis
nomodulatory drug, leflunomide: mecha-
hort. Arthritis Rheum 2002;46:906-12.
nisms of action. Biochem Pharmacol 1999;
Felson DT, Anderson JJ, Boers M, et al.
Lipsky PE, van der Heijde DMFM, St
Preliminary definition of improvement in
Clair EW, et al. Infliximab and methotrexate
14. Burger D, Begue-Pastor N, Benavent S,
rheumatoid arthritis. Arthritis Rheum 1995;
in the treatment of rheumatoid arthritis.
et al. The active metabolite of leflunomide,
N Engl J Med 2000;343:1594-602.
A77 1726, inhibits the production of prosta-
Pincus T, Stein CM. ACR 20: clinical or
Bathon JM, Martin RW, Fleischmann
glandin E(2), matrix metalloproteinase 1 and
statistical significance? Arthritis Rheum
RM, et al. A comparison of etanercept and
interleukin 6 in human fibroblast-like syn-
methotrexate in patients with early rheuma-
oviocytes. Rheumatology (Oxford) 2003;42:
Felson DT, Anderson JJ, Lange ML,
toid arthritis. N Engl J Med 2000;343:1586-
Wells G, LaValley MP. Should improvement
93. [Errata, N Engl J Med 2001;344:76, 240.]
15. Breedveld FC, Dayer JM. Leflunomide:
in rheumatoid arthritis clinical trials be de-
10. Kremer JM. Methotrexate and lefluno-
mode of action in the treatment of rheuma-
fined as fifty percent or seventy percent im-
mide: biochemical basis for combination
toid arthritis. Ann Rheum Dis 2000;59:841-
provement in core set measures, rather than
therapy in the treatment of rheumatoid ar-
twenty percent? Arthritis Rheum 1998;41:
thritis. Semin Arthritis Rheum 1999;29:14-
16. Rozman B. Clinical pharmacokinetics
of leflunomide. Clin Pharmacokinet 2002;
Sharp JT, Strand V, Leung H, Hurley F,
11. Manna SK, Mukhopadhyay A, Aggarwal
Loew-Friedrich I. Treatment with lefluno-
BB. Leflunomide suppresses TNF-induced
17. Beaman JM, Hackett LP, Luxton G, Illett
mide slows radiographic progression of
cellular responses: effects on NF-kappa B,
KF. Effect of hemodialysis on leflunomide
rheumatoid arthritis: results from three ran-
activator protein-1, c-Jun N-terminal protein
plasma concentrations. Ann Pharmacother
domized controlled trials of leflunomide in
kinase, and apoptosis. J Immunol 2000;165:
patients with active rheumatoid arthritis.
18. Lim V, Pande I. Leflunomide can poten-
The New England Journal of Medicine
Downloaded from nejm.org on February 3, 2011. For personal use only. No other uses without permission.
Copyright 2004 Massachusetts Medical Society. All rights reserved.
tiate the anticoagulant effect of warfarin.
32. Auer J, Hinterreiter M, Allinger S, Kirch-
alpha in the knee-joints of patients with
BMJ 2002;325:1333.
gatterer A, Knoflach P. Severe pancytopenia
rheumatoid arthritis. Inflamm Res 1995;44:
19. Smolen JS, Kalden JR, Scott DL, et al. Ef-
after leflunomide in rheumatoid arthritis.
ficacy and safety of leflunomide compared
Acta Med Austriaca 2000;27:131-2.
46. Keffer J, Probert L, Cazlaris H, et al.
with placebo and sulphasalazine in active
33. Marchesoni A, Arreghini M, Panni B,
Transgenic mice expressing human tumour
rheumatoid arthritis: a double-blind, ran-
Battafarano N, Uziel L. Life-threatening re-
necrosis factor: a predictive genetic model
domised, multicentre trial. Lancet 1999;
versible bone marrow toxicity in a rheuma-
of arthritis. EMBO J 1991;10:4025-31.
toid arthritis patient switched from lefluno-
47. Williams RO, Feldmann M, Maini RN.
20. Perez-Ruiz F, Nolla JM. Influence of
mide to infliximab. Rheumatology (Oxford)
Anti-tumor necrosis factor ameliorates joint
leflunomide on renal handling of urate and
disease in murine collagen-induced arthri-
phosphate in patients with rheumatoid ar-
34. Aventis statement in response to report-
tis. Proc Natl Acad Sci U S A 1992;89:9784-
thritis. J Clin Rheumatol 2003;9:215-8.
ed cases of interstitial pneumonitis in Japan.
