Doi:10.1016/j.antiviral.2006.05.002

Antiviral Research 71 (2006) 154–163
Antiviral drugs for cytomegalovirus diseases
Department of Clinical Virology, Division of Virology, GlaxoSmithKline Inc., RTP, NC, United States
Received 15 March 2006; accepted 4 May 2006
Dedicated to Prof. Erik De Clercq on the occasion of reaching the status of Emeritus-Professor
at the Katholieke Universiteit Leuven in September 2006
Cytomegalovirus infections are associated with severe morbidity and mortality is patients at risk for disease because of immune system disabilities;
in particular, recipients of stem cell (HSCT) or solid organ (SOT) transplants. There are three systemic drugs approved for CMV treatment:ganciclovir, or its prodrug valganciclovir, foscarnet, and cidofovir. An anti-sense therapeutic, ISIS 2922, is also approved specifically as inintravitreal treatment for CMV retinitis. Ganciclovir, and more recently, valganciclovir, have been useful in proactive approaches of CMV diseasemanagement; in both prophylactic and preemptive regimens in HSCT and SOT populations. The major anti-herpes agent valacyclovir has also beenapproved for prophylaxis of renal transplant recipients, or SOTs outside of the US. These drugs have provided major advances in CMV diseasemanagement, although they are limited by intolerable toxicities, oral bioavailability and efficacy, and risk of drug resistance with extended use.
Several drugs are in early clinical development which may address these limitations; this review will provide an overview of our current arsenalof available drugs, and of those in the early clinical development pipeline.
2006 Elsevier B.V. All rights reserved.
Keywords: CMV; Nucleoside analog; Nucleotide analog; Pyrophosphate analog; Novel benzimidazole riboside antiviral drugs; Alkoxyalkyl esters of cidofovir;Ganciclovir; Valganciclovir; Valaciclovir; Foscarnet
∗ Present address: GlaxoSmithKline, 5 Moore Drive, RTP, NC 27709, United States. Tel.: +1 919 483 9310; fax +1 919 315 5243.
E-mail address:
0166-3542/$ – see front matter 2006 Elsevier B.V. All rights reserved.
doi:
K.K. Biron / Antiviral Research 71 (2006) 154–163
Human cytomegalovirus (CMV) is an opportunistic pathogen
3. CMV-associated disease in transplant recipients
associated with significant morbidity and mortality in suscep-tible populations; i.e. those with immature or immunocom-
CMV infection is the leading viral cause of morbidity and
promised immune systems. Numerous antiviral agents with in
mortality facing patients who receive hematopoietic stem cell
vitro activity against the various human herpesviruses have been
transplant (HSCT) or solid organ transplant (SOT), with both
described over the past three decades, yet only a few have been
direct adverse effects resulting from viral invasion of organ sys-
approved for the treatment or prophylaxis of CMV diseases. This
tems and indirect effects on the immune systems that increase
article will provide an overview of the diseases caused by this
the risk of other infections and promote acute graft rejection
ubiquitous virus and a description of approved drug products,
(reviewed in CMV viremia is a
and will briefly describe several drug candidates that are in early
significant predictor for organ involvement and progression to
stages of clinical development.
Risk of CMV-associated complications is increased with
1. CMV infection and CMV disease
more potent immunosuppressive regimens, such as many ofthose required for HSCT, and transplant patients are at great-
CMV is a double-stranded DNA virus of approximately
est risk for CMV-associated disease within the first 100 days
220 kb and is a member of the beta class of human herpesviruses.
post-transplant. For recipients of SOTs, the most vulnerable
Cytomegalovirus is easily transmitted, usually through contact
patients ("high-risk patients") are CMV-seronegative recipients
with bodily fluids or by placental transfer. Seroprevalence rates
who receive an organ from a CMV-seropositive donor (D+/R−).
vary by socioeconomic class and geographic location, but the
CMV-seropositive recipients of allogeneic stem cell transplants
overall seroprevalence in developed countries is estimated to
are at risk for reactivation of latent CMV infection.
be in the range of 30–70% (Primary infection
In high-risk patients without symptomatic CMV disease, two
in immunocompetent individuals is usually benign, with mini-
common strategies of disease management are prophylactic and
mal or no clinical manifestations (although approximately 10%
preemptive therapy, both of which are designed to prevent CMV
of mononucleosis syndromes are a result of CMV infection).
disease. In the prophylactic approach, therapy is usually initiated
Following primary infection, the virus establishes latency, and
at the time of stem cell engraftment or solid organ transplant.
viremia is mainly controlled by cell-mediated immunity. Virus
The suppressive doses used for prophylaxis are generally lower
reactivation occurs when this protective immune surveillance
than those instituted for induction treatment of active disease,
fails; e.g. as a result of chemotherapy or in patients who have
and the suppression of CMV reactivation in specific transplant
AIDS or who are immunosuppressed for transplantation pur-
populations can be successfully accomplished with a less potent
poses. Such reactivation or primary infection in the context of a
antiviral agent than would be used for treatment. In the pre-
disabled immune system can lead to overt disease. In the case of
emptive approach, therapy is initiated in asymptomatic high-risk
vertical transmission of CMV to the developing fetus, adverse
patients based on diagnostic test results indicating primary CMV
outcomes are most commonly associated with primary infec-
infection or reactivation of latent virus to a threshold level that
tion of the mother, although significant morbidity has also been
signals the potenial for disease escalation (blood CMV DNA
associated with secondary infection.
