Microsoft word - avandamet_pm-2013-04-18.doc
PRODUCT MONOGRAPH
rosiglitazone maleate/metformin hydrochloride
2 mg/500 mg Tablets
2 mg rosiglitazone (as rosiglitazone maleate) and 500 mg metformin hydrochloride
4 mg/500 mg Tablets
4 mg rosiglitazone (as rosiglitazone maleate) and 500 mg metformin hydrochloride
2 mg/1000 mg Tablets
2 mg rosiglitazone (as rosiglitazone maleate) and 1000 mg metformin hydrochloride
4 mg/1000 mg Tablets
4 mg rosiglitazone (as rosiglitazone maleate) and 1000 mg metformin hydrochloride
Antidiabetic Agent
GlaxoSmithKline Inc.
Date of Revision:
7333 Mississauga Road
Mississauga, Ontario L5N 6L4
Submission Control No:
2013 GlaxoSmithKline, All Rights Reserved ®AVANDAMET is a registered trademark, used under license by GlaxoSmithKline Inc. ®AVANDIA is a registered trademark, used under license by GlaxoSmithKline Inc.
®
GLUCOPHAGE is a registered trademark of MERCK SANTÉ
April 18, 2013
Page 1 of 57
Table of Contents
PART I: HEALTH PROFESSIONAL INFORMATION .3
SUMMARY PRODUCT INFORMATION .3 INDICATIONS AND CLINICAL USE .3 CONTRAINDICATIONS .4 WARNINGS AND PRECAUTIONS .5 ADVERSE REACTIONS .15 DRUG INTERACTIONS .21 DOSAGE AND ADMINISTRATION .24 OVERDOSAGE .26 ACTION AND CLINICAL PHARMACOLOGY .26 STORAGE AND STABILITY .34 DOSAGE FORMS, COMPOSITION AND PACKAGING .34
PART II: SCIENTIFIC INFORMATION .35
PHARMACEUTICAL INFORMATION .35 CLINICAL TRIALS .36 DETAILED PHARMACOLOGY .41 TOXICOLOGY .42 REFERENCES .45
PART III: CONSUMER INFORMATION .53
April 18, 2013
Page 2 of 57
rosiglitazone maleate/metformin hydrochloride
PART I: HEALTH PROFESSIONAL INFORMATION
Note: for additional information on rosiglitazone and metformin, consult the individual
Product Monographs.
SUMMARY PRODUCT INFORMATION
Dosage Form / Strength
Clinically Relevant
Nonmedicinal Ingredients
Tablet / lactose monohydrate 2 mg/500 mg, 4 mg/500 mg,
For a complete listing see
2 mg/1000 mg, 4 mg/1000 mg
Dosage Forms, Composition
and Packaging section.
INDICATIONS AND CLINICAL USE AVANDAMET® (rosiglitazone maleate/metformin hydrochloride) is indicated as an
adjunct to diet and exercise to improve glycemic control in patients with type 2 diabetes
mellitus for whom all other oral antidiabetic agents, in monotherapy or in combination,
do not result in adequate glycemic control or are inappropriate due to contraindications or
intolerance. (See
WARNINGS AND PRECAUTIONS, Serious Warnings and
Precautions Box and Cardiovascular).
Prior to prescribing AVANDAMET®, physicians must:
• Document the eligibility of patients to meet the above criteria; • Counsel each patient on the risks and benefits of AVANDAMET®, including the
cardiovascular risks; and
• Obtain the patient's written informed consent to take the drug.
Caloric restriction, weight loss, and exercise improve insulin sensitivity and are essential for the proper treatment of a diabetic patient. These measures are important not only in the primary treatment of type 2 diabetes, but also in maintaining the efficacy of drug therapy. Prior to initiation of therapy with AVANDAMET®, secondary causes of poor glycemic control (e.g. infection) should be investigated and treated.
April 18, 2013
Page 3 of 57
Geriatrics (≥
65 years of age):
Rosiglitazone maleate
Evidence from clinical studies and experience suggest that use in the geriatric population may be associated with differences in safety (see WARNINGS & PRECAUTIONS, Cardiovascular).
Metformin hydrochloride
Limited data from controlled pharmacokinetic studies of metformin hydrochloride in healthy elderly subjects suggest that total plasma clearance of metformin is decreased, the half-life is prolonged and Cmax is increased, compared to healthy young subjects. From
these data, it appears that the change in metformin pharmacokinetics with aging is primarily accounted for by a change in renal function. Metformin treatment and therefore treatment with AVANDAMET® should not be initiated in patients 80 years of age or older unless measurement of creatinine clearance demonstrates that renal function is not reduced (see WARNINGS AND PRECAUTIONS and DOSAGE AND ADMINISTRATION).
Pediatrics (< 18 years of age):
The safety and effectiveness of rosiglitazone and metformin have not been established in
patients younger than 18 years of age. Furthermore, thiazoledinediones promote the
maturation of preadipocytes and have been associated with weight gain. Therefore,
AVANDAMET® is not indicated in patients younger than 18 years of age (see
WARNINGS AND PRECAUTIONS, Special Populations).
CONTRAINDICATIONS AVANDAMET® is contraindicated in:
• Patients with New York Heart Association (NYHA) Class I to IV heart failure.
• Patients with renal impairment or for whom renal function is not known, in
patients with serum creatinine levels above the upper limit of normal range, and in patients with renal disease or renal dysfunction (e.g., as suggested by serum creatinine levels ≥136 µmol/L (males), ≥124 µmol/L (females) or abnormal creatinine clearance) (<60 mL/min) which may result from conditions such as cardiovascular collapse (shock), acute myocardial infarction, and septicemia (see WARNINGS AND PRECAUTIONS).
• Patients with known hypersensitivity to this product (rosiglitazone maleate or
metformin hydrochloride), or any of its ingredients.
• Patients with acute or chronic metabolic acidosis, including diabetic ketoacidosis,
with or without coma, history of ketoacidosis with or without coma. Diabetic ketoacidosis should be treated with insulin.
• Patients with a history of lactic acidosis, irrespective of precipitating factors.
April 18, 2013
Page 4 of 57
• Patients with serious hepatic impairment (see WARNINGS AND
• Patients with Type 1 diabetes mellitus. • Pregnancy. Insulin is recommended during pregnancy to control blood glucose
levels. Oral antidiabetic agents should not be given (see WARNINGS AND PRECAUTIONS, Special Populations, Pregnant Women).
• Breastfeeding.
• Excessive alcohol intake, acute or chronic. • In cases of cardiovascular collapse and in disease states associated with
hypoxemia such as cardiorespiratory insufficiency, which are often associated with hyperlactacidemia.
• During stress conditions, such as severe infections, trauma or surgery and the
recovery phase thereafter.
• In patients suffering from severe dehydration.
AVANDAMET® should be temporarily discontinued in patients undergoing radiologic
studies involving intravascular administration of iodinated contrast materials, because use
of such products may result in acute alteration of renal function (see WARNINGS AND
PRECAUTIONS).
WARNINGS AND PRECAUTIONS
Serious Warnings and Precautions
Rosiglitazone, like other thiazolidinediones, can cause fluid retention and
congestive heart failure (See
Cardiovascular below).
Rosiglitazone may be associated with an increased risk of cardiac ischemia.
AVANDAMET® is not recommended in patients with a history of ischemic
heart disease, particularly those with myocardial ischemic symptoms. (See
Cardiovascular below).
AVANDAMET® should be used only when all other oral antidiabetic agents, in
monotherapy or in combination, do not result in adequate glycemic control or are
inappropriate due to contraindications or intolerance (See
Cardiovascular below).
AVANDAMET®
Administration with other drugs: For safety reasons, the use of AVANDAMET® in
combination with insulin is not indicated (see CLINICAL TRIALS).
April 18, 2013
Page 5 of 57
The use of AVANDAMET® in combination with a sulfonylurea (triple therapy) is not
indicated. An increase in reporting of fluid retention related events (including congestive
heart failure) has been seen in patients receiving rosiglitazone in combination with
metformin AND a sulfonylurea.
Close monitoring of glycemic control and dose adjustment of the rosiglitazone or
metformin components may be needed when AVANDAMET® is co-administered with
CYP2C8 inhibitors or inducers or cationic drugs that are eliminated by renal tubular
excretion (see DRUG INTERACTIONS).
Rosiglitazone maleate
Due to its mechanism of action, rosiglitazone is active only in the presence of
endogenous insulin. Therefore, AVANDAMET® should not be used in patients with
type 1 diabetes.
Metformin hydrochloride
Radiologic studies involving the use of intravascular iodinated contrast materials
(for example, intravenous urogram, intravenous cholangiography, angiography, and
computed tomography (CT) scans with contrast materials): Intravascular contrast
studies with iodinated materials can lead to acute alteration of renal function and have
been associated with lactic acidosis in patients receiving metformin (see
CONTRAINDICATIONS). Therefore, in patients in whom any such study is planned,
AVANDAMET® should be temporarily discontinued at the time of or prior to the
procedure, and withheld for 48 hours subsequent to the procedure and reinstituted only
after renal function has been re-evaluated and found to be normal.
Change in clinical status of previously controlled diabetic: A diabetic patient
previously well controlled on AVANDAMET® who develops laboratory abnormalities or
clinical illness (especially vague and poorly defined illness) should be evaluated
promptly for evidence of ketoacidosis or lactic acidosis. Evaluation should include
serum electrolytes and ketones, blood glucose and, if indicated, blood pH, lactate,
pyruvate and metformin levels. If acidosis of either form occurs, AVANDAMET® must
be stopped immediately and appropriate corrective measures initiated (see WARNINGS
AND PRECAUTIONS, Lactic Acidosis).
Cardiovascular
Rosiglitazone maleate
Rosiglitazone can cause fluid retention, congestive heart failure, and may be associated
with an increased risk of cardiac ischemia. Some studies have reported an increased
cardiovascular risk with rosiglitazone compared to another member of the
thiazolidinedione class, pioglitazone.
AVANDAMET® should be used only when all
other oral antidiabetic agents, in monotherapy or in combination, do not result in
April 18, 2013
Page 6 of 57
adequate glycemic control or are inappropriate due to contraindications or
intolerance.
Congestive heart failure: Thiazolidinediones, like rosiglitazone, alone or in
combination with other antidiabetic agents, can cause fluid retention, which can
exacerbate or lead to congestive heart failure. The fluid retention may very rarely present
as rapid and excessive weight gain. All patients should be monitored for signs and
symptoms of adverse reactions relating to fluid retention and heart failure (see
ADVERSE REACTIONS). An increase in reporting of fluid retention related events
including congestive heart failure has been seen in patients receiving rosiglitazone in
combination with metformin and a sulfonylurea. This triple therapy regimen is not an
approved indication.
Treatment with thiazolidinediones has been associated with cases of congestive heart
failure, some of which were difficult to treat unless the medication was discontinued.
AVANDAMET® should be discontinued if any deterioration in cardiac status occurs.
AVANDAMET® is contraindicated in patients with NYHA Class I, II, III and IV heart
failure. Patients with severe heart failure (including NYHA Class III and IV cardiac
status) were not studied during the clinical trials.
Edema and heart failure have been reported more frequently in elderly patients using
rosiglitazone. Caution should be exercised in patients over 75 years because of the
limited experience in this patient group.
Ischemic heart disease: In a retrospective analysis of data from pooled clinical studies
(n=14,237), which included patients on combination therapy with insulin as well as
patients with NYHA Class I and II heart failure, the overall incidence of events typically
associated with cardiac ischemia was higher for rosiglitazone containing regimens, 2.00%
versus comparators, 1.53% [Hazard ratio 1.30 (95% confidence interval 1.004 – 1.69)].
In a subgroup analysis of these data, this risk was further increased in patients receiving
nitrates with approximately twice as many events in patients receiving rosiglitazone
versus comparators.
The use of AVANDAMET® is therefore not recommended for
patients being treated with nitrates.
In a meta-analyses of 52 double-blind, randomized, controlled clinical trials (mean
duration 6 months) (n=16,995) statistically significant increases in myocardial infarction
(Odds ratio (OR)= 1.80; 95% CI= [1.03, 3.25]), serious myocardial ischemic events
(OR= 1.46; 95% CI= [1.06, 2.03]) and total myocardial ischemic events (OR= 1.34; 95%
CI= [1.07, 1.70]) were demonstrated. A nearly statistically significant increase was
shown for major adverse cardiovascular events (MACE) (OR= 1.44; 95% CI= [0.95,
2.20]). Non-statistically significant increases were also shown for CV death (OR= 1.46;
95% CI= [0.60, 3.77]) and all-cause death (OR=1.38; 95% CI= [0.72, 2.72]). The odds
ratios for congestive heart failure and stroke were OR=1.93; 95% CI= [1.30, 2.93] and
OR= 0.86; 95% CI= [0.40, 1.83], respectively.
April 18, 2013
Page 7 of 57
Patients with a history of Ischemic Heart Disease: There are limited clinical trial data
in patients with ischemic heart disease. In a subgroup of rosiglitazone users with a history
of Ischemic Heart Disease of a large cardiovascular outcomes trial (383 out of
2220 patients) there was a non-significant increase in the primary endpoint of
cardiovascular death or cardiovascular hospitalization (Hazard Ratio 1.26; 95% CI [0.95,
1.68]).
AVANDAMET® is not recommended in patients with a history of ischemic
heart disease, particularly those with myocardial ischemic symptoms. Edema: AVANDAMET® should be used with caution in patients with edema. In
healthy volunteers who received rosiglitazone 8 mg once daily as monotherapy for
8 weeks, there was a statistically significant increase in median plasma volume
(1.8 mL/kg) compared to placebo. In controlled clinical trials of patients with Type 2
diabetes, mild to moderate edema was observed at a greater frequency in patients treated
with rosiglitazone, and may be dose related (see ADVERSE REACTIONS). For
information on macular edema, see WARNINGS AND PRECAUTIONS,
Ophthalmologic.
Edema and heart failure have been reported more frequently in elderly patients using
rosiglitazone. Caution should be exercised in patients over 75 years because of the
limited experience in this patient group.
Metformin hydrochloride
Hypoxic states: Cardiovascular collapse (shock) from whatever cause, acute congestive
heart failure, acute myocardial infarction and other conditions characterized by
hypoxemia have been associated with lactic acidosis and may also cause prerenal
azotemia. When such events occur in patients receiving AVANDAMET®, the drug
should be promptly discontinued.
Endocrine and Metabolism
AVANDAMET®
Loss of control of blood glucose: When a patient stabilized on any diabetic regimen is
exposed to stress such as fever, trauma, infection, or surgery, a temporary loss of
glycemic control may occur. At such times, it may be necessary to withhold
AVANDAMET® and temporarily administer insulin. AVANDAMET® may be
reinstituted after the acute episode is resolved.
Rosiglitazone maleate
Weight Gain: Dose-related weight gain was seen with rosiglitazone alone and in
combination with other hypoglycemic agents. Treatment should be re-evaluated in
patients with excessive weight gain (see ACTION AND CLINICAL
PHARMACOLOGY and ADVERSE REACTIONS).
April 18, 2013
Page 8 of 57
Metformin hydrochloride
Lactic acidosis: Lactic acidosis is a rare, but serious, metabolic complication that occurs
due to metformin accumulation during treatment with AVANDAMET®; when it occurs,
it is fatal in approximately 50% of cases. Lactic acidosis may also occur in association
with a number of pathophysiologic conditions, including diabetes mellitus, and whenever
there is significant tissue hypoperfusion and hypoxemia. Lactic acidosis is characterized
by elevated blood lactate levels (>5 mmol/L), decreased blood pH, electrolyte
disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. When
metformin is implicated as the cause of lactic acidosis, metformin plasma levels
> 5 μg/mL are generally found.
The reported incidence of lactic acidosis in patients receiving metformin is very low
(approximately 0.03 cases/1000 patient-years, with approximately 0.015 fatal cases/1000
patient-years). Reported cases have occurred primarily in diabetic patients with
significant renal insufficiency, including both intrinsic renal disease and renal
hypoperfusion, often in the setting of multiple concomitant medical/surgical problems
and multiple concomitant medications. Patients with congestive heart failure requiring
pharmacologic management, in particular those with unstable or acute congestive heart
failure who are at risk of hypoperfusion and hypoxemia, are at increased risk of lactic
acidosis. In particular, treatment of the elderly should be accompanied by careful
monitoring of renal function. AVANDAMET® treatment should not be initiated in
patients 80 years of age or older, unless measurement of creatinine clearance
demonstrates that renal function is not reduced, as these patients are more susceptible to
developing lactic acidosis. The risk of lactic acidosis increases with the degree of renal
dysfunction and the patient's age. The risk of lactic acidosis may, therefore, be
significantly decreased by regular monitoring of renal function in patients taking
AVANDAMET® and by use of the minimum effective dose of AVANDAMET®.