21. Cohen S, Cannon GW, Schiff M, et al.
(Accessed April 26, 2004, at http://www.
48. Elliott MJ, Maini RN, Feldmann M, et al.
Two-year, blinded, randomized, controlled
Treatment of rheumatoid arthritis with chi-
trial of treatment of active rheumatoid ar-
35. Brent RL. Teratogen update: reproduc-
meric monoclonal antibodies to tumor ne-
thritis with leflunomide compared with
tive risks of leflunomide (Arava): a pyrimi-
crosis factor alpha. Arthritis Rheum 1993;
methotrexate. Arthritis Rheum 2001;44:
dine synthesis inhibitor: counseling women
taking leflunomide before or during preg-
49. Elliott MJ, Maini RN, Feldmann M, et al.
22. Strand V, Cohen S, Schiff M, et al. Treat-
nancy and men taking leflunomide who are
Repeated therapy with monoclonal antibody
ment of active rheumatoid arthritis with
contemplating fathering a child. Teratology
to tumour necrosis factor alpha (cA2) in pa-
leflunomide compared with placebo and
tients with rheumatoid arthritis. Lancet
methotrexate. Arch Intern Med 1999;159:
36. Sokka T. Pincus T. Contemporary disease
modifying antirheumatic drugs (DMARD)
50. Korth-Bradley JM, Rubin AS, Hanna
23. Schuna AA, Megeff C. New drugs for the
in patients with recent onset rheumatoid ar-
RK, Simcoe DK, Lebsack ME. The pharma-
treatment of rheumatoid arthritis. Am J
thritis in a US private practice: methotrexate
cokinetics of etanercept in healthy volun-
Health Syst Pharm 2000;57:225-34.
as the anchor drug in 90% and new DMARD
teers. Ann Pharmacother 2000;34:161-4.
24. American College of Rheumatology. Re-
in 30% of patients. J Rheumatol 2002;29:
51. Lee H, Kimko HC, Rogge M, Wang D,
ports of leflunomide hepatotoxicity in pa-
Nestorov I, Peck CC. Population pharmaco-
tients with rheumatoid arthritis. Hotline.
37. Mladenovic V, Domljan Z, Rozman B, et
kinetics and pharmacodynamics modeling
(Accessed April 26, 2004, at http://www.
al. Safety and effectiveness of leflunomide in
of etanercept using logistic regression
the treatment of patients with active rheu-
analysis. Clin Pharmacol Ther 2003;73:348-
matoid arthritis: results of a randomized,
25. Idem. Hotline. FDA meeting March
placebo-controlled, phase II study. Arthritis
52. Keystone EC, Schiff MH, Kremer JM, et
2003: update on the safety of new drugs for
al. Once-weekly administration of 50 mg
rheumatoid arthritis. Part III: safety and effi-
38. Bazzoni F, Beutler B. The tumor necro-
etanercept in patients with active rheuma-
cacy update on leflunomide (Arava) (Ac-
sis factor ligand and receptor families.
toid arthritis: results of a multicenter, ran-
cessed April 26, 2004, at http://www.
N Engl J Med 1996;334:1717-25.
domized, double-blind, placebo-controlled
39. Taylor PC, Peters AM, Paleolog E, et al.
trial. Arthritis Rheum 2004;50:353-63.
