Titel der lehrveranstaltung:
Effects of a basic
micronutrient supplement
on physiological
parameters
Institute of science of sports
University of Salzburg
Mag. Michael G. Eder
Responsible person: Univ.-Prof. Dr. Erich Müller
St. Michael, 2005
Preface Who wants to build high towers, have to spend a lot of his time on the foundation.
I want to thank Mr. Mag. Norbert Fuchs, who gave me a lot of inspiration, know how
and his time for making this investigation possible.
Thank you too, for being so patient with me and with my questions.
For my brother Klaus
2.3.7 Errors _ 77
Problem- and task setting
1 Problem- and task setting
1.1 Introduction
It is well known that human nutrition at the moment is not well balanced. The German
society of nutrition (DGE) started an investigation about the daily intake of
micronutrients. The result was a too high rate of macronutrients and a too low intake
of micronutrients (Berg, König, Keul 1996, S. 316). The terminus macronutrient
concerns the necessary substances for building and energy supplying processes like
carbohydrates, protein and fat. The terminus micronutrient concerns the or- and
anorganic modulators like vitamins, trace elements and trace minerals
(Ohlenschläger 1998, S. 30). Metabolism implies a permanent change of substances.
The quantity and quality of food and nutrients is strongly related to physical
performance, health and well-being. Imagine that the human is like a clockwork. It
consists of different gear wheels in different areas which are precisely tuned. Fuchs
(2001, S. 23) points out that all this big and little gear wheels of the human clockwork
consists of nutrients which are daily consumed with food.
So the composition of the food determines also the composition and the quality of the
human clockwork.
The consequence of the oversupply of macronutrients contains a poor developed
amount of micronutrients. Keul (1987, S. 88) summarize that there is now doubt, that
there is a big interdependence between the vitamin status and the physiological
performance. Clear is also that a vitamin deficiency influences unfavorably the
physiological and mental human performance.
So we can assume that there is a connection between vitamin status and every kind
of performance, f.e. coordination, concentration, energy production and energetic
Brubacher (1987, S. 188) created a 7 step diagram for the classification of the states
of vitamin, mineral and trace elements deficiencies.
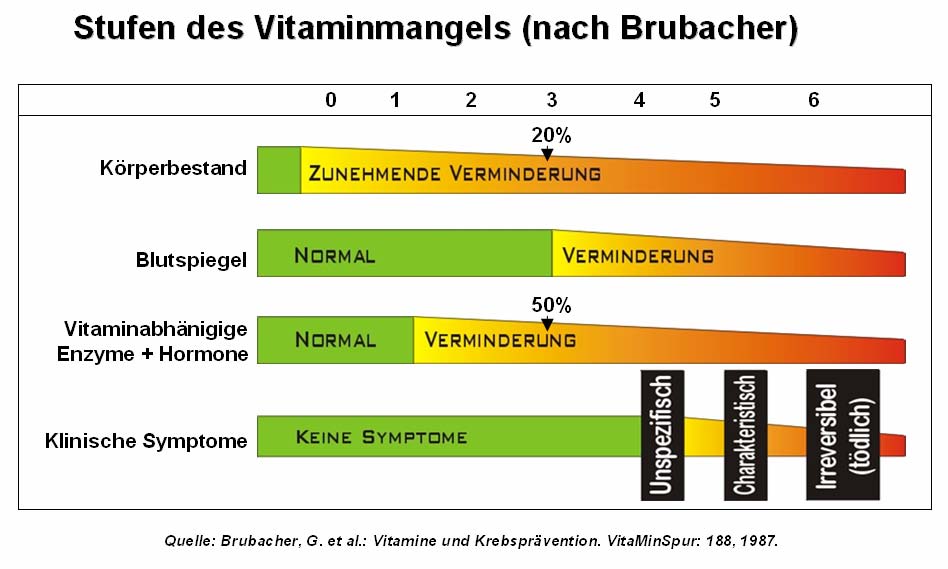
Problem- and task setting
Especially for us the stages 1 to 3 have a big relevance. Many scientific nutrition
reports executed by public institutes determine a latent nutrient deficiency on different
kind of persons: schoolkids, women, men, pregnant women, sportsmen and older
persons (vgl. Fuchs 2001, S. 35). The terminus "latent" means in this relationship
"hidden", because it is not measurable by blood analyses or other kind of symptoms.
The consequence of this reduction of the bodystorage of nutrients (in amounts from
10 to 25 % of the ideal quantity), causes drastic reductions in the metabolism of up to
50 % and decrease in the first deficiency phase the affected enzyme capacities.
The goal of the supplementation with the specially selected micronutrient supplement
was to fill up the stores of the body so far, that the nutrients dependent enzymes and
hormones can furnish 100 % of their capacity.
There is a big difference between micronutrients and pharmaceuticals, which is not
well known: Because nutrients are interacting as body-own regulators of the
metabolism and therefore obey physiological regularities, pharmaceuticals obey
pharmacological regularities, because the extraneous active substances try to block
or to stimulate biologic receptors.
Well known scientists are often writing about the pharmacological influence of
vitamins (vgl. Hamm 1990, S. 27) in human performance. Because micronutrients
Problem- and task setting
obey physiological and under no circumstances pharmacological regularities this kind
of question is scientifically not durable and has to be considered again.
Pharmacology (in Greek: pharmacon (φάρμακον) is drug, and logos (λόγος) is
science) is the study of how chemical substances interact with living systems. If these
substances have medicinal properties, they are referred to as pharmaceuticals (vgl.
Kurz 1998, S. 21) The field encompasses drug composition, drug properties,
interactions, toxicology, and desirable effects that can be used in therapy of diseases
Î body-strangely
Physiology (in Greek physis = nature and logos = word) is the study of the
mechanical, physical, and biochemical functions of living organisms. It is the science
of body functions and functional interaction (www.aerztekammer-
The aim of this distinction is not to make pharmaceuticals generally bad and
micronutrients healthy. The aim is that the supplementation of nutrients or
pharmaceuticals pursues different goals and effects.
All kinds of sport beverages on the market are useful only for substitution. This
micronutrient supplement (in form of powder, which is dissolved in water) goes one
step further. Riedl (2001, S. 711) summarize that substitution is the replacement of
something which is lost, gone, missing or converted. Substitution is the basic safety
device of the performance to maintain it and keeping of a status quo.
The goal of supplementation is to supply the human body with necessary considered
substances in a above averaged recognized amount. The aim is to enhance human
performance (ergogenic effect).
Ergogenic means that there is a direct influence of the physiological capacity of a
particular body system thereby improving performance and increase the speed of
recovery from training and competition.
At this point it its important to note that the intervention supplement is absolutely legal
and does not contain any doping substances.
Problem- and task setting
Goal of the study was to investigate, that a useful arranged micronutrient
supplement could fill up the body own stores in this this way, that the nutrient
dependent enzymes and hormones could unfold their whole power and performance
so that there is a lasting and measurable effect in human performance.
Based on the basic knowledge of the muscle metabolism physiology the different
ways of ATP replenishment will be worked out and in consequence the biochemical
effect and influence of the micronutrients in the energy metabolism will be
represented and explained.


Problem- and task setting
1.2 Boosting energy and power – the muscle
Every kind of movement involves energy, which exists in six forms in nature
Nuclear Fission
(Williams 2001, S. 20)
A key principle of energy is that one form can be converted into another. The body
also can convert one form of energy into another. Two principal forms of energy that
are important to sport are mechanical energy and chemical energy. Sport involves
movement, which is mechanical energy. Chemical energy is stored in our bodies in a
variety of forms and is used to produce movement.
In order to understand how to enhance sport performance, it is important to
understand how the human body stores and uses energy, and the possible causes of
impairment, such as fatigue or inefficient utilizitation.
The sophisticated racing car must have
The world-class sprinter needs
a powerful engine;
powerful muscles, the engines
of human movement;
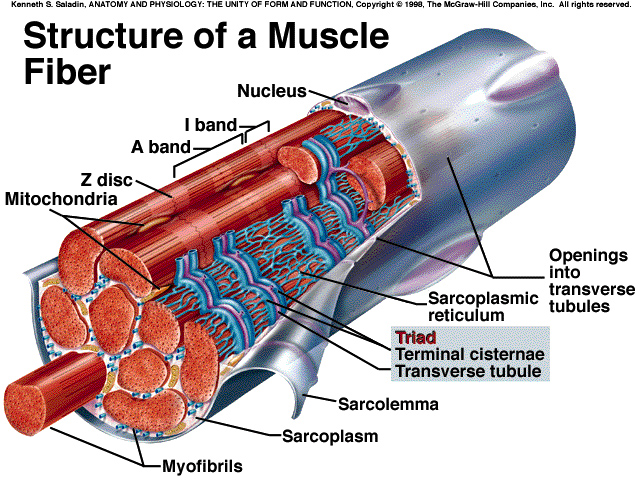
Problem- and task setting
Cardiac muscle tissue forms the bulk of the wall of the heart. Like skeletal muscle
tissue, it is striated. Unlike skeletal muscle tissue its contraction is usually not under
conscious control (involuntary).
Smooth muscle tissue is located in the walls of hollow internal structures such as
blood vessels, the stomach, intestines, and urinary bladder. Smooth muscle fibers
are usually involuntary, and they are nonstriated (smooth). Smooth muscle tissue,
like skeletal and cardiac muscle tissue, can undergo hypertrophy. In addition, certain
smooth muscle fibres, such as those in the uterus, retain their capacity for division
and can grow by hyperplasia.
Skeletal muscle tissue is named for its location - attached to bones. It is striated; that
is, the fibers (cells) contain alternating light and dark bands (striations) that are
perpendicular to the long axes of the fibers. Skeletal muscle tissue can be made to
contract or relax by conscious control (voluntary).
Problem- and task setting
All skeletal muscle fibres are not alike in structure or function. For example, skeletal
muscle fibres vary in colour depending on their content of myoglobin (myoglobin
stores oxygen until needed by mitochondria). Skeletal muscle fibres contract with
different velocities, depending on their ability to split Adenosine Triphosphate (ATP).
Faster contracting fibres have greater ability to split ATP. In addition, skeletal muscle
fibres vary with respect to the metabolic processes they use to generate ATP. They
also differ in terms of the onset of fatigue. On the basis of various structural and
functional characteristics, skeletal muscle fibres are classified into three types: Type I
fibres, Type II B fibres and type II A fibres.
These fibres, also called slow twitch or slow oxidative fibres, contain large amounts of
myoglobin, many mitochondria and many blood capillaries . Type I fibres are red, split
ATP at a slow rate, have a slow contraction velocity, very resistant to, fatigue and
have a high capacity to generate ATP by oxidative metabolic processes. Such fibres
are found in large numbers in the postural muscles of the neck.
Type II A Fibres
These fibres, also called fast twitch or fast oxidative fibres, contain very large
amounts of myoglobin, many mitochondria and many blood capillaries. Type II A
fibres are red, have a very high capacity for generating ATP by oxidative metabolic
processes, split ATP at a very rapid rate, have a fast contraction velocity and are
resistant to fatigue. Such fibres are infrequently found in humans.
Type II B Fibres
These fibres, also called fast twitch or fast glycolytic fibres, contain a low content of
myoglobin, relatively few mitochondria, relatively few blood capillaries and large
amounts glycogen. Type II B fibres are white, geared to generate ATP by anaerobic
metabolic processes, not able to supply skeletal muscle fibres continuously with
sufficient ATP, fatigue easily, split ATP at a fast rate and have a fast contraction
velocity. Such fibres are found in large numbers in the muscles of the arms. (vgl.
Karlson 1994, S. 524)
Problem- and task setting
1.2.1 Energy Systems and ATP
The rate at which a muscle fiber contracts depends on its ability to convert is
chemical energy into mechanical energy, the latter being the actual shortening of the
muscle cell. The muscles contain three distinct system that determine the rate of
energy production for movement. One is called the ATP-CP energy system, the
second is the lactic acid energy system and the third is the oxygen energy system.
Each muscle fiber posseses all three energy systems, but the dominance of one
system over another determines the primary energy characteristics of the individual
Although your muscles posseses three totally different energy systems, only one
form of energy is utilized to cause the muscle contraction. This form is ATP (adenosin
triphosphate) a high energy chemical compound found in all muscle cells. Without
ATP your muscle cannot contract.
The muscle contains only a very small amount of ATP (only energy for 1 second).
Additional ATP must be supplied if muscle contraction is to continue. The intensity of
work of the muscle (outside workload) determines the way of ATP-resynthesis (inside
Problem- and task setting
1. anaerobic alactazide (Creatinphosphateshuttle)
(Williams 1997, S. 24)
The ATP-CP energy system consists of ATP and another high-energy phosphate
compound, CP (creatine phosphate). ATP is the immediate source of energy for
muscle contraction. It can release energy very rapidly , but as known, it is in very
limited supply. CP also may break down and release energy very rapidly, but this
energy cannot be used directly for contract the muscle cell. Its role is to resynthesize
ATP rapidly. CP supply is also limited in muscle and may resynthesize ATP for only
an additional for 5 to 10 seconds. This energy system does not need oxygen in order
to perform, and thus is an anaerobic source of energy.
2. anaerobic lactazide (anaerobic glycolyses)
The lactic acid energy system uses carbohydrate as fuel, primarily in the form of
glycogen stored in muscles. Glycolysis may occur both in the presence and
absence of oxygen. This leads to a process called glycolysis, in which ATP can
be produced rapidly, although not as rapidly as in the breakdown of CP.
Glycolysis that occurs and produces ATP witout oxygen is called the anaerobic
glycolysis. Through a serie of chemical reactions in the muscle cell, the formation
of lactic acid allows anaerobic glycolysis to continue. The accumulation of lactic
acid, however, has been associated with fatigue processes within the msucle
Problem- and task setting
cell, limiting it´s effectiveness during exercise. This energy system works fairly
rapid, but it cannot produce energy for prolonged periods.
3. aerobic (Oxygen Energy System)
The oxygen energy system uses a variety of fuels to produce ATP, but depends
primarily on carbohydrates and fats. The main source of carbohydrate for muscular
energy during exercise is glucose that, as noted, is stored in limited rates in the
muscle as glycogen. The main source of fat for muscular energy during exercise is
free fatty acids (FFA). Some fats, known as triglycerides, are stored in limited supply
in the muscle and break down into FFA for entry into the oxygen energy system. The
oxygen energy system, needs an adequate supply of oxygen delivered to the
muscles to help release the chemical energy stored in carbohydrate and fats Î
aerobic pathway. Although the oxygen system cannot produce ATP as rapidly as can
the two anaerobic systems, but it can produce much greater quantities of ATP at a
somewhat slower rate (Williams 2001, S. 26).
Problem- and task setting
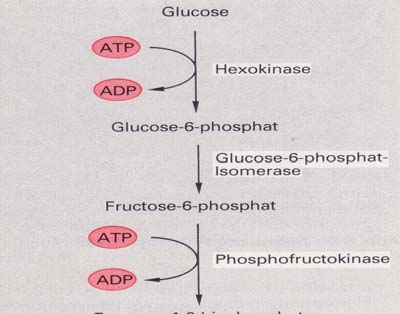
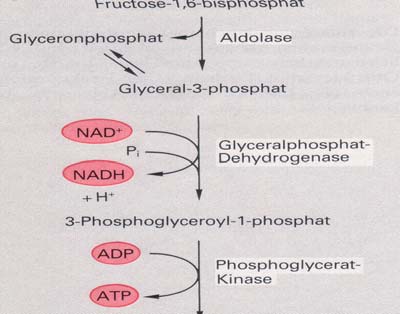
1.2.2 Glycolysis
Glycolysis is a metabolic pathway that is found in all living organisms and does not
require oxygen. The process converts one molecule of glucose into two molecules of
pyruvate, and makes energy in the form of two molecules of ATP. Glycolysis takes
place in the cytoplasm of the cell. The overall reaction can be expressed this way:
The individual steps of the conversion of
glucose into pyruvate are:
A glucose molecule from the hydrolysis of
starch or glycogen is phosphorylated using one
ATP molecule to give glucose-6-phosphate.