load by PCR or pp65 antigenemia). This latter strategy ofteninvolves intermittent therapy, creating conditions thought to pose
2. Congenital CMV infection
a greater risk of selection of resistant virus. However, this riskmay be balanced by the protective effect of restoration of T-
In developed countries, congenital CMV infection occurs in
cell responses to CMV afforded by the delay in treatment with
approximately 1% of live births. The majority of the cases are
potent antivirals, particularly with myelosuppressive agents. On
asymptomatic, but approximately 5–10% of infants with con-
the other hand, the longer duration of drug exposure in the pro-
genital CMV will have symptomatic disease, associated with
phylactic approach also poses risk of resistance emergence.
profoundly deleterious effects on the central nervous system(CNS), including microcephaly, intracranial calcifications, and
4. CMV retinitis in AIDS patients
ventriculomegaly. Prognosis for neonates with symptomatic dis-ease is poor, with a high likelihood of mental defecits, hearing
Although CMV retinitis is a relatively rare manifestation of
loss and psychomotor and perceptual handicaps (reviewed in
CMV disease in other immunocompromised populations, it is
the primary manifestation of CMV infection in patients with
It is now recognized that even asymptomatic congenital CMV
AIDS, usually resulting from reactivation of latent virus. CMV
is associated with increased risk of sensorineural hearing loss
retinitis is a disease characterized by progressive, necrotizing
(SNHL) (an observation that high-
retinitis that can lead to retinal detachment and blindness. Initial
lights the importance of identifying infants with congenital
symptoms are non-specific, but may include blurred or distorted
CMV infection and conducting periodic auditory assessments.
vision, floaters, light flashes, and loss of peripheral vision.
The morbidity and mortality associated with congenital CMV
CMV retinitis and other manifestations of CMV disease in
infection underscores the need for a vaccine to prevent CMV
individuals with HIV-1 infection are opportunistic infections,
infection. CMV vaccines currently in preclinical and clinical
occurring when CD4+ cell counts are profoundly suppressed
development are reviewed in
(e.g. <50 cells/l). Since the advent of highly active antiretro-
K.K. Biron / Antiviral Research 71 (2006) 154–163
viral therapy for treatment of HIV-1 infection, CMV retinitis is
(PCV). Ganciclovir has become the gold standard for manage-
a condition rarely seen in developed countries, although aymp-
ment of CMV diseases in the majority of patient settings. A
tomatic CMV viremia remains a significant risk factor for death
series of nucleotide analogs with broad activity across viruses
was discovered by DeClercq and colleagues; from this class,cidofovir (CDV) was evaluated for anti-CMV activity. Otherantiviral agents designed to exploit the unusual characteristics
5. Antiviral therapies for CMV
of the herpesviral DNA polymerases are the pyrophosphate ana-log phosphonoacetic acid (PAA) and its analog foscarnet (PFA),
The nucleoside analog class of compounds has historically
which have broad inhibitory activity across the herpesviruses.
provided the richest source of antiviral agents, originating from
These agents have facilitated the management of CMV infec-
basic cancer research programs into purine and pyrimidine
tions; the next section of this review will highlight their thera-
metabolic pathways. These nucleoside analogs have been highly
peutic applications and advantages based on key pivotal studies
successful due to the potential for chemical diversity within the
and experience in broader clinical practice.
class, and the differentiation of target viral DNA polymerasesor reverse transcriptases from host enzymes. The herpesviral-encoded nucleoside kinases (HSV, VZV and EBV thymidine
6. Currently marketed antiviral agents
kinases, and the CMV protein kinase) provided added selectivityin the initial phosphorylation of the various nucleoside analogs.
Three of the antiviral agents mentioned earlier, GCV, CDV,
The triphoshorylated forms ultimately served as competitive
and FOS have received marketing approval for the
inhibitors of, and substrates for, the viral DNA polymerases, thus
systemic treatment of CMV infection. ACV has also received
reducing the amount of viral DNA synthesized in infected cells.
marketing approval in various European countries for prophy-
Nucleoside analogs with variable anti-tumor cell activity,
laxis of CMV disease in solid organ transplant (SOT) recipients,
notably adenosine arabinoside (ara A), cytosine arabinoside
but lacks sufficient potency to be used for treatment of active
(ara C), and trifluorothymidine (TFT), were among those first
CMV disease. An anti-sense RNA (fomivirsen) is approved for
described to have anti-HSV and anti-VZV activity. Acyclovir (9-
local treatment of CMV retinitis by intraocular injection.