In addition, AVANDAMET® should be promptly withheld in the presence of any
condition associated with hypoxemia, dehydration or sepsis. Because impaired hepatic
function may significantly limit the ability to clear lactate, AVANDAMET® should
generally be avoided in patients with clinical or laboratory evidence of hepatic disease.
Patients should be cautioned against excessive alcohol intake, either acute or chronic,
when taking AVANDAMET®, since alcohol potentiates the effects of metformin on
lactate metabolism.
The onset of lactic acidosis often is subtle, and accompanied only by nonspecific
symptoms such as malaise, myalgias, respiratory distress, increasing somnolence and
nonspecific abdominal distress. There may be associated hypothermia, hypotension and
resistant bradyarrhythmias with more marked acidosis. The patient and the patient's
physician must be aware of the possible importance of such symptoms and the patient
should be instructed to notify the physician immediately if they occur (see General).
AVANDAMET® should be withdrawn until the situation is clarified. Serum electrolytes,
ketones, blood glucose and, if indicated, blood pH, lactate levels and even blood
April 18, 2013
Page 9 of 57
metformin levels may be useful. Once a patient is stabilized on any dose level of
AVANDAMET®, gastrointestinal symptoms, which are common during initiation of
therapy, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms
could be due to lactic acidosis or other serious disease. Levels of fasting venous plasma
lactate above the upper limit of normal but less than 5 mmol/L in patients taking
AVANDAMET® do not necessarily indicate impending lactic acidosis and may be
explainable by other mechanisms, such as poorly controlled diabetes or obesity, vigorous
physical activity or technical problems in sample handling. Lactic acidosis should be
suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis
(ketonuria and ketonemia).
Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a
patient with lactic acidosis who is taking AVANDAMET®, the drug should be
discontinued immediately and general supportive measures promptly instituted. Because
metformin hydrochloride is dialyzable (with a clearance of up to 170 mL/min under good
hemodynamic conditions), prompt hemodialysis is recommended to correct the acidosis
and remove the accumulated metformin. Such management often results in prompt
reversal of symptoms and recovery (see Cardiovascular, Renal and Hepatic, and
CONTRAINDICATIONS).
If acidosis of any kind develops, AVANDAMET® should be discontinued immediately.
Vitamin B12 levels: Impairment of vitamin B12 and folic acid absorption has been
reported in some patients on metformin. Therefore, measurements of serum vitamin B12
and folic acid are advisable at least every one to two years in patients on long-term treatment with AVANDAMET®. A decrease to subnormal levels of previously normal serum vitamin B12 levels, without
clinical manifestations, is observed in approximately 7% of patients receiving metformin hydrochloride in controlled clinical trials of 28 weeks duration. Such a decrease, possibly due to interference with B12 absorption from the B12 -intrinsic factor complex, is,
however, very rarely associated with anemia and appears to be rapidly reversible with discontinuation of metformin or vitamin B12 supplementation. Measurement of
hematologic parameters on an annual basis is advised in patients on AVANDAMET® and any apparent abnormalities should be appropriately investigated and managed (see Monitoring and Laboratory Tests). Certain individuals (those with inadequate vitamin B12 or calcium intake or absorption) appear to be predisposed to developing subnormal
vitamin B12 levels.
Hypoglycemia: Hypoglycemia does not occur in patients receiving metformin alone
under usual circumstances of use, but could occur when caloric intake is deficient, when
strenuous exercise is not compensated by caloric supplementation, or during concomitant
use with hypoglycemic agents (such as sulfonylureas) or ethanol. Elderly, debilitated or
malnourished patients, and those with adrenal or pituitary insufficiency or alcohol
intoxication are particularly susceptible to hypoglycemic effects. Hypoglycemia may be
April 18, 2013
Page 10 of 57
difficult to recognize in the elderly, and in people who are taking beta-adrenergic
blocking drugs.
Hematologic Rosiglitazone maleate
In controlled trials, there were dose-related decreases in hemoglobin and hematocrit. The
magnitude of the decreases (≤ 11 g/L for hemoglobin and ≤ 0.034 for hematocrit) was
small for rosiglitazone alone and rosiglitazone in combination with other hypoglycemic
agents. The changes occurred primarily during the first 3 months of therapy or following
an increase in rosiglitazone dose and remained relatively constant thereafter. Decreases
may be related to increased plasma volume observed during treatment with rosiglitazone
and have not been associated with any significant hematologic clinical effects (see
ADVERSE REACTIONS, Abnormal Hematologic and Clinical Chemistry Findings).
Patients with a hemoglobin value of <110 g/L for males and <100 g/L for females were
excluded from the clinical trials.
Hepatic
Rosiglitazone maleate
Therapy with AVANDAMET® should not be initiated in patients with increased
baseline liver enzyme levels (ALT >2.5 times the upper limit of normal). Rare cases of severe hepatocellular injury have been reported with thiazolidinediones.
In postmarketing experience with rosiglitazone, reports of hepatitis and of hepatic
enzyme elevations to three or more times the upper limit of normal have been received.
Very rarely, these reports have involved hepatic failure with and without fatal outcome,
although causality has not been established (see ADVERSE REACTIONS, Post-Market
Adverse Drug Reactions).
Liver enzymes should be checked prior to the initiation of therapy with AVANDAMET®
in all patients and periodically thereafter per the clinical judgement of the healthcare
professional. Patients with mildly elevated liver enzymes (ALT levels ≤2.5 times the
upper limit of normal) at baseline or during therapy with AVANDAMET® should be
evaluated to determine the cause of the liver enzyme elevation.
Initiation of, or continuation of, therapy with AVANDAMET® in patients with mild liver
enzyme elevations should proceed with caution and include appropriate close clinical
follow-up, including more frequent liver enzyme monitoring, to determine if the liver
enzyme elevations resolve or worsen. If at any time ALT levels increase to >3 times the
upper limit of normal in patients on therapy with AVANDAMET®, liver enzyme levels
should be rechecked as soon as possible. If ALT levels remain >3 times the upper limit
April 18, 2013
Page 11 of 57
of normal, therapy with AVANDAMET® should be discontinued (see DOSAGE AND
ADMINISTRATION).
If any patient develops symptoms suggesting hepatic dysfunction, which may include
unexplained nausea, vomiting, abdominal pain, fatigue, anorexia and/or dark urine, liver
enzymes should be checked. If jaundice is observed, drug therapy should be
discontinued. In addition, if the presence of hepatic disease or hepatic dysfunction of
sufficient magnitude to predispose to lactic acidosis is confirmed, therapy with
AVANDAMET® should be discontinued.
Metformin hydrochloride
Impaired hepatic function: Since impaired hepatic function has been associated with
some cases of lactic acidosis, AVANDAMET® should generally be avoided in patients
with clinical or laboratory evidence of hepatic disease.
Musculoskeletal
Rosiglitazone maleate
In post-marketing experience, there have been very rare cases of creatinine kinase (CK)
elevation, myalgia, and rhabdomyolysis reported with the use of rosiglitazone.
Fractures: Long-term studies showed an increased incidence of bone fractures in
patients taking rosiglitazone. In females, this increased incidence was noted after the first
year of treatment and persisted during long-term treatment. The majority of the fractures
have occurred in the upper limbs and distal lower limbs (see ADVERSE REACTIONS).
The risk of fracture should be considered in the care of all patients treated with
rosiglitazone.
Decreases in spine and hip bone mineral density have been reported in men and women
taking rosiglitazone in epidemiological and randomized clinical trials.
Ophthalmologic
Rosiglitazone maleate
New onset and/or worsening macular edema with decreased visual acuity has been
reported rarely in postmarketing experience with AVANDAMET®. In some cases, the
visual events resolved or improved following discontinuation of AVANDAMET®.
Physicians should consider the possibility of macular edema if a patient reports
disturbances in visual acuity (see Post-Market Adverse Drug Reactions).
April 18, 2013
Page 12 of 57
Peri-Operative Considerations
Metformin hydrochloride
Surgical procedures: Use of AVANDAMET® should be temporarily suspended for any
surgical procedure (except minor procedures not associated with restricted intake of food
and fluids). AVANDAMET® should be discontinued 2 days before surgical intervention
and should not be restarted until the patient's oral intake has resumed and renal function
has been evaluated as normal.
Renal
Metformin hydrochloride
Use of concomitant medications that may affect renal function or metformin
disposition: Concomitant medication(s) that may affect renal function or result in
significant hemodynamic change or may interfere with the disposition of metformin, such
as cationic drugs that are eliminated by renal tubular secretion (see DRUG
INTERACTIONS), should be used with caution.
Sexual Function/Reproduction
Rosiglitazone maleate
Ovulation: As with other thiazolidinediones, rosiglitazone may result in resumption of
ovulation in premenopausal, anovulatory women with insulin resistance (e.g., patients
with polycystic ovary syndrome).
As a consequence of their improved insulin
sensitivity, these patients may be at risk of pregnancy if adequate contraception is
not used.
Although hormonal imbalance has been seen in preclinical studies (see TOXICOLOGY,
Carcinogenesis, Mutagenesis, Impairment of Fertility), no significant adverse experiences
associated with menstrual disorders have been reported in clinical trial participants,
including premenopausal women. If unexpected menstrual dysfunction occurs, the
benefits of continued therapy should be reviewed.
Special Populations Pregnant Women: There are no controlled trials of AVANDAMET® in pregnant
women. Rosiglitazone has been reported to cross the human placenta and to be
detectable in fetal tissues. AVANDAMET® is contraindicated for use in pregnant
women. Because current information strongly suggests that abnormal blood glucose
levels during pregnancy are associated with a higher incidence of congenital anomalies as
well as increased neonatal morbidity and mortality, most experts recommend that insulin
be used during pregnancy to maintain blood glucose levels as close to normal as possible.
In animal studies, rosiglitazone was not teratogenic but treatment during mid-late
April 18, 2013
Page 13 of 57
gestation caused fetal death and growth retardation in both rats and rabbits at 19- and 73-
fold clinical systemic exposure, respectively (see TOXICOLOGY, Teratogenic Effects).
Labour and Delivery: The effect of AVANDAMET® or its components on labour and
delivery in humans is unknown.
Nursing Women: No studies have been conducted with the combined components of
AVANDAMET®. In studies performed with the individual components, both
rosiglitazone-related material and metformin were detectable in milk from lactating rats.
It is not known whether rosiglitazone and/or metformin is excreted in human milk.
Because many drugs are excreted in human milk, AVANDAMET® should not be
administered to a nursing woman. If AVANDAMET® is discontinued, and if diet alone
is inadequate for controlling blood glucose, insulin therapy should be considered.
Pediatrics (< 18 years of age): There are no data on the use of AVANDAMET® in
patients under 18 years of age; therefore, AVANDAMET® is not indicated for use in
patients under 18 years of age. Thiazolidinediones promote the maturation of
preadipocytes and have been associated with weight gain. Obesity is a major problem in
adolescents with type 2 diabetes.
Geriatrics (≥
65 years of age):
Rosiglitazone maleate
Evidence from clinical studies and experience suggest that use in the geriatric population may be associated with differences in safety (see WARNINGS & PRECAUTIONS, Cardiovascular).
Metformin hydrochloride
Metformin is known to be substantially excreted by the kidney and because the risk of
serious adverse reactions to the drug is greater in patients with impaired renal function,
AVANDAMET® should only be used in patients with normal renal function (see
CONTRAINDICATIONS and WARNINGS AND PRECAUTIONS). Because aging is
associated with reduced renal function, AVANDAMET® should be used with caution as
age increases. Care should be taken in dose selection and should be based on careful and
regular monitoring of renal function. Generally, elderly patients should not be titrated to
the maximum dose of AVANDAMET® (see WARNINGS, DOSAGE AND
ADMINISTRATION).
Monitoring and Laboratory Tests Periodic fasting blood glucose and A1C measurements should be performed to monitor
therapeutic response.
Liver enzyme monitoring is recommended prior to initiation of therapy with
AVANDAMET® in all patients and periodically thereafter (see WARNINGS AND
PRECAUTIONS, Hepatic).
April 18, 2013
Page 14 of 57
Initial and periodic monitoring of hematologic parameters (e.g., hemoglobin/hematocrit and red blood cell indices) should be performed, at least on an annual basis. While megaloblastic anemia has rarely been seen with metformin therapy, if this is suspected, vitamin B12 deficiency should be excluded.
Monitoring of renal function: Metformin is known to be substantially excreted by the
kidney, and the risk of metformin accumulation and lactic acidosis increases with the
degree of impairment of renal function. Thus, patients with serum creatinine levels
above the upper limit of normal for their age should not receive AVANDAMET® (see
Endocrine and Metabolism, Geriatrics (≥ 65 years of age) and DOSAGE AND
ADMINISTRATION).
Before initiation of therapy with AVANDAMET® and every 6 months while on
AVANDAMET® therapy, renal function should be assessed and verified as being within
normal range. In patients in whom development of renal dysfunction is anticipated, renal
function should be assessed more frequently and AVANDAMET® discontinued if
evidence of renal impairment is present.
ADVERSE REACTIONS
Adverse Drug Reaction Overview
Rosiglitazone maleate
In clinical trials, anemia and edema were generally dose-related, mild to moderate in
severity and usually did not require discontinuation of treatment with rosiglitazone.
In clinical trials, edema was reported in 4.8% of patients taking rosiglitazone compared to
1.3% on placebo, and 2.2% on metformin monotherapy and 4.4% on rosiglitazone in
combination with maximum doses of metformin. Treatment was required for 1.2% of
patients on rosiglitazone monotherapy with an adverse event of edema. These adverse
experiences rarely led to withdrawal. In these clinical trials, few patients (1.0%) were
enrolled with a presenting medical condition of congestive heart failure (NYHA Class
I/II). Edema was more frequently observed when rosiglitazone was used in combination
with insulin (see WARNINGS AND PRECAUTIONS, General and CLINICAL
TRIALS).
In double blind studies where rosiglitazone was administered for up to one year, serious
adverse experiences of ischemic heart disease were reported in 1.3% of patients taking
rosiglitazone maleate compared to 0.5% on placebo,1.3% on metformin and 1.2% on
rosiglitazone in combination with maximum doses of metformin.
April 18, 2013
Page 15 of 57
In a retrospective analysis of data from pooled clinical studies, which included patients on combination therapy with insulin as well as patients with NYHA Class I and II heart failure, the overall incidence of events typically associated with cardiac ischemia was higher for rosiglitazone containing regimens, 2.00% versus comparators, 1.53% [Hazard ratio 1.30 (95% confidence interval 1.004 -1.69)]. In a subgroup analysis of this data, this risk was further increased in patients receiving nitrates with approximately twice as many events in patients receiving rosiglitazone versus comparators (see WARNINGS AND PRECAUTIONS, Cardiovascular, Rosiglitazone maleate, Ischemic heart disease). In a meta-analyses of 52 double-blind, randomized, controlled clinical trials (mean duration 6 months) (n=16,995) statistically significant increases in myocardial infarction (Odds ratio (OR)= 1.80; 95% CI= [1.03, 3.25]), serious myocardial ischemic events (OR= 1.46; 95% CI= [1.06, 2.03]) and total myocardial ischemic events (OR= 1.34; 95% CI= [1.07, 1.70]) were demonstrated. A nearly statistically significant increase was shown for major adverse cardiovascular events (MACE) (OR= 1.44; 95% CI= [0.95, 2.20]). Non-statistically significant increases were also shown for CV death (OR= 1.46; 95% CI= [0.60, 3.77]) and all-cause death (OR=1.38; 95% CI= [0.72, 2.72]). The odds ratios for congestive heart failure and stroke were OR=1.93; 95% CI= [1.30, 2.93] and OR= 0.86; 95% CI= [0.40, 1.83], respectively. In a subgroup of rosiglitazone users with a history of Ischemic Heart Disease of a large cardiovascular outcomes trial (383 out of 2220 patients) there was a non-significant increase in the primary endpoint of cardiovascular death or cardiovascular hospitalization (Hazard Ratio 1.26; 95% CI [0.95, 1.68]) (see WARNINGS AND PRECAUTIONS, Cardiovascular, Rosiglitazone maleate, Ischemic heart disease, Patients with a history of Ischemic Heart Disease). In clinical trials, dose-related weight gain was seen with rosiglitazone alone and in combination with other hypoglycemic agents (see ACTION AND CLINICAL PHARMACOLOGY and WARNINGS AND PRECAUTIONS). Hypoglycemia was commonly observed and generally mild to moderate in nature and was dose-related when rosiglitazone was used in combination with metformin. Patients receiving rosiglitazone in combination with oral hypoglycemic agents may be at risk for hypoglycemia, and a reduction in the dose of rosiglitazone may be necessary. In double blind studies, anemia was reported in 1.9% of patients taking rosiglitazone compared to 0.7% on placebo, and 2.2% on metformin and 7.1% on rosiglitazone in combination with maximum doses of metformin. Treatment was required for 0.3% of patients with an adverse event of anemia. These adverse experiences rarely led to withdrawal. Lower pre-treatment hemoglobin/hematocrit levels in patients enrolled in the metformin combination clinical trials may have contributed to the higher reporting rate of anemia in these studies (see ADVERSE REACTIONS, Abnormal Hematologic and Clinical Chemistry Findings).