The glucose-6-phosphate is converted to
fructose-6-phosphate by isomerisation.
Fructose-6-phosphate is again phosphorylated
to give fructose-1,6-diphosphate with the use
of another ATP molecule.
Next, the fructose-1,6-diphosphate is then
lysed into two molecules of 3-carbon sugar
(dihydroxyacetone phosphate and
glyceraldehyde-3-phosphate) which are
interconvertible.
The 3-carbon sugars are dehydrogenated and
inorganic phosphate is added to them, forming
two molecules of 1,3 diphosphoglycerate.
Problem- and task setting
The hydrogen is used to reduce two molecules of NAD, a hydrogen carrier, to give
NADH+H+. NADH+H+ later proceeds to the mitochondria for use in the electron
transport chain.
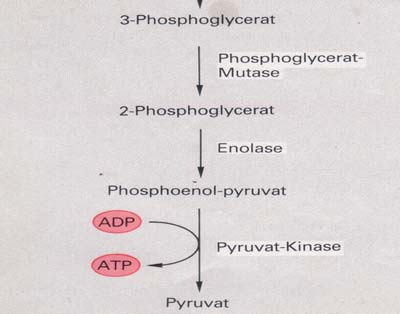
The two molecules of 1,3 diphosphoglycerate lose two phosphate groups to form two
molecules of glycerate-3-phosphate (3-phosphoglycerate), converting two molecules
The two molecules of glycerate-3-phosphate again lose phosphate forming two
molecules of pyruvate, with the production of another two ATP molecules (for a net
Breakdown of pyruvate
There are now two ways to break down the resulting pyruvate:
Aerobic respiration (Cellular Respiration)
Aerobic respiration requires oxygen in order to generate energy. It is the preferred
method of pyruvate breakdown. As molecules of pyruvate travel into a mitochondrion
entering the Krebs cycle. In this process it is broken down producing energy in the
form of ATP (which travels to the cell), NADH and FADH2 which travel to the electron
transport chain. In this process, an electron is transferred from an energy-rich atom
(such as a carbon atom in an organic molecule) to an oxygen atom, via an electron
transport chain. Oxygen serves as the "terminal electron acceptor" in the electron
transport chain. In the process, it yields 30 ATP molecules via the diffusion of
hydrogen atoms through an ATP synthase, as well as carbon dioxide and water. This
makes for a total gain of 32 ATP molecules during cellular respiration under optimal
conditions; however, such conditions are generally not realized due to such losses as
the cost of moving pyruvate into mitochondria. This takes place in the mitochondria in
eukaryotic cells, and at the cell membrane in prokaryotic cells. The gain of ATP in the
oxdiative phosphorylation is not so sure, because of the existing of stöchimetries
(Stryer 1999, S. 580).
Aerobic metabolism is rather more efficient than anaerobic metabolism. It actually
starts off with the Glycolysis process of anaerobic metabolism, and then continues
with the krebs cycle and oxydative phosphorylation.
Problem- and task setting
Anaerobic respiration
"Anaerobic respiration" does not require oxygen. True anaerobic respiration involves
an electron acceptor other than oxygen.
Abb.: Lactatdehydrogenase (vgl. Stryer 1999, S. 517)
Glukose + 2i + 2 ADP
2Laktat + 2ATP + 2 H20
Bacteria are capable of using a wide variety of compounds as terminal electron
acceptors in respiration: nitrogenous compounds (such as nitrates and nitrites),
sulphur compounds (such as sulphates, sulphites, sulphur dioxide, and elemental
sulphur), carbon dioxide, iron compounds, manganese compounds, cobalt
compounds, and uranium compounds.
However, none of these alternative electron acceptors yields as much energy from
respiration as does oxygen. In environments where oxygen is present, typically only
aerobic respiration will occur.
Problem- and task setting
The anaerobic oxydation of nutrients occurs only in cytplasma. Anaerobic glycolysis
leads that the pyruvate and NADH rests in the cytoplasma. NADH in the mitochdrium
cannot be converted in energy. So it has to be reduced to pyruvate by producing
lactic acid (Bereiter-Hahn 2001).
NADH-accumulation (vgl. Bereiter-Hahn, 2001)
Problem- and task setting
The pH (potentio hydrogenii) is a value of the activity of hydrogen ions (H+) in a
solution and, therefore, its acidity or alkalinity.
In aqueous systems, the hydrogen ion activity is dictated by the dissociation constant
of water (Kw) = 1.011 × 10−14 at 25 °C and interactions with other ions in solution.
Due to this dissociation constant a neutral solution (hydrogen ion activity equals
hydroxide ion activity) has a pH of approximately 7. Aqueous solutions with pH
values lower than 7 are considered acidic, while pH values higher than 7 are
considered alkaline.
Though a pH value has no unit, it is not an arbitrary scale; the number arises from a
definition based on the activity of hydrogen ions in the solution.
Problem- and task setting
1.2.3 Lipid metabolism
Nearly all of the energy needed by the human body is provided by the oxidation of
carbohydrates and lipids. Whereas carbohydrates provide a readily available source
of energy, lipids function primarily as an energy reserve. The amount of lipids stored
as an energy reserve far exceeds the energy stored as glycogen since the human
body is simply not capable of storing as much glycogen compared to lipids. Lipids
yield 9 kcal of energy per gram while carbohydrates and proteins yield only 4 kcal of
energy per gram.
It is interesting to compare the relative amounts of energy provided by various
biochemicals in a typical 154 lb male. The free glucose in the blood provides only a
40 kcal energy reserve -- only enough to maintain body functions for a few minutes.
Glycogen remaining stored in the liver and muscles after an overnight fast, amounts
to about 600 kcal energy. Glycogen reserves can maintain body functions for about
one day without new inputs of food. Protein (mostly in muscle) contains a substantial
energy reserve of about 25,000 kcal.
Finally, lipid reserves containing 100,000 kcal of energy can maintain human body
functions without food for 30-40 days with sufficient water. Lipids or fats represent
about 24 pounds of the body weight in a 154 pound male. Lipids provide the sole
source of energy in hibernating animals and migrating birds. Fortunately, lipids are
more compact and contain more energy per gram than glycogen, otherwise body
weight would increase approximately 110 pounds if glycogen were to replace fat as
the energy reserve.
Lipids or fats are stored in cells throughout the body principle in special kinds of
connective tissue called adipose tissue or depot fat. Whereas many cells contain
phospholipids in the bilayer cell membranes, adipose tissue cells consist of fat
globules of triglycerides which may occupy as much as 90% of the cell volume.
In addition to energy storage, depot fat provides a number of other functions. Fat
serves as a protective cushion and provides structural support to help prevent injury
to vital organs such as the heart, liver, kidneys, and spleen. Fat insulates the body
Problem- and task setting
from heat loss and extreme temperature changes. At the same time, fat deposits
under the skin may be metabolized to generate heat in response to lower skin
Lipids ingested as food are digested in the small intestine where bile salts are used
to emulsify them and pancreatic lipase hydrolyzes lipids into fatty acids, glycerol,
soaps, or mono- and diglycerides. There is still some dispute about the lipid form that
passes through the intestinal wall -- whether as fatty acids or as glycerides. In either
case, triglycerides are found in the lymph system and the blood.
Excess lipids in the blood are eventually converted into adipose tissue. If lipid levels
in the blood become too low, the body synthesizes lipids from other foods, such as
carbohydrates, or removes lipids from storage. The body also excretes some lipids in
the form of fats, soaps, or fatty acids as a normal component of feces.
Abnormally high levels of triglycerides and cholesterol are thought to be involved in
hardening of the arteries. Lipids may be deposited on the walls of arteries as a partial
consequence of their insolubility in the blood.
free fatty acids
Free fatty acid in the cytoplasm is activited by consuming ATP (Acyl-CoA-
Synthases) with Coenzyme A before they can be carried through the mebrane to the
matrix of the mitochondria. The necessary carrier for this reaction is L-Carnitine.
Problem- and task setting
"Beta Oxidation is the process of converting fatty Acids to Acetyl-CoA" (Powers 2001,
Abb.: Betaoxidation im Mitochondrium (Bereiter-Hahn 2001)
Acetyl-CoA is in the tricarbon cycle reduced to Coenzyme A. This process creates
reduction equivalences like ATP, NADH wich is used in the respiratory chain in the
mitochondria. (gl. Bereiter-Hahn, 2001).
Problem- and task setting
1.2.3.1 Oxdidative Phosphorylation
The reductive power generated by the citric acid cycle in form of NADH/H+ can be
utilized by the inner mitochondrial membrane to generate ATP. Reducing equivalents
harnessed in the Krebs cycle, fatty acid oxidation, and pyruvate dehydrogenase
activity are transferred to membrane bound electron transport chain. Four classes of
electron transport complexes segregate electron and protons promoting proton
pumping across the inner membrane using molecular oxygen as a final electron
acceptor ('sink') reducing oxygen to water. The proton gradient across the inner
membrane drives the ATP synthase to regenerate ATP from ADP and inorganic
phosphate. This process is also known as respiration.
The Mitochondrion
Mitochondria are small organelles with possible common origin to bacteria
(endosymbiotic theory). They contain a double membrane system. The outer
membrane is relatively permeable to small metabolites. This is due to the presence of
a porin channel type VDAC (voltage dependent anion channel) with a high copy
number (thousands of channel in a single mitochondrion) with preferences for
negatively charged ions (nucleotides, phosphorylated compounds). Large molecules
like proteins, however, are not able to cross the outer membrane. The size limit for
permeable molecules is about 1,000 Dalton.
Problem- and task setting
The mitochondrial inner membrane functions as electrical insulator and capacitor and
is impermeable for ions and small hydrophilic metabolites. This membrane maintains
the ion (proton) gradient essential for ATP synthesis. The matrix side is negatively
charged relative to the outside (inter-membrane space; connected to cytoplasm
through VDAC) because of the proton gradient (high out; low in) generated during the
electron transport chain reaction. This membrane contains many of the substrate
specific transport systems like citrate, glycerol, and malate shuttles, but also the ATP-
ADP exchange protein. It is the latter exchange transport that controls the speed of
citric acid cycle, oxidative phosphorylation and thus all precursor pathways like
glycolysis, protein degradation and fatty acid oxidation.
Problem- and task setting
Electron carriers in oxidative phosphorylation
Electron carriers along the electron transfer chain come in two forms; they bind
reducing equivalents or electrons only. Combining both types within a chain forces
the separation of protons from electrons. While the electrons stay within the
membrane, protons are captured and released from and into the surrounding
compartments. This process is unidirectional and always picks up protons in the
matrix compartments and releases them in the inter-membrane space thus creating a
proton gradient which stores energy in form of electrochemical potential.
The first category of reducing equivalent acceptors are riboflavin (vitamin B2) and
nicotinamine (vitamin B3 or Niacin). Riboflavin is part of FAD and FMN and is
analogous to that of nicotinamide in NAD(H) by accepting hydrogens and electrons
into its heterocyclic ring structures. Two important differences to NAD(H), however,
exist. First, flavin-adenine dinucleotide (FAD; or FADH2, the reduced flavin- adenine-
dinucleotide; C01352) and flavin mono nucleotide (FMN oxidized C00061; reduced
FMN C01847) are prosthetic groups (covalent link to enzyme) and do not carry
reducing equivalents by diffusion. Second, the reduction of FAD and FMN by NADH
is not reversible. Flavoproteins participate at several points where electrons are first
funneled into the respiratory chain:
Problem- and task setting
NADH + H+ + Enz-FMN Þ NAD+ + Enz-FMNH2
The reduction potential of this reaction D E0' = 0.3V corresponding to about -
46kcal/mol of standard free energy change, enough for the synthesis of two to three
mols of ATP per mol of oxidized NADH.
The second category of reducing equivalent carriers is the benzoquinone (oxidized)
conatining compound coenzyme Q. Like flavoproteins they accept two hydrogens
along with two electrons upon reduction of the hydroquinone ring structure to
ubiquinole or CoQH2. The quinone-hydroquinone redox couple serves as a diffusible
transport system within the inner membrane of mitochondria coupling the electron
flux with a proton flux across the dielectric barrier by providing a non-charged carrier
system. Quinones serve as a collector molecule of reducing equivalents (e-/H+) from
NADH and succinate donors.
Redox systems of the respiratory chain that only bind electrons are the Fe-S
complexes and heme groups (Fe-proto-porphyrin ring; protoheme C00032). The
irons of the heme groups serve as redox partners by reversibly changing their redox
state between the reduced Fe(II) and the oxidized Fe(III) form. Synthesis of heme
can be found in KEGG as MAP00860 (Porphyrin and chlorophyll metabolism).
Table of individual protein complex systems in oxidative phosphorylation
NADH dehydrogenase (complex I)
Complex I is the first coupling site in the mitochondrial membrane meaning that the
redox reaction is coupled to a proton pumping activity across the membrane. It is the
energy stored in the electrochemical proton gradient that is used for ATP synthesis
by the H+-ATPase or complex V. Complex I is the entry point for NADH reducing
power and involves FMN, Fe-S complexes, and ubiquinone.
Succinate dehydrogenase (complex II) is the mitochondrial succinate dehydro-
genase and unlike complex I does not directly contribute to proton pumping. The
reducing equivalents from succinate are transferred by FAD to ubiquinone.
Problem- and task setting
Cytochrome-c reductase (complex III) Complex III or cytochrome bc1 complex
transfers electrons from quinones to cytochrome c, a small peripheral membrane
protein (cytochrome c; C00524) in the inter-membrane space. This complex
contributes to proton pumping in a mechanism known as Q-cycle. A similar
mechanism is also hypothesized to work in complex I.
Cytochrome-c oxidase (complex IV)
Cytochrome c transfer single electrons from
complex III to complex IV, or cytochrome-c oxidase. As can be seen from the KEGG
entry for cytochrom c oxidase, the human complex IV contains up to eight subunits.
For some subunits, cell type specific homologues exists such as COX7A1 in muscle
and COX7A2 in liver. Cytochrome oxidase catalyzes a cyclic reaction in which the
electrons extracted from metabolites are finally transferred to molecular oxygen in the
presence of 4 protons to form 2 molecules of H2O. This is a carefully controlled
mechanism because the intermediate oxygen scavengers are highly reactive and
damaging to the cell. Oxygen is tightly bound to an iron-copper containing coenzyme.
H+-ATPase (complex V) The mitochondrial ATP synthase is a multi-subunit protein
complex that couples a proton channel (F0 portion, integral membrane protein
complex) with an ATP synthesizing unit (F1 portion, soluble mitochondrial matrix
component) and is a member of the so called F-type ATPases.
Problem- and task setting
1.3 Micronutrients and their effects
As we have seen, carbohydrates, proteins and fats are all nutriment substances; the
absorable components of food, from which energy is derived. Collectively they are
referred to as the macronutrients. The micronutrients, vitamins, trace elements and
minerals, are necessary for organ function, food utilisation cell growth and are
regulating and modulating the metabolism. Unlike the micronutrients, however, they
themselves do not provide energy; only the macronutrients can do that. But, without
sufficient quantities of micronutrients available the energy of the macronutrients
cannot be released as vitamins and minerals energize and regulate their metabolism.
(Ohlenschläger 1998, S. 30).
In such an interacting system like our body, it should be obviously to know that
vitamins or minerals alone can do nothing for our health because they cannot be
assimilated and functioned without the aid of one another. Nor should it be a surprise
that certain combinations of vitamins and minerals are antagonistic; they work
against each other, while still other combinations are synergistic; that is, they
enhance the activity of one another. Macro- and micronutrients are working together
as catalysts in the intermediar metabolism as a part of enzymes.
A Vitamin is an organic molecule required by a living organism in minute amounts for
proper health. An organism deprived of all sources of a particular vitamin will
eventually suffer from disease symptoms specific to that vitamin.