Reduction of chemokine levels and leuko-
53. Moreland LW, Baumgartner SW, Schiff
26. Food and Drug Administration, Center
cyte traffic to joints by tumor necrosis factor
MH, et al. Treatment of rheumatoid arthritis
for Drug Evaluation and Research, Arthritis
alpha blockade in patients with rheumatoid
with a recombinant human tumor necrosis
Advisory Committee meeting, Wednesday
arthritis. Arthritis Rheum 2000;43:38-47.
factor receptor (p75)-Fc fusion protein.
March 5, 2003. (Accessed April 26, 2004, at
40. Black RA, Rauch CT, Kozlosky CJ, et al.
N Engl J Med 1997;337:141-7.
A metalloproteinase disintegrin that releas-
54. Moreland LW, Schiff MH, Baumgartner
es tumour-necrosis factor-alpha from cells.
SW, et al. Etanercept therapy in rheumatoid
27. Weinblatt ME, Kremer JM, Coblyn JS, et
arthritis: a randomized, controlled trial.
al. Pharmacokinetics, safety, and efficacy of
41. Newton RC, Solomon KA, Covington
Ann Intern Med 1999;130:478-86.
combination treatment with methotrexate
MB, et al. Biology of TACE inhibition. Ann
55. Weinblatt ME, Kremer JM, Bankhurst
and leflunomide in patients with active
Rheum Dis 2001;60:Suppl 3:iii25-iii32.
AD, et al. A trial of etanercept, a recombi-
rheumatoid arthritis. Arthritis Rheum 1999;
42. Smith CA, Davis T, Anderson D, et al. A
nant tumor necrosis factor receptor:Fc fu-
receptor for tumor necrosis factor defines
sion protein, in patients with rheumatoid
28. Kremer JM, Genovese MC, Cannon GW,
an unusual family of cellular and viral pro-
arthritis receiving methotrexate. N Engl J
et al. Concomitant leflunomide therapy in
teins. Science 1990;248:1019-23.
Med 1999;340:253-9.
patients with active rheumatoid arthritis de-
43. Saxne T, Palladino MA Jr, Heinegard D,
56. Maini R, St Clair EW, Breedveld F, et al.
spite stable doses of methotrexate: a ran-
Talal N, Wollheim FA. Detection of tumor
Infliximab (chimeric anti-tumour necrosis
domized, double-blind, placebo-controlled
necrosis factor alpha but not tumor necrosis
factor alpha monoclonal antibody) versus
trial. Ann Intern Med 2002;137:726-33.
factor beta in rheumatoid arthritis synovial
placebo in rheumatoid arthritis patients re-
29. Weinblatt ME, Dixon JA, Falchuk KR.
fluid and serum. Arthritis Rheum 1988;31:
ceiving concomitant methotrexate: a ran-
Serious liver disease in a patient receiving
domised phase III trial. Lancet 1999;354:
methotrexate and leflunomide. Arthritis
44. Chu CQ, Field M, Feldmann M, Maini
RN. Localization of tumor necrosis factor al-
57. Humira (adalimumab). Abbott Labora-
30. Coblyn JS, Shadick N, Helfgott S.
pha in synovial tissues and at the cartilage-
tories (package insert). (Accessed April 26,
Leflunomide-associated weight loss in rheu-
pannus junction in patients with rheuma-
2004, at http://www.fda.gov/cder/foi/label/
matoid arthritis. Arthritis Rheum 2001;44:
toid arthritis. Arthritis Rheum 1991;34:
58. Weinblatt ME, Keystone EC, Furst DE,
31. Rozman B, Praprotnik S, Logar D, et al.
45. Neidel J, Schulze M, Lindschau J. Asso-
et al. Adalimumab, a fully human anti-
Leflunomide and hypertension. Ann Rheum
ciation between degree of bone-erosion and
tumor necrosis factor alpha monoclonal
Dis 2002;61:567-9.
synovial fluid-levels of tumor necrosis factor
antibody, for the treatment of rheumatoid
n engl j med 350;21
The New England Journal of Medicine
Downloaded from nejm.org on February 3, 2011. For personal use only. No other uses without permission.
Copyright 2004 Massachusetts Medical Society. All rights reserved.