April 18, 2013
Page 16 of 57
Constipation was commonly observed and generally mild to moderate in nature in
clinical trials of rosiglitazone with metformin.
Long-term studies showed an increased incidence of bone fracture in patients taking
rosiglitazone (see WARNINGS AND PRECAUTIONS, Fractures, and ADVERSE
REACTIONS, Clinical Trial Drug Adverse Reactions).
Metformin hydrochloride
Gastrointestinal Reactions: Gastrointestinal symptoms (diarrhea, nausea, vomiting,
abdominal bloating, flatulence, and anorexia) are the most common reactions to
metformin and are approximately 30% more frequent in patients on metformin
monotherapy than in placebo-treated patients, particularly during initiation of metformin
therapy. These symptoms are generally transient and resolve spontaneously during
continued treatment. Occasionally, temporary dose reduction may be useful.
Because gastrointestinal symptoms during therapy initiation appear to be dose-related,
they may be decreased by gradual dose escalation and by having patients take
AVANDAMET® with meals (see DOSAGE AND ADMINISTRATION).
Special Senses: During initiation of AVANDAMET® therapy, approximately 3% of
patients may complain of an unpleasant or metallic taste, which usually resolves
spontaneously.
Dermatologic Reactions: The incidence of rash/dermatitis in controlled clinical trials
was comparable to placebo for metformin monotherapy.
Clinical Trial Adverse Drug Reactions
Because clinical trials are conducted under very specific conditions the adverse reaction rates observed in the clinical trials may not reflect the rates observed in practice and should not be compared to the rates in the clinical trials of another drug. Adverse drug reaction information from clinical trials is useful for identifying drug-related adverse events and for approximating rates.
Controlled Clinical Trials: The incidence and types of adverse events reported in
clinical trials of rosiglitazone as monotherapy or in combination with maximum doses of
metformin of 2500 mg/day are shown in Table 1.
April 18, 2013
Page 17 of 57
Adverse Events (≥ 5% in Any Treatment Group) Reported by Patients in Double-blind
Clinical Trials with Rosiglitazone as Monotherapy or in Combination with Metformin
Rosiglitazone
Metformin
Rosiglitazone plus
metformin
Upper respiratory tract
infection Injury*
* includes cuts, burns, sprains, fractures, falls, accidents and surgical procedures
In clinical trials, reports of hypoglycemia in patients treated with rosiglitazone added to
maximum metformin monotherapy were more frequent than in patients treated with
rosiglitazone or metformin monotherapies. In double-blind studies, hypoglycemia was
reported by 0.6% of patients receiving rosiglitazone as monotherapy compared to 0.2%
on placebo and by 3.0% of patients receiving rosiglitazone in combination with
maximum doses of metformin compared to 1.3% on metformin monotherapy.
Long-term Trials of Rosiglitazone: In a 4 to 6 year monotherapy study, fractures were
reported in a greater number of females with rosiglitazone (9.3%, 2.7/100 patient-years)
compared to glyburide (3.5%, 1.3/100 patient-years) or metformin (5.1%, 1.5/100
patient-years). The majority of the fractures in the females who received rosiglitazone
were reported in the upper arm, hand and foot (see WARNINGS AND PRECAUTIONS,
Fractures and Adverse Drug Reaction Overview).
In a multi-centre, randomized, open -label study with a mean follow-up of 5.5 years,
there was an increased incidence of bone fractures for subjects randomized to
rosiglitazone in addition to metformin or sulfonylurea compared to those randomized to
metformin plus sulfonylurea (see WARNINGS AND PRECAUTIONS, Fractures). The
risk of fracture was higher in females relative to control than in males relative to control.
April 18, 2013
Page 18 of 57
Summary of Bone Fractures by Overall Rate, Gender and Relative Risk During CV
Follow-up (ITT Population)
Bone fracture (female and male);
Relative risk
n (%) subjects [no. of events]
(N=2220)
(N=2227)
Any event
1.57 (1.26, 1.97)
1.57 (1.12, 2.19)
Distal lower limb
2.60 (1.67, 4.04)
1.25 (0.50, 3.17)
1.56 (0.68, 3.60)
1.00 (0.58, 1.74)
Bone fracture in female
subjects, n (%) subjects [no. of
Relative risk
(N=1078)
(N=1075)
Any event
124 (11.5) [154]
1.82 (1.37, 2.41)
1.75 (1.17, 2.61)
Distal lower limb
2.93 (1.67, 5.13)
1.00 (0.35, 2.83)
1.99 (0.60, 6.60)
1.10 (0.46, 1.94)
Bone fracture in male subjects,
Relative risk
n (%) subjects [no. of events]
(N=1142)
(N=1152)
Any event
1.23 (0.85, 1.77)
1.22 (0.67, 2.23)
Distal lower limb
2.11 (1.03, 4.31)
3.03 (0.32, 29.05)
1.21 (0.37, 3.96)
0.94 (0.46, 1.94)
Abnormal Hematologic and Clinical Chemistry Findings
Hematological: Small decreases in hematological parameters were more common in the
patients treated with rosiglitazone than in placebo-treated patients. Leukopenia was
reported in 0.4% of rosiglitazone patients compared to 0.2% of patients on placebo, 0%
on metformin and 0.3% on rosiglitazone in combination with maximum doses of
metformin. Decreases may be related to increased plasma volume observed with
treatment with rosiglitazone. The mean decrease in hemoglobin in patients treated with
rosiglitazone was approximately 10 to 12 g/L; the decrease in hematocrit was 0.03 to
0.04.
During controlled clinical trials of 29 weeks duration, approximately 9% of patients on
metformin monotherapy developed asymptomatic subnormal serum vitamin B12 levels;
serum folic acid levels did not decrease significantly. However, only five cases of megaloblastic anemia have been reported with metformin administration (none during U.S. clinical studies) and no increased incidence of neuropathy has been observed.
April 18, 2013
Page 19 of 57
Therefore, serum vitamin B12 levels should be appropriately monitored or periodic
parenteral B12 supplementation considered (see WARNINGS AND PRECAUTIONS).
Lipids: Small increases in total cholesterol and LDL have been observed following
treatment with rosiglitazone (see ACTION AND CLINICAL PHARMACOLOGY,
Pharmacodynamics and Clinical Effects).
Serum Transaminase Levels: In clinical studies in 4598 patients treated with
rosiglitazone encompassing approximately 3600 patient years of exposure, there was no
evidence of drug-induced hepatotoxicity or elevated ALT levels.
In the controlled trials (including patients with ALT/AST of up to 2.5 times the upper
limit of the reference range at study entry), 0.2% of patients treated with rosiglitazone
had reversible elevations in ALT >3 times the upper limit of the reference range
compared to 0.2% on placebo and 0.5% on active comparators. Hyperbilirubinemia was
found in 0.3% of patients treated with rosiglitazone compared with 0.9% treated with
placebo and 1% in patients treated with active comparators. Overall, there was a
decrease in mean values for ALT, AST, alkaline phosphatase and bilirubin over time in
patients treated with rosiglitazone (see WARNINGS AND PRECAUTIONS, Hepatic).
In the clinical program including long-term, open-label experience, the rate per 100 patient years exposure of ALT increase to >3 times the upper limit of normal was 0.35 for patients treated with rosiglitazone, 0.59 for placebo-treated patients, and 0.78 for patients treated with active comparator agents.
In pre-approval clinical trials, there were no cases of idiosyncratic drug reactions leading
to hepatic failure.
Post-Market Adverse Drug Reactions
In postmarketing experience with rosiglitazone, as monotherapy and in combination with
other antidiabetic agents, adverse events potentially related to volume expansion (e.g.,
congestive heart failure, pulmonary edema, and pleural effusions) have been reported
(see WARNINGS AND PRECAUTIONS, Cardiovascular).
Reports of events related to cardiovascular ischemia including myocardial infarction, and
hypertension or hypertension accelerated have been received.
Reports of new onset and/or worsening macular edema with decreased visual acuity
occurring with the use of rosiglitazone have been received rarely. These patients
frequently reported concurrent peripheral edema. In some cases, symptoms improved
following discontinuation of rosiglitazone (see WARNINGS AND PRECAUTIONS,
Ophthalmologic).
Reports of anaphylactic reaction (such as angioedema and urticaria), rash and pruritus
have been received very rarely.
April 18, 2013
Page 20 of 57
In post-marketing experience, there have been very rare cases of creatinine kinase (CK)
elevation, myalgia, and rhabdomyolysis reported with the use of rosiglitazone.
Long-term post-market studies have shown an increased incidence of bone fracture in
patients taking rosiglitazone (see WARNINGS AND PRECAUTIONS, Fractures; and
ADVERSE REACTIONS, Clinical Trial Drug Adverse Reactions).
Reports of hepatitis and of hepatic enzyme elevations to three or more times the upper
limit of normal have been received. Very rarely, these reports have involved hepatic
failure with and without fatal outcome, although causality has not been established.
Postmarketing reports of parotid gland enlargement have been associated with
rosiglitazone and approximately one third of the reports resolved or improved following
discontinuation of rosiglitazone.
DRUG INTERACTIONS Overview Rosiglitazone maleate
Drugs Metabolized by Cytochrome P450: It has been shown
in vitro that rosiglitazone
does not inhibit any of the major P450 enzymes at clinically relevant concentrations.
In vitro studies demonstrate that rosiglitazone is predominantly metabolized by CYP2C8, with CYP2C9 as only a minor pathway.
In vitro studies have shown that montelukast is an inhibitor of CYP 2C8 and may inhibit the metabolism of drugs primarily metabolized by CYP 2C8 (e.g. paclitaxel, rosiglitazone, repaglinide). No
in vivo interaction studies have been performed with the CYP 2C8 inhibitor, montelukast; or, with CYP2C8 substrate paclitaxel. Although rosiglitazone is not anticipated to affect the pharmacokinetics of paclitaxel, concomitant use is likely to result in inhibition of the metabolism of rosiglitazone. Co-administration of rosiglitazone with CYP2C8 inhibitors (e.g. gemfibrozil) resulted in increased rosiglitazone plasma concentrations. Since there is a potential for an increase in the risk of dose-related adverse reactions, a decrease in rosiglitazone may be needed when CYP2C8 inhibitors are co-administered. Co-administration of rosiglitazone with a CYP2C8 inducer (e.g. rifampin) resulted in decreased rosiglitazone plasma concentrations. Therefore, close monitoring of glycemic control and changes in diabetic treatment should be considered when CYP2C8 inducers are co-administered. Clinically significant interactions with CYP2C9 substrates or inhibitors are not anticipated.
April 18, 2013
Page 21 of 57
CYP3A4 Substrates: Rosiglitazone (8 mg once daily) was shown to have no clinically
relevant effect on the pharmacokinetics of nifedipine and oral contraceptives
(ethinylestradiol and norethindrone), which are predominantly metabolized by CYP3A4.
The results of these two drug interaction studies suggest that rosiglitazone is unlikely to
cause clinically important drug interactions with other drugs metabolized via CYP3A4.
Metformin hydrochloride
In healthy volunteers, the pharmacokinetics of propranolol and ibuprofen were not
affected by metformin when co-administered in single-dose interaction studies.
Metformin is negligibly bound to plasma proteins and is therefore, less likely to interact
with highly protein-bound drugs such as salicylates, sulfonamides, chloramphenicol and
probenecid.
Alcohol intake: Alcohol is known to potentiate the effect of metformin on lactate
metabolism. Patients, therefore, should be warned against excessive alcohol intake, acute
or chronic, while receiving AVANDAMET®.
Drug-Drug Interactions
AVANDAMET®
Concurrent administration of rosiglitazone (2 mg twice daily) and metformin (500 mg twice daily) in healthy volunteers for 4 days had no effect on the steady-state pharmacokinetics of either metformin or rosiglitazone.
Rosiglitazone maleate
Oral Contraceptives: In 32 healthy women, rosiglitazone maleate (8 mg once daily)
was shown to have no statistically significant effect on the pharmacokinetics of oral
contraceptives (ethinylestradiol and norethindrone). Breakthrough bleeding occurred in
5 individuals when rosiglitazone was co-administered with an oral contraceptive. In one
of these subjects a 40% decrease in ethinylestradiol exposure (AUC) was recorded. This
was not correlated with a reduction in exposure to norethindrone, nor was there a
consistent relationship between the occurrence of breakthrough bleeding and the
pharmacokinetics of either ethinylestradiol or norethindrone in individual subjects.
Digoxin: Repeat oral dosing of rosiglitazone (8 mg once daily) for 14 days did not alter
the steady-state pharmacokinetics of digoxin (0.375 mg once daily) in healthy volunteers.
However, metformin has the potential for interaction with digoxin (see DRUG
INTERACTIONS, Cationic Drugs).
Warfarin: Coadministration of rosiglitazone (4 mg twice daily for 7 days) did not alter
the anticoagulant response of steady-state warfarin in healthy volunteers with baseline
values of INR of <2.75. Repeat dosing with rosiglitazone had no clinically relevant
effect on the steady-state pharmacokinetics of warfarin.
April 18, 2013
Page 22 of 57
Fibrates: Some epidemiologic studies and case reports suggest that markedly decreased
HDL-C in some patients involve the interaction of rosiglitazone with fenofibrate or
bezafibrate. Laboratory findings in some case reports demonstrated that, in some cases, it
is the combination of rosiglitazone and fenofibrate, and neither agent alone that lowers
HDL-C.
A study conducted in normal healthy volunteers showed that gemfibrozil (an inhibitor of
CYP2C8) administered as 600 mg twice daily, increased rosiglitazone systemic exposure
two-fold at steady state (see WARNINGS AND PRECAUTIONS, General).
Rifampin: A study conducted in normal healthy volunteers showed that rifampin (an
inducer of CYP2C8) administered as 600 mg daily, decreased the rosiglitazone systemic
exposure three-fold (see WARNINGS AND PRECAUTIONS, General).
Methotrexate: An interaction study of 22 adult patients with psoriasis examined the
effect of repeat doses of rosiglitazone (8 mg daily as a single dose for 8 days) on the
pharmacokinetics of oral methotrexate administered as single oral doses of 5 to 25 mg
weekly. Following 8 days of rosiglitazone administration, the Cmax and AUC(0-inf) of
methotrexate increased by 18% (90% CI: 11% to 26%) and 15% (90% CI: 8% to 23%),
respectively, when compared to the same doses of methotrexate administered in the
absence of rosiglitazone.
Metformin hydrochloride
Furosemide: A single-dose, metformin-furosemide drug interaction study in healthy
subjects demonstrated that pharmacokinetic parameters of both compounds were affected
by co-administration. Furosemide increased the metformin plasma and blood Cmax by
22% and blood AUC by 15%, without any significant change in metformin renal clearance. When administered with metformin, the Cmax and AUC of furosemide were
31% and 12% smaller, respectively, than when administered alone, and the terminal half-
life was decreased by 32%, without any significant change in furosemide renal clearance.