[(2-hydroxyethoxy)methyl]guanine) was the first really selec-tive nucleoside analog (with potent activity
6.1. Ganciclovir
against HSV 1 and 2, VZV and EBV, and moderate activityagainst CMV in vitro. Acyclovir (ACV) and its prodrug, the l-
GCV was the first antiviral agent approved for treatment of
valyl ester valacyclovir, have become the standard of care for
CMV disease, and remains the first-line treatment for CMV
prophylaxis and treatment of the most common diseases caused
infection and CMV disease in transplant recipients
by HSV and VZV, and both have provided benefit in CMV dis-
GCV is an acyclic nucleoside analogue of
eases in certain transplant populations.
2�-deoxyguanosine (In a multi-step process dependent
The discovery of ACV was quickly followed by discovery
on both viral and cellular enzymes, ganciclovir is converted
of related purine analogs ganciclovir (GCV) and penciclovir
to ganciclovir triphosphate, the chemical form that is active
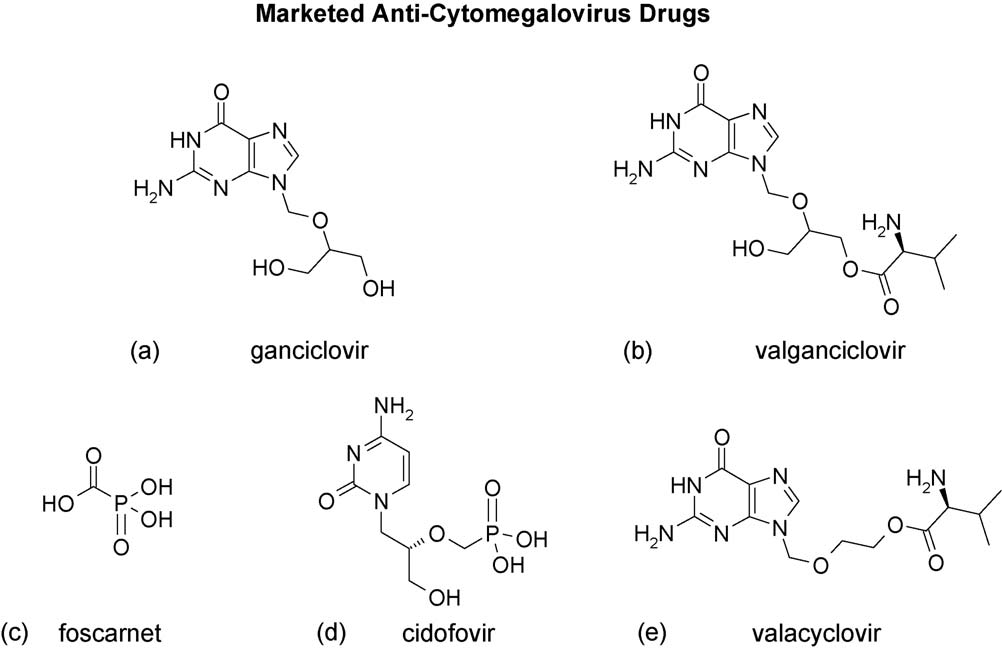
Fig. 1. Approved anti-CMV drugs. (a) Ganciclovir; (b) valganciclovir; (c) foscarnet; (d) cidofovir; (e) valacyclovir. Not shown is fomivirsen.
K.K. Biron / Antiviral Research 71 (2006) 154–163
against CMV. The initial phosphorylation is catalyzed by an
1994 for treatment of CMV retinitis, but only as maintenence
unusual protein kinase homolog encoded by the CMV UL97
therapy, as the low bioavailability (approximately 5%) of the oral
open reading frame (Cellular enzymes
formulation was considered insufficient for induction therapy.
generate the triphosphate form. Ganciclovir triphosphate com-
A sustained-release GCV intraocular implant (Vitrasert®;
petitively inhibits DNA synthesis catalyzed by the viral DNA
developed by Chiron, now marketed by Bausch and Lomb) for
polymerase (encoded by the UL54 gene), with slower chain
treatment of CMV retinitis in AIDS patients was approved in
elongation resulting from incorporation of ganciclovir triphos-
1996. In a clinical study comparing the implant to IV GCV in
phate in place of dGTP into the growing viral DNA chain.
HIV-1-infected patients with CMV retinitis, the implant was
Resistance to GCV arises from mutations in either the UL97
significantly more efficacious in treating CMV retinitis in the
or the UL54 genes. The point mutations or small deletions in the
affected eye, but patients treated with the implant alone were
UL97 protein kinase gene lead to changes at codons 460 or 520,
at significantly greater risk for developing CMV disease in the
or changes clustered in codons 590–607 (regions attributed to
contralateral eye or in other organ systems than were patients
ATP-binding and substrate recognition, respectively) that appar-
who received systemic GCV (The addition
ently do not prevent the protein kinase function (
of oral ganciclovir to the implant controlled the systemic CMV
The resistance mutations associated with GCV
in the pol gene UL54 generally occur in specific conserved
Oral GCV represented a major advance in treatment options
subdomains, and may confer cross-resistance to CDV or less
for maintenence therapy and prophylaxis. However, the low
commonly, to FOS (
bioavailability and the high pill burden from the t.i.d. regi-
The side effects of GCV include hematologic abnormali-
men were limitations. In addition, there were concerns that
ties (primarily neutropenia, anemia, and thrombocytopenia) and,
inadequate viral suppression resulting from the lower systemic
based on preclinical toxicologic studies, probable long-term
exposure from oral GCV could lead to emergence of drug resis-
reproductive toxicity (In
tance. Development of prodrugs has been one valuable strategy
animal studies GCV was both carcinogenic and teratogenic and
in circumventing problems of poor solubility or low bioavail-
ability (reviewed in Based on the
GCV as an intravenous (IV) formulation (Cytovene-IV®,
model exemplified by valacyclovir (see below), a prodrug was
Roche) was approved in 1989 for treatment of CMV retinitis
developed to improve the bioavailability of oral GCV.