Vitamins can either be classified as water soluble, which means they dissolved easily
in water or fat soluble, which means they are absorbed through the intestinal tract
with the help of fats.
In general, an organism must obtain vitamins or their metabolic precursors from
outside the body, most often from the organism's diet. Examples of vitamins that the
human body can derive from precursors include vitamin A, which can be produced
from beta carotene; niacin from the amino acid, tryptophan; and vitamin D through
exposure of skin to ultraviolet light.
Problem- and task setting
The term, vitamin, does not encompass other essential nutrients such as dietary
minerals, essential fatty acids or essential amino acids, nor is it used for the large
number of other nutrients that are merely health promoting, but not strictly essential.
The word vitamin was coined by the Polish biochemist Casimir Funk in 1912. Vita in
Latin is life and the -amin suffix is short for amine; at the time it was thought that all
vitamins were amines. Though this is now known to be incorrect, the name has stuck.
(vgl. Mindell 1985, S. 19).
The value of eating certain foods to maintain health was recognized long before
vitamins were identified. The ancient egyptians knew that feeding a patient liver
would help cure night blindness, now known to be caused by a Vitamin A deficiency.
In 1747, the Scottish surgeon James Lind discovered that citrus foods helped prevent
scurvy, a particularly deadly disease characterized by bleeding and severe pain. In
1753, Lind published his Treatise on the Scurvy.
In 1905, William Fletcher discovered that eating unpolished rice instead of polished
helped prevent the disease beriberi. The following year, Frederick Hopkins postulated
that foods contained "accessory factors"—in addition to proteins, carbohydrates, fats,
etc.—that were necessary to the human body. When Casimir Funk isolated the
water-soluble complex of micronutrients whose bioactivity Fletcher had identified, he
proposed that it be named "Vitamine". The name soon became synonymous with
Hopkins' "accessory factors", and by the time it was shown that not all vitamins were
amines, the word was ubiquitous. In 1920, Jack Cecil Drummond proposed that the
final "e" be dropped, to deemphasize the "amine" reference, after the discovery that
Vitamin C had no amine component, and the name has been "vitamin" ever since.
Throughout the early 1900s, scientists were able to isolate and identify a number of
vitamins by depriving animals of them. Initially, lipid from fish oil was used to cure
rickets in rats, and the fat-soluble nutrient was called "antirachitic A". The irony here
is that the first "vitamin" bioactivity ever isolated, which cured rickets, was initially
called vitamine A, this bioactivity is now called vitamin D which is subject to the
semantic debate that vitamin D is not truly a vitamin. What we now call "vitamin A"
was identified in fish oil because it was inactivated by ultraviolet light. Most of what
Problem- and task setting
we now recognize as the water-soluble organic micronutrients were initially referred
to as just one entity, "vitamin B".
(Hamm 1990, S. 127).
1.3.2 Vitamins – important part of enzymes
Enzymes are protein catalysts that carry out the chemical reactions of metabolism.
All chemical reactions require activation energy to break chemical bonds and begin
the reaction. The need for activation energy acts as a barrier to the chemical reaction
occurring and/or to the speed at which it occurs.
Enzymes lower the barriers that normally prevent chemical reactions from occurring
(or slow them down) by decreasing the required activation energy. Thus, in the
presence of enzymes, reactions proceed and/or proceed at a faster rate.
Enzyme names end with the -ase suffix, unless they were named prior to adoption of
the -ase naming system. Often when enzymes are named, the -ase suffix is added to
the substrate name. For example, sucrase is the enzyme that breaks down the
substrate sucrose, a disaccharide, into the monosaccharides glucose and fructose.
Problem- and task setting
Enzymes carry out their function of lowering activation energy by temporarily
combining with the chemicals involved in the reaction. These chemicals are called
the substrate. Enzymes are specific for their substrate: A particular substrate
molecule will combine temporarily with one enzyme type, and the active site of a
particular enzyme will fit only one kind of substrate. For example, the enzyme
sucrase will attach only to the substrate sucrose. The combination is called the
enzyme- substrate complex. When the enzyme and substrate combine, the substrate
is changed to a different chemical called the product. The enzyme is not consumed
or altered by the reaction.
Enzymes are responsible for
• Digesting process
• Muscle contraction
• Energy production
• Bloodgas transport
• Growth
• Blood clotting
So enzymes are regulating metabolism procedures, without being involved with the
result in the reaction, thus without being changed itselfs (vgl. Williams 1997, S. 220).
Vitamins are an extremely diverse range of biochemical compounds which have
been classified by legislative rather than biochemical functions. For example,
vitamins were originally supposed to be coenzyme factors for essential enzyme
funcitons. However, vitamins A, C. D, and E function via noncoenzymatic or
hormonal mechanisms for their major roles (vgl. Wolinsky 1996, S. 2).
Problem- and task setting
Classification of enzymes
These enzyme catalyse oxidation and reduction reactions involving the transfer of
hydrogen atoms or electrons. The following are of particular importance in the design
of enzyme electrodes. This group can be further divided into 4 main classes.
dehydrogenases catalyse hydrogen transfer from the substrate to a nicotinamide
adenine dinucleotide cofactor (NAD+). An example of this is lactate dehydrogenase
which catalyses the following reaction:
Lactate + NAD+ = Pyruvate + NADH + H+
oxidases catalyse hydrogen transfer from the substrate to molecular oxygen
producing hydrogen peroxide as a by-product. An example of this is FAD dependent
glucose oxidase which catalyses the following reaction:
b-D-glucose + O2 = gluconolactone + H2O2
peroxidases catalyse oxidation of a substrate by hydrogen peroxide. An example of
this type of enzyme is horseradish peroxidase which catalyses the oxidation of a
number of different reducing substances (dyes, amines, hydroquinones etc.) and the
concomitant reduction of hydrogen peroxide. The reaction below illustrates the
oxidation of neutral ferrocene to ferricinium in the presence of hydrogen peroxide:
2[Fe(Cp)2] + H2O2 + 2H+= 2[Fe(Cp)2]+ + 2 H2O
oxygenases catalyse substrate oxidation by molecular oxygen. The reduced product
of the reaction in this case is water and not hydrogen peroxide. An example of this is
the oxidation of lactate to acetate catalysed by lactate-2-monooxygenase.
lactate + O2 = acetate + CO2 + H2O
These enzymes transfer C, N, P or S containing groups (alkyl, acyl, aldehyde, amino,
phosphate or glucosyl) from one substrate to another. Transaminases,
transketolases, transaldolases and transmethylases belong to this group.
Problem- and task setting
Hydrolases
These enzymes catalyse cleavage reactions or the reverse fragment condensations.
According to the type of bond cleaved, a distinction is made between peptidases,
esterases, lipases, glycosidases, phosphatases and so on. Examples of this class of
enzyme include; cholesterol esterase, alkaline phosphatase and glucoamylase.
These enzymes non-hydrolytically remove groups from their substrates with the
concomitant formation of double bonds or alternatively add new groups across
Isomerases
These enzymes catalyse intramolecular rearrangements and are subdivided into;
cis-trans-isomerases
An example of this class of enzyme is glucose isomerase which catalyses the
isomerisation of glucose to fructose.
Ligases split C-C, C-O, C-N, C-S and C-halogen bonds without hydrolysis or
oxidation. The reaction is usually accompanied by the consumption of a high energy
compound such as ATP and other nucleoside triphosphates. An example of this type
of enzyme is pyruvate carboxylase which catalyses the following reaction:
pyruvate + HCO3- + ATP = Oxaloacetate + ADP + Pi
An important aspect of catalytic action is the requirement by certain enzymes of
either co-factors or prosthetic groups. Co-factors receive redox equivalents, protons
or chemical groups from the substrate during the course of the enzymatic reaction.
Problem- and task setting
They tend to associate with the enzyme in a transient manner and can diffuse away
from the active site. Examples of this type of molecule include NAD+ and NADP+.
Prosthetic groups have similar function to co-factors with the exception that they are
tightly bound to the enzyme. When they are released, the enzyme is mostly
denatured. Flavin nucleotides and hemes are the most important examples of this
class of molecule.
(vgl. Hamm 1990, S. 159).
1.3.3 Vitamins – biochemical effects
Vitamin B1 (Thiamin) The classic deficiency state of thiamin is beriberi. An analogous disorder in fowl is
called polyneuritis. The term beriberi is derived from the Sinhalese word meaning
extreme weakness. Beriberi was very common during the early part of the last
century in those whose diets consisted principally of highly polished rice.
Interestingly, those who ate parboiled rice—partially boiled rice—did not develop
beriberi. Milling removes the husk, which contains most of the thiamin, while
parboiling the rice before husking disperses thiamin throughout the grain. Beriberi still
occurs in those whose diet mainly consists of polished rice. Thiamin deficiency is also
associated with alcoholism and occurs in some cases of malnutrition, those receiving
total parenteral nutrition without thiamin, malabsorption syndromes, increased
carbohydrate intake, major catabolic and physiologic stress states, acute infection,
folate deficiency, thyrotoxicosis and those on long-term loop diuretics (furosemide,
ethacrynic acid, bumetanide). Subclinical thiamin deficiency may not be uncommon.
Key roles of thiamin in energy metabolism and biosynthetic processes are outlined in
the seven metabolic pathways. Thiamin diphosphate (TDP), also referred to as
thiamin pyrophoshate (TPP), is required for energy transformations and
transketolases of the pentose phosphate pathway. In the citric acid cycle (CAC), TDP
is required for oxidative decarboxylation of pyruvic and alpha-keto-ß-methylvaleric
acids. In each case, BCKA is further degraded to metbolites that can enter the CAC.
Only the dephosphoorylatet form of the BCKA-dehydrogenase comples (BCKAD) is
Problem- and task setting
active for these oxidative dacarboxylations. Biosynthesis of fatty acids is one
example of a NADPH-dependent reductive-biosynthetic process. Pentoses generated
by this pathway are utilized for biosynthesis of tissue nucleotides (vgl. Pfeifer 1998,
Various reports suggest a definitve link between high-carbohydrate intakes, physical
exertion, and nutritional-metabolic demands for thiamin in humans. Moderate
physical acitivities do not appear to significantly deplete thiamin reserves of healthy
There are few studies investigating the effect of large doses of thiamin as an aid to
exercise performance. In one such study, carbohydrate-loaded mice administered
very high doses of thiamin demonstrated an improvement in swim time to exhaustion.
In another study, experienced cyclists administered 900 milligram daily of thiamin for
three days were found to have lower exercise heart rates, blood glucose and blood
lactate concentrations. In still another study, thiamin supplementation at 100
milligram/day was found to decrease exercise-induced fatigue in male athletes. A
recent study, however, using a thiamin derivative, thiamin tetrahydrofurfuryl disulfide,
which is better absorbed than thiamin, showed no effect on high-intensity exercise
Further studies have to be occurred to determine the real influence of Thiamin in the
muscle metabolism.
Problem- and task setting
Vitamin B2 (Riboflavin) Riboflavin, also known as vitamin B2, is an easily absorbed, water-soluble
micronutrient with a key role in maintaining human health. Riboflavin is an orange
powder, and water solutions have intense greenish yellow fluorescence. Vitamin B2
is a water-soluble vitamin, one that cannot be stored by the body except in
insignificant amounts. It must be replenished daily.
Riboflavin is an integral component of the coenzymes that participate in many
energy-yielding metabolic pathways. Like its close relative vitamin B1 (thiamine),
riboflavin plays a crucial role in certain metabolic reactions, particularly the
conversion of carbohydrates into sugar, which is "burned" to produce energy. They
promote the first steps in the metabolism (breakdown and production) of glucose and
of fatty acids. The metabolism of some vitamins and minerals also require riboflavin.
Riboflavin is essential for tissue respiration and the generation of energy from the
carbohydrates, acids and fats. It is important for body growth and red blood cell
production and helps in releasing energy from carbohydrates.
Vitamin B2 is an important part of the aerobic enzymes, the flavoproteins, which are taken part in the energy production from carbohydrates and fatty acids. (vgl. Williams
In addition to the key roles of flavin coenzymes in the TCA Cycle and the electron
transport chain, FAD is reduced in a flavin-dependent acyl-CoA dehydrogenase
reaction, the first step of ß-oxidation in which acyl-Coa is converted to enoyl-CoA.
The electrons generated are also transferred to ubiquinonen with the electron
transport system.
With increased energy expenditure there is an accelerated use of carbohydrate and
fat for energy. Based on the above roles of the flavin coenzymes in respiratory
metabolism, one might expect increased needs associated with the enhanced
oxidative potential of skeletal muscle with aerobic training (vgl. Pfeifer 1998, S. 58).
Problem- and task setting
Beside NAD, FAD is the most important electron carrier in the oxidation of nutrients.
The FAD/FADH System posseses a standard-redoxpotential from – 190 mV. So it is
one of the most effective antioxidants in human metabolism (Fuchs 2001, S. 381).
Abb.: FAD-FADH-System
Problem- and task setting
Vitamin B3 (Niacin) Niacin or vitamin B3, also referred to as nicotinic acid and nicotinamide, is a watersoluble vitamin. Nicotinamide serves as a precursor of nictotinamide adenine
dinucleotide (NAD) and NAD phosphate (NADP) the two active coenzyme forms.
The coenzyme form of nictoinamide have a major role in intermediary metaboism as
they serve as carriers of reducing equivalents in glycolysis, the pentose hunt, the
TCA cycle, and electron transport and also in ß-oxidation and fat and protein
biosynthesis. Indeed there are at least 200 enzymes requiring NAD or NADP. In
glycolysis once reducing equivalents are generated in the 3-phospho-glyceraldehyde
dehydrogenase reaction, the hydrogens can be utilized for the synthesis of lactate
under anaerobic conditions or transferred to the electron transport chain if oxygen is
available. In the TCA cycle, several enzymes including pyruvate dehydrogenase,
isocitrate dehydrogenase, alpha-ketoglutarate dehydrogenase and malate
dehyrogenase require NAD as an electron acceptor. The primary function of NAD in
these cycles is to accept two electrons and a proton from intermediary substrates,
becoming NADH. The hydrogens are then transferred to the electron transport
system via the NADH complex. Where NADH is reoxidized to NAD and ATP is
generated. The regneration of NAD is essential to continued ATP synthesis (vgl.
Lewis 1998, S. 68).
Problem- and task setting
Much of what we know about the role of niacin in exercise performance has been
learned through studies investigating the relative contributions of fat and
carbohydrate substrates during short-term and prolonged exercise. Nicotinic acid
administration has been utilized in the experimental designs of these studies
because of the pronounced effects the vitamin has on free fatty acid availability at
rest and during exercise.
Because of the metabolic roles of NAD in glycolysis and respiratory metabolism, a
deficiency of the vitamin would be expected to impare performance by limiting
substrate oxidation during prolonged exercise.
The citric cycle does not deliver energy (ATP). In this cycle there are formed however
the highly reative reduction equivalents. (NADH und FADH).
Is in the sceletal muscle to less reducing NAD+ (Niacin deficiency) it comes to an
NADH accumulation in the cell. The only one way out is the anaerobic metabolism
pathway on which NADH transfer his electron on pyruvate, along with the production
Problem- and task setting
Vitamin B6 (Pyrodoxin) This water soluble vitamin functions as a coenzyme in several pathways involved in
substrate utilization during exercise. Many studies in animals and humans have
shown that exercise alters vitamin B6 metabolism, and conversely that poor vitamin B6 nutritional status compromises exercise performance. (Stryer 1999, S. 683).
Several aspects of energy metabolism during exercise require pyridoxal phosphate
(PLP) dependent enzymes.
Vitamin B6, also called pyridoxine, is one of eight water-soluble B vitamins. The B vitamins help the body to convert carbohydrates into glucose (sugar), which is
"burned" to produce energy. These vitamins, often referred to as the B complex, are
also essential in the metabolism of fats and protein. B complex vitamins also play an
important role in maintaining muscle tone in the gastrointestinal tract and promoting
the health of the nervous system, skin, hair, eyes, mouth, and liver.