No information is available about the interaction of metformin and furosemide when co-
administered chronically.
Nifedipine: A single-dose, metformin-nifedipine drug interaction study in normal
healthy volunteers demonstrated that co-administration of nifedipine increased plasma
metformin Cmax and AUC by 20% and 9%, respectively, and increased the amount
excreted in the urine. Tmax and half-life were unaffected. Nifedipine appears to enhance
the absorption of metformin. Metformin had minimal effects on nifedipine.
Cationic Drugs: Cationic drugs (e.g., amiloride, digoxin, morphine, procainamide,
quinidine, quinine, ranitidine, triamterene, trimethoprim, and vancomycin) that are
eliminated by renal tubular secretion, theoretically have the potential for interaction with
metformin by competing for common renal tubular transport systems. Such an
interaction has been observed between metformin and oral cimetidine in normal healthy
volunteers in both single- and multiple-dose, metformin-cimetidine drug interaction
April 18, 2013
Page 23 of 57
studies. These studies showed a 60% increase in peak metformin plasma and whole
blood concentrations and a 40% increase in plasma and whole blood metformin AUC.
There was no change in elimination half-life in the single-dose study. Metformin had no
effect on cimetidine pharmacokinetics. Therefore, careful patient monitoring and dose
adjustment of AVANDAMET® or the interfering drug is recommended in patients who
are taking cationic medications that are excreted via the proximal renal tubular secretory
system.
Other: Other drugs tend to produce hyperglycemia and may lead to a loss of blood sugar
control. These include thiazides and other diuretics, corticosteroids, phenothiazines,
thyroid products, estrogens, estrogen plus progestogen, oral contraceptives, phenytoin,
nicotinic acid, sympathomimetics, calcium channel blocking drugs, and isoniazid. When
such drugs are administered to patients receiving AVANDAMET®, the patient should be
closely observed to maintain adequate glycemic control.
Drug-Food Interactions Interactions with food have not been established.
Drug-Herb Interactions Interactions with herbal products have not been established.
Drug-Laboratory Test Interactions Interactions with laboratory tests have not been established.
DOSAGE AND ADMINISTRATION Dosing Considerations The management of antidiabetic therapy with AVANDAMET® should be individualized
on the basis of effectiveness and tolerability while not exceeding the maximum
recommended daily dose of 8 mg rosiglitazone/2000 mg metformin.
Consistent with the dosing of metformin (i.e., in divided doses), AVANDAMET® should
be given in divided doses with meals, with gradual dose escalation. This reduces GI side
effects (largely due to metformin) and permits determination of the minimum effective
dose for the individual patient.
Sufficient time should be given after initiation of AVANDAMET® therapy or any dose
increase to assess adequacy of therapeutic response. Fasting plasma glucose (FPG)
should be used to determine the therapeutic response to AVANDAMET®. After an
increase in metformin dosage, dose titration is recommended if patients are not
adequately controlled after 1-2 weeks. After an increase in rosiglitazone dosage, dose
titration is recommended if patients are not adequately controlled after 8-12 weeks.
Increases in the rosiglitazone component to 8 mg/day should be undertaken cautiously
following appropriate clinical evaluation to assess the patient's risk of developing
April 18, 2013
Page 24 of 57
adverse reactions relating to fluid retention (see WARNINGS AND PRECAUTIONS;
ADVERSE REACTIONS and CLINICAL TRIALS).
No studies have been performed specifically examining the safety and efficacy of
AVANDAMET® in patients previously treated with other oral hypoglycemic agents and
switched to AVANDAMET®. Any change in therapy of type 2 diabetes should be
undertaken with care and appropriate monitoring as changes in glycemic control can
occur.
Specific Patient Populations AVANDAMET® is not recommended for use in pregnancy or for use in pediatric
patients.
The initial and maintenance dosing of AVANDAMET® should be conservative in
patients with advanced age, due to the potential for decreased renal function in this
population. Any dosage adjustment should be based on a careful assessment of renal
function. Generally, elderly, debilitated, and malnourished patients should not be titrated
to the maximum dose of AVANDAMET®. Monitoring of renal function is necessary to
aid in prevention of metformin-associated lactic acidosis, particularly in the elderly (see
WARNINGS AND PRECAUTIONS).
Therapy with AVANDAMET® should not be initiated if the patient exhibits clinical
evidence of active liver disease or increased serum transaminase levels (ALT >2.5 times
the upper limit of normal at start of therapy) (see WARNINGS AND PRECAUTIONS,
Hepatic and ACTION AND CLINICAL PHARMACOLOGY, Special Populations and
Conditions, Hepatic Insufficiency). Liver enzyme monitoring is recommended in all
patients prior to initiation of therapy with AVANDAMET® and periodically thereafter.
AVANDAMET® is contraindicated in patients with serious hepatic impairment (see
CONTRAINDICATIONS, WARNINGS AND PRECAUTIONS, Hepatic).
Recommended Dose and Dosage Adjustment
For patients inadequately controlled on metformin monotherapy: the usual starting
dose of AVANDAMET® is 4 mg rosiglitazone (total daily dose) plus the dose of
metformin already being taken (see Table 3).
Table 3
AVANDAMET® Starting Dose
PRIOR THERAPY
Usual AVANDAMET® Starting Dose
Total Daily Dose
Tablet Strength
Number of tablets
* For patients on 1500, 1700 or 2550 mg/day of metformin, initiation of AVANDAMET® requires individualization of therapy.
April 18, 2013
Page 25 of 57
When switching from combination therapy of rosiglitazone plus metformin as
separate tablets: the usual starting dose of AVANDAMET® is the dose of rosiglitazone
and metformin already being taken.
If additional glycemic control is needed: the daily dose of AVANDAMET® may be
increased by increments of 4 mg rosiglitazone and/or 500 mg metformin, up to the
maximum recommended total daily dose of 8 mg/2000 mg.
Missed Dose
If a dose of AVANDAMET® is missed, the patient should be advised to take one dose as
soon as they remember and the next dose at the usual time. Three doses should never be
taken in one day to make up for a missed dose the day before. If a whole day of
AVANDAMET® is missed, the usual dosing schedule should be followed the next day
without making up for the missed doses.
OVERDOSAGE In the event of an overdose, appropriate supportive treatment should be initiated as
dictated by the patient's clinical status.
No data are available with regard to overdosage of AVANDAMET®. In clinical studies
in volunteers, rosiglitazone has been administered at single oral doses of up to 20 mg and
was well tolerated.
Hypoglycemia has not been seen even with ingestion of up to 85 grams of metformin
hydrochloride, although lactic acidosis has occurred in such circumstances (see
WARNINGS AND PRECAUTIONS). Metformin is dialyzable with a clearance of up to
170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be
useful for removal of accumulated drug from patients in whom metformin overdosage is
suspected.
For management of a suspected drug overdose, contact your regional Poison Control Centre.
ACTION AND CLINICAL PHARMACOLOGY Mechanism of Action AVANDAMET® tablets combine two antidiabetic agents with different but
complementary mechanisms of action to improve glycemic control while reducing
circulating insulin levels in patients with type 2 diabetes: rosiglitazone maleate, a
member of the thiazolidinedione class and metformin hydrochloride, a member of the
biguanide class. Thiazolidinediones are insulin sensitizing agents that act primarily by
enhancing peripheral glucose utilization, whereas biguanides act primarily by decreasing
endogenous hepatic glucose production.
April 18, 2013
Page 26 of 57
Rosiglitazone maleate is an oral antidiabetic agent which acts primarily by increasing insulin sensitivity in type 2 diabetes. Rosiglitazone, a member of the thiazolidinedione class of antidiabetic agents, improves glycemic control while reducing circulating insulin levels. It improves sensitivity to insulin in muscle and adipose tissue and inhibits hepatic gluconeogenesis. Rosiglitazone is not chemically or functionally related to the sulfonylureas, the biguanides or the alpha-glucosidase inhibitors. Rosiglitazone is a highly selective and potent agonist for the peroxisome proliferator- activated receptor- gamma (PPARγ). In humans, PPAR receptors are found in key target tissues for insulin action such as adipose tissue, skeletal muscle and liver. Activation of PPARγ nuclear receptors regulates the transcription of insulin-responsive genes involved in the control of glucose production, transport, and utilization. In addition, PPARγ-responsive genes also participate in the regulation of fatty acid metabolism and in the maturation of preadipocytes, predominantly of subcutaneous origin. Insulin resistance is a primary feature characterizing the pathogenesis of type 2 diabetes. Rosiglitazone maleate results in increased responsiveness of insulin-dependent tissues and significantly improves hepatic and peripheral (muscle) tissue sensitivity to insulin in patients with type 2 diabetes. Clinical studies in patients with type 2 diabetes treated with rosiglitazone either as monotherapy or in combination with metformin showed improved beta-cell function and decreased fasting plasma glucose, insulin and C-peptide values following 26 weeks of treatment. A homeostasis model assessment (HOMA) was conducted using fasting plasma glucose and insulin or C-peptide levels as a measure of insulin sensitivity and beta-cell function. In these studies, reductions in mean plasma pro-insulin and pro-insulin split product concentrations were also observed. Rosiglitazone significantly reduced hemoglobin A1C (A1C, a marker for long term glycemic control), and fasting blood glucose (FBG) in patients with type 2 diabetes. Inadequately controlled hyperglycemia is associated with an increased risk of diabetic complications, including cardiovascular disorders and diabetic nephropathy, retinopathy and neuropathy. Studies between 8 and 26 weeks with rosiglitazone have shown a statistically significant reduction in markers of inflammation, C-reactive protein (CRP) and matrix metalloproteinase-9 (MMP-9). The clinical significance of these effects are still unknown. Further long term clinical trials are needed. Estimates of LDL particle size can be determined by the LDL cholesterol (LDL) to apolipoprotein B (Apo B) ratio. In controlled clinical trials, rosiglitazone has been shown to increase the LDL cholesterol to Apo B ratio consistent with a beneficial change in LDL particle size from small dense LDL particles to larger more buoyant particles. This change has been confirmed by measuring LDL particle buoyancy (Rf) following 8 weeks treatment with rosiglitazone in an open-label study. Metformin hydrochloride is an antihyperglycemic agent, which improves glucose tolerance in type 2 diabetes subjects, lowering both basal and postprandial plasma glucose. Metformin is not chemically or pharmacologically related to the oral
April 18, 2013
Page 27 of 57
sulfonylureas, thiazolidinediones, or alpha-glucosidase inhibitors. Metformin decreases
hepatic glucose production, decreases intestinal absorption of glucose and improves
insulin sensitivity by increasing peripheral glucose uptake and utilization. Unlike
sulfonylureas, metformin does not produce hypoglycemia in either patients with type 2
diabetes or normal subjects (except in special circumstances, see WARNINGS AND
PRECAUTIONS) and does not cause hyperinsulinemia. With metformin therapy, insulin
secretion remains unchanged while fasting insulin levels and day-long plasma insulin
response may actually decrease.
Pharmacodynamics and Clinical Effects
In clinical studies, treatment with rosiglitazone resulted in an improvement in glycemic
control, as measured by fasting plasma glucose (FPG) and haemoglobin A1C (HbA1C),
with a concurrent reduction in insulin and C-peptide. Postprandial glucose and insulin
were also reduced. This is consistent with the mechanism of action of rosiglitazone as an
insulin sensitizer. The improvement in glycemic control was durable. In open-labelled
extension studies sustained improvements in glycemic control (as measured by A1C
levels) were observed in patients receiving rosiglitazone monotherapy for 36 months.
Rosiglitazone is believed to act primarily on muscle and adipose tissue whereas
metformin acts primarily on the liver to decrease hepatic glucose output. The co-
administration of rosiglitazone with metformin resulted in significantly improved
glycemic control compared to either of these agents alone. These results are consistent
with a synergistic effect on glycemic control when rosiglitazone is used in combination
with metformin. In patients whose type 2 diabetes was inadequately controlled with
metformin monotherapy, the addition of rosiglitazone led to reductions in A1C levels that
were sustained for over 30 months of treatment, in open-labelled studies.
Weight gain has been observed in clinical studies with rosiglitazone (see Table 4). In
addition, rosiglitazone significantly decreased visceral (abdominal) fat stores while
increasing subcutaneous abdominal fat. The reduction in visceral fat correlates with
improved hepatic and peripheral tissue insulin sensitivity. Weight gain with
thiazolidinediones can result from increases in subcutaneous adipose tissue and/or from
fluid retention. Treatment should be re-evaluated in patients with excessive weight gain
(see WARNINGS AND PRECAUTIONS and ADVERSE REACTIONS).
April 18, 2013
Page 28 of 57
Weight Changes (kg) from Baseline During Clinical Trials with Rosiglitazone
Treatment
Duration
(25th, 75th
(25th, 75th
(25th, 75th
percentile)
percentile)
percentile)
Monotherapy
Combination Therapy
Rosiglitazone +
Patients with lipid abnormalities were not excluded from clinical trials of rosiglitazone. In all 26-week controlled trials, across the recommended dose range, rosiglitazone as monotherapy was associated with increases in total cholesterol, LDL, and HDL and decreases in free fatty acids. These changes were statistically significantly different from controls. Increases in LDL occurred primarily during the first 1 to 2 months of therapy with rosiglitazone and LDL levels remained stable, but elevated above baseline throughout the trials. In contrast, HDL continued to rise over time. As a result, the LDL/HDL ratio peaked after 2 months of therapy and then appeared to decrease over time. The pattern of LDL and HDL changes following therapy with rosiglitazone in combination with metformin was generally similar to those seen with rosiglitazone in monotherapy. The changes in triglycerides during therapy with rosiglitazone were variable and were generally not statistically different from controls. The long term significance of the lipid changes is not known.
April 18, 2013
Page 29 of 57
Pharmacokinetics
Bioavailability
AVANDAMET
In a bioequivalence and dose proportionality study of AVANDAMET® 4 mg/500 mg,
both the rosiglitazone component and the metformin component were bioequivalent to
coadministered 4 mg rosiglitazone maleate tablet and 500 mg metformin hydrochloride
tablet under fasted conditions (see Table 5). In this study, dose proportionality of
rosiglitazone in the combination formulations of 1 mg/500 mg and 4 mg/500 mg was
demonstrated.
Table 5
Mean (SD) Pharmacokinetic Parameters for Rosiglitazone and Metformin
Pharmacokinetic Parameter
AUC (0-inf)
(ng.h/mL)
Metformin
* = Median and range presented for Tmax
Regimen A = 4 mg/500 mg AVANDAMET®
Regimen B = 4 mg rosiglitazone maleate tablet + 500 mg metformin hydrochloride tablet
Regimen C = 1 mg/500 mg AVANDAMET®
Administration of AVANDAMET® 4 mg/500 mg with food resulted in no change in overall exposure (AUC) for either rosiglitazone or metformin. However, there were decreases in Cmax of both components (22% for rosiglitazone and 15% for metformin,
respectively) and a delay in Tmax of both components (1.5 hrs for rosiglitazone and 0.5 hrs
for metformin, respectively). These changes are not likely to be clinically significant. The pharmacokinetics of both the rosiglitazone component and the metformin component of AVANDAMET® when taken with food were similar to the pharmacokinetics of rosiglitazone and metformin when administered concomitantly as separate tablets with food.
April 18, 2013
Page 30 of 57
Absorption
Rosiglitazone maleate
Rosiglitazone is rapidly and completely absorbed after oral administration with negligible
first-pass metabolism. The absolute bioavailability of rosiglitazone is 99%. Peak plasma
concentrations are observed by 1 hour after dosing. Maximum plasma concentration
(Cmax) and the area under the curve (AUC0-inf) of rosiglitazone increase in a dose-
proportional manner over the therapeutic dose range. The elimination half-life is 3 to
4 hours and is independent of dose.
Metformin hydrochloride
Metformin absorption is relatively slow and may extend over about 6 hours. The
absolute bioavailability of a 500 mg metformin hydrochloride tablet given under fasting
conditions is approximately 50-60%. Studies using single oral doses of metformin tablets
of 500 mg and 1500 mg, and 850 mg to 2550 mg, indicate that there is a lack of dose
proportionality with increasing doses, which is due to decreased absorption rather than an
alteration in elimination.
Distribution
Rosiglitazone maleate
The mean (SD) volume of distribution (Vss) of rosiglitazone after intravenous
administration to healthy subjects is approximately 14.1 (3.1) litres. Rosiglitazone is
approximately 99.8% bound to plasma proteins, primarily albumin.