The new england journal of medicine
arthritis in patients taking concomitant
71. Warris A, Bjørneklett A, Gaustad P. In-
cidal granulomas during BCG infection.
methotrexate: the ARMADA trial. Arthritis
vasive pulmonary aspergillosis associated
Rheum 2003;48:35-45. [Erratum, Arthritis
with infliximab therapy. N Engl J Med 2001;
86. Mohan VP, Scanga CA, Yu K, et al. Ef-
Rheum 2003;48:855.]
fects of tumor necrosis factor alpha on host
59. American College of Rheumatology
72. Kamath BM, Mamula P, Baldassano RN,
immune response in chronic persistent tu-
subcommittee on Rheumatoid Arthritis
Markowitz JE. Listeria meningitis after
berculosis: possible role for limiting pathol-
Guidelines. Guidelines for the management
treatment with infliximab. J Pediatr Gastro-
ogy. Infect Immun 2001;69:1847-55.
of rheumatoid arthritis: 2002 update. Ar-
enterol Nutr 2002;34:410-2.
87. Keane J, Gershon S, Wise RP, et al. Tu-
thritis Rheum 2002;46:328-46.
73. Kashyap AS, Kashyap S. Infliximab-
berculosis associated with infliximab, a tu-
60. Knight DM, Trinh H, Le J, et al. Con-
induced aseptic meningitis. Lancet 2002;
mor necrosis factor a–neutralizing agent.
struction and initial characterization of a
N Engl J Med 2001;345:1098-104.
mouse-human chimeric anti-TNF antibody.
74. McCain ME, Quinet RJ, Davis WE. Etan-
88. Ekstrom K, Hjalgrim H, Brandt L, et al.
Mol Immunol 1993;30:1443-53.
ercept and infliximab associated with cuta-
Risk of malignant lymphomas in patients
61. Scallon BJ, Moore MA, Trinh H, Knight
neous vasculitis. Rheumatology (Oxford)
with rheumatoid arthritis and in their first-
DM, Ghrayeb J. Chimeric anti-TNF-alpha
degree relatives. Arthritis Rheum 2003;48:
monoclonal antibody cA2 binds recombi-
75. Nakelchik M, Mangino JE. Reactivation
nant transmembrane TNF-alpha and acti-
of histoplasmosis after treatment with in-
89. Baecklund E, Ekbom A, Sparen P, Felte-
vates immune effector functions. Cytokine
fliximab. Am J Med 2002;112:78.
lius N, Klareskog L. Disease activity and risk
76. Mohan N, Edwards ET, Cupps TR, et al.
of lymphoma in patients with rheumatoid
62. St Clair EW, Wagner CL, Fasanmade AA,
Demyelination occurring during anti-tumor
arthritis: nested case-control study. BMJ
et al. The relationship of serum infliximab
necrosis factor alpha therapy for inflamma-
concentrations to clinical improvement in
tory arthritides. Arthritis Rheum 2001;44:
90. Colombel JF, Loftus EV Jr, Tremaine WJ,
rheumatoid arthritis: results from ATTRACT,
et al. The safety profile of infliximab in pa-
a multicenter, randomized, double-blind,
77. Brown SL, Greene MH, Gershon SK,
tients with Crohn's disease: the Mayo Clinic
placebo-controlled trial. Arthritis Rheum
Edwards ET, Braun MM. Tumor necrosis
experience in 500 patients. Gastroenterolo-
factor antagonist therapy and lymphoma
gy 2004;126:19-31.
63. Elliott MJ, Maini RN, Feldmann M, et al.
development: twenty-six cases reported to
91. Baert F, Noman M, Vermeire S, et al. In-
Randomised double-blind comparison of
the Food and Drug Administration. Arthritis
fluence of immunogenicity on the long-
chimeric monoclonal antibody to tumour
term efficacy of infliximab in Crohn's dis-
necrosis factor alpha (cA2) versus placebo
78. Shakoor N, Michalska M, Harris CA,
ease. N Engl J Med 2003;348:601-8.