in AIDS patients. The IV formulation was later approved for
Valganciclovir (Valcyte®, Roche) is the l-valyl ester of gan-
prevention of CMV disease in SOT recipients and in individu-
ciclovir (After oral administration, valganciclovir is
als with advanced HIV infection at risk for CMV disease. The
rapidly metabolized to the active form (ganciclovir) in the intesti-
pivotal clinical trials for the transplant indication included two
nal wall and liver. Valganciclovir has an oral bioavailability of
double-blind, placebo-controlled trials evaluating the incidence
around 60% Once-daily admin-
of CMV disease in D+/R− or D+/R+ heart transplant recipients
istration of valganciclovir 900 mg produces systemic exposure
(or in allogeneic bone marrow transplant
to GCV that is equivalent to that produced with once-daily
recipients with positive CMV cultures
administration of IV GCV 5 mg/kg, and 1.7-fold greater than
In the heart transplant study, patients were given study drug
the systemic exposure produced by oral GCV 1000 mg given
for 28 days post-transplant. The incidence of CMV illness at
t.i.d. (In principle, the val-
post-transplant day 120 was assessed, with the results strati-
ganciclovir formulation could be used to deliver the exposures
fied based on the recipient's serostatus. For seropositive recip-
demonstrated to be efficacious in various transplant populations
ients, significantly fewer patients given GCV had CMV illness
treated with IV or high-dose oral GCV, barring absorption defi-
during the first 120 days post-transplant compared to patients
ciencies associated with GI disruption, as in graft-versus-host-
given placebo (5/56 patients, 9% versys 26/56 patients, 46%;
disease. Indeed, registrational studies and multiple comparative
p < 0.001). For D+/R− transplant recipients there was no sig-
trials have been reported (
nificant difference among treatment groups in the incidence of
Valganciclovir was approved in 2000 for treatment of
CMV disease during the first 120 days.
CMV retinitis in AIDS patients (reviewed in
In the bone marrow transplant study, patients were given
and was later approved for prophylactic treat-
study drug from the time of engraftment until 100 days post-
ment of CMV in certain SOT recipients. The first study of
transplant. The incidence of CMV disease occuring within the
valganciclovir for CMV prophylaxis in SOT recipients com-
first 100 days post-transplant was significantly less in the GCV
pared valganciclovir to oral GCV in a randomized, double-blind,
arm (1/37 patients, 3%) compared to the placebo arm (15/35
double-dummy study of 364 D+/R− SOT recipients (
patients, 43%; p < 0.00001). In addition, patients receiving GCV
Patients were randomized 2:1 to receive valganciclovir
had significantly greater overall survival than the placebo group
900 mg once-daily or oral GCV 1000 mg t.i.d. for 100 days post-
at both 100 and 180 days post-transplantion (p = 0.041 and 0.027,
transplant. The study was designed to show equivalence, rather
than superiority of valganciclovir compared to oral GCV. The
To circumvent the risks and inconvenience associated with
incidence of CMV disease by 12 months were comparable in the
the need for an indwelling catheter for intravenous administra-
two treatment groups, and the safety profiles were similar
tion, an oral formulation was developed. Oral GCV (250 and
Valganciclovir has now replaced oral ganciclovir
500 mg GCV capsules; Cytovene®, Roche) was approved in
in clinical practice.
K.K. Biron / Antiviral Research 71 (2006) 154–163
Debate continues over the most appropriate antiviral treat-
disorders, including seizures, that in several cases resulted in
ment for prevention of CMV disease in high-risk SOT recipients
(D+/R−) and whether prophylactic therapy or preemptive ther-
FOS is considered second-line therapy, but is the preferred
apy should be used for asymptomatic high-risk patients
drug for patients who are failing GCV therapy due to viral
In one study late-onset CMV disease was more preva-
resistance, or those who cannot be treated with GCV due to
lent in in D+/R− liver transplant recipients who had received
dose-limiting neutropenia or leucopenia
prophylaxis versus preemptive therapy, suggesting that perhaps
In one study, FOS was compared to IV GCV as a pre-
prophylactic treatment with a potent antiviral agent interfered
emptive therapy in a large, prospective, randomized, open-label
with development of cell-mediated immune response
study in HSCT patients. FOS and IV GCV were equally effective
Similar outcomes have been reported in HSCT popula-
in prevention of CMV disease and mortality within 180 days of
tions (The International
HSCT FOS has also been used in combi-
Herpes Management Forum issued a set of guidelines in 2004
nation with IV GCV, each at half dose, and the combination was
for the use of ganciclovir and valganciclovir as either prophylac-
compared to IV GCV alone in SOT patients. The outcome was
tic or preemptive therapy in SOT and HSCT patients (
unfavorable for the combination in terms of virologic response
This consensus opinion provided recommen-
dations for quantitative monitoring of CMV load to optimize thetiming, duration and intensity of therapy. The guidelines also
6.3. Cidofovir
compare the efficacy of several regimens for first- and second-line viral control, as well as for treatment of established disease.