Vitamins B12, B6, and B9 (folic acid) work closely together to control blood levels of the amino acid homocysteine. Elevated levels of this substance appear to be linked
to heart disease. Plus, vitamin B6 is essential for normal brain development and function, participating in the process of making important brain chemicals called
neurotransmitters.
Pyridoxine is an especially important vitamin for maintaining healthy nerve and
muscle cells and it aids in the production of DNA and RNA, the body's genetic
material. It is necessary for proper absorption of vitamin B12 and for the production of red blood cells and cells of the immune system. Pyridoxine has also been called the
"woman's vitamin" because it may help relieve symptoms of premenstrual syndrome
In addition to other B complex vitamins, pyridoxine is considered an "anti-stress"
vitamin because it is believed to enhance the activity of the immune system and
improve the body's ability to withstand stressful conditions.
Symptoms of pyridoxine deficiency include muscle weakness, nervousness,
irritability, depression, difficulty concentrating, and short-term memory loss. (vgl.
Williams 1997, S. 236).
Problem- and task setting
Vitamin B12 (Cyanocobalamin) Vitamin B12 is a member of the vitamin B complex. It contains cobalt, and so its also known as cobalamin. It is exclusively synthesised by bacteria and is found primarily
in meat, eggs and dairy products. There has been considerable research into
proposed plant sources of vitamin B12. Fermented soya products, seaweeds, and algae such as spirulina have all been suggested as containing significant B12. However, the present consensus is that any B12 present in plant foods is likely to be unavailable to humans and so these foods should not be relied upon as safe
sources. Many vegan foods are supplemented with B12. Vitamin B12 is necessary for the synthesis of red blood cells, the maintenance of the nervous system, and growth
and development in children. Deficiency can cause anemia. Vitamin B12 neuropathy, involving the degeneration of nerve fibres and irreversible neurological damage, can
also occur (vgl. Williams 1997, S. 238).
Cobalamins act as coenzymes in humans in only two reactions, methylcobalamin in
methionine synthase and adenosylcobalamin in methyl malonyl CoA mutase.
Because of the link with the folate system, vitamin B12´s importance for athlets also lies in proper erythropoiesis to maintain oxygen transport in the blood. Athletes, who
depend on central coordination of movement, timing, strength, etc., would probably
be dependent on sufficient cobalamin to maintain proper CNS function(vgl. Glaesel
Matter et al. studied the effects of folate supplementation on a subgroup fo 23 female
marathoners with low serum folate levels compared to a group with normal folate
status given placebo similarly. Various physiologcial parameters were measured and
no one was due to the supplementation significantly changed.
In contrast to this negative report, Sehadri and Malhotra showed signifant effects on
physical performance in a placebo-controlled study.
Problem- and task setting
Folate (Vitamin M)
Folate is a water-soluble B vitamin that occurs naturally in food. Folic acid is the
synthetic form of folate that is found in supplements and added to fortified foods
Folate gets its name from the Latin word "folium" for leaf. A key observation of
researcher Lucy Wills nearly 70 years ago lead to the identification of folate as the
nutrient needed to prevent the anemia of pregnancy. Dr. Wills demonstrated that the
anemia could be corrected by a yeast extract. Folate was identified as the corrective
substance in yeast extract in the late 1930s, and was extracted from spinach leaves
Folate helps produce and maintain new cells . This is especially important during
periods of rapid cell division and growth such as infancy and pregnancy. Folate is
needed to make DNA and RNA, the building blocks of cells. It also helps prevent
changes to DNA that may lead to cancer. Both adults and children need folate to
make normal red blood cells and prevent anemia . Folate is also essential for the
metabolism of homocysteine, and helps maintain normal levels of this amino acid.
Cardiovascular disease is the most common cause of death in industrialized
countries such as the US, and is on the rise in developing countries. The National
Heart, Lung, and Blood Institute of the National Institutes of Health has identified
many risk factors for cardiovascular disease, including an elevated LDL-cholesterol
level, high blood pressure, a low HDL-cholesterol level, obesity, and diabetes. In
recent years, researchers have identified another risk factor for cardiovascular
disease, an elevated homocysteine level. Homocysteine is an amino acid normally
found in blood, but elevated levels have been linked with coronary heart disease and
stroke. Elevated homocysteine levels may impair endothelial vasomotor function,
which determines how easily blood flows through blood vessels. High levels of
homocysteine also may damage coronary arteries and make it easier for blood
clotting cells called platelets to clump together and form a clot, which may lead to a
A deficiency of folate, vitamin B12 or vitamin B6 may increase blood levels of homocysteine, and folate supplementation has been shown to decrease
Problem- and task setting
homocysteine levels and to improve endothelial function. At least one study has
linked low dietary folate intake with an increased risk of coronary events. The folic
acid fortification program in the U. S. has decreased the prevalence of low levels of
folate and high levels of homocysteine in the blood in middle-aged and older adults.
Daily consumption of folic-acid fortified breakfast cereal and the use of folic acid
supplements has been shown to be an effective strategy for reducing homocysteine
concentrations (vgl. Williams 1997, S. 239).
Problem- and task setting
Pantothenic acid
Pantothenic acid (PA), a B-complex vitamin, is essential for humans and animals for
growth, reproduction, and normal physiological functions. It is a precursor of the
coenzymes, CoA and acyl carrier protein of fatty acid synthase, which are involved in
more than 100 different metabolic pathways including energy metabolism of
carbohydrates, proteins and lipids, and the synthesis of lipids, neurotransmitters,
steroid hormones, porphyrins and hemoglobin.
Pantothenic acid serve as a regulator for several metabolic processes, many of which
are important for exercise performance (Thomas 1998, S. 97).
The biologically active forms of pantothenic acid, coenzyme A (CoA) an acyl carrier
protein, are cofactors for acetylation reactions which are essential in many
biosynthetic pathways, as well as in energy production. Pantothenic acid-containing
coenzymes are involved in acylation of alcohols, amines, and amino acids.
Abb.: Chemische Struktur Coenzym A
Pantothenic acid derivative called pantethine has been reported by a number of
investigators to have a cholesterol lowering effect. Pantethine is actually two
molecules of pantetheine joined by a disulfide bond (chemical bond between two
Problem- and task setting
molecules of sulfur). In the synthetic pathway of coenzyme A (CoA), pantethine is
closer to CoA than pantothenic acid, and is the functional component of CoA and
acyl carrier proteins. Several studies found doses of 900 mg of pantethine daily (300
mg, three times daily) to be significantly more effective than placebo in lowering total
cholesterol and triglyceride levels in the blood of both diabetic and non-diabetic
individuals . Pantethine was also found to lower cholesterol and triglyceride levels in
diabetic patients on hemodialysis without adverse side effects. The low side effect
profile of pantethine was especially attractive for hemodialysis patients because of
the increased risk of drug toxicity in patients with renal (kidney) failure. Pantethine is
not a vitamin; it is a derivative of pantothenic acid. The decision to use pantethine to
treat elevated blood cholesterol or triglycerides should be made in collaboration with
a qualified health care provider, who can provide appropriate follow up. (Stryer 1999,
Problem- and task setting
Biotin (Vitamin H, Vitamin B7) Biotin was first recognized as an essential nutrient factor in mammals in 1936. Ten
years earlier, the inclusion of large amounts of raw egg whites in experimental diets
in rats had produced symptoms of toxicity. The symptoms appeared within a few
weeks of initiation of the diet containing raw egg whites. In 1926, Boas referred to
these symptoms of toxicity as egg-white injury syndrome. The major findings included
severe dermatitis, loss of hair, and lack of muscular coordination. Boas also noted
that yeast, liver, and several other foodstuffs contained a substance that protected
rats from egg-white injury syndrome. Later, the protective compound in the foodstuffs
was identified as biotin.
The biochemical basis for egg-white injury syndrome was quickly elucidated when
raw egg whites were found to contain the glycoprotein avidin, which has a
remarkable affinity for biotin. Once a biotin-avidin complex forms, the bond is
essentially irreversible; the biotin-avidin complex is not broken during passage of the
food bolus through the stomach and intestines. As a result, biotin is not liberated from
food, and the biotin-avidin complex is lost in the feces. The final step in solving the
mystery of egg-white injury syndrome was the demonstration that the syndrome
could be prevented by heating the egg whites, a process that denatures avidin and
destroys its affinity for biotin. (Williams 1997, S. 240).
Biotin is a bicyclicfe molecule composed of a ureido ring fused with a
tetrahydrothiophene ring. A valeric acid substituent is attached to 1 of the 2 carbon
atoms of the tetrahydrothiophene ring. Through this carboxyl group, biotin is linked
covalently to the e-amino group of lysine in 4 carboxylases that play critical roles in
intermediary metabolism.
The 4 enzymes are propionyl coenzyme A (CoA) carboxylase (PCC), pyruvate
carboxylase (PC), b-methylcrotonyl CoA carboxylase (b-MCC), and acetyl CoA
carboxylase (ACC). PCC is required for the complete catabolism of several
branched-chain amino acids and all odd-chain fatty acids. In the absence of PCC, a
severe clinical disease (characterized by acidosis, hypoglycemia, hyperammonemia,
coma, and death) develops. b-MCC is required for the complete catabolism of the
Problem- and task setting
amino acid leucine. In absence of b-MCC, a severe clinical illness (similar to that of
PCC deficiency) develops. ACC is required for the catalysis of the first step in fatty
acid synthesis. PC is an essential enzyme of gluconeogenesis. In the absence of PC,
severe fasting hypoglycemia develops.
In all 4 carboxylases, biotin functions as a coenzyme or prosthetic group that serves
as a carrier for CO2 in a multistep reaction. In the first reaction, the biotin moiety of a
carboxylase is carboxylated at the nitrogen atom diagonally across from the valeric
acid substituent. In the second reaction, the CO2 moiety is transferred to the
substrate (causing it to be carboxylated in the process), and the original carboxylase
is liberated intact, ready to perform another carboxylation.
Due to the fact that reduced pyruvate carboxylases activity (biotin dependend) is
responsible for gluconeogenases leads to increase the rate of pyruvate and lactate
as the same time to decrease the energy production rate.
Problem- and task setting
Vitamin C (Ascorbic Acid)
Vitamin C, also known as ascorbic acid, is a water-soluble vitamin. Unlike most
mammals, humans do not have the ability to make their own vitamin C. Therefore, we
must obtain vitamin C through our diet.
Vitamin C is required for the synthesis of collagen, an important structural component
of blood vessels, tendons, ligaments, and bone. Vitamin C also plays an important
role in the synthesis of the neurotransmitter, norepinephrine. Neurotransmitters are
critical to brain function and are known to affect mood. In addition, vitamin C is
required for the synthesis of carnitine, a small molecule that is essential for the
transport of fat to cellular organelles called mitochondria, for conversion to energy.
Recent research also suggests that vitamin C is involved in the metabolism of
cholesterol to bile acids, which may have implications for blood cholesterol levels and
the incidence of gallstones.
Vitamin C is also a highly effective antioxidant. Even in small amounts vitamin C can
protect indispensable molecules in the body, such as proteins, lipids (fats),
carbohydrates, and nucleic acids (DNA and RNA) from damage by free scavengers
and reactive oxygen species that can be generated during normal metabolism as well
as through exposure to toxins and pollutants (e.g. smoking). Vitamin C may also be
able to regenerate other antioxidants such as vitamin E. (Fuchs 2001, S. 167).
Selected functions of vitamin C that could affect Physical Performance
Chemical Reaction Requiring Vitamin C
Body Function
Lysine Î Hydroxylysine
Needed for normal collagen (cartilage, connecitve
Proline Î Hydroxyproline
tissue, ligaments, tendons)
Necessary for normal fatty oxidation in muscle cell
Lysine Î Carnitin (liver, kidney)
Phenylalanine Î Dopamine, Norephinephrine
Needed for normal neurotransmitter formation
Ascorbic acid Î dehydroascorbic acid
Normal antioxidant function
(vgl. Keith 1997, S. 31)
Problem- and task setting
Vitamin E (Tocopherol)
Physical activity has been recognized as an important lifestyle factor which contribute
to good health and delays the onset of many diseases later in life. Vitamin E is an
essential fat-soluble vitamin which includes a group of eight naturally occurring
compounds in two classes designated as tocopherols and tocotrienols with different
biological activities (vgl. Meydani et al, 1998, S. 119).
The main function of alpha-tocopherol in humans appears to be that of an
antioxidant. Free scavengers are formed primarily in the body during normal
metabolism and also upon exposure to environmental factors such as cigarette
smoke or pollutants. Fats, which are an integral part of all cell membranes, are
vulnerable to destruction through oxidation by free scavengers. The fat-soluble
vitamin, alpha-tocopherol, is uniquely suited to intercepting free scavengers and
preventing a chain reaction of lipid destruction. Aside from maintaining the integrity
of cell membranes throughout the body, alpha-tocopherol also protects the fats in low
density lipoproteins (LDLs) from oxidation. Lipoproteins are particles composed of
lipids and proteins, which are able to transport fats through the blood stream. LDL
transport cholesterol from the liver to the tissues of the body. Oxidized LDLs have
been implicated in the development of cardiovascular diseases. When a molecule of
alpha-tocopherol neutralizes a free scavenger, it is altered in such a way that its
antioxidant capacity is lost. However, other antioxidants, such as vitamin C, are
capable of regenerating the antioxidant capacity of alpha-tocopherol.
Several other functions of alpha-tocopherol have been identified, which likely are not
related to its antioxidant capacity. Alpha-tocopherol is known to inhibit the activity of
protein kinase C, an important cell signaling molecule, as well as to affect the
expression and activity of immune and inflammatory cells. Additionally, alpha-
tocopherol has been shown to inhibit platelet aggregation and to enhance
vasodilation (vgl. Williams 1997, S. 229).
Problem- and task setting
Vitamin K (Phyllochinone)
Vitamin K is a fat-soluble vitamin. The "K" is derived from the German word
"koagulation". Coagulation refers to blood clotting, because vitamin K is essential for
the functioning of several proteins involved in blood clotting There are two naturally
occurring forms of vitamin K. Plants synthesize phylloquinone, also known as vitamin
K1. Bacteria synthesize a range of vitamin K forms, using repeating 5-carbon units in
the side chain of the molecule. These forms of vitamin K are designated
menaquinone-n (MK-n), where n stands for the number of 5-carbon units. MK-n are
collectively referred to as vitamin K2. MK-4 is not produced in significant amounts by
bacteria, but appears to be synthesized by animals (including humans) from
phylloquinone. MK-4 is found in a number of organs other than the liver at higher
concentrations than phylloquinone. This fact, along with the existence of a unique
pathway for its synthesis, suggests there is some unique function of MK-4 that is yet
to be discovered.
The only known biological role of vitamin K is that of the required coenzyme for a
vitamin K-dependent carboxylase that catalyzes the carboxylation of the amino acid,
glutamic acid, resulting in its conversion to gamma-carboxyglutamic acid (Gla).
Although vitamin K-dependent gamma-carboxylation occurs only on specific glutamic
acid residues in a small number of proteins, it is critical to the calcium-binding
function of those proteins
The ability to bind calcium ions (Ca2+) is required for the activation of the 7 vitamin
K-dependent clotting factors in the coagulation cascade. The term, coagulation
cascade, refers to a series of events, each dependent on the other that stops
bleeding through clot formation.