Metformin hydrochloride
The apparent volume of distribution (V/F) of metformin following single oral doses of
850 mg metformin hydrochloride averaged 654 ± 358 L. Metformin is negligibly bound
to plasma proteins. Metformin partitions into erythrocytes, most likely as a function of
time. At usual clinical doses and dosing schedules of metformin, steady state plasma
concentrations of metformin are reached within 24-48 hours and are generally < 1 μg/mL.
During controlled clinical trials, maximum metformin plasma levels did not exceed
5 μg/mL, even at maximum doses.
Metabolism
Rosiglitazone maleate
Rosiglitazone is extensively metabolized with no unchanged drug excreted in the urine.
The major routes of metabolism were N-demethylation and hydroxylation, followed by
conjugation with sulfate and glucuronic acid. All the circulating metabolites are
considerably less potent than the parent drug and, therefore, are not expected to
contribute to the insulin-sensitizing activity of rosiglitazone.
In vitro data demonstrate
April 18, 2013
Page 31 of 57
that rosiglitazone is predominantly metabolized by cytochrome P450 isoenzyme CYP2C8,
with CYP2C9 contributing as only a minor pathway.
Metformin hydrochloride
Intravenous single-dose studies in normal subjects demonstrate that metformin is
excreted unchanged in the urine and does not undergo hepatic metabolism (no
metabolites have been identified in humans) nor biliary excretion. Renal clearance is
approximately 3.5 times greater than creatinine clearance which indicates that tubular
secretion is the major route of metformin elimination.
Excretion
Rosiglitazone maleate
Following oral or intravenous administration of [14C]rosiglitazone maleate,
approximately 64% and 23% of the dose was eliminated in the urine and in the feces,
respectively. The plasma half-life of [14C] related material ranged from 103 to 158 hours.
Metformin hydrochloride
Following oral administration, approximately 90% of the absorbed drug is eliminated via
the renal route within the first 24 hours, with a plasma elimination half-life of
approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours,
suggesting that the erythrocyte mass may be a compartment of distribution.
Special Populations and Conditions Pediatrics: The safety and effectiveness of rosiglitazone and metformin have not been
established in patients younger than 18 years of age, therefore, AVANDAMET® is not
indicated in patients younger than 18 years of age. Thiazolidinediones promote the
maturation of preadipocytes and have been associated with weight gain. Obesity is a
major problem in adolescents with type 2 diabetes.
Geriatrics: Results of the population pharmacokinetic analysis (n=716 <65 years; n=331
≥65 years) showed that age does not significantly affect the pharmacokinetics of
rosiglitazone.
However, limited data from controlled pharmacokinetic studies of metformin
hydrochloride in healthy elderly subjects suggest that total plasma clearance of
metformin is decreased, the half-life is prolonged and Cmax is increased, compared to
healthy young subjects. From these data, it appears that the change in metformin pharmacokinetics with aging is primarily accounted for by a change in renal function. Metformin treatment and therefore treatment with AVANDAMET® should not be initiated in patients 80 years of age or older unless measurement of creatinine clearance demonstrates that renal function is not reduced (see WARNINGS AND PRECAUTIONS and DOSAGE AND ADMINISTRATION).
April 18, 2013
Page 32 of 57
Gender: Results of the population pharmacokinetic analysis showed that the mean oral
clearance of rosiglitazone in female patients (n=405) was 15% lower compared to male
patients (n=642), primarily related to lower body weight in females. In rosiglitazone and
metformin combination studies, efficacy was demonstrated with no gender differences in
glycemic response.
Metformin pharmacokinetic parameters did not differ significantly between normal
subjects and patients with type 2 diabetes when analyzed according to gender (males =
19, females = 16). Similarly, in controlled clinical studies in patients with type 2
diabetes, the antihyperglycemic effect of metformin hydrochloride tablets was
comparable in males and females.
Race: Results of a population pharmacokinetic analysis including subjects of white,
black, and other ethnic origins indicate that race has no influence on the
pharmacokinetics of rosiglitazone.
No studies of metformin hydrochloride pharmacokinetic parameters according to race
have been performed. In controlled clinical studies of metformin in patients with type 2
diabetes, the antihyperglycemic effect was comparable in whites (n = 249), blacks (n =
51) and Hispanics (n = 24).
Hepatic Insufficiency: Unbound oral clearance of rosiglitazone was significantly lower
in patients with moderate to severe liver disease (Child-Pugh Class B/C) compared to
healthy subjects. As a result, unbound Cmax and AUC0-inf were increased 2- and 3-fold,
respectively. Elimination half-life for rosiglitazone was about 2 hours longer in patients
with liver disease, compared to healthy subjects. Therapy with AVANDAMET® should
not be initiated if the patient exhibits clinical evidence of active liver disease or increased
serum transaminase levels (ALT > 2.5 times the upper limit of normal) at baseline (see
WARNINGS AND PRECAUTIONS, Hepatic).
No pharmacokinetic studies have been conducted in subjects with hepatic insufficiency
with metformin.
Renal Insufficiency: In subjects with decreased renal function (based on measured
creatinine clearance), the plasma and blood half-life of metformin is prolonged and the
renal clearance is decreased in proportion to the decrease in creatinine clearance (see
WARNINGS AND PRECAUTIONS).
Since metformin is contraindicated in patients with renal impairment, administration of
AVANDAMET® is contraindicated in these patients.
April 18, 2013
Page 33 of 57
STORAGE AND STABILITY Store at controlled room temperature 15°C to 30°C.
Special Instructions
Dispense in a tight, light-resistant container.
DOSAGE FORMS, COMPOSITION AND PACKAGING AVANDAMET® tablets contain rosiglitazone maleate and metformin hydrochloride
equivalent to: 2 mg rosiglitazone with 500 mg metformin hydrochloride (2 mg/500 mg),
4 mg rosiglitazone with 500 mg metformin hydrochloride (4 mg/500 mg), 2 mg
rosiglitazone with 1000 mg metformin hydrochloride (2 mg/1000 mg), and 4 mg
rosiglitazone with 1000 mg metformin hydrochloride (4 mg/1000 mg).
Each tablet contains rosiglitazone as the maleate and metformin hydrochloride as
follows:
2 mg/500 mg:
pale pink, film coated oval tablet, debossed with gsk on one side and 2/500 on the other.
orange, film coated oval tablet, debossed with gsk on one side and 4/500 on the other.
yellow, film coated oval tablet, debossed with gsk on one side and 2/1000 on the other.
pink, film coated oval tablet, debossed with gsk on one side and 4/1000 on the other.
Non-medicinal Ingredients: hydroxypropyl methylcellulose, lactose monohydrate,
magnesium stearate, microcrystalline cellulose, polyethylene glycol 400, povidone 29-32,
sodium starch glycolate, titanium dioxide and one or more of the following: red and
yellow iron oxides.
Presentations: 2 mg/500 mg, 4 mg/500 mg, 2 mg/1000 mg and 4 mg/1000 mg in bottles
of 100 tablets.
April 18, 2013
Page 34 of 57
PART II: SCIENTIFIC INFORMATION
PHARMACEUTICAL INFORMATION Drug Substance Proper
methyl]-2,4-thiazolidinedione,
(Z)-2-butenedioate
C18H19N3O3S•C4H4O4
473.52 (357.44 free base)
Structural formula:
Physicochemical properties Description:
A white to off-white solid
Readily soluble in ethanol and buffered aqueous
solution with pH 2.3; solubility decreases with increasing
pH value of a saturated solution of rosiglitazone maleate in
water is 3.3, and in 0.9% saline is 3.4.
pKa1=6.1, pKa2=6.8
Partition Coefficient: The distribution coefficient of rosiglitazone maleate, was
measured by the shake-flask method, using a pH 6.5
phosphate buffer. In n-octanol/water the distribution
to be 194 (logD = +2.29). In
cyclohexane/water
determined to be 0.32 (logD = - 0.49).
Range of 122°C to 123°C
April 18, 2013
Page 35 of 57
Drug Substance Proper
N,N-dimethyl biguanide hydrochloride
Structural formula:
Physicochemical properties:
Metformin hydrochloride is a white crystalline powder
in 95% ethyl alcohol and practically
insoluble in ether and chloroform.
The pH of a 1% aqueous solution of metformin hydrochloride is 6.68.
CLINICAL TRIALS There have been no clinical efficacy trials conducted with AVANDAMET®
(rosiglitazone maleate/metformin hydrochloride) tablets. However, studies utilizing the
separate components have established the effective and safe use, and the additive benefits
of the combination. Bioequivalence of AVANDAMET® with co-administered
rosiglitazone tablets and metformin tablets was demonstrated (see ACTION AND
CLINICAL PHARMACOLOGY, Pharmacokinetics).
A total of 670 patients with type 2 diabetes participated in two 26-week, randomized,
double-blind, placebo/active-controlled studies designed to assess the efficacy of
rosiglitazone in combination with metformin. Rosiglitazone maleate, administered in
either once-daily or twice-daily dosing regimens, was added to the therapy of patients
April 18, 2013
Page 36 of 57
who were inadequately controlled on a maximum dose (2.5 grams/day) of metformin
hydrochloride.
In one study, patients inadequately controlled on 2.5 grams/day of metformin
hydrochloride (mean baseline FPG 12.0 mmol/L and mean baseline A1C 0.088) were
randomized to receive rosiglitazone 4 mg once daily, rosiglitazone 8 mg once daily, or
placebo in addition to metformin. A statistically significant improvement in FPG and
A1C was observed in patients treated with the combinations of metformin and
rosiglitazone 4 mg once daily and rosiglitazone 8 mg once daily, versus patients
continued on metformin alone (Table 6).
Table 6
Glycemic Parameters in a 26-Week Rosiglitazone maleate + Metformin hydrochloride
Combination Study
Metformin
2.5 g/day
4 mg once daily
8 mg once daily
+ metformin
+ metformin
2.5 g/day
2.5 g/day
FPG (mmol/L)
Change from baseline (mean)
Difference from metformin alone
(adjusted mean)
Responders (≥ 1.7 mmol/L
decrease from baseline)
A1C (ratio)
Change from baseline (mean)
Difference from metformin alone
-0.010* -0.012*
(adjusted mean)
Responders (≥ 0.007 decrease in
ratio from baseline)
*<0.0001 compared to metformin In a second 26-week study, patients with type 2 diabetes inadequately controlled on 2.5 grams/day of metformin who were randomized to receive the combination of rosiglitazone 4 mg twice daily and metformin (N=105) showed a statistically significant improvement in glycemic control with a mean treatment effect for FPG of -3.1 mmol/L and a mean treatment effect for A1C of -0.008 over metformin alone. The combination of metformin and rosiglitazone resulted in lower levels of FPG and A1C than either agent alone. In a third 24 week double blind study, the efficacy of rosiglitazone in combination with 1.0 gram/day of metformin hydrochloride was compared with continued titration to 2.0 grams/day of metformin hydrochloride. Patients with type 2 diabetes inadequately
April 18, 2013
Page 37 of 57
controlled on 1.0 gram/day of metformin hydrochloride were randomized to receive rosiglitazone 4 mg twice daily in addition to metformin 1.0 gram/day or to receive 2.0 grams/day of metformin monotherapy. Patients receiving rosiglitazone received an initial dose of 2 mg twice daily for 8 weeks, followed by 4 mg twice daily for the remainder of the study. Patients receiving metformin monotherapy received 1.5 grams/day of metformin for 8 weeks, followed by 2.0 grams/day for the remainder of the study. At the end of week 24, the addition of rosiglitazone to 1.0 gram/day of metformin was at least as effective as 2.0 grams/day of metformin in improving A1C (mean reduction of A1C of 0.0093 and 0.0071, respectively). At the end of week 24, the reduction from baseline in FPG was significantly greater with rosiglitazone added to 1.0 gram/day (mean reduction of 2.29 mmol/L) compared to 2.0 grams/day of metformin (mean reduction of 1.12 mmol/L). Significantly more patients receiving rosiglitazone plus 1.0 gram/day of metformin achieved a 0.007 or greater reduction from baseline in A1C (59.5%) compared to patients receiving 2.0 grams/day of metformin (49.5%) (p = 0.0247). Open-labelled extension studies of rosiglitazone in combination with metformin double-blind, placebo-controlled trials showed a decrease in baseline A1C levels from 0.087 in the 4 mg bd group and 0.084 in the 8 mg od group to 0.071 and 0.077 respectively at month 30. In addition, FPG open-labelled baseline values decreased from 10.52 mmol/L in the 4 mg bd group and 10.36 mmol/L in the 8 mg od group to 7.55 mmol/L and 8.28 mmol/L respectively at month 30. Figures 1 and 2 show that the decreases in mean A1C and mean FPG values achieved during the treatment months were sustained in those patients who remained in the study.
April 18, 2013
Page 38 of 57
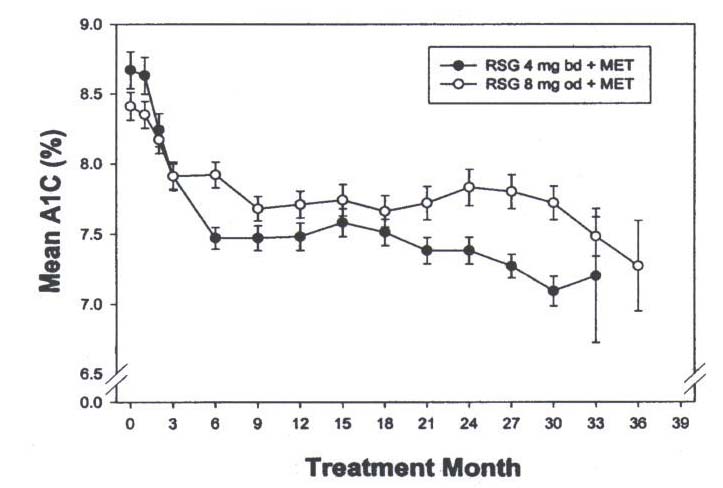
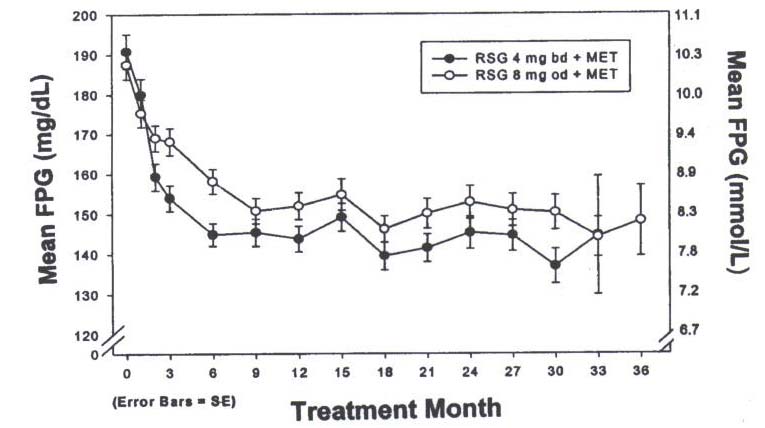
Mean A1C Values Over Time
Mean FPG Values Over Time
April 18, 2013
Page 39 of 57
Cardiovascular Studies:
Two echocardiography studies in 437 type 2 diabetic patients (a 52-week study with
rosiglitazone 4 mg twice daily and a 26-week study with 8 mg once daily), designed to
detect a change in left ventricular mass of 10% or more, showed no deleterious alteration
in cardiac structure or function. Compared to placebo, there was a small, statistically
significant increase in median plasma volume (1.8 mL/kg) in healthy volunteers treated
with rosiglitazone 8 mg once daily for 8 weeks. See ADVERSE REACTIONS for
experience concerning serious cardiovascular adverse events.
Patients with congestive heart failure (CHF) New York Heart Association (NYHA) Class
I and II treated with rosiglitazone have an increased risk of cardiovascular events. A 52-
week, double-blind, placebo-controlled echocardiographic study was conducted in 224
patients with type 2 diabetes mellitus and NYHA Class I or II CHF (ejection fraction
≤ 45%) on background antidiabetic and CHF therapy. An independent committee
conducted a blinded evaluation of fluid-related events (including congestive heart failure)
and cardiovascular hospitalizations according to predefined criteria (adjudication).