Cidofovir (Vistide®, Gilead) is an acyclic nucleoside phos-
To date (2006), no anti-CMV agent has been approved for
phonate, with the chemical name 1-[(S)-3-hydroxy-2-(phos-
treatment of congenital CMV disease, although the Collabora-
phonomethoxy)propyl]cytosine dihydrate (HPMPC,
tive Antiviral Study Group (CASG) of the National Institute of
CDV is a broad-spectrum antiviral agent with potency against
Allergy and Infectious Diseases has conducted Phase II and III
both herpesviruses and other DNA viruses, such as smallpox
trials evaluating twice-daily dosing of 6 mg/kg IV GCV for treat-
virus (Host kinases convert CDV to
ment of infants with symptomatic congenital CMV involving
the active diphosphoryl form, and cidofovir disphosphate then
the CNS. Study results showed clear benefits that for neonates
acts as a competitive inhibitor of the viral DNA polymerase,
with severe CMV disease outweighed the risks of acute tox-
causing premature chain termination in viral DNA synthesis.
icities and long-term reproductive toxicities associated with
Resistance to CDV has been difficult to select in the laboratory
intravenous ganciclovir (
(Resistant isolates have not been reported
A large multicenter trial is under-
in the clinic, although this may reflect the shorter treatment reg-
way to assess the safety and efficacy of valganciclovir syrup on
imens and the limited use of this agent. Several GCV-resistant
infants with symptomatic congenital CMV disease. However,
CMV clinical isolates or laboratory-selected strains with specific
while the oral formulation would avoid the considerable risks
pol gene mutations are clearly cross-resistant to CDV (
and disadvantages associated with IV administration, the hema-
tologic and reproductive toxicities would remain, limiting the
CDV received US marketing approval in 1996 for treatment
usefulness of the therapy for all but the most severely affected
of CMV retinitis in AIDS patients. CDV is available only as an
IV formulation; its oral bioavailability is less than 5%. One ofthe distiguishing features of CDV, and others in this nucleotide
6.2. Foscarnet
analog class, is the stability of the active form in cells. Theintracellular half-life of CDV-DP is reported to be >24 h, and
In 1991 foscarnet (Foscavir®, AstraZeneca) became the sec-
efficacy in both animal models and in humans can be achieved
ond drug approved for treatment of CMV retinitis in AIDS
with infrequent dosing (Recom-
patients. Foscavir®, or foscarnet sodium, is the trisodium salt
mended treatment for CMV retinitis in AIDS patients consists
of phosphormophonic acid, a pyrophosphonate analogue. Fos-
of 5 mg/kg administered over a 1-h period once a week for two
carnet (FOS) inhibits activity of the viral DNA polymerase by
consecutive weeks (induction phase), followed by 5 mg/kg once
binding to the pyrophosphate binding site and blocking cleav-
every 2 weeks (maintenance phase).
age of pyrophosphate from the terminal nucleoside triphosphate
The major limitation of CDV as an antiviral agent is severe
added to the growing DNA chain. Resistance to FOS in the lab-
renal toxicity An anion trans-
oratory or clinical setting has been mapped to point mutations in
porter located in the convoluted proximal tubules binds to
the pol gene UL54. Cross-resistance has been observed between
CDVwith high affinity, leading to the accumulation of CDV in
GCV and FOS in several laboratory and clinical isolates with
the renal cortex The major route of drug elim-
both phenotypic and genotypic resistance.