Some people are at risk of forming clots, which could block the flow of blood in
arteries of the heart, brain, or lungs, resulting in heart attack, stroke, or pulmonary
embolism, respectively. Some oral anticoagulants, such as warfarin, inhibit
coagulation through antagonism of the action of vitamin K. Although vitamin K is a
fat-soluble vitamin, the body stores very little of it, and its stores are rapidly depleted
without regular dietary intake. Perhaps, because of its limited ability to store vitamin
K, the body recycles it through a process called the vitamin K cycle. The vitamin K
cycle allows a small amount of vitamin K to function in the gamma-carboxylation of
proteins many times, decreasing the dietary requirement. Warfarin prevents the
Problem- and task setting
recycling of vitamin K by inhibiting two important reactions and creating a functional
vitamin K deficiency (see diagram). Inadequate gamma-carboxylation of vitamin K-
dependent coagulation proteins interferes with the coagulation cascade, and inhibits
blood clot formation. Large quantities of dietary or supplemental vitamin K can
overcome the anticoagulant effect of vitamin K antagonists, so patients taking these
drugs are cautioned against consuming very large or highly variable quantities of
vitamin K in their diets.
Williams (1998, S. 231) postulates that there is no evidence that vitamin K have any
ergogenic effects on human performance. Against this statement Fuchs (2001, S.
348) says: "Vitamin K could because of the same biochemical similarity like Vitamin E
and Q 10 have a role in oxidative phosphorilisation and in production and storing of
Energie (ATP) in the mitochondrium."
Problem- and task setting
1.3.4 Minerals – the anorganic regulators
Minerals are elements that originate in the Earth and cannot be made by living
systems. Plants obtain minerals from the soil, and most of the minerals in our diets
come from directly from plants or indirectly from animal sources. Minerals may also
be present in the water we drink, but this varies with geographic locale. Minerals from
plant sources may also vary from place to place, because soil mineral content varies
geographically. (vgl. Williams 1997, S. 260).
In order to discuss why exercise might increase a person´s need for minerals, we
must first explain the role of minerals in energy metabolism, their exercise-related
functions and their role in the maintenance of good health. Zinc, magnesium, iron,
and copper are especially important for the metabolic pathways involved in energy
metabolism and for the maintenance, building, and repair of muscle tissues.
Chromium is important for glucose metabolism and optimal insulin action (vgl.
Manore 2000, S. 306).
Problem- and task setting
Magnesium, an essential mineral, may be classified as a nutritional sports ergogenic.
Magnesium is a natural constituent of various foods. Magnesium supplements have
been studied in attempts to increase physical power in the ATP-CP energy system,
primarily by increasing muscle mass. Additionally, magnesium supplements have
been studied in attempts to increase aerobic power and endurance for events
dependent on the oxygen energy system (Williams 1998, S. 221).
The most important exercise-related functions of magnesium are energy metabolism,
protein synthesis and neuromuscular transmission and activity. Magnesium is
required for the glycolytic pathway, the synthesis and oxidation of fatty acids and
proteins, adenosine triphospate (ATP) hydrolysis, and for the formation fo cyclic
adenosine monophosphate (cAMP). Several key enzymes in the glycolytic pathway
require magnesium (hexokinase, phosphofructokinsase, and pyruvate kinase) (vgl.
Manore 2000, S. 308).
Two studies have reported that magensium supplementation improved the energy
efficiency for running and rowing, as evidence by either a low oxygen cost or less
lactic acid production, but no effect on performance was noted.
Problem- and task setting
Zinc, an essential mineral, may be classified as a nutritional sports ergogenic
(Williams 1998, S. 275).
Zinc is a cofactor for over 300 enzymes in the human body. Zinc is important in the
structure and function of biomembranes and helps to stabilize the structures of
ribonucleic acid (RNA), deoxyribonucleic acid (DNA) and ribosomes. Zinc is also
important for growth and repair for tissue, maintenance of the immune response, and
energy metabolism during exercise (Manore 2000, S. 306).
Zinc supplementation has been studied primarily in attempts to increase muscle
mass and physical power, particularily explosive power, high power and power
Theoretically, zinc supplements could enhance muscle protein synthesis, increasing
strength and power. Zinc also is needed for lactic acid dehydrogenase (LDH), an
enzyme important to the lactic acid energy system. Enhanced LDH activity could
benefit anaerobic exercise performance (Williams 1998, S. 276).
Problem- and task setting
Selenium (Se) is a trace element which is essential, yet toxic when consumed in
excessive quantities (Wolinsky 1998, S. 200).
Selenium, an essential mineral, may be classified as a nutritional sports ergogenic.
Selenium supplementation is used in attempt to increase aerobic power and
endurance for sport events that derive energy primarily from the oxygen energy
system. Selenium is a cofactor for glutathione peroxidase (Gpx), a naturally occurring
antioxidant enzyme in the tissues. Selenium also works closely with vitamin E,
another antioxidant.
Syndromes related to selenium deficiency are often somewhat nonspecific, may be
multifactorial and are rare.
Keshan disease is a dariomyopathy, more common in women and children than in
men, which is characterized by multifocal necrosis in the myocardium. Individuals
with Keshan disease have been reported to have signifanctly lower levels of blood Se
and GSHPx activity than normal subjects living in the same area in China.
Problem- and task setting
"In a recent survey of magazines targeted to bodybuilders, chromium was one of the
top two dietary supplements advertised" (Willams 2001, S. 171).
Unlike the other minerals discussed so far, the role of chromium in exercise is less
well defined. According to current research, chromium´s primary bilogical role is to
potentiate the effect of insulin, thereby enhancing the uptake and utilization of protein
fat, and carbohydrate (Manore 2000, S. 311).
Chromium supplementation has been studied primarily in attempts to increase
muscle mass and decrease body fat for enhanced strength and power or for a more
aesthetic physical appearance in sports such as bodybuilding. Chromium also may
be used in attempts to improve performance in prolonged aerobic endurance
Chromium is part of a biologically active organic complex in the body known as the
glucose tolerance factor (GTF) that is believed to enhance insulin sensitivity.
Theoretically, chromium supplementation will enhance the anabolic activity of insulin,
increasing muscle mass by promoting the transfer of amino acids into the muscle
cell, stimulating protein synthesis, and decreasing the rate of muscle protein
breakdown. Increased insulin sensitivity may enhance the storage of muscle and liver
glycogen and may improve utilization of glucose during exercise, factors that
theoretically could enhance performance in prolonged aerobic endurance events.
Problem- and task setting
1.3.5 Biochemical characteristics of the supplement
Nutritional sports ergogenics primarily serve to increase muscle tissue, muscle
energy supplies, and the rate of energy production in the muscle. Although most
nutritional sports ergogenics are designed to increase physical power, some also
may contribute to mental strength or mechanical edge.
This product was made to obtain all the nutrients you need for optimal sports
performances. This product theoretically helps to increase all types of physical power
from the three human energy stems, the ATP-CP, lactic acid, and the oxygen energy
systems. Additionally, thiamin and several other B vitamins may be used in attempts
to improve mental strength by inducing a calmative effect.
As we have noted vitamins and minerals are involved in almost all metabolic
processes in the human body. Functioning as coenzymes or in other metabolic roles
important to exercise performance and any kind of physiological work. They are
essential for the
optimal functioning of all three human energy systems
needed for the formation of red blood cells to transport oxygen
involved in the formation of muscle protein
required for the formation of various neurotransmitters
(Williams 2001, S. 255)
Problem- and task setting
Theoretically, a supplementation with this product could enhance these metabolic
functions, almost every type of sports performance could be improved.
During high-intensity exercise, the accumulation of lactic acid in the muscle cell is
believed to cause fatigue. The hydrogen ion released from lactic acid in the muscle
cell is thought to inhibit the activity of various enzymes necessary for energy
production. One theory suggests that by increasing the alkaline reserve, sodium
bicarbonate supplementation will facilitate the removal of hydrogen ions from the
muscle cell, reducing its acidity and delaying the onset of fatigue.
This supplement contains also alkaline salts. Bicarbonates may be classified as a
physiologic sport ergogenic. Sodium bicarboante is an alkaline salt that is part of the
natural alkaline reserve in the body that helps neutralize metabolic acids. Studies
with bicarbonates have primarily tried to enhance power endurance for sport events
that derive energy primarily from the lactic acid energy system.
Problem- and task setting
Contents of one dose:
Vitamins
Trace elements
Pantothenic acid
Amino acid
204,20 mg Carbohydrates
Minerals
Further ingredients
Hydrogencarbonate
1.3.5.1 Further ingredients
L-Carnitine is synthesized primarily in the liver and also in the kidneys, and must be
transported to other tissues. It is most concentrated in tissues that use fatty acids as
their primary dietary fuel, such as skeletal and cardiac (heart) muscle. In this regard,
L-carnitine plays an important role in energy production by chaperoning activated
fatty acids (acyl-CoA) into the mitochondrial matrix for metabolism and chaperoning
intermediate compounds out of the mitochondrial matrix to prevent their
The transport of long-chain fatty acids by L-carnitine into the mitochondrial matrix
where they can be metabolized to generate energy requires three enzymes located
on the mitochondrial outer and inner membranes. On the outer mitochondrial
membrane of skeletal and cardiac muscle cells, carnitine-palmitoyl transferase I
Problem- and task setting
(CPTI) catalyzes the formation of acylcarnitine (a fatty acid + L-carnitine) from acyl-
CoA (a fatty acid + coenzyme A). A transporter protein called carnitine:acylcarnitine
translocase (CT) transports acylcarnitine across the inner mitochondrial membrane.
Carnitine-palmitoyl transferase II (CPTII) is associated with the inner mitochondrial
membrane and catalyzes the formation of acyl-CoA within the mitochondrial matrix
where it can be metabolized through a process called beta-oxidation, ultimately
yielding propionyl-CoA and acetyl-CoA (2, 3).
Flavonoids have been referred to as "nature's biological response modifiers" because
of their ability to modify the body's reaction to other compounds such as allergens,
viruses, and carcinogens. They show anti-allergic, anti-inflammatory, and anti-cancer
activity. In addition, flavonoids act as powerful antioxidants, providing remarkable
protection against oxidative and free scavenger damage.
As a result, consumers and food manufacturers have become increasingly interested
in flavonoids for their healthful properties, especially its potential beneficial role in the
prevention of cancers and cardiovascular disease. The beneficial effects of fruits and
vegetables are now often attributed to flavonoid compounds rather than to known
nutrients and vitamins.
Problem- and task setting
1.4 Physiological parameters
It is well known in sporting circles that in 2001 the world record for the 100m for men
was 9.79 seconds (Maurice Green).
What is less well known is the accuracy of a tennis pro (shots that would have gone
in), or of a baseball pitcher.
How far does a rugby union player run in a match versus a rugby league player?
One of the biggest key questions not only in sports, but also in human being: "How
can we get better?"
Getting better is a process, which takes time – but the fact is that you have to fix a
status quo (making a test) – intervention – retest – development.
The cardiac system
Heart rate is a term used to describe the frequency of the cardiac cycle. It is
considered one of the four vital signs. Usually it is calculated as the number of
contractions (heart beats) of the heart in one minute. As such heart rate is usually
expressed as "beats per minute" (bpm). When resting, the adult human heart beats
at about 70 bpm (males) and 75 bpm (females), but this rate varies between people.
However, the reference range is taken between 60 bpm (if less termed bradycardia)
and 100 bpm (if greater, termed tachycardia).
The body can increase the heart rate in response to a wide variety of conditions in
order to increase the cardiac output (the amount of blood ejected by the heart per
unit time). Exercise causes a normal person's heart rate to increase above the
resting heart rate. As the physical activity becomes more vigorous, the heart rate
increases more. With very vigorous exercise, a maximum heart rate can be reached.
As an economic effect, effective training can decrease the heart rate as response to
cardiac adaption.
Problem- and task setting
The maximum O2-intake rate
Fitness can be measured by the volume of oxygen you can consume while exercising
at your maximum capacity. VO2 max is the maximum amount of oxygen in milliliters, one can use in one minute per kilogram of body weight. Those who are more fit have
higher VO2 max values and can exercise more intensely than those who are not as well conditioned. Numerous studies show that you can increase your VO2 max by working out at an intensity that raises your heart rate to between 65 and 85% of its
maximum for at least 20 minutes three to five times a week. A mean value of VO2 max for male athletes is about 3.5 litres/minute and for female athletes it is about 2.7
„The oxygen intake (V02) is a directly gauge for the capacity of the aeorbic metabolism" Grosser (1998, S. 126)
Normrates of the VO2
Lactic acid (α-hydroxypropanoic acid) is a chemical compound that plays a role in
several biochemical processes. Lactate is its ionic equivalent.
Lactic acid is a carboxylic acid with a chemical formula of C3H6O3. Its structure is
reflected in its systematic name, 2-hydroxypropanoic acid. In solution, it can lose a
proton from the COOH carboxy group, turning into the lactate ion CH3CHOHCOO-.
There are two optical isomers of lactic acid (and of lactate) since the central carbon
atom is bound to four different groups. The first isomer is known as L(+)-lactic acid or
(S)-lactic acid and the second is D(-)-lactic acid or (R)-lactic acid (vgl. Grosser 1997,
S. 77). Lactic acid accumulates in skeletal muscles during extensive anaerobic
exercise, causing temporary muscle pain. Lactic acid is quickly removed from
muscles when they resume aerobic metabolism. While lactic acid accumulation is
one of the limiting factors in exercise, delayed onset muscle soreness, which
Problem- and task setting
becomes apparent more than 24 hours after exercising, is not (as was once thought)
caused by lactic acid buildup.
During one form of anaerobic glycolysis or fermentation, L-lactate is produced from
pyruvate via the enzyme lactate dehydrogenase. This conversion also oxidizes one
molecule of NADH to NAD+, furthering the goal of generating energy. NAD+ has to
be regenerated so that glycolysis can continue.
This lactic acid fermentation occurs in red blood cells since they don't have
mitochondria, and in skeletal muscle during intense exertion when sufficient amounts
of oxygen cannot be supplied fast enough. This lactate is released into the
bloodstream. The typical lactate concentration in the blood is 1-2 mmol/l. The liver
takes up about 60% of the lactate and reoxidizes it to pyruvate, which is then
reconverted to glucose in a process known as gluconeogenesis. The glucose enters
the bloodstream and can be used by the tissues. This glucose → lactate → glucose
cycle, originally described by Carl and Gerty Cori, is known as the Cori cycle. About
40% of the lactate is taken up by well oxygenated muscle cells and oxidized to
pyruvate, which is then directly used to fuel the citric acid cycle.
Lactate threshold
Defined the breakpoint – The highest VO2 that can be attained during incremental exercise before an elevation in blood lactate is observed. This occurs just before the
curvilinear increase in blood lactate observed when blood lactate is plotted versus
VO2 from exercise tests in which subjects perform a series of increasing bouts of exercise intensities at work increments of at least 3 min. This phenomenon has also
been called the lactate breaking point, the onset of plasma lactate accumulation, the
anaerobic threshold and the aerobic threshold (Weltmann 1995, S. 3).
The aerobic threshold 2.0 mmol/l lactate (AT)
The aerobic threshold (AT) is an exercise intensity at which lactate (lactic acid) starts
to build into the blood stream. The AT is defined as a lactate concentration of 2
mmols. It is approximately 20 bpm less than the anaerobic threshold. The dominant
metabolism under 2.0 mmol/l lactate is the utilization of free fatty acids (vgl. Astrand
Problem- and task setting
The anaerobic threshold 4.0 mmol/l lactate (ANT)
The anerobic threshold (ANT) is an exercise intensity at which lactate (lactic acid)
starts to accumulate into the blood stream faster than it can be metabolized. Below
the ANT any lactate produced by the muscles is removed by the body without it
The only accurate way to measure anaerobic threshold is via blood samples. The
ANT is defined as a lactate concentration of 4 mmols (at rest it is around 1 mmol).
There are ways of estimating AT based on heart rate but these can be very
A person's anerobic threshold can be improved by exercising, however the threshold
will never exceed the person's VO2max - the point at which the body cannot supply any more oxygen to the muscles. The relation between the two is reliant on the
amount of training - an untrained individual's ANT is approximately 55% of VO2max, but elite endurance athletes are about 80-90% of VO2max (Weltman 1995, S. 10).
The fat metabolism basic threshold (FMBT)
Since now in sports medicine and science the FMBT was not really considered.