in rheumatoid arthritis. Lancet 1994;344:
Block JA. Drug-induced systemic lupus
92. Food and Drug Administration. Adali-
erythematosus associated with etanercept
mumab — for use in the treatment of rheu-
64. Maini RN, Breedveld FC, Kalden JR, et al.
therapy. Lancet 2002;359:579-80.
matoid arthritis. Abbott biologic licensing
Therapeutic efficacy of multiple intravenous
79. Food and Drug Administration, Center
application. (Accessed April 26, 2004, at
infusions of anti-tumor necrosis factor alpha
for Drug Evaluation and Research, Arthritis
monoclonal antibody combined with low-
Advisory Committee. Safety update meeting
dose weekly methotrexate in rheumatoid ar-
on TNF blocking agents. Tuesday, March 4,
93. Charles PJ, Smeenk RJ, De Jong J, Feld-
thritis. Arthritis Rheum 1998;41:1552-63.
2003. (Accessed April 26, 2004, at http://
mann M, Maini RN. Assessment of antibod-
65. den Broeder A, van de Putte LBA, et al. A
ies to double-stranded DNA induced in rheu-
single dose, placebo controlled study of the
transcripts/ 3930T1.htm.)
matoid arthritis patients following treatment
fully human anti-tumor necrosis factor-a
80. Kroesen S, Widmer AF, Tyndall A,
with infliximab, a monoclonal antibody to
antibody adalimumab (D2E7) in patients
Hasler P. Serious bacterial infections in pa-
tumor necrosis factor alpha: findings in
with rheumatoid arthritis. J Rheumatol
tients with rheumatoid arthritis under anti-
open-label and randomized placebo-con-
TNF-alpha therapy. Rheumatology (Oxford)
trolled trials. Arthritis Rheum 2000;43:
66. Velagapudi RB, Noertershauser P, Bank-
mann Y, et al. Pharmacokinetics of adali-
81. Lee JH, Slifman NR, Gershon SK, et al.
94. Mohan AK, Edwards ET, Cote TR, Siegel
mumab (D2E7), a fully human anti-TNF-a
Life-threatening histoplasmosis complicat-
JN, Braun MM. Drug-induced systemic lu-
monoclonal antibody, following a single in-
ing immunotherapy with tumor necrosis fac-
pus erythematosus and TNF-alpha block-
travenous injection in rheumatoid arthritis
tor alpha antagonists infliximab and etaner-
ers. Lancet 2002;360:646.
patients treated with methotrexate. Arthritis
cept. Arthritis Rheum 2002;46:2565-70.
95. TNF neutralization in MS: results of a
Rheum 2002;46:Suppl:S133. abstract.
82. Helbling D, Breitbach TH, Krause M.
randomized, placebo-controlled multicenter
67. van de Putte LBA, Atkins C, Malaise M,
Disseminated cytomegalovirus infection in
study. Neurology 1999;53:457-65.
et al. Adalimumab (D2E7) monotherapy in
Crohn's disease following anti-tumour ne-
96. Francis GS. TNF-alpha and heart fail-
the treatment of patients with severely active
crosis factor therapy. Eur J Gastroenterol
ure: the difference between proof of princi-
rheumatoid arthritis. Arthritis Rheum 2002;
ple and hypothesis testing. Circulation 1999;
83. Fleischmann RM, Baumgartner SW,
68. Abbott Laboratories. Humira (adalimu-
Tindall EA, et al. Response to etanercept
97. Fichtlscherer S, Rossig L, Breuer S, Vasa
mab). Advisory Committee briefing docu-
(Enbrel) in elderly patients with rheumatoid
M, Dimmeler S, Zeiher AM. Tumor necrosis
ment, February 4, 2003. (Accessed April
arthritis: a retrospective analysis of clinical
factor antagonism with etanercept improves
26, 2004, at http://www.fda.gov/ohrms/
trial results. J Rheumatol 2003;30:691-6.