ination occurs through the kidney. Patients receiving IV CDV
The major dose-limiting toxicity of FOS is renal impair-
must be given oral probenecid to protect against kidney failure,
ment, underscoring the importance of adequate hydration and
and must be prehydrated before infusion. Neutropenia is another
frequent monitoring of serum creatinine levels in patients receiv-
toxicity associated with CDV, and CDV was shown to be both
ing FOS. Mineral and electrolyte abnormalities resulting from
carcinogenic and teratogenic in preclinical toxicological studies
renal impairment can lead to a number of cardiac or neurologic
K.K. Biron / Antiviral Research 71 (2006) 154–163
Due to the risks of renal damage, CDV remains a second-line
Valacyclovir prophylaxis significantly
therapy. In the transplant setting, CDV has been used primary
reduced the incidence of CMV disease among transplant recip-
in preemptive strategies to rescue allogeneic HSCT patients
ients at 6 months post-transplant, at 1% versus 6% for seropos-
following therapy with GCV, FOS, or both drugs, with suc-
itive patients and 16% versus 45% for seronegative patients
cesses ranking from 62% to 66% In
randomized to valacyclovir versus placebo, respectively. Sim-
this same retrospective analysis, patients with established CMV
ilar results were seen in incidence of active CMV infection
disease were also treated, and 50% (of a total of 20 subjects)
and graft rejection. Although not potent enough for treatment
responded clinically and virologically. However, 25% of these
of established CMV disease, valacyclovir has been approved
patients experienced renal toxicity, which was irreversible in
in several countries for prophylaxis of CMV infection and
approximately half of the affected patients. More recently, sim-
CMV disease in renal or heart transplant recipients or SOT
ilar efficacy was seen following preemptive administration of
CDV in pediatric HSCT patients failing GCV or FOS, with rea-sonable success achieved in the management of renal function
6.5. Fomivirsen
in these patients (
The usual treatment regimen in these studies involves an
Fomivirsen (Vitravene®; developed by Isis Pharmaceuticals,
induction dosage of 1–5 mg/kg/week, followed by a mainte-
licensed to Novartis Ophthalmics) is a 21-nucleotide anti-sense
nance dose every other week. The toxicities of CDV or the
RNA (5�-GCG TTT GCT CTT CTT CTT GCG-3�), specifi-
adjunct probenicid limit the utility of this interesting and potent
cally targeted against the mRNA from the major immediate-
antiviral drug. The potential of CDV prodrugs to avoid renal
early transcriptional unit of CMV. Fomivirsen is administered
tubular uptake and concentration is under evaluation.
by intraocular injection. Fomivirsen was approved in 1998 asa second-line therapy for local treatment of CMV retinitis in
6.4. Acyclovir
AIDS patients. Recommended treatment consists of a 4-weekinduction phase, with a single injection every other week (i.e.
Acyclovir is an analogue of 2�-deoxyguanosine. Like GCV,
two doses), followed by a maintenence phase, in which a sin-
acyclovir must be phosphorylated in a multi-step process in the
gle injection is administered every 4 weeks. The most frequent
host cell to the active triphosphate form. The CMV-encoded pro-
adverse effect is ocular inflammation (uveitis), which can be
tein kinase pUL97 catalyzes the initial phosphorylation step of
managed by treatment with topic corticosteroids and by delay-
this purine analog, which, like GCV monophosphate, is subse-
ing additional injections.
quently di- and tri-phosphorylated by host kinases. ACV is a lessefficient substrate than GCV, which in part explains the lower
7. Anti-CMV drugs in clinical development
in vitro potency of ACV compared to GCV in CMV-infectedcells. Another factor that clearly differentiates ACV and GCV
New drugs, preferably in oral formutions, are needed for
is the four- to five-fold shorter half-life of ACV-TP compared
treatment of CMV disease, especially congenital disease in
to GCV-TP in infected cells, resulting in the lower intracellular
neonates. The currently approved systemic drugs have an unfa-
levels of the active ACV-TP. As with GCV, drug resistance to
vorable safety profile, with severe acute and long-term toxi-
ACV results from mutations in the viral DNA polymerase or
cities. Of special concern for a pediatric population is long-
term reproductive toxicity and carcinogenicity. Moreover, the
Oral acyclovir has approximately 6–10% bioavailability,
approved systemic drugs share a similar mechanism of action,
which increases to approximately 55% with administration
targeting the viral DNA polymerase. As a consequence, viral
of valacyclovir (Valtrex®, GlaxoSmithKline), the l-valyl ester
cross-resistance is a potential problem with the current drug
of acyclovir The primary use
of high-dose oral ACV or its prodrug valacyclovir in trans-
Despite the medical need for new drugs, relatively few
plant patients has been for suppression of HSV reactivation.
research programs currently focus on anti-CMV drug develop-
However, prophylactic treatment can also significantly reduce
ment. One reason may be the reduction in CMV retinitis in AIDS
the incidence of CMV infection and CMV disease in SOT
patients following the introduction of HAART.