Separate from the adjudication, other cardiovascular adverse events were reported by
investigators. Although no treatment difference in change from baseline of ejection
fractions was observed, more cardiovascular adverse events were observed with
rosiglitazone treatment compared to placebo during the 52-week study (see Table 7).
Table 7
Emergent Cardiovascular Adverse Events in Patients with congestive Heart Failure
(NYHA Class I and II) treated with Rosiglitazone or Placebo (in addition to Background
Antidiabetic and CHF Therapy
Adjudicated
Cardiovascular Deaths
With overnight hospitalization
Without overnight hospitalization
New or Worsening Edema
New or Worsening Dyspnea
Increases in CHF Medication
Cardiovascular Hospitalization*
Investigator-reported, Non-adjudicated
Ischemic Adverse Events
Myocardial Infarction
* Includes hospitalization for any cardiovascular reason
Rosiglitazone in Combination with Insulin
For safety reasons, the use of rosiglitazone in combination therapy with insulin is not
indicated.
April 18, 2013
Page 40 of 57
In two 26-week U.S. trials involving 611 patients with type 2 diabetes, rosiglitazone
maleate plus insulin therapy was compared with insulin therapy alone. These trials
included patients with long-standing diabetes and a high prevalence of pre-existing
medical conditions, including peripheral neuropathy (34%), retinopathy (19%), ischemic
heart disease (14%), vascular disease (9%), and congestive heart failure (2.5%). In these
clinical studies, an increased incidence of cardiac failure and other cardiovascular adverse
events were seen in patients on rosiglitazone and insulin combination therapy compared
to insulin and placebo. Patients who experienced heart failure were on average older, had
a longer duration of diabetes, and were mostly on the higher 8 mg daily dose of
rosiglitazone. In this population, however, it was not possible to determine specific risk
factors that could be used to identify all patients at risk of heart failure on insulin
combination therapy. Three of 10 patients who developed cardiac failure on insulin
combination therapy during the double blind part of the fixed dose studies had no known
prior evidence of congestive heart failure, or pre-existing cardiac condition.
In 26-week double-blind fixed dose studies, edema was reported with higher frequency in
the rosiglitazone plus insulin combination trials (insulin, 5.4%; and rosiglitazone in
combination with insulin, 14.7%). Reports of new onset or exacerbation of congestive
heart failure occurred at rates of 1% for insulin alone, and 2% (4 mg) and 3% (8 mg) for
insulin in combination with rosiglitazone (see WARNINGS AND PRECAUTIONS). In
these studies, approximately 2.5% of the patients were enrolled with a presenting medical
condition of congestive heart failure (NYHA Class I/II). Patients with NYHA Class III
and IV heart failure were excluded from all clinical trials.
In the retrospective analysis of data from pooled clinical studies, a greater increased risk
of myocardial ischemic events was observed in studies where rosiglitazone was added to
insulin.
DETAILED PHARMACOLOGY
The antidiabetic activity of rosiglitazone has been demonstrated in a number of animal
models of type 2 diabetes in which hyperglycemia and/or impaired glucose tolerance is a
consequence of insulin resistance in target tissues. Rosiglitazone normalizes blood
glucose concentrations and reduces hyperinsulinemia in the ob/ob obese mouse, db/db
diabetic mouse and fa/fa fatty Zucker rat. Rosiglitazone also prevents the development of
overt diabetes in both the db/db mouse and Zucker fa/fa Diabetic Fatty (ZDF) rat models.
In addition, rosiglitazone prevents the development of systolic hypertension, proteinuria,
renal morphologic abnormalities and renal dysfunction in the Zucker rat and prevents the
deleterious changes in pancreatic morphology seen in untreated db/db mice, ZDF rats and
Zucker fa/fa rats.
In animal models, rosiglitazone's antidiabetic activity was shown to be mediated by
increased sensitivity to insulin's action in the liver, muscle and adipose tissues. The
expression of the insulin-regulated glucose transporter GLUT-4 was increased in adipose
tissue. Rosiglitazone did not induce hypoglycemia in animal models of type 2 diabetes
and/or impaired glucose tolerance.
April 18, 2013
Page 41 of 57
TOXICOLOGY
No animal studies have been conducted with the combined products in AVANDAMET®.
The following data are based on findings in studies performed with rosiglitazone or
metformin individually.
Rosiglitazone maleate
Teratogenic Effects
There was no effect on implantation or the embryo with rosiglitazone treatment during
early pregnancy in rats, but treatment during mid-late gestation was associated with fetal
death and growth retardation in both rats and rabbits. Teratogenicity was not observed.
Rosiglitazone caused placental pathology (labyrinth congestion and increased weight) in
rats (≥3 mg/kg/day) but not in rabbits at 100 mg/kg/day. Treatment of rats during
gestation through lactation reduced litter size, neonatal viability and postnatal growth
with growth retardation reversible after puberty. For effects on the placenta,
embryo/fetus and offspring, the no-effect dose was 0.2 mg/kg/day (AUC=11.94 μg.h/mL)
in rats and 15 mg/kg/day (AUC=12.5 μg.h/mL) in rabbits.
Impairment of Fertility
Rosiglitazone had no effects on mating or fertility of male rats given up to 40 mg/kg/day.
Rosiglitazone altered estrous cyclicity (≥2 mg/kg/day) and reduced fertility
(40 mg/kg/day) of female rats in association with lower plasma levels of progesterone
and estradiol with no such effects at 0.2 mg/kg/day (AUC=11.94 μg.h/mL). In monkeys,
rosiglitazone (0.6 and 4.6 mg/kg/day [AUCs of 8.21 and 44.14 μg.h/mL]) diminished the
follicular phase rise in serum estradiol with consequential reduction in the luteinizing
hormone surge, lower luteal phase progesterone levels, and amenorrhea. The mechanism
for these effects appears to be direct inhibition of ovarian steroidogenesis, apparently a
thiazolidinedione class effect.
Carcinogenesis and Mutagenesis:
Two-year carcinogencity studies were conducted in Charles River CD-1 mice at doses of
0.4, 1.5 and 6 mg/kg/day in the diet and in Sprague-Dawley rats at oral gavage doses of
0.05, 0.3 and 2 mg/kg/day (top doses equivalent to approximately 10 to 20 times human
AUC at the maximum recommended human dose of 8 mg/day). Rosiglitazone was not
carcinogenic in the mouse. There was an increase in incidence of adipose hyperplasia in
the mouse at doses >1.5 mg/kg/day (approximately 2 times human AUC). In rats, there
was a significant increase in the incidence of benign adipose tissue tumors (lipomas) at
doses >0.3 mg/kg/day (approximately 2 times human AUC). These proliferative changes
in both species are considered due to the persistent pharmacological overstimulation of
adipose tissue and appear to be rodent-specific.
Rosiglitazone was not mutagenic or clastogenic in the in vitro bacterial assays for gene mutation, the in vitro chromosome aberration test in human lymphocytes, the in vivo mouse micronucleus test and the in vivo/in vitro rat UDS assay. There was a small (about 2-fold) increase in mutation in the in vitro mouse lymphoma assay at toxic
April 18, 2013
Page 42 of 57
concentrations of 150 to 200 μg/mL, but this was regarded as system-specific with no
general relevance.
Cardiovascular-Renal
Heart weights were increased in mice (≥3 mg/kg/day), rats (≥5 mg/kg/day), and dogs
(≥2 mg/kg/day) with rosiglitazone treatments. There were increases in wet and dry
cardiac weight and total protein content. Morphometric analysis showed left ventricular
hypertrophy, and echocardiographic assessments revealed an increase in left ventricular
mass with a proportional increase in left ventricular wall area and lumen volume. The
no-effect dose for cardiac hypertrophy was 0.5 mg/kg to 2 mg/kg among mice, rats and
dogs in studies of up to 1 year duration.
In preclinical studies, thiazolidinediones caused plasma volume expansion and pre-load-
induced cardiac hypertrophy. The cardiac hypertrophy was an adaptive consequence of
an increase in preload, as shown by an increase in diastolic wall stress, with no
contribution from afterload. The increase in preload derives from plasma volume
expansion due to increased renal sodium and fluid retention in response to increased
blood flow to specific tissues (particularly adipose, skin and gastrointestinal) and mild
vasorelaxation.
Liver
There was a small increase in liver weight in female rats (≥5 mg/kg/day) but no effects in
male rats (40 mg/kg) or mice of either sex (20 mg/kg). Only in the dog were there
increases in plasma enzyme activity (principally alanine aminotransferase, ALT) at doses
of 0.5 mg/kg or greater. There was evidence of hepatocellular regeneration and oxidative
stress in dogs with raised ALT. Species-specific hepatotoxicity in dogs may be attributed
to toxic metabolites formed to a greater extent in this species rather than to parent drug
exposure.
Endocrine System
In rats only, ovary weight was decreased in association with a reduction/absence of
corpora lutea at doses ≥5 mg/kg, and there was increased pituitary weight with lactotroph
hyperplasia at doses ≥0.2 mg/kg. These changes in the ovary and pituitary of female rats
were attributed to reduced ovarian synthesis of estradiol and progesterone to a greater
extent, with a net increase in the ratio of plasma estradiol to progesterone concentrations.
Whereas such changes in steroid hormone levels causing persistent vaginal estrus and
lactotroph hyperplasia in female rats are sex and species-specific outcomes, lower levels
of estradiol and progesterone in the cynomolgus monkey were associated with
amenorrhea. The frequency of reports relating to menstrual dysfunction in clinical trials
was low and similar to placebo (0.4% on rosiglitazone and placebo).
April 18, 2013
Page 43 of 57
Metformin hydrochloride
Carcinogenesis and Mutagenesis:
Long-term carcinogenicity studies have been performed in rats (dosing duration of 104
weeks) and mice (dosing duration of 91 weeks) at doses up to and including
900 mg/kg/day and 1500 mg/kg/day, respectively. These doses are both approximately
four times the maximum recommended human daily dose of 2000 mg of the metformin
component of AVANDAMET® based on body surface area comparisons. No evidence of
carcinogenicity with metformin was found in either male or female mice. Similarly,
there was no tumorigenic potential observed with metformin in male rats. There was,
however, an increased incidence of benign stromal uterine polyps in female rats treated
with 900 mg/kg/day.
There was no evidence of a mutagenic potential of metformin in the following in vitro
tests: Ames test (S. typhimurium), gene mutation test (mouse lymphoma cells), or
chromosomal aberrations test (human lymphocytes). Results in the in vivo mouse
micronucleus test were also negative.
Impairment of Fertility
Fertility of male or female rats was unaffected by metformin when administrated at doses
as high as 600 mg/kg/day, which is approximately three times the maximum
recommended human daily dose of the metformin component of AVANDAMET® based
on body surface area comparisons.
Teratogenic Effects
Metformin was not teratogenic in rats and rabbits at doses up to 600 mg/kg/day. This
represents an exposure of about two and six times the maximum recommended human
daily dose of 2000 mg based on body surface area comparisons for rats and rabbits,
respectively. Determination of fetal concentrations demonstrated a partial placental
barrier to metformin.
April 18, 2013
Page 44 of 57
REFERENCES
1. Alberti KG, Nattrass M. Lactic Acidosis, Lancet 1977; 2(8027):25-29.
2. American Diabetes Association. Detection and management of lipid disorders in
diabetes. Diabetes Care 1993; 16(5):828-834.
3. Arlt W, Auchus RJ, Miller WL. Thiazolidinediones but not metformin directly inhibit
the steroidogenic enzymes P450c17 and 3beta-hydroxysteroid hydrogenase. J Biol Chem. 2001; 276(20):16767-71.
4. AVANDIA® Product Monograph, 2001 5. Bailey CJ, Turner RC. Metformin. N Eng J Med 1996; 334(9):574-79. 6. Balfour JA, Plosker GL. Rosiglitazone. Drugs: Adis New Drug Profile,
Rosiglitazone. Drugs 1999; 57(6):921-930
7. Beckmann, R.: Absorption, distribution in the organism and elimination of
metformin. Diabetologia 1969; 5(5):318-324.
8. Benoit R, Lacroix A, D'Amico P, Pesant P, Matte R. Lactic acidosis and phenformin.
Union Med Can 1976; 105(12): 1810-1814.
9. Berger W, et al. Problèmes d'actualité concemant le méchanisme d'action des
biguanides. Jour. Diab. Hôtel-Dieu Paris, 239.258, 1975.
10. Bermond, P. The coefficient of insulin efficacy. Effect of Metformin on this
parameter. Xième Congrès Fédération Int. Diabétologie, Stockholm, 1967. Ed. Excerpa Medica F. Amsterdam, 1968.
11. Biron, P. Metformin monitoring. Can Med Assoc J 1980; 123(1):11-12. 12. Bouaziz, PI. Apport à l'étude de l'épreuve d'hyperglycémie provoquée par voie
veineuse sous thérapie diabétique. Thése de doctorat en Médecine, Paris, 1966.
13. Campbell IW. Antidiabetic drugs present and future: will improving insulin resistance
benefit cardiovascular risk in type 2 diabetes mellitus? Drugs 2000; 60(5):1017-1028.
14. Carey DG, Cowen GJ, Galloway GJ, Jones NP, Richards JC, Biswas N et al. Effect
of Rosiglitazone on Insulin Sensitivity and Body Composition in Type 2 Diabetic Patients. Obesity Research October 2002; 10(10):1008-1015.
15. Chan LY, Yeung JH, Lau TK. Placental transfer of rosiglitazone in the first trimester
of pregnancy. Fertility and Sterility 2005; 83(4):955-8.
April 18, 2013
Page 45 of 57
16. Cohen RD. The relative risks of different biguanides in the causation of lactic
acidosis. Research and Clinical Forums, l:(4) 125-134, 1979.
17. Cohen Y et al. Etude autoradiographique chez la souris d'un antidiabétique oral, le
N.N. Diméthylbiguanide, marqué au C14 Thérapie 109-120, 1961.
18. Cohen Y, Hirsch C. Etude autoradiographique chez la souris d'un antidiabétique oral
marqué au C14, le N.N. Diméthylbiguanide, après administrations répétées. Thérapie XXIII, 1185-1191, 1968.
19. Cusi K, Defronzo RA. Metformin: a review of its metabolic effects. Diabetes
Reviews 1998; 6(2):89-131.
20. Daubresse, JC et al: Acidose lactique et thérapeutique par biguanides. Méd. et Hyg.
21. The DCCT Research Group. The effect of intensive treatment of diabetes on the
development and progression of long-term complications in insulin-dependent diabetes mellitus. N Eng J Med. 1993; 329(14): 977-986.
22. Debry G, et al. Etude du mode d'excrétion du N.N. Diméthylbiguanide chex le
diabétique adulte. Thérapie 1963 ; XX: 351-358.
23. Derot M. et al. Retrospective study of the cardiovascular fate of 190 patients treated
for 5 year or more with biguanides alone. Abstracts, 11th Annual Meeting, Munich Sept., 1975.
24. DiCicco R, Allen A, Jorkasky D, Carr A, Freed M. Lack of pharmacokinetic drug
interaction between rosiglitazone (BRL49653) and metformin. Clin Pharmacol Ther 1998;63(2):Abs P1-75.
25. DiCicco R, Allen A, Carr A, Fowles S, Jorkasky D, Freed M. Rosiglitazone does not
alter the pharmacokinetics of metformin. J Clin Pharmacol 2000; 40:1280-1285.
26. Duval D. Contribution à l'étude de l'action hypoglycémiante des biguanides. Thèse
de Doctorat en Médecine, Paris, 1960.
27. Duwoos H, Bertrand CM, Husson A, Cramer J, Tayot J. Hyperlactacidémie réversible
induite par la phenformine avec asthénie musculaire et signes cardio-respiratoires. Presse Med. 78: 23-26, 1970.
28. Finegood DT, McArthur MD, Dunichand-Hoedl A et al. The PPAR[gamma]
Agonist, Rosiglitazone, Reverses Hyperinsulinemia and Promotes Growth of Islet ß-cell Mass. Diabetes 1998; 47(suppl 1):A47. Abstract 0182.
April 18, 2013
Page 46 of 57
29. Finegood DT, McArthur MD, Kojwang D, Thomas MJ, Topp BG, Leonard T et al. β-
Cell Mass Dynamics in Zucker Diabetic Fatty Rats, Rosiglitazone Prevents the Rise in Net Cell Death. Diabetes 2001; 50(5):1021-1029.