systemic endothelial vasoreactivity in pa-
84. Doran MF, Crowson CS, Pond GR,
tients with advanced heart failure. Circula-
O'Fallon WM, Gabriel SE. Frequency of
69. Moreland LW, Cohen SB, Baumgartner
infection in patients with rheumatoid ar-
98. Bozkurt B, Torre-Amione G, Warren MS,
SW, et al. Long-term safety and efficacy of
thritis compared with controls: a popula-
et al. Results of targeted anti-tumor necro-
etanercept in patients with rheumatoid ar-
tion-based study. Arthritis Rheum 2002;46:
sis factor therapy with etanercept (ENBREL)
thritis. J Rheumatol 2001;28:1238-44.
in patients with advanced heart failure. Cir-
70. Day R. Adverse reactions to TNF-alpha
85. Kindler V, Sappino AP, Grau GE, Piguet
inhibitors in rheumatoid arthritis. Lancet
PF, Vassalli P. The inducing role of tumor ne-
99. Chung ES, Packer M, Lo KH, Fasan-
crosis factor in the development of bacteri-
made AA, Willerson JT. Randomized, dou-
The New England Journal of Medicine
Downloaded from nejm.org on February 3, 2011. For personal use only. No other uses without permission.
Copyright 2004 Massachusetts Medical Society. All rights reserved.
ble-blind, placebo-controlled, pilot trial of
108. Bresnihan B, Alvaro-Gracia JM, Cobby
115. Husni ME, Maier AL, Mease PJ, et al.
infliximab, a chimeric monoclonal antibody
M, et al. Treatment of rheumatoid arthritis
Etanercept in the treatment of adult patients
to tumor necrosis factor-alpha, in patients
with recombinant human interleukin-1 re-
with Still's disease. Arthritis Rheum 2002;
with moderate-to-severe heart failure: re-
ceptor antagonist. Arthritis Rheum 1998;
sults of the anti-TNF Therapy Against Con-
116. Reiff A, Takei S, Sadeghi S, et al. Etan-
gestive Heart Failure (ATTACH) trial. Circu-
109. Cohen S, Hurd E, Cush J, et al. Treat-
ercept therapy in children with treatment-
ment of rheumatoid arthritis with anakinra,
resistant uveitis. Arthritis Rheum 2001;44:
100. Furst DE, Cush J, Kaufmann S, Siegel J,
a recombinant human interleukin-1 receptor
Kurth R. Preliminary guidelines for diag-
antagonist, in combination with metho-
117. Stone JH, Uhlfelder ML, Hellmann DB,
nosing and treating tuberculosis in patients
trexate: results of a twenty-four-week, mul-
Crook S, Bedocs NM, Hoffman GS. Etaner-
with rheumatoid arthritis in immunosup-
ticenter, randomized, double-blind, placebo-
cept combined with conventional treatment
pressive trials or being treated with biologi-
controlled trial. Arthritis Rheum 2002;46:
in Wegener's granulomatosis: a six-month
cal agents. Ann Rheum Dis 2002;61:Suppl
open-label trial to evaluate safety. Arthritis
110. Nuki G, Bresnihan B, Bear MB, Mc-
101. Dinarello CA. Biologic basis for inter-
Cabe D. Long-term safety and maintenance
118. Yocum DE, Furst DE, Kaine JL, et al. Ef-
leukin-1 in disease. Blood 1996;87:2095-
of clinical improvement following treat-
ficacy and safety of tacrolimus in patients
ment with anakinra (recombinant human
with rheumatoid arthritis: a double-blind
102. Horai R, Saijo S, Tanioka H, et al. De-
interleukin-1 receptor antagonist) in pa-
trial. Arthritis Rheum 2003;48:3328-37.
velopment of chronic inflammatory arthrop-
tients with rheumatoid arthritis: extension
119. Silverman GJ, Weisman S. Rituximab
athy resembling rheumatoid arthritis in in-
phase of a randomized, double-blind, place-
therapy and autoimmune disorders: pros-
terleukin 1 receptor antagonist-deficient
bo-controlled trial. Arthritis Rheum 2002;
pects for anti-B cell therapy. Arthritis
mice. J Exp Med 2000;191:313-20.