patients. In a meta-analysis of 12 randomized trials enrolling1574 SOT patients, a 56% decrease
7.1. Maribavir
in the risk of CMV infection (p < 0.001), a 59% reductionin CMV disease (p < 0.001), and a 30% reduction in oppor-
One of the most promising anti-CMV drugs in clinical devel-
tunistic infections (p < 0.009) in patients receiving prophy-
opment is maribavir (1-(-l-ribofuranosyl)-2-isopropylamino-
lactic therapy with high-dose oral acyclovir or valacyclovir,
5,6-dichlorobenzimidazole), also known as GW1263W94
compared to patients receiving placebo or no prophylactic
Although this drug is a riboside analog, it does not act
as such: it is not anabolized in infected cells, nor do its phospho-
The safety and efficacy of valacyclovir for prevention of
rylated forms directly inhibit the viral DNA polymerase (
CMV disease was evaluated in 408 R+ and 208 R−/D+ renal
Maribavir is a potent and selective, orally bioavail-
transplant patients, randomly assigned to treatment with either
able drug with a novel mechanism of action against only two
2000 g valacyclovir or placebo q.i.d. for 90 days post-transplant
of the human herpesviruses: CMV and EBV. Maribavir inhibits
K.K. Biron / Antiviral Research 71 (2006) 154–163
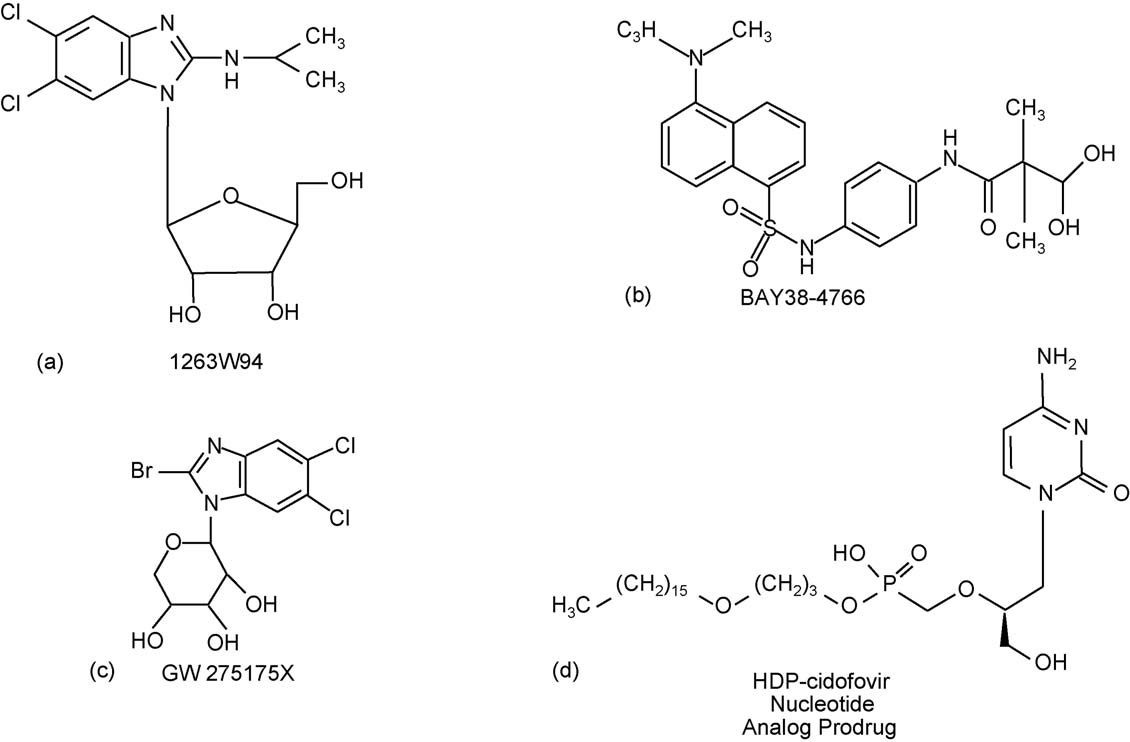
Fig. 2. Anti-CMV drugs in clinical development. (a) Maribavir; (b) BAY 38-4766; (c) GW275175X; (d) CMX001.
the replication of both CMV and EBV in cell culture by interfer-
7.2. BAY 38-4766
ing with viral DNA synthesis (In CMV-infected cells, maribavir has also been shown
BAY 38-4766 (Bayer Pharmaceuticals), or 3-hydroxy-2,2-
to interfere with viral nucleocapsid egress from the nucleus,
thereby reducing the yield of infectious CMV
no)-phenyl]propanamide, represents a novel class of non-
A key target for maribavir's action in the CMV life cycle
nucleoside antiviral agents. BAY 38-4766 is a highly selective
is the viral-encoded protein kinase, pUL97, a finding that was
inhibitor of CMV in vitro (
based on the genetics of resistance, direct protein kinase inhi-
In an immunodeficient mouse model the compound shows
bition studies (and on phenotypic similarity
anti-CMV activity against human CMV similar to that of GCV
of maribavir-treated, CMV-infected cells and cells infected with
(BAY 38-4766 had a favorable safety and
the pUL97-deleted virus (
efficacy profile in a guinea pig model of CMV, and measurable
Inhibition of viral DNA synthesis is likely a consequence of a
amounts of drug were detected in fetal blood, indicating that
block in the phosphorylation of the polymerase accessory pro-
the compound crosses the placenta in pregnant guinea pigs
tein, pUL44, by the pUL97 protein kinase (
BAY 38-4766 was active against strains resistant to currently
Clearly the mechanism of action of maribavir against CMV
approved anti-CMV agents and no
has not been fully elucidated, since the role of the pUL97 protein
cross-resistance to these agents was seen in virus selected for
kinase in CMV replication or disease pathogenesis is still under
resistance to BAY 38-4766 (Antivi-
study. Moreover, laboratory-generated resistant mutations also
ral activity of BAY 38-4766 results from inhibition of DNA
map to the CMV UL27 gene, a protein of unknown function
maturation, and mutations conferring drug resistance map in the
UL89 and UL56 genes, which encode subunits of the viral termi-
Maribavir preclinically shows advantages over existing anti-
nase (This mechanism of action is similar
CMV drugs in its in vitro potency, bioavailability, safety profile
to that of the original benzimidazole riboside leads, BDCRB and
in acute, chronic and genetic toxicology testing, and the lack of
cross-resistance inherent in its novel mechanism of action. The
vation that is interesting in view of the differences in structures
drug has completed several Phase 1 clinical studies
between these unrelated chemical series and the complexity of
and its potential for efficacy
the drug target.