30. Fonseca V, Rosenstock J, Patwardhan R, Salzman A. Effect of metformin and
rosiglitazone combination therapy in patients with type 2 diabetes mellitus. JAMA 2000;283(13): 1695-1702. Erratum published due to incorrect wording and data presentation - JAMA 2000; 284(11)1384.
31. Fonseca V, Biswas N, Salzman A. Once-daily rosiglitazone (RSG) in combination
with metformin (MET) effectively reduces hyperglycemia in patients with type 2 diabetes. Diabetes 1999; 48(S1):A100, Abs 431. (Poster 431, 59th American Diabetes Association meeting, San Diego, California. June 19-22, 1999.)
32. Fonseca V, Biswas N, Salzman A. Rosiglitazone in combination with metformin
effectively reduces hyperglycemia in patients with type 2 diabetes. Diabetologia 1999; 42(S1):A230, Abs 864.
33. Freed MI, Millet A, Inglis AM, et al. Rosiglitazone, a PPAR-gamma Agonist, Does
Not Alter the Pharmacokinetics of Nifedipine, a Cytochrome P450 3A4-Substrate. Diabetes 1998; 47(suppl 1):A94. Abstract 0368.
34. Freed MI, Ratner R, Marcovina SM, Kreider MM, Biswas N, Cohen BR et al.
Effects of rosiglitazone alone and in combination with atorvastatin on the metabolic abormalities in type 2 diabetes mellitus. Am J Cardiol 2002; 90(9): 947-952.
35. Freed MI, Miller A, Jorkasky D, et al. Rosiglitazone Pharmacokinetics Are Not
Affected by Coadministration of Ranitidine. Diabetes. 1998; 47(suppl 1): A353.
36. Gasic S, Bodenburg Y, Nagamani M, Green A, Urban RJ. Troglitazone Inhibits
Progesterone Production in Porcine Granulosa Cells. Endocrinology 1998; 139(12): 4962-4966.
37. GLUCOPHAGE® Product Monograph, 2006. 38. GLUCOPHAGE® U.S. Prescribing Information, 2001 39. Gomez-Perez FJ, Fanghannel-Salmon G, Berry RA, Warsi G, Gould EM.
Rosiglitazone-metformin combination therapy improves glycemic control in Mexican patients with type 2 diabetes. Diabetes 2001; 50(Suppl 2): A436 , Abs 1818-PO.
40. Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI. Effect of
Rosiglitazone Treatment on Nontraditional Markers of Cardiovascular Disease in Patients with Type 2 Diabetes Mellitus. Circulation 2002; 106(6):679-684.
April 18, 2013
Page 47 of 57
41. Hermann LS. Metformin: A review of its pharmacological properties and therapeutic
use. Diabete et Metabolisme 1979; 5(3):233-245.
42. Hermann LS. Metabolic effects of Metformin in relation to clinical effects and side
effects in Biguanide Therapy Today. International Congress and Symposium, series published by the Royal Society of Medicine 1981; 48:17-43.
43. Hermans MP, Levy JC, Morris RJ, Turner R. Comparison of tests of beta-cell
function across a range of glucose tolerance from normal to diabetes. Diabetes 1999; 48(9): 1779-1786.
44. Hermans MP, Levy JC, Morris RJ, Turner R. Comparison of insulin sensitivity tests
across a range of glucose tolerance from normal to diabetes. Diabetologia 1999; 42(6): 678-687.
45. Holle A, Mangeis W, Dreyer M, Kuhnau J, Rudiger HW. Biguanide treatment
increases the number of insulin receptor sites on human erythrocytes. The New Engl J Med 1981; 305(10):563-566.
46. Hunt J A, Catellier C, Dupre J, Gardiner RJ, McKendry JB, Toews CJ et al: The use
of phenformin and metformin. Can Med Assoc J 1977; 117(5):429-430.
47. Inglis AML, Miller AK, Thompson KA. Coadministration of rosiglitazone and
acarbose (A): lack of clinically relevant pharmacokinetic drug interaction. Diabetes 1998; 47(suppl 1):A353. Abstract 1366.
48. Irsigler, K. Glucoseutilisation and Plasmaliporide bei adiposen Patienten unter dem
Einfluss von Dimethylbiguanide (GLUCOPHAGE®). wiener med. Wsch.1969; 119:191-194.
49. Isnard F, Lavieuville M. Acidose lactique et biguanides. Etat actuel de la question en
France. Journ Annu Diabetol Hotel Dieu 1977; 362-375.
50. Iozzo P, Hallsten K, Oikonen V, Virtanen KA, Parkkola R, Kemppainen J et al.
Effect of Metformin and Rosiglitazone Monotherapy in Insulin-mediated Hepatic Glucose Update and Their Relation to Visceral Fat in Type 2 Diabetes. Diabetes Care 2003; 26(7): 2069-2074.
51. Joncas F. Evaluation clinique de GLUCOPHAGE® pour le traitement du diabète de
l'adulte. Hôpital Maisonneuve, Montréal. L'Union Médicale du Canada, Jan. Issue, 1972.
52. Jones T, Jones NP, Sautter M. Addition of rosiglitazone to metformin is effective in
obese, insulin resistant patients with type 2 diabetes. Diabetologia 2000; 43(S1):A191, Abs 735.
April 18, 2013
Page 48 of 57
53. Jones T, Jones NP, Sautter M. Rosiglitazone: Effective when added to metformin in
obese, insulin-resistant patients with type 2 diabetes. Diab Res Clin Pract 2000;
50(suppl 1):P308. Poster 308 presented at the 17th IDF (International Diabetes
Foundation) meeting, Mexico City, Mexico, November 5-10, 2000.
54. Jones NP, Mather R, Owen S, Porter LE, Patwardhan R. Rosiglitazone: Long term
efficacy in combination with metformin or as monotherapy. Poster 307 presented at the 17th IDF (International Diabetes Foundation) meeting, Mexico City, Mexico, November 5-10, 2000. Diab Res Clin Pract 2000; 50(suppl 1):P307.
55. Kannel WB, McGee DL. Diabetes and Glucose Tolerance as Risk Factors for
Cardiovascular Disease: The Framingham Study. Diabetes Care 1979; 2(2):120-126.
56. Kinosian B, Glick H, Garland G. Cholesterol and coronary heart disease: predicting
risks by levels and ratios. Ann Intern Med 1994; 121(9):641-647.
57. Kreider M, Miller E, Patel J. Rosiglitazone is safe and well tolerated as monotherapy
or combination therapy in patients with type II diabetes mellitus. Diabetes 1999; 48(Suppl 1):A117. Poster 506 presented at 59th American Diabetes Association meeting, San Diego, California. June 19-22, 1999.
58. Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PF et al.
Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in Men: Prospective results form the Quebec cardiovascular study. Circulation 1997; 95(1): 69-75.
59. Laurendeau E, et al: Traitement du diabète sucré chez des patients âgés, hospitalisés
avec le N.N. Diméthylbuguanide (GLUCOPHAGE®). Hôpital Notre-Dame de Ia Merci. Montréal, 1970. Ref Lab. Franca (non publié).
60. Lefebvre P, et al. Le mécanisme d'action des biaguanides. Biguanides et sécrétion
insulinique. Congrès International de Diabétologie de Rémini, 1968.
61. Leonard T, Bakst A, Warsi G, Bonora E. Rosiglitazone may reduce insulin resistance-
related cardiovascular disease risk in type 2 diabetes patients. Poster 852 presented at 37th Annual EASD Meeting. Glasgow, Scotland. September 9-13, 2001.
62. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment
(HOMA) evaluation uses the computer program. Diabetes Care 1998; 21(12): 2191-2192.
63. Maheux P, Berry RA, Warsi GG, Gould EM. Rosiglitazone-Metformin combination
improves glycemic control in patients with type 2 diabetes. Poster 168 presented at the CDA/CSEM Professional Conference and Annual Meeting. Edmonton, Alberta. October 17-20, 2001.
April 18, 2013
Page 49 of 57
64. Mainguet P, Lauvaux JP, Franckson JR. Intestinal glucose absorption in diabetic
patients after acute administration of dimethylbiguanide. Diabete 1972; 20(1) :39-42.
65. Marx N, Froehlich J, Siam L, Ittner J, Wierse G, Schmidt A et al. Antidiabetic
PPAR(-Activator Rosiglitazone Reduces MMP-9 Serum Levels in Type 2 Diabetic Patients With Coronary Artery Disease. Arterioscler Thromb Vasc Biol 2003; 23(2): 283-288.
66. McKlish A. Toxicité du N.N. Diméthylbiguanide chez le chien Beagle. Centre de
recherches Laval, Quebec (1970). Ref. Laboratoires Franca Inc. (non publié) 1970.
67. Meyer F, Ipaktchi M, Clauser H. Données nouvelles sur le mécanisme d'action des
biguanide hypoglycémiants. Journ Annu Diabetol Hotel Dieu 1967; 341-347.
68. Miller E, Patel J, Reichek N, Granett J. BRL 49653 (a thiazolidinedione) is Well
Tolerated and Has No Effect on LV Mass Following 12 Weeks Treatment in NIDDM patients. Diabetes 1997; 46(suppl 1):96A. Abstract 0377.
69. Oakes ND, Kennedy CJ, Jenkins AB, Laybutt DR, Chisholm DJ, Kraegen EW. A
New Antidiabetic Agent, BRL 49653, Reduces Lipid Availability and Improves Insulin Action and Glucoregulation in the Rat. Diabetes 1994; 43(10):1203-1210.
70. Patel J, Miller E, Patwardhan R, The Rosiglitazone 011 Study Group. Rosiglitazone
(BRL49653) Monotherapy Has Significant Glucose Lowering Effect in Type 2 Diabetic Patients. Diabetes 1998; 47(suppl 1):A17. Abstract 0067.
71. Patwardhan R, Porter LE, Jones NP. Long-term efficacy of rosiglitazone as
monotherapy or combination therapy in patients with type 2 diabetes mellitus. Poster 1826. Presented at the US Society of Endocrinology Meeting June 2000.
72. Pelletier G, et al. Etude de toxicité chronique de N.N. Diméthylbiguanide chez le rat.
Centre de recherche Laval, Québec. Ref. Laboratoires Franca Inc. (non publieé).
73. Pelletier G, et al. Etude tératologique avec le N.N. Diméthylbiguanide chez le rat.
Centre de Recherche Laval, Québec. Ref. laboratoires Franca Inc. (non publieé) 1970.
74. Pignard, P. Dosage spectrotométrique du N.N. Dimethylbiguanide dans le sang et
l'urine. Annales de Biologie Clinique 1962; 20:225-233.
75. Sidhu JS, Cowan D, Kaski JC. The Effect of Rosiglitazone a Peroxisome Proliferator-
Activated Receptor-gamma Agonist, on Markers of Endothelial Cell Activation, C-Reactive Protein, and Fibrinogen Levels in Non-Diabetic Coronary Artery Disease Patients. J Am Coll Cardiol 2003; 42(10): 1757-1763.
April 18, 2013
Page 50 of 57
76. Smith S, Boam D, Bretherton-Watt D. Rosiglitazone Increases Pancreatic Islet Area,
Density and Insulin Content, but not Insulin Gene Expression. Diabetes 1998; 47(suppl 1):A18. Abstract 0072.
77. Smith S, Boam D, Cawthorne M. Rosiglitazone Improves Insulin Sensitivity and
Reduces Hyperexpression of Insulin and Amylin mRNA's in Pancreatic Islets. Diabetes 1998; 47(suppl 1):A94. Abstract 3065.
78. Smith SA, Cawthorne MA, Coyle PJ. BRL 49653 Normalises Glycaemic Control in
Zucker fa/fa Rats by Improving Hepatic and Peripheral Tissue Sensitivity to Insulin. Diabetologia 1993; 36(suppl 1):A184. Abstract 707.
79. Sterne J. Oral Hypoglycemic agents. Medicinal Chemistry 1969; 9(5):193-294. 80. Sterne J.M, et al: Oral hypoglycemic agents: Clinical pharmacology and therapeutic
use. Drugs 1977; 14:41-56.
81. Sterne J. Pharmacology and mechanism of action of the antidiabetic biguanides.
Paper read in Moscow, April 1977, Unpublished.
82. Stowers JM. Long-term therapy with biguanides in: Biguanide Therapy Today,
International Congress and Symposium, Series published by the Royal Society of Medecin, 48:49-57, 1981.
83. Stowers JM, Borthwick LJ. Oral hypoglycemic agents: Clinical pharmacology and
therapeutic use. Drugs 1977; 14(1):41-56.
84. Tiikkainen M, Hakkinen AM, Korsheninikova E, Nyman T, Makimattila S, Yki-
Jarvinen H. Effect of Rosiglitazone and Metformin on Liver Fat Content, Hepatic Insulin Resistance, Insulin Clearance, and Gene Expression in Adipose Tissue in Patients With Type 2 Diabetes. Diabetes 2004; 53:2169-2176.
85. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control
with sulfonylureas or insulin compared with conventional gtreatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837-853.
86. UK Prospective Diabetes Study (UKPDS) Group Association of glycaemia with
macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000; 34: 405-412.
87. Virtanen KA, Hallsten K, Parkkola R, et al. Differential Effects of Rosiglitazone and
Metformin on Adipose Tissue Distribution and Glucose Uptake in Type 2 Diabetic Subjects. Diabetes. 2003; 52: 283-290.
April 18, 2013
Page 51 of 57
88. Wang TD, Chen WJ, Lin JW et al. Effects of Rosiglitazone on Endothelial Function,
C-Reactive Protein, and Components of the Metobolic Syndrome in Nondiabetic Patients With the Metabolic Syndrome. Am J Cardiol. 2004; 93: 362-365.
89. Vague P. Effet d'une dose unique de metformine sur la tolérance au glucose des
sujets normaux ou obèses. Le Diabète 1970; 18:35-39.
90. Vermulen A, Rottiers R. Influence of dimethylbiguanide (metformin) on
carbohydrate metabolism in obese, non diabetic women. Diabetologia 1972; 8:8-11.
91. Werner AL, Travaglini MT. A Review of Rosiglitazone in Type 2 Diabetes Mellitus.
Pharmacotherapy 2001; 21(9):1082-1099.
92. Young PW, Cawthorne MA, Coyle PJ, Holder JC, Holman GD, Kozka IJ et al.
Repeat Treatment of Obese Mice With BRL 49653, a New and Potent Insulin Sensitizer, Enhances Insulin Action in White Adipocytes. Diabetes 1995; 44(9):1087-1092.
April 18, 2013
Page 52 of 57
IMPORTANT: PLEASE READ
PART III: CONSUMER INFORMATION
What it does:
AVANDAMET® combines two glucose-lowering medicines,
rosiglitazone (AVANDIA®) and metformin, together in one
rosiglitazone maleate/metformin hydrochloride tablets
tablet. These two medicines work together to help you achieve
better blood sugar control. Rosiglitazone helps your body use its
This leaflet is part III of a three-part "Product Monograph" for
own insulin better by making the tissues more sensitive to
AVANDAMET® and is designed specifically for Consumers.
insulin. The tissues are better able to "hear" the signals insulin
This leaflet is a summary and will not tell you everything
sends out. That means the tissues will absorb sugar more easily.
about AVANDAMET®. Contact your doctor or pharmacist if
Metformin helps to lower the amount of sugar made by the liver.
you have any questions about the drug.
Together, these medicines keep the amount of sugar in your
Keep this leaflet until you have finished all your tablets as you
blood at a more normal level.
may need to read it again.
When it should not be used:
ABOUT THIS MEDICATION
• If you have or have had heart problems or heart failure (the
heart cannot pump enough blood to the body's other
organs), talk to your doctor. One of the two medicines that
What the medication is used for:
make up AVANDAMET®, rosiglitazone, can cause your
AVANDAMET® (ah-VAN-duh-met) is a medicine used in
body to keep extra fluid (fluid retention), which can make
addition to diet and exercise to lower blood sugar in patients
some heart problems worse, lead to heart failure, swelling
with type 2 diabetes (non-insulin dependent) when all other
and weight gain.
diabetes medicines taken orally (by mouth) have not lowered
blood sugar enough or are not appropriate.
If you have kidney disease or impairment (reduced kidney function).