Bergmüller (2003, S. 75) introduced after 1000 of tests this kind of threshold on 1.0
mmo/l – it seems to be a good indicator for the capacity of the dominant aerobic fat
metabolism – espacially for untrained persons and patients in rehabilitation but also
for endurance trained athletes.
Problem- and task setting
Bioelectrical Impedance Analysis (BIA)
Body fat percentage is the proportion of fat in a person's body. Excess body fat has
previously been determined by measuring weight against height, but body fat is not
always visible and cannot be measured on an ordinary scale. Obesity, which
indicates a high degree of excess body fat, has been linked to high blood pressure,
heart disease, diabetes, cancer, and other disabling conditions.
Body fat scales uses the BIA (Bioelectrical
Impedance Analysis) technique. This method
measures body composition by sending a low, safe
electrical current through the body. The current
passes freely through the fluids contained in muscle
tissue, but encounters difficulty/resistance when it
passes through fat tissue. This resistance of the fat
tissue to the current is termed 'bioelectrical
impedance', and is accurately measured by body fat
scales. When set against a person's height and
weight, the scales can then compute their body fat
Impedance analysis is based upon relating the measured electrical values of a
subject to their physiologic equivalents as determined when the subject is the only
unknown part of a safe and controlled electrical circuit. The properties of the circuit
are well-defined and don't change over time. The method is precise, sensitive and
specific in its ability to illustrate the changes inherent in the biological subject. Of
particular benefit is these changes occur at a level of physiology that precedes those
seen through biochemical assays, cell counts, imaging techniques and physical
signs. This 'snapshot' of cellular level dynamics and architecture provide valuable
additional data to clinical practice and medical research.
BCM (Body cell mass)
Body cell mass: The total mass of all the cellular elements in the body which
constitute all the metabolically active tissue of the body. There is depletion of the
Problem- and task setting
body cell mass (BCM) that is characteristic of wasting of the body common with
chronic diseases such as AIDS and terminal cancer.
The BCM includes muscle tissue, organ tissue, intracellular and extracellular water,
and bone tissue. In the normally nourished individual, muscle tissue accounts for
approximately 60% of the BCM, organ tissue accounts for 20% of BCM, with the
remaining 20% made up of red cells and tissue cells. The BCM also contains the
large majority (98-99%) of the body's potassium.
The preferred method for assessing BCM depletion is bioelectrical impedance
analysis (BIA) which can be performed with portable equipment in the office setting. It
involves no radiation, is inexpensive, painless, and has a high degree of accuracy.
The results of BIA are combined with other statistics (height, weight, sex, and age) to
calculate the BCM, the fat-free mass, and other body composition measurements.
Phase angle
The phase angle is calculated by finding the ratio of reactance divided by resistance,
and taking the arctangent of that ratio. Phase angle is normally expressed in
degrees. In addition, the ratio of reactance to resistance can be multiplied by 100 to
yield a percent grade, similar to that often seen on mountainous highway signs for
truckers. The greater the grade the more work or energy is required to ascend the
mountain or brake in going down the mountain. The truck would have a specific
phase angle (trigonometry) depending on the engine size and efficiency, and weight
of the truck. The energy of all living things comes from cells that consume oxygen
and nutrients and expel carbon dioxide and waste. The quantity and efficiency of
cells directly affect phase angle. The outer boundary of the cell is a plasma
membrane of phospholipid molecules that are a dielectric to form an electrical
capacitor when a radio frequency signal is introduced to the cells environment.
Capacitance is fundamental to any human phase angle measurement, the higher the
capacitance the greater the phase angle. An elite athlete would have a higher phase
angle than a sedentary person. It has been well documented that phase angle
declines with disease, age and reduce activity level.
Problem- and task setting
ECM/BCM Ratio
The ECM/BCM ratio is the ratio of extracellular mass to body cell mass. Fat-free
mass consisting of 50% extracellular mass and 50% body cell mass has an
ECM/BCM ratio of 1.0. ECM/BCM typically ranges from 0.8 to 2.0 or a 55% to 33%
proportion of body cell mass to fat-free mass respectively.
Practitioners use ECM/BCM to relate the amount of body cell mass to total fat-free
mass. In this way, the ECM/BCM ratio can be used as an alternative to phase angle.
Meta-Index
Meta-Index is the ratio of resistance to body mass index. Calculating this index one
can receive a prediction of electrical conductivity and the content of water and
electrolytes of lean body mass. Reduction of the Meta-Index as found in the study
argues for the accumulation of sodium bonded water.
Problem- and task setting
What is limiting sports performance? The ultimate barrier is the inability to achieve
optimal production, control, and efficient use of energy. Three general types of
barriers to optimal performance in sport that can be controlled to some degree are
physilogical, psychological, and biomechanical barriers. But all this skills leads us to
the result that energy (muscle contraction, ATP-replenishment) play the key role in
human performance.
(vgl. Williams 1998)
„Theoretically multivitamin/mineral supplementation could enhance every type of
sports performance" (Williams 1997, S. S226).
Problem- and task setting
1.5.1 Task setting
This study will be executed to investigate the effect of a basic micronutritient
supplement on physiological parameters.
1.5.2 Hypothesis
Working hypothesis:
A supplemention with a basic micronutrient supplement influences specially selected
physiological parameters.
Null hypothesis:
A supplemention with a basic micronutrient supplement does not influence specially
selected physiological parameters.
A supplementation with the micronutritient supplement has an effect on:
the performance of the muscle metabolism (concentration of blood lactate on
submaximal work loads eg. 1.0, 2.0, 4.0 mmol/lactate)
the performance of the cardio-pulmonary system(VO2 max)
S3: the performance of the cardio-vascular system (heart rate) S4:
the body composition (BIA)
2 Investigation
methodology
2.1 Characteristics of the study
Placebo controlled double blind study.
The claim of the declaration of Helsiniki for the biological research on humans has
been considered. The test person has been advertised about this project orally and
written and gave their consents. All test persons are informed about the target of this
research and the goal. While the verum supplement consists of a useful mix of
vitamins, minerals and trace elements and bioflavonoids, the placebo supplement
contains only a mix of fructoses, glucose, citric-acid, orange flavour, carrot extraction
and aspartum. Both supplements are according to the calculation of isotonie the
osmotic pressure considered, lightly hypotonic.
Vitamins
Trace elements
Pantothenic acid
Amino acid
204,20 mg Carbohydrates
Minerals
Further ingredients
Hydrogencarbonate
Abb.: Consistence of the verum
2.2 Design of the study
Group comparison, randomised, double blind, placebo controlled, monocentric.
• Selection of 30 female test persons, who are informed about the goal of this
intervention. During the study, neither the leader of the study, nor the test
persons know what kind of supplement (placebo, verum) they get. At the
beginning of the investigation there were also considered men in the group of
test persons. But the reason not to mix the group was the possibility of
comparability of the small intervention groups.
• Selection of 30 female test persons • Measurement of the body composition • Execution of a test on a cycle ergometer with the goal to win physiological
parameters. Subsequent to the cycle test, beginning of the intervention with
the supplements.
• A compliance of at least 80 % is the precondition for remaining in the group • After 8 weeks – final test – comparison of the results – longitudinal section
2.3 Data winning
Before to participating in the study, every test person has to bring a medical attest of
his physical suitability. The first test started with the body composition analyse, then
the maximum test on the cycle, on which relevant parameters like lactate, heart
frequence and oxygen intake has been measured.
2.3.1 Random test
The test persons are comprised of 30 female persons within an age of 20 – 35 years.
The precondition of the participation on this project was that at least 2 months no
supplements or medicines has been taken. The decision of remaining in the
investigation group was not possible until the first test (cycle test) was executed.
Criterion of the decision to remain in the group is the grade of the physical fitness.
The goal of the study is to research the influence of a micronutritient supplement on
sports performances. The test persons are not allowed to do any kind of training. So
every kind of movement that the test person has done during the investigation has to
be recorded exactly. Criterium to stay in group or not was the physical fitness of the
test persons. The maximum oxygen intake was not allowed to be higher than 35
ml/min/kg, because Grosser assumes that this is a sign of untrained condition.
Therefore we get a dependent sample which is supplemented over 2 months with
verum or placebo. The test persons were allowed to execute moderate movement
like walking, but no physiological training were allowed. The test persons were
required not to change their alimentation habitualness during the period of
intervention, and they have to record exactly the daily supplementation.
First execution of the bio electrical impendance analyse (BIA). In consequence
starting with the performance diagnostic on the cycle ergometer with the following
rectangular-triangulare
2. Increment of workload:
Increments of the watts
3. Initial workload:
4. Steps of workload:
5. period of 1 step:
The guidelines of the steps of workloads are so intensive, that on subminimal steps
lactate rate from 1 to 8 mmo/l can be reached. This includes the biological orientation
helps: Aerobic threshold (2.0 mmol/l lactate), anaerobic threshold (4.0 mmol/l lactate)
and the aerobic-anaerobic crossing zone (Pansold et. al 1994, S. 48).
The combination of the parameter lactate from the cycle test to the incremental steps
of workload leads to the lactate-performance-curve. This curve has to be calculated
with a non linear regression with the formula y = a * e bx .
As feature size for performance the adduced individual power on the submaximum
steps, like 1.0 (fat metabolism threshold), 2.0 (aerobic threshold), 3.0 (steady-state),
4.0 mmol/l (anaeribic threshold) are calculated. The measurement of this feature
sizes is the precondition for producing interpretation models and basic for the
development of the performance.
2.3.3 Testing procedures
The first evaluation is subdivided in two step. First in condition of silence and no
stress the BIA (bioelectrical Impendance analyse) is executed. Subsequent to this the
maximum power test on the cycle with parallel measurement of all relevant
parameters will be occurred. Blood lactate is measured after every step and
increment. During the whole investigation heart frequence for the reaction of the
cardiac system was measured. With the measuring of lactate you get information
about how your muscle metabolism is working, the heart rate tells us what is
happening in the cardiac system and the oxygen intake is important to predict the
capacity of the pulmonary system.
2.3.4 Testing instruments
B.I.A. (Bioelectrical impendance analysis)
Bioelectrical Impendance Analysis, or BIA, is considered one of the most exact and
accessible methods of screening body composition. In conventional BIA, a person is
weighed, then height, age, gender and weight or other physical characteristics such
as body type, physical activity level, ethnicity, etc. are entered in a computer. While
the person is lying down, electrodes are attached to various parts of the body and a
small electric signal is circulated.
Simply explained, BIA measures the impedance or resistance to the signal as it
travels through the water that is found in muscle and fat. The more muscle a person
has, the more water their body can hold. The greater the amount of water in a
person's body, the easier it is for the current to pass through it. The more fat, the
more resistance to the current. BIA is safe and it does not hurt. In fact, the signal
used in body fat monitors can not be felt at all either by an adult or child.
Heart rate
Heart monitors are tools that provide feedback specific to your body. As a result,
heart monitor training can only be effective if you use that information to design and
implement a workout that is tailored to your body and fitness level. Heart monitors
are devices that are designed for wear during strenuous exercise, and serve the
purpose of measuring and recording your heart rate, while giving you instant
feedback about the work level of your heart. The fitness of the heart is the key to
one's aerobic endurance - sometimes called 'cardiovascular respiratory endurance'.
Both for health and racing reasons, aerobic endurance is a point of focus for almost
any runner. Heart monitors are one of the most effective aids for tracking and
developing your progress on the path to increased aerobic endurance.
The monitoring of blood lactate can play a key role. Nowadays most of the blood gas
instruments are equipped with additional ion-selective electrodes for the analysis of
electrolytes and metabolites like glucose and lactate.
In this study the Radiometer ABL 700 (Radiometer, Kopenhagen), which uses ion-
selective electrodes was evaluated for the measurement of lactate in plasma and
whole blood. The ABL-700-method uses an ion-selective electrode with lactate
oxidase as enzyme.
Oxygen intake
K4b2 is the first portable system designed by COSMED to measure gas exchange on
a true breath by breath basis. Its technology enables the exploration of physiological
responses in the field during very fast and brief events, or while recording data over a
period of many hours. K4b² accurately measures over 30 physiological parameters
including VO2, VCO2, Heart Rate and Ventilation. K4b² is an indispensable device
for the researcher. Testing and calibration are easy procedures that can be carried
out either via a PC or from the on-board key pad of the Portable Unit
K4b² has been designed to be accurate and reliable during any condition. K4b² has
been validated and used within a significant amount of researchers published in the
most important scientific journals.
2.3.5 Criterion of the test
The multivariat dependence and interdependence of physiological parameters, is
correlated with the performance on maximum and submaximum steps. Therefore the
reaction of the cardiac, pulmonary, and muscle metabolism systems should be
analysed. This is a precondition to see how both supplements (verum/placebo) are
2.3.6 Criterion for maximum load
Grosser (1998, S. 163) describes the following situations:
1. Heart rate > 190 / min.
2. „Levelling off" of the oxygen intake
3. Blood lactate at least 8 – 10 mmol/l
4. The respiratory quotient is the ratio of carbon dioxide diffusing from blood into
alveolar of the lung to oxygen diffusing in the opposite direction (> 1,1)
5. Blood pressure of (systolic) over 220 mmHg is a criterion for an immediate
stop of the test.
Invariably the reliability and validity are redeemed because of the standardized labor
tests. Only the objectivity depends on the testleader. Extrem important for getting real
and reliable results is the routine and skillfulness. When you are measuring blood
lactate you have to care about the cleanliness. Because for example perspiration
could change the results. Further the test leader depends on the function of whole
technical instruments, for this only branded articles where used.
One possible error could probably be also the day time of test execution. For
example when a cycle test is occurs in the evening the carbohydrate stores are
emptier then when you test in the very early morning. For this it was important only to
execute the test in the late morning, but not later, because less lactate production
seemed to be the consequence.
2.4 Statistical analysis
Descriptive statistic
All relevant physiological parameters like lactate, heart rate, oxygen intake and watt-
performance were featured in diagrams by microsoft excel. With this method a
longitudinal section of the change and evolution of the results through the
intervention was illustrated.
Analytical statistic
The analytical statistic was performed with the program SPSS (Statistical Package
for Social Sciences) 13.0
1. Testing of normal distribution
The testing of the normal distribution of the results is the precondition for further
statistical steps. The interval scaled data was tested regarding the normal distribution
with the Kolmogorov-Smirnov test.
2. Onefactorial variance analysis
In the pre- and retest there were constructed mean value differences and they have
been brought to dependence of the factor supplementation (1 = verum / 2 = placebo).
So a global significance was calculated. This gives an information about the fact, that
the change of a result in dependence of the kind of supplementation is casual or
The t-test is the most commonly used method to evaluate the differences of means
between two groups. The groups can be independent (e.g., blood pressure of
patients who were given a drug vs. a control group who received a placebo) or
dependent (e.g., blood pressure of patients "before" vs. "after" they received a drug,
see below). Theoretically, the t-test can be used even if the sample sizes are very
small (e.g., as small as 10; some researchers claim that even smaller n's are
possible), as long as the variables are approximately normally distributed and the
variation of scores in the two groups is not reliably different. The t-test for dependent
samples was used in this investigation.
3 Results
The following result are first featured descriptive in forms of diagrams (development)
and then analytically (significance).
3.1 Changes in the metabolic area
1.0 mmol/l Lactate
0,60 0,5 1,10 0,4
0,65 0,5 0,67 0,4
The onefactorial variance analysis shows a significant dependence on the
supplementation. The verum group were fulfilling a statistically significant change
(p = 0,003) in performance.
2mmol/l Aerobic threshold
The onefactorial variance analysis shows a significant dependence on the
supplementation. The results of the verum group were statistically significant (p =
Lactate steady-state 3 mmol/l
The development of the performance has not shown any significant influence through
the kind of supplementation (p = 0,607).
The development of the performance has not shown any significant influence through
the kind of supplementation (p = 0,952).
Lactate 6 mmol/l
The development of the performance has not shown any significant influence through
the kind of supplementation (p = 0,225).