103. Firestein GS, Boyle DL, Yu C, et al. Syn-
111. Fleischmann RM, Schechtman J, Ben-
120. Choy EH, Isenberg DA, Garrood T, et
ovial interleukin-1 receptor antagonist and
nett R, et al. Anakinra, a recombinant hu-
al. Therapeutic benefit of blocking interleu-
interleukin-1 balance in rheumatoid arthri-
man interleukin-1 receptor antagonist (r-
kin-6 activity with an anti-interleukin-6 re-
tis. Arthritis Rheum 1994;37:644-52.
metHuIL-1ra), in patients with rheumatoid
ceptor monoclonal antibody in rheumatoid
104. Arend WP, Malyak M, Guthridge CJ,
arthritis: a large, international, multicenter,
arthritis: a randomized, double-blind, pla-
Gabay C. Interleukin-1 receptor antagonist:
placebo-controlled trial. Arthritis Rheum
cebo-controlled, dose-escalation trial. Ar-
role in biology. Annu Rev Immunol 1998;
thritis Rheum 2002;46:3143-50.
112. Schiff MH, Bulpitt K, Weaver AA, et al.
121. Kremer JM, Westhovens R, Leon M, et
105. Bresnihan B, Cunnane G. Interleukin-1
Safety of combination therapy with anakinra
al. Treatment of rheumatoid arthritis by se-
receptor antagonist. Rheum Dis Clin North
and etanercept in patients with rheumatoid
lective inhibition of T-cell activation with fu-
Am 1998;24:615-28.
arthritis. Arthritis Rheum 2001;44:Suppl:
sion protein CTLA4Ig. N Engl J Med 2003;
106. Yang BB, Baughman S, Sullivan JT.
S79. abstract.
Pharmacokinetics of anakinra in subjects
113. Mease PJ, Goffe BS, Metz J, Vander-
122. Smolen JS, Steiner G. Therapeutic
with different levels of renal function. Clin
Stoep A, Finck B, Burge DJ. Etanercept in
strategies for rheumatoid arthritis. Nat Rev
Pharmacol Ther 2003;74:85-94.
the treatment of psoriatic arthritis and pso-
Drug Discov 2003;2:473-88.
107. Campion GV, Lebsack ME, Lookabaugh
riasis: a randomised trial. Lancet 2000;356:
Copyright 2004 Massachusetts Medical Society.
J, Gordon G, Catalano M. Dose-range and
dose-frequency study of recombinant human
114. Gorman JD, Sack KE, Davis JC Jr.
interleukin-1 receptor antagonist in patients
Treatment of ankylosing spondylitis by inhi-
with rheumatoid arthritis. Arthritis Rheum
bition of tumor necrosis factor a. N Engl J
n engl j med 350;21
The New England Journal of Medicine
Downloaded from nejm.org on February 3, 2011. For personal use only. No other uses without permission.
Copyright 2004 Massachusetts Medical Society. All rights reserved.
Source: http://www.fundapoyarte.org/contenidos/Nuevas%20ttxos%20AR%20NEJM.pdf
¿SE PUEDEN FRACCIONAR O TRITURAR LOS COMPRIMIDOS? ¿SE PUEDEN ABRIR LAS CÁPSULAS? Como es conocido, existen varias vías para poder administrar las especialidades farmacéuticas y según la vía de administración, a los principios activos se les formula de una forma galénica diferente. La vía oral es en general la más habitual para la administración de los medicamentos y siendo las formas farmacéuticas sólidas orales de las presentaciones más utilizadas.
Nevirapine- Versus Lopinavir/Ritonavir-Based InitialTherapy for HIV-1 Infection among Women in Africa: ARandomized Trial Shahin Lockman1,2,3*, Michael Hughes2, Fred Sawe4, Yu Zheng2, James McIntyre5, Tsungai Chipato6, Aida Asmelash3, Mohammed Rassool7, Sylvester Kimaiyo8, Douglas Shaffer4, Mina Hosseinipour9, Lerato Mohapi10, Francis Ssali11, Margret Chibowa12, Farida Amod13, Elias Halvas14, Evelyn Hogg15,