Before starting AVANDAMET®, your doctor will discuss the
If you are allergic to AVANDAMET® or any of its
possible benefits and possible side effects of AVANDAMET®
to decide if AVANDAMET® is right for you. Your doctor will
• If you have diabetic ketoacidosis (dangerously high levels
ask you to read and sign a form indicating you understand the
of ketones, which signals the body doesn't have enough
cardiovascular risks of AVANDAMET®.
• If you have had a condition called lactic acidosis. Lactic
In order for AVANDAMET® to be effective, you should
acidosis is caused by a build-up of lactic acid in the blood.
continue to exercise and follow the diet recommended for
Because of the possibility of lactic acidosis, you should not
your diabetes while taking AVANDAMET®.
take AVANDAMET® if:
• You drink alcohol excessively (all the time or
People who have diabetes have problems with insulin. Insulin
short-term "binge" drinking).
is produced by an organ called the pancreas (PAN-kree-us).
• You are seriously dehydrated (have lost a large
Inside the pancreas are special cells called beta-cells that
amount of body fluids).
actually make insulin. Insulin is a hormone (body's own
• You have a severe infection or experience serious
natural chemical) that allows the body's tissues to absorb
physical trauma, including surgery or while
glucose (known as "sugar") from the bloodstream to provide
recovering from surgery.
the body energy.
• You develop a serious condition such as a heart
attack, stroke or have severe heart or breathing
People with Type 2 diabetes do not make enough insulin, or
the body tissues become less sensitive to insulin. When the
• You are 80 years of age or older and have NOT
tissues do not respond normally to insulin, it is as if they
had your kidney function tested.
cannot "hear" the signals insulin sends out – this is called
• If you have serious liver problems.
"insulin resistance."
• If you have Type I diabetes – this needs different treatment.
• If you are pregnant or breastfeeding.
With diabetes, sugar (glucose) builds up in the blood. This can
lead to serious medical problems including kidney damage,
AVANDAMET® therapy will need to be stopped temporarily if
heart disease, loss of limbs, and blindness. The main goal of
you are going to have certain x-ray procedures with injectable
treating diabetes is to lower your blood sugar to a normal
contrast agents.
level. Lowering and controlling blood sugar may help prevent
or delay complications of diabetes such as heart disease, kidney disease or blindness.
April 18, 2013
Page 53 of 57
IMPORTANT: PLEASE READ
What the medicinal ingredients are:
• you are going to have any surgery or specialized x-ray
AVANDAMET® tablets contain two active ingredients,
procedures that require injection of contrast agents (substances
rosiglitazone maleate and metformin hydrochloride, in one
that help physicians see the tissues more clearly).
AVANDAMET® therapy will need to be stopped temporarily
in such instances.
What the nonmedicinal ingredients are:
hydroxypropyl methylcellulose, lactose monohydrate,
Broken bones usually in the hand, upper arm or foot, have been
magnesium stearate, microcrystalline cellulose, polyethylene
seen in people taking rosiglitazone, one of the active ingredients
glycol 400, povidone 29-32, sodium starch glycolate, titanium
of AVANDAMET®. Talk to your doctor about the risk of
dioxide and one or more of the following: red and yellow iron
Decreases in spine and hip bone mineral density (a measure of
What dosage forms it comes in:
bone strength, based on the amount of calcium and other
rosiglitazone maleate/metformin hydrochloride tablets
minerals in your bones) have been reported in men and women
2 mg/500 mg, 4 mg/500 mg, 2 mg/1000 mg, 4 mg/1000 mg.
taking rosiglitazone.
WARNINGS AND PRECAUTIONS
Muscle problems, including muscle tenderness, weakness, or pain that you cannot explain, have been seen in people taking
rosiglitazone, one of the active ingredients of AVANDAMET®.
Serious Warnings and Precautions
Talk with your doctor if you experience these symptoms. If you
• AVANDAMET®, which contains rosiglitazone, may
experience brownish or discoloured urine with your muscle
increase the risk of serious heart problems, including:
problems, stop taking AVANDAMET® and call your doctor
• heart failure
right away.
• angina (chest pain)
• heart attack (myocardial infarction)
The safety and effectiveness of AVANDAMET® have not been
• fluid retention (with or without rapid weight gain)
established in children under 18 years of age, therefore
AVANDAMET® is not recommended for use in these patients.
• AVANDAMET® should not be used if you have or have
had heart problems.
AVANDAMET® is not approved for use with insulin therapy,
therefore AVANDAMET® is not recommended for use with
Before you use AVANDAMET®, talk to your doctor
about other options to treat your diabetes.
AVANDAMET® is not approved for use with a sulfonylurea,
therefore AVANDAMET® is not recommended for use with a
Before, or while taking AVANDAMET®, talk to your doctor
about all your medical conditions, including if:
• you have experienced edema (swelling in the wrists, hands,
INTERACTIONS WITH THIS MEDICATION
feet or ankles).
you have been diagnosed with angina (chest pain) or have
AVANDAMET® may affect how other medicines work, and
had a heart attack.
some medicines may affect how AVANDAMET® works. Drugs
• you have heart-related risks, including cigarette smoking,
that may interact with the two active ingredients in
high blood pressure, high cholesterol, or a family history of
AVANDAMET® (rosiglitazone and metformin) include: digoxin
and quinidine (used to treat heart failure and arrhythmias),
• you are taking nitrate medicines (such as nitroglycerin or
gemfibrozil (used to lower cholesterol and triglyceride levels in
isosorbide dinitrate).
your blood), methotrexate (used to treat psoriasis and
• you have a type of diabetic eye disease called macular
rheumatoid arthritis), morphine (used to relieve severe pain),
edema (swelling in the back of the eye).
ranitidine (used to treat ulcers and gastroesophageal reflux
• you have liver problems.
disease (GERD)), rifampin (used to treat tuberculosis).
• you are breastfeeding.
• you are pregnant or planning to become pregnant.
Keep a list of all the medicines you take and tell your doctor and
• you are not near menopause but not ovulating (e.g., you are
pharmacist about every medication you take. This means both
a patient with polycystic ovary syndrome).
prescription medications (the ones your doctor writes for you)
AVANDAMET® could make you ovulate again, which
and over-the-counter medications (the ones you buy in the
means you could get pregnant. Talk to your doctor about
drugstore, like cold or allergy medicines), or natural health
effective methods of birth control (e.g., hormonal
products (herbal medicines).
contraceptive pills).
April 18, 2013
Page 54 of 57
IMPORTANT: PLEASE READ
PROPER USE OF THIS MEDICATION
Very common side effects (could affect one in 10 people or more):
• Symptoms of an upset stomach such as nausea, vomiting,
Usual dose:
diarrhea, and stomach pain. If these side effects occur, they
The usual starting dose of AVANDAMET® depends on your
usually occur during the first few weeks of therapy. Taking
previous treatment with metformin (GLUCOPHAGE®) and
your AVANDAMET® with meals can help reduce these
rosiglitazone (AVANDIA®). Your doctor will decide on the
dose of AVANDAMET® that is suitable for you.
Common side effects (could affect up to one in 10 people):
AVANDAMET® should be taken by mouth and with meals.
Your doctor may need to adjust your dose until your blood
Anemia (low red blood cell count) which may make you feel very weak or tired.
sugar is better controlled. AVANDAMET® can begin to work
1 or 2 weeks after you start taking it. It may take 2-3 months
Chest pain (angina).
to see the optimal effects.
• Heart failure or pulmonary edema (fluid accumulation in the
Test your blood sugar regularly as your doctor tells you.
lungs). Symptoms of heart failure include shortness of
breath, getting tired easily after light physical activity such
Remember: This medicine has been prescribed only for
as walking, unusual tiredness, waking up short of breath at
you. Do not give it to anybody else.
night, swollen ankles or feet, and an unusually rapid
increase in weight. Symptoms of fluid in the lungs are
Take your AVANDAMET® each day, as instructed by your
breathlessness, which may be very severe and usually
doctor. AVANDAMET® can help control your blood sugar
worsens on lying down. Stop taking AVANDAMET® and
levels only if you take it regularly.
call your doctor right away if you experience these
• Constipation.
Overdose:
• Edema (fluid retention or swelling) which could lead to or
Taking too much of any medicine can be dangerous.
worsen heart failure. If you notice swelling in your
extremities (arms and legs, hands and feet), an unusually rapid increase in weight, or if you experience unusual
In case of drug overdose, contact a health care practitioner,
tiredness, trouble breathing or shortness of breath, call your
hospital emergency department or regional Poison Control
doctor. These symptoms, although not specific, may signal
Centre immediately, even if there are no symptoms.
heart problems or heart failure. Pay closer attention to these
symptoms if you are using the higher dose of rosiglitazone
Missed Dose:
(8 mg) in AVANDAMET® as fluid retention is more
Take one dose as soon as you remember. Then take the next
dose at the usual time. Never take extra doses in one day to
• Broken bones usually in the hand, upper arm or foot. Talk to
make up for a missed dose the day before. If you miss a
your doctor about the risk of fracture.
whole day of AVANDAMET®, just take your dose as usual
• A small increase in total cholesterol levels. Total cholesterol
the next day. Don't try to make it up by taking extra tablets.
is made up of "good cholesterol" (HDLc) and "bad
cholesterol" (LDLc) and it is the balance of these that is
Recommended clinical and laboratory tests while taking
more important than the total level. AVANDAMET® does
not affect the balance of good and bad cholesterol. If you
have any concerns about your cholesterol levels, you should
Your doctor may do additional blood sugar tests to see how
speak to your doctor.
well AVANDAMET® is working.
• Low blood sugar (hypoglycemia). There is a low risk of
hypoglycemia with AVANDAMET®. Dizziness, lack of
Your doctor may conduct various blood or laboratory tests to
energy, drowsiness, headache, trembling, sweating, or
monitor your health and liver before you start
hunger may mean that your blood sugar is too low. This can
AVANDAMET® and repeat the tests periodically while you
happen if you skip meals, drink alcohol, use another
are on AVANDAMET®.
medicine that lowers blood sugar, exercise (particularly hard
or long), or if you have certain medical problems. Call your
Your doctor should check your eyes regularly. Rarely, some
doctor if you feel that your symptoms of low blood sugar
patients have experienced vision changes due to swelling in
are uncomfortable.
the back of the eye while taking AVANDAMET®.
• A strange or metallic taste in the mouth.
• Increased weight. Tell your doctor if you gain a lot of
SIDE EFFECTS AND WHAT TO DO ABOUT THEM
weight in a short period of time.
Rare side effects (could affect up to one in 1,000 people):
April 18, 2013
Page 55 of 57
IMPORTANT: PLEASE READ
• Liver problems. If you experience nausea, vomiting,
SERIOUS SIDE EFFECTS, HOW OFTEN THEY
stomach pain, lack of appetite, tiredness, dark urine, or
HAPPEN AND WHAT TO DO ABOUT THEM
yellowing of the skin, stop taking AVANDAMET® and call your doctor right away.
Symptom / effect
Talk with your
Stop taking
Blurred vision due to swelling (or fluid) in the back of the
AVANDAMET®
and call your
immediately
Very rare side effects (could affect up to one in 10,000 people):
Fluid retention or
extremities (arms
Metformin, one of the active ingredients in
and legs, hands and
AVANDAMET®, can cause a serious side effect
feet) without signs
called lactic acidosis. This is caused by a build-up of
of heart failure or fluid in the lungs
lactic acid in your blood. This build-up can cause
serious damage. Lactic acidosis is a medical
emergency that must be treated in a hospital.
• Allergic reactions, which may include hives or rash
(which may be itchy), or more serious symptoms which
Dizziness, lack of
energy, drowsiness,
may occur suddenly, such as swelling of the face, lips,
mouth, tongue or throat (which may cause difficulty in
trembling, sweating
swallowing or breathing). Stop taking AVANDAMET®
and call your doctor right away if you experience these
Heart failure or
symptoms.
fluid in the lungs
Breakthrough bleeding (unexpected vaginal bleeding or
spotting) while using oral contraceptives, or generally, if
you experience any symptoms that persist or become
shortness of breath,
troublesome, these should be discussed with your doctor.
getting tired easily
after light physical
Muscle problems. If you experience muscle tenderness,
activity, unusual
weakness, or pain that you cannot explain, talk with your
tiredness, waking
doctor. If you experience brownish or discoloured urine
up short of breath at
with your muscle problems, stop taking AVANDAMET®
night, an unusually rapid increase in
and call your doctor right away.
weight. Fluid may
• Problems related to Vitamin B12 deficiency.
also cause swollen
You may experience swelling of the parotid gland (salivary
Chest pain (angina).
glands located over the jaw, in front of the ears).
Nausea, vomiting,
SERIOUS SIDE EFFECTS, HOW OFTEN THEY
stomach pain, lack
HAPPEN AND WHAT TO DO ABOUT THEM
tiredness, dark urine, or yellowing
Symptom / effect
Talk with your
Stop taking
AVANDAMET®
Lactic Acidosis:
and call your
Feeling very weak,
immediately
(nausea, vomiting,
breathing, unusual
diarrhea, stomach
discomfort, feeling
Low red blood cell
cold, feeling dizzy
or light-headed,
Feeling very weak
unusual fatigue and
drowsiness, or suddenly developing a slow or irregular heartbeat.
April 18, 2013
Page 56 of 57
IMPORTANT: PLEASE READ
SERIOUS SIDE EFFECTS, HOW OFTEN THEY
REPORTING SUSPECTED SIDE EFFECTS
HAPPEN AND WHAT TO DO ABOUT THEM
You can report any suspected adverse reactions
Symptom / effect
Talk with your
Stop taking
associated with the use of health products to the Canada
AVANDAMET®
Vigilance Program by one of the following 3 ways:
and call your
immediately
• Report online at
Blurred vision or
decreased vision
• Call toll free at 1-866-234-2345
[which may be due
• Complete a Canada Vigilance Reporting Form
fluid) in the back of the eye].
- Fax toll-free to 1-866-678-6789, or
- Mail to: Canada Vigilance Program
Allergic reactions: Hives or rash
Health Canada
Postal Locator 0701C
Ottawa, ON K1A 0K9
serious symptoms
Very rare
suddenly, such as
Postage paid labels, Canada Vigilance Reporting Form
swelling of the face,
and the adverse reaction reporting guidelines are
lips, mouth, tongue
available on the MedEffect™ Canada Web site at
cause difficulty in swallowing or
NOTE: Should you require information related to the
management of side effects, contact your health
Muscle tenderness or weakness,
professional. The Canada Vigilance Program does not
Very rare
muscle pain that
provide medical advice.
you cannot explain.
Very rare
MORE INFORMATION
especially if you do
This document plus the full product monograph, prepared for
Very rare
discoloured urine.
health professionals can be found at:
http://www.gsk.ca
or by contacting the sponsor,
This is not a complete list of side effects. If you experience
GlaxoSmithKline Inc.
any unexpected effects while taking AVANDAMET®, contact
7333 Mississauga Road
your doctor or pharmacist.
Mississauga, Ontario
HOW TO STORE IT
Store AVANDAMET® at room temperature (15º C to 30º C),
This leaflet was prepared by GlaxoSmithKline Inc.
out of the reach of children.
Last revised: April 18, 2013
2013 GlaxoSmithKline Inc. All Rights Reserved.
®AVANDAMET is a registered trademark, used under license by GlaxoSmithKline Inc.
®AVANDIA is a registered trademark, used under license by GlaxoSmithKline Inc.
®GLUCOPHAGE is a registered trademark of MERCK SANTÉ
April 18, 2013
Page 57 of 57
Source: http://www.gsk.ca/english/docs-pdf/product-monographs/Avandamet.pdf
Q&A: 2009 Prohibited List What major changes does the 2009 List of Prohibited Substances and Methods include compared to the 2008 List? • The 2009 List includes modifications in relation to the status of specified substances in order to align the 2009 List with the more flexible sanctions, able to be imposed in cases involving "specified substances", set forth in the revised World Anti-Doping Code (2009 Code) to come into effect on January 1, 2009.
A Production Guidefor North Carolina North Carolina Cooperative Extension ServiceNorth Carolina State University North Carolina State University Description and Stages of Growth . 3 General Culture . 4 Site Selection . 4 Shade Requirements . 5 Site Preparation . 5 Transplanting Roots . 7 Shade Management . 8 Fertilization . 8 Pest Management. 8 Seed Production, Harvesting, and Handling. 9