The development of the performance has not shown any significant influence through
the kind of supplementation (p = 0,322).
3.2 Changes in the pulmonary system
33,2 4,3 37,6 6,2
32,7 5,7 33,7 7,3
The onefactorial variance analysis shows a significant dependence on the
supplementation. The results of the verum group were statistically significant (p =
3.3 Changes in the cardiac system
Heart rate - 1 mmol/l lactate
101 14 108 15 0,110
The development of the performance has not shown any significant influence through
the kind of supplementation (p = 0,110).
Heart rate - 2.0 mmol/l lactate
The development of the heart rate on 2.0 mmol/l lactate has shown a significant
dependence on the kind of supplementation (p = 0,097).
Heart rate 3.0 mmol/l lactate
145 19 150 14 0,775
The development of the performance has not shown any significant influence through
the kind of supplementation (p = 0,775).
Heart rate 4.0 mmol/l lactate
157 19 161 14 0,920
The development of the performance has not shown any significant influence through
the kind of supplementation (p = 0,322).
Heart rate - 6.0 mmol/l lactate
170 14 175 13 0,763
The development of the performance has not shown any significant influence through
the kind of supplementation (p = 0,763).
Maximum Heart rate
182 13 182 14 0,465
The development of the performance has not shown any significant influence through
the kind of supplementation (p = 0,465).
3.4 Changes in the body composition
Phase angle
The onefactorial variance analysis shows a significant dependence on the
supplementation. The results of the verum group were statistically significant (p =
BCM (body cell mass)
36,3 6,9 34,9 8,2
The onefactorial variance analysis shows a significant dependence on the
supplementation. The results of the verum group were statistically significant (p =
Relationship ECM / BCM (extra cellular mass /
body cell mass)
The onefactorial variance analysis shows a significant dependence on the
supplementation. The results of the verum group were statistically significant (p =
24,4 4,6 23,4 4,5
19,5 4,4 19,8 4,1
The development of the performance has not shown any significant influence through
the kind of supplementation (p = 0,156).
4 Interpretation of the results
Metabolic changes
Beside the maximum oxygen intake and the heart rate, the lactate concentration is
one of the most important parameters in the evaluation of musculuar capability
(Stegmann 1985, S. 13).
Lactat threshold concepts, based on submaximum steps of workload, are the
precondition for the information about the response of intensity in muscle
The fat metabolism basic threshold was introduced by Bergmüller 2003. This is not a
scientifically threshold (no ventilatory changes) – but the performance on this
submaximum step gives us information and feedback about the capability of the fat
metabolism and the peculiarities of the aerobic enzymes. Often small stairs for
untrained persons leads to an overextending of the aerobic metabolism. The theory
of Bergmüller is when the performance at 1.0 mmol/ lactate (fat metabolism basic
threshold – FMBT) is high, the capacity of the fat metbalism is well developed.
It is a fact that the performance of the verum supplemented group on this
submaximum step was increased significantly (p = 0,003). This is without doubt a
sign of the optimized capacity of the aerobic enzymes. "To the aerobic enzymes
belongs citricsynthases, alpha-ketoglutaratedehydrogenases, succinate-
dehydrogenases, malatdehydrogenases, … . Only their increasing activity is
responsible for a stout aerobic performance" (Neumann et al. 2001, S. 95).
So we conclude that through the effect of the basic micronutrient supplement the
systems functions, especially the capacity of the muscle metabolism, can be
increased on moderate submaximum steps.
We can speak of the aerobic metabolism when the lactic acid concentration is under
2.0 mmol/l (vg. Neumann 2001, S. 204).
Equally the performance of the verum group at the aerobic 2.0 mmol/l lactate
threshold was improved significantly. The aerobic threshold is limiting the dominant
aerobic metabolism. Beyond this threshold lactic acid is accumulating in the blood
(Schulz 1994, S. 12). The performance on the aerobic threshold was increased from
1,4 to 1,9 Watt/kg. This is the confirmation of the known result (FMBT) and allows the
conclusion that an "optimization of cellular enzyme capacities through aimed and
controlled supplementation in the high-grade aerobic metabolism" was possible.
Niacin for example is the key enzyme for any reaction that occurs with NADH and
Riboflavin interacts as FAD in citric acid cycle. Before pyruvate can get into the citric
acid cycle, sufficient vitamin B1 is necessary für the decarboxylisation from pyruvate to acetyl-CoA. Therefore is Vitamin B1 an important key enzyme (Thiaminpyrophosphate TPP) to avoid an pyruvate accumulation and to keep the
aerobic energy production upright (vgl. Diebschalg 1991, S. 36).
A further theoretically trial of interpretation is that increased performance on this
submaximum steps was due the effect of the alcalic salts (Schek 1995, S. 243).
The production of lactic acid occurs with a parallel appearing of hydrogen ions. Those
are decreasing the pH-rate in the cell. This leads to an impairment of the enzymes
functions (phosphofructokinases-inhibition) and to a slow down in the energy
production – appearance of fatigue is the consequence. The supplementation of
alcalic salts should minimize the sloping pH-rate in the cell and help to surrender
hydrogen ions to the blood.
Pansold & Zinner assumes (1993, S. 28), that between 2 and 4 mmol/l lactic acid ist
the aerobic-anaerobic crossing zone. Important is to see the capability in this
metabolism zone, what kind of performance can one keep up on 3.0 mmol/l lactic
acid. Are the aerobic and anaerobic thresold scientifically proved; is the lactic acid
steady state (3.0 mmol/l) only a practical solution for results. In this state of
metabolsim the lactate production and –elimination are balanced. The performance
of both groups (verum/placebo) did not change statistical significant. The same
result we can also determine for the anaerobic threshold (4.0 mmo/l lactate). The
anaerobic threshold is also called the onset of blood lactate accumulation (OBLA),
because if someone wants to keep the performance on this step, more and more
lactate is accumulating in the blood and leads to acidosis (vgl. Diebschlag 1991, S.
12). The supplementation shows no effect in optimizing the anaerobic key enzymes
e.g. LDH (lactatedehydorgenases), PDH (pyruvatedehydorgenases), PGK
(phosphoglyceratedehydrogenases) and PFK (phosphofructokinases).
Also the theory of the optimization of the buffers through alcalic salts can not be
assumed to be right in this intensity of metabolism. We can conclude that the basic
micronutritient supplement improves the high-graded aerobic metabolism
(performance on 1.0 – 2.0 mmol/l lactic acid). This also means that the capacity of
the aerobic fat metabolism did increase and this possibly leads to an effect of
economization of the carbohydrate stores. Because of the importance for this
metabolic pathway it is possible to assume, that this micronutritient supplement has
positive effects on the concentrative and coordinative system and enhance also
Respiratory changes
Outgoing therefrom, that the performance is a complex structure of factors, only the
interpretation of lactate can not describe every cause. For this is imporant to see the
changes and reactions of the respiratory systems. The improvement of the aerobic
capacity is due to the increment of metabolism and circuit economy on submaximum
steps. "The physiological basic of the increment of V02max is an improvement of muscular oxygen utilization (Neumann et al. 2001, S.74). The V02max was increased in the verum supplemented group significantly from 33,2 to 37,6 ml/kg/min. This is
the expression of the fact, that the muscle cell is able to use the existing oxygen
more efficiently. Also possible is that the activity of important key enzymes in the
citric acid cycle and in the respiratory transport chain could have been improved.
Perhaps it was also due to the fact that more free fatty acids where mobilized and
utilized in the muscle cell. The improvement of the maximum oxygen intake should
not be confounded with the maximum performance (Pmax). Important is that parallel
to the significant change of the V02max also the changes in the muscle metabolsim (aerobic threshold, ….) were increased significantly.
Zintl (1994, S. 57) and Weineck (2002, S. 182) assume, that the oxygen intake is
only improvable from 15-20 %. But they performed their investigations on trained
The reason why the test persons were not performing a controlled training is simple.
We don t want to mix the effect of supplementation with the training. Any
improvement has been due to training or supplementation. Nobody was able to make
an exact and right analyzation.
Cardial change
Obviously there is no evidence that a micronutrient supplement influences directly the
heart rate system. But there is a strong relationship and interdependence between
the cardial and the metabolic system. We can conclude that lactate is the traffic jam,
that the heart rate has to regulate. It is absolutly possible that the economization in
the metabolic pathway on submaximum steps of workload induces lower heart rate
on this intensity. This theory can not be assumed and confermed in this investigation.
At 2.0 mmol/l lactate the heart rate in the verum supplemented group was even
significantly 12 beats/min higher. This is a negative effect, which could be relativized
with the following explanation: At the second test, the test persons were more tensed
than at the first test – because every one wanted to perform a good diagnostic.
Particular the heart rate under tranquility conditions (initial heart rate) was also
higher. The heart rate is a parameter which is effected from many sides – day time,
psycho-physical condition, day condition. Also the heart rate in the placebo group
was increased, but not significantly. So we can conclude that the results about the
individual development was without any measurable effect for this system.
Changes in body composition
BIA is much more than a method for fat determination. Because BIA enables you to
measure several nutritionally relevant bodily compartments, thus providing a more
sophisticated analysis of bodily composition and nutritional status. Bio-electric
impedance analysis is based on measuring the electrical resistance of the human
Via electrodes on each hand and foot, a weak, imperceptible electrical field is
generated inside the body by means of an impedance analysis device. 2 different
electrical resistances are measured here:
The water resistance R. The electrolyte water of the human body is a good conductor
of electric current. The water resistance is used to determine the body water, the lean
body mass (fat-free mass) and the body fat.
State-of-the-art, scientific BIA devices feature what is called „phase-sensitive
metrology", enabling the cell resistance Xc to be measured. This means the body cell
mass (BCM) can be determined – an examination that is not possible with other
simple methods for measuring the bodily composition, such as the infra-red
reactance method.
In order to be able to differentiate between the two components resistance and
reactance from the measured total resistance, modern BIA devices have phase
sensitive electronics.The principle of measurement is based upon the fact that the
condensers in the alternating current circuit lead to a time delay ∆t: the current
maximum is in advance of the voltage maximum. Every metabolically active cell of
the body has an electrical potential difference at the cell membrane of about 50-100
mV. This membrane potential allows the cell to act in an alternating electrical field like
a spherical condenser. As alternating current has a sinus wave, this shift is measured
in ° (degrees) and is described as a phase angle ∏ (phi) or α (alpha). Expressed
figuratively, well nourished, "plump"cells with stabile membrane potentials have a
large phase angle, whereas poorly nourished, you could say "withering" cells with low
membrane potentials have correspondingly small phase angles.
The phase angle is most meaningful at a frequency of 50 kHz. A pure cell membrane
mass would have a phase angle of 90 degrees, pure electrolyte water has a phase
angle of 0 degrees. The phase angle is thus directly proportional to the body cell
mass BCM. In contrast to cells of the body cell mass, fat cells, which are purely
storage cells, have hardly any metabolic activity, only posses a minimal membrane
potential and cannot be detected by phase sensitive measurements. The phase
angle being a direct measurement parameter or "basic value" is less dependent on
problems incurred with measuring technology or other sources of error. It is a general
measure of the membrane integrity of the cells and allows a judgement to be made
about the state of the cell and the overall condition of the body (vgl. Handbuch Data
The phase angel in the verum supplemented group was enhanced to 6,7 %. This
significant change leads us to the conclusion that this micronutritient supplement
could also enhance the nutritional status of the cells.
(vgl. Handbuch Data Input, S. 11)
The BCM is the sum of all cells actively involved in the metabolic processes. It is not
an anatomically, but rather a functionally defined compartment and consists above all
of the cells of the muscles and inner organs. Each tissue of the human organism
contains a certain proportion of the BCM. Connective tissue with low fibrocyte content
contains only a small percentage of the entire BCM, whereas the muscles have a
high percentage and, therefore, constitute the largest part of the BCM. The
adipocytes are not considered as belonging to the BCM due to their low energy
metabolism. The sum of adipocyte cells consequently constitutes its own
compartment in the body. The BCM includes the following types of tissues: the cells
of the skeletal muscle system, the cardiac muscles, the smooth muscles, the inner
organs, the gastrointestinal tract, the blood, the glands and the nervous systems.
BCM is the central parameter in the assessment of the nutritional state of a patient,
as all metabolic work in the body is carried out within the cells of the BCM. The BCM
is also the standard parameter for assessing energy consumption and determines the
calorific requirement of the body. Apart from catabolism, the work performance of the
BCM includes the anabolism to maintain cell structures and synthesis for the ECM,
such as e.g. the formation of connective tissue fibres, bone and cartilage tissues,
transportation proteins and enzymes. The body cell mass of an individual is a partial
component of the lean body mass. Genetic factors (type of constitution), age and
physical condition play a role in individual available BCM. Young people with high
physical activity (e.g. competitive athletes) train their muscles in the maturation phase
of the body. Frequently, a higher proportion of body cell mass in the lean body mass
is found in these individuals throughout their lives. BCM can amount to up to 60 % of
the lean body mass in competitive athletes. The body cell mass is dependent upon
age. In children and young people the cell mass of the body is not fully developed.
The proportion of the cell mass in the lean body mass is mostly less than 50%. The
muscle cells finally differentiate after longitudinal growth has been completed. Adults
with a normal nutritional status have more than 50% BCM in the lean body mass. At
a great age, the BCM normally decreases due to physical inactivity, quite often down
to 45 - 40%. In physically active older persons, however, the BCM is retained to a
large extent. Normal values for the body cell mass can be defined from the proportion
of the cell mass in the lean body mass. In the age range 18 - 75 years, men should
have approx. 53 - 59%, women approx. 50 - 56% BCM in the lean body mass (ideal
values). If one looks at the simple measuring methods for determining the body
composition, then only phase sensitive B.I.A. can be considered for determining the
BCM. Maintenance of the BCM is the central objective in all forms of nutritional
therapy. Even in reducing diets, the loss of BCM should under no circumstances be
more than 20% of the BCM, as a BCM reduction – if at all – is much more slowly
compensated for by the body than e.g. a reduction of body fat. A reduction of the
body cell mass in B.I.A. can arise through a genuine substantial loss of body cell
mass, but also by temporary intracellular water loss. A genuine loss of BCM is only
then the case, if at the same time the phase angle goes down the reactance drops
and/or the cell density falls 4 %. The BCM (body cell mass) was statistically
significant increased in this study, this is a further indication for the effect of the
supplement and changes in the results of VO2 max.
The ECM/BCM index is the second most important parameter for assessing
nutritional status. In healthy individuals, the body cell mass BCM is always distinctly
larger than the extra-cellular mass ECM, so that the index is smaller than 1. The early
stages of malnutrition are characterized by a decrease of BCM with a concomitant
enlargement of the extra-cellular mass; lean body mass and weight can thereby
remain constant. An increase in the ECM/BCM index points to the first signs of a
deterioration of nutritional status. Results higher than 1 in the ECM/BCM are a clear
indicator for malnutrition.
Contrarily to the used literature, the intervention with this basic micronutrient
supplement had no effect on anaerobic energy productions:
The aerobic way of producing energy is the most important. The limiting factor is the
capacity of enzymes in the cellular area. The following model of interpretation should
explain the fact of optimizing cellular capacities with a very special kind of
supplementation (micronurtritients):
• fresh tools (in form of vitamins) • optimal cell protection (in form of antioxidants) • cleaning substances (alcalic electrolytes)
The following graphic should this theory explain:
The replenishment of ATP is the most important key reaction in the energy
metabolism. So much the better enzyme capacity is, so much more economically can
the muscle cell regenerate ATP. The limiting factors of aerobic enzyme production
The capability of those different enzyme systems, who control, steer and catalyze the
chemical reaction and the oxygen supply in the target cell. Nearly every kind of
biochemical metabolism is catalyzed by enzymes.
, or speeds up, a
comes from ένζυμο, énsymo, which comes from én ("at" or "in") and simo
because most chemical reactions in
would occur too slowly, or would lead to different products, without enzymes. A
malfunction (mutation, overproduction, underproduction or deletion) of a single critical
enzyme can lead to a severe disease. A mutation could occur in any part of the
caused by an enzyme malfunction in the enzyme
catalyses the first step in the degradation of phenylalanine. If this enzyme does not
function, the resulting build-up of phenylalanine leads to
Like all catalysts, enzymes work by lowering the ion, thus
allowing the reaction to proceed much faster. Enzymes may speed up reactions by a
factor of many millions. An enzyme, like any catalyst, remains unaltered by the
completed reaction and can therefore continue to function. Because enzymes, like all
catalysts, do not affect the relative energy between the products and reagents, they
of a reaction. However, the advantage of enzymes
compared to most other catalysts is their sterio-, regio- and chemoselectivity and
It is possible that through the intervention with this supplement the body own energy
stores and enzymestores are filled up and optimized. We conclude that the
performance on submaximum steps (aerobic metabolic pathways) did increase
through an optimization of enzymatic capacity reserves.
Not investigated was in this study the effect of supplementation on the regeneration.
Probably it is possible to get a positve effect. When oxygen is used in the
mitochondriens 5 % in the oxidative transport chain can not be converted and have to
rest as scavengers. Antioxidants can not enhance directly the performance, but they
can influence the rate of muscle damage as enzymatic part of antioxidative systems.
Alcalic salts (cleaning substrates) are regulating the base-access homäostases.
They are important for the buffering of lactate, which is produced in anaerobic
glucolyses from muscle glycogen (Schek 1995, S. 243).
With the production of lactate also hydrogen ions accumulate in the muscle cell. The
effect of the supplement was that on submaximum steps, the lactate did not increase
and the base-access did not lower. So on submaximum steps (aerobic metabolism)
the rate of acidity can be reduced significantly. The supplement has also its limits:
Beyond the aerobic (2.0 mmol/l lactate) threshold there was not any ergogenic effect
measurable. We can conclude that the investigated micro nutrient supplement has a
positive ergogenic effect on relevant physiological parameters.
5 Summary
Different studies demonstrated that there is a strong relationship between the vitamin
status and the physiological performance (vgl. Berg 1996 et al. S. 316, Keul 1987 et
al. S.884). All those studies conclude that there is an excess offer of macronutrients
(carbohydrates, protein, fat) and parallel a deficiency of micronutrients (vitamins,
minerals, trace elements). This can lead to a "latent" deficiency. "Latent" means that
through blood-analyses the deficiency can not be verified. This inconspicuous
deficiencies (from 10 – 25 % of the ideal body own nutritient-store) causes a drastic
reduction of the enzyme capacity from up to 50 % (vgl. Fuchs 2001, S. 35).
The available sportsdrinks are trying only to substitute the lost nutrients. The goal of
this studie was to go one step further and not only to substitute, but also to
supplement with vitamins, trace elements, minerals, alcalic salts the body own
enzyme and energy stores that there could be an ergogenic effect on sports
At first we have explained the different kinds of ATP-replenishment in the muscle cell.
Then our goal was to explain the biochemical effect of the micronutritient supplement.
This was important to give us an impression of the interdependence of energy
metabolism and micronutrients. The science about an ergogenic effect of
micronutrient supplements is divided. 50 % of the studies certificate a positive effect
and the other 50 % alleged the contrary (vgl. Studies of Keul 1987 et al., S. 884 and
Noakes, T. 1988, S. 192).
30 female test persons were involved in this placebo controlled double blind study in
the initial test. At the beginning the body composition was anaylized an then they
executed a maximum test on the cycle ergometer. Parallel to this were measured
relevant physiological parameters (lactate, heart rate, maximum-oxygen intake).
The criteria for remaining in the group was that the test person don t have a higher
maximum intake than 38 ml//kg/min (vgl. Grosser 1998, S. 126).
Then the intervention with the daily supplementation of 20 g of the verum and
placebo started. The test persons were not allowed to participate on a systematic and
controlled training to enhance their performance. A daily compliance of 80 % was the
precondition for the approval for the second test. After the second test the results
were compared and analyzed.
The results of the body composition and the performances on the cycle ergometer on
submaximum and maximum steps was set in dependence with the relevant
physiological parameters (heart rate, lactate, VO2 max) and featured in diagram description. Subsequent to this the analytical statistic was performed. With the
Kolmorogov-Smirnov test the results were tested for their normal distribution. Then a
onefactorial variance analysis determined the global significance, and in
consequence the t-Test for dependent samples was performed. The most important
- significant change (verum group) of the watt performance on 1.0
- significant change (verum group) of the watt performance on the
aerobic threshold (2.0 mmol/l lactate)
- significant change of the V02 max (verum group) - significant higher heart rate (verum group) at 2.0 mmol/l lactate
- significant changes of different parameters of the body composition
4. Interpretation
In the dominant aerobic energy metabolism the verum supplemented group could
enhance their perforamance on 1.0 and 2.0 mmol/l lactate significantly. This can be
the expression of optimized enzyme capacities and be considered as a sustainable
A negative influence in the cardial system at 2.0 mmol/l was measured. The heart
rate was significantly higher (9 bpm). This circumstance should be related because
the heart rate is a very sensitive parameter, which is influenced by very many factors
(temperature, psycho-physical condition, individual pressure to perform,….)
Positive and statistically significant changes where measured in the oxygen intake of
the verum supplemented group. This is the expression that the oxygen transporting,
intaking and converting system of the organism has been optimized. The phase
angel gives information about the nutrition condition of the cells (BIA). This angel was
increased significantly in the verum group. Equally the BCM (body cell mass) was
increased significantly. The BCM includes the potassium containing metabolism
So we can conclude, that the supplementation with this basic micronutrient
supplement has beside the optimization of the quality of the cell substance also a
positive ergogenic effect in sports performance.
6 Bibliography
Anderson, R. (1988). Trace minerals and exercise. In: Exercise, Nutrition and Energy
Metaboism. New York: Macmillan.
Appell, H. (1992). Messen und Erfassen sportlicher Leistungsfähigkeit. Schorndorf:
Karl Hofmann-Verlag.
Astrand, P.-O. (1993). Ausdauer im Sport. Eine Veröffentlichung des IOC in
Zusammenarbeit mit der FIMS. Köln: Deutscher Ärzte-Verlag.
Bereiter, K. (2001). Die Zelle. Membranpotential und Energiebereitstellung. CD-Rom.
Berg, A. (1996) et al. Sport und Ernährung 1996. In: Aktuelle Ernährungs-Medizin.
Nr. 21. S. 315 – 322. Stuttgart: Georg Thieme Verlag.
Bergmüller, H. (2003). Das Hermann Maier Trainingsprogramm. St. Pölten: NP-
Brubacher, G. (1988). Bestimmung von Vitaminen und Beurteilung von Normwerten.
In: Vitamine und Leistung, 1988, S. 15 – 25.
Diebschlag, W. (1991). Leistungskontrolle bei Ausdauersportlern. Blutlaktat als
Indikator. Stuttgart: Schwer-Verlag.
Dietl, H. (1998). Handbuch der Orthomolekularen Medizin. Prävention und Therapie
durch körpereigene Substanzen. Heidelberg: Haug-Verlag.
Dietl, H. (1998). Handbuch der Orthomolekularen Medizin. Prävention und Therapie
durch körpereigene Substanzen. Heidelberg: Haug-Verlag.
Deutsche Apotheker Zeitung (1997). Nr 21. S. 34.
Döll, M. (1996). Körperliche Belastung und sportliche Aktivitäten – erhöhter oxidativer
Streß durch freie Radikale. In: Journal für Orthomolekulare Medizin. Nr. 4. S. 317.
Evans, G. (1993). Chromium picolinate is an efficacious and a safe supplement. In:
Int. J. Sport Nutr. Nr 3. S. 117.
Fuchs, N. (2001). Mit Nährstoffen heilen. Eine Einführung in die komplexe
Orthomolekulare Nährstoff-Therapie. Köln: Ralf Reglin Verlag.
Glaesel, K. (1994). Heilung ohne Wunder und Nebenwirkungen. Gesundheit
biologisch gesteuert. Konstanz: Labor-Glaesel-Verlag.
Grosser, M. (1998). Das neue Konditionstraining für alle Sportarten. München: BLV-
Hamm, M. (1990). Handbuch Sportlerernährung. Hamburg: Rowohlt-Verlag.
Handbuch (2002) – Grundlagen der Bioelektrischen Impendanz Analyse (B.I.A.) Data
Heck, H. (1990), Laktat in der Leistungsdiagnostik, Schorndorf: Karl
Heck, H. (1990). Energiestoffwechsel und medizinische Leistungsdiagnostik.
Schorndorf: Hofmann-Verlag.
Jansen, P. (1996), Ausdauertraining, Balingen: Perimed-Spitta
Karlson, P. (1994). Biochemie für Mediziner und Naturwissenschaftler. Stuttgart:
Keith, R. (1997). Ascorbic Acid. In: In: Wolinsky, I. Sports Nutrition. Vitamins and
Trace Elements. Boca Raton: CRC Press.
Keul, J. et al (1987). Zur Wirkung von Vitaminen und Eisen auf die Leistungs- und
Erholungsfähigkeit des Menschen und die Sportanämie. In: Ernährungswissenschaft,
Band 26 Heft 1, S. 21 – 42.
Kußmaul, B., Döring, A. und Filipiak, B. (1996): Bioelektrische Impedanz- analyse
(BIA) in einer epidemiologischen Studie.Ernährungs-Umschau 43,46-48
Koch, H. (2003). Österreichisches Journal für Sportmedizin 04/2003.
Löffler, (1998). Basiswissen Biochemie mit Pathobiochemie. Berling: Springer Verlag.
Lukaski, H. (1995). Micronutrients (magnesium, zing and copper): are mineral
supplements needed?. In: Int. J. Sports Nutr. Nr. 5. S. 74.
Neumann, G. (2001) et. al. Optimiertes Ausdauertraining. Aachen: Meyer & Meyer
Mindell, E. (1985). Die Vitaminbibel. Bausteine für ein gesundes langes Leben.
München: Heyne Verlag
Manore M. (2000) et al. Sports nutrition for health and performance. Champaign:
Matter, M (1987) et al. The effect of iron and folate therpy on maximal exercise
performance in female marathon runners with iron folate deficience. Clin. Sci. Nr 72.
Moshen, M. (1997) et al. Vitamin E. In : In: Wolinsky, I. Sports Nutrition. Vitamins and
Trace Elements. Boca Raton: CRC Press.
Noakes, T. (1988) et al. Vitamin and mineral supplementation : effect on the running
performance of trained athletes. In: Am. J. Clinical Nutrition Nr. 47. S192 – 195.
National Cancer Institute (1996). Beta Carotene and Vitamin A Haltet in Lung Cancer
Prevention Trial, Pressemttteilung vom 18.01.
Pansold, B. (1977). Leistungsphysiologische Untersuchung unter besonderer
Berücksichtigung des Informatiosngehaltes der Laktatkonzentration im Blut an
Leistungssportlern der Sportart Schwimmen. Dissertation A. E.-M.-Arndt-Universität.
Pansold, B. (1985). Untersuchungen zur komplexen Leistungsbewertung unter
Berücksichtigung der Spezifität des Testverfahrens an ausgewählten Gruppen und
Probanden von Leistungssportlern. Dissertation B. E.-M.-Arndt-Universität.
Pansold, B. (1993). Leistungsdiagnostik mit Latktat und Mini 8. Handbuch – Dr.
Lange LOX-PAP-Methode/Miniphotometer 8.
Pansold, B. (1994) et al. Die Laktat-Leistungskurve – ein Analyse- und
Interpretationsmodell. In: Clasing, D. et al. Stellenwert der Laktatbestimmung in der
Leistungsdiagnostik. Stuttgart : Gustav Fischer Verlag.
Patenschrift (2001). Basenhältige Mikronährsttmischung. Ökopharm Forschungs-
und Entwicklungs- GmbH, Moosham, Unternberg (AT).
Powers, S. (2001). Applied physiology: theory and application to fitness and
performance. Boston: Ullstein-Mosby-Verlag.
Riedl, T. (2001). Supplementierung & Substitution. Leistungssteigerung im
Breitensport. In: Österreichische Apotheker Zeitung. Nr 15. S. 710.
Rokitzki L. (1989). Ernährung, Vitamin-Substitution und sportliche Leistungsfähigkeit.
In: Ernährung und Vitamine, 1989, 9, S. 1 – 6.
Röthig, P. (1992) et al. Sportwissenschaftliches Lexikon. Schorndorf: Verlag K.
Scheck, A. (1995). Ernährungsbezogene Leistungsförderer versus
leistungsbezogene Ernährung. In: Ernährungs-Umschau Heft 7.
Seshadri, S. (1984) et al. The effect of hematicincs on the pysical work capacity in
anemics. In: Indian Pediatr. Nr 21. S 529.
Schulz, R. (1994). Laktat und seine Tauglichkeit als leistungsdeterminierender
Parameter. Seminararbeit. Wien.
Smekal, G. (2000) et al. Das « Kraftwerk » Mensch. Energiebereitstellung und
Energiebevorratung unter besonderer Berücksichtigung von
Langzeitausdauerbelastungen. In: Österreichisches Journal für Sportmedizin Nr. 3.
Stroh, S. (1995): Methoden zur Erfassung der Körperzusammensetzung.
Ernährungs-Umschau 42, 88-94
Stryer, L. (1999). Biochemie. Braunschweig: Spektrum-Verlag. Thomas, E. (1997). Pantothenic Acid and Biotin. In: Wolinsky, I. Sports Nutrition.
Vitamins and Trace Elements. Boca Raton: CRC Press.
Weineck, J. (2002). Optimales Training.11. Auflage. Balingen: Spitta – Verlag.
Weltman, A. (1995). The blood lactate response to exercise. Champaign: Human
Williams M. (1997). Ernährung, Fitness und Sport. Deutsche Ausgabe
Williams M. (1998). The ergogenics edge: pushing the limits of sports performance.
Champaign: Human Kinetics.
Wolinsky, I. (1997). Sports Nutrition. Vitamin and Trace Elements. Boca Raton: CRC
Zintl, F. (1994), Ausdauertraining, 3. Auflage, München: BLV.
Zintl, F. (2001). Ausdauertraining. Grundlagen – Methoden – Trainingssteuerung.
7 Appendix
Source: http://www.happylonglife.at/images/sport/background_thesis_powersports.pdf
Autumn 2015– News and information from the Department of Dermatology Letter from our Chairman"If I have seen further, it is by standing on the UConn Dermatology shoulders of giants." – Isaac Newton, 1676. granD roUnDs, 8 am Over 35 years Dr. Jane Grant-Kels has built a city in Farmington. Avenues October 7, November 4
ORIGINAL SUBMISSION Spirulina platensis GRAS self affirmation 7-1-2011 Executive Summary The objective of this Generally Recognized as Safe (GRAS) determination is to summarize the available safety information on Spirulina platensis, which is used as an ingredient in foods and beverages. We, the undersigned expert panel members, Susan Cho, Ph.D., Joanne Slavin, Ph.D., and George C. Fahey, Jr., Ph.D., have individually and collectively critically evaluated the materials summarized in the Spirulina platensis GRAS report. We conclude that Spirulina platensis is safe and GRAS for its intended use in food. There is broad-based and widely disseminated knowledge concerning the chemistry and health benefits of Spirulina platensis in both human and animals. Pursuant to 21 CFR § 170.30, this GRAS determination for Spirulina platensis is based on scientific procedures. There are no indications of significant adverse effects related to Spirulina platensis in the publicly available literature, and the manufacturing process of Spirulina platensis does not employ any treatments with organic solvents. In the United States, Spirulina platensis has been already recognized as a GRAS substance since 2003 (FDA, GRN 000127). Since that time, several toxicity and human clinical studies have been published to report higher values of safe intake levels than the previously reported. This GRAS notice captures the findings from recent studies.