was demonstrated in a 28-day study in HIV-infected subjects
BAY 38-4766 entered clinical development and showed a
that showed a reduction in viral shedding in the semen and urine
favorable safety profile in healthy male volunteers at single oral
This drug is currently in a prophylaxis
doses up to 2000 mg. However, no recent reports have revealed
study in allogeneic stem cell transplants, with results expected
the current status of clinical development of this compound or
related compounds in the series.
K.K. Biron / Antiviral Research 71 (2006) 154–163
7.3. GW275175X
drug has provided a reduction in the risk of diseases caused byother herpesviruses, as well as bacterial and fungal infections,
Another interesting clinical candidate to emerge from the
which could reflect the direct activity of the broad spectrum anti-
novel benzimidazole riboside class of CMV inhibitors is the -d-
herpetic, as well as the indirect benefits of suppressing CMV
pyranosyl sugar analog of the original leads BDCRB and TCRB
Additional progress in reducing the consequences of CMV
infection in all the susceptible populations will be made with the
benzimidazole), this molecule addressed the in vivo lability of
introduction of new drugs with greater efficacy and safety, and
the glycosidic linkage of BDCRB by substitution of the six-
long term management of infection will be facilitated by drugs
membered sugar ring for the -d-ribose moiety. GW275175X
with non-overlapping mechanisms of action.
retains the mechanism of action of the parent compoundBDCRB; that of blocking the maturational cleavage of highmolecular weight CMV DNA by interaction with pUL56
and pUL89, the two subunits of the viral terminase complex
Balfour Jr., H.H., 1979. Cytomegalovirus: the troll of transplantation [edito-
rial]. Arch. Intern. Med. 139, 279–280.
GW275175X was advanced through a Phase 1 single-escal-
Beadle, J.R., Hartline, C., Aldern, K.A., Rodriguez, N., Harden, E., Kern,
ating dose trial of safety, tolerability and pharmacokinetics, but
E.R., Hostetler, K.Y., 2002. Alkoxyalkyl esters of cidofovir and cyclic
was then shelved in favor of the advancement of maribavir. The
cidofovir exhibit multiple-log enhancement of antiviral activity against
clinical potential of this early candidate is yet to be determined.
cytomegalovirus and herpesvirus replication in vitro. Antimicrob. AgentsChemother. 46, 2381–2386.
Biron, K.K., Harvey, R.J., Chamberlain, S.C., Good, S.S., Smith III,
7.4. Cidofovir esters
A.A., Davis, M.G., Talarico, C.L., Miller, W.H., Ferris, R., Dornsife,R.E., Stanat, S.C., Drach, J.C., Townsend, L.B., Koszalka, G.W., 2002.
Renal toxicity associated with CDV treatment limits the use-
Potent and selective inhibition of human cytomegalovirus replication by
ful of the drug, despite its efficacy as an anti-CMV agent.
1263W94, a benzimidazole l-riboside with a unique mode of action.
Antimicrob. Agents Chemother. 46, 2365–2372.
However, recent reports describe a promising series of CDV
Boeckh, M., Leisenring, W., Riddell, S.R., Bowden, R.A., Huang, M.L.,
derivatives that overcome this limitation. Alkoxyalkyl esters of
Myerson, D., Stevens-Ayers, T., Flowers, M.E., Cunningham, T., Corey,
CDV have been developed that retain the efficacy of the parent
L., 2003. Late cytomegalovirus disease and mortality in recipients of
compound (without the associated renal
allogeneic hematopoietic stem cell transplants: importance of viral load
toxicity (Moreover, the derivatives showed
and T-cell immunity. Blood 101, 407–414.
Boivin, G., Goyette, N., Gilbert, C., Roberts, N., Macey, K., Paya, C.,
improved uptake and absorption, and had oral bioavailabilities
Pescovitz, M.D., Humar, A., Dominguez, E., Washburn, K., Blumberg,
in mice in the range of 88–97%, compared to less than 5% for
E., Alexander, B., Freeman, R., Heaton, N., Covington, E., 2004. Absence
of cytomegalovirus-resistance mutations after valganciclovir prophylaxis,
CMX001, or hexadecyloxypropyl-cidofovir (HDP-CDV), is
in a prospective multicenter study of solid-organ transplant recipients. J.
currently under development by Chimerix as an oral drug for the
Infect. Dis. 189, 1615–1618.
Bradford, R.D., Cloud, G., Lakeman, A.D., Boppana, S., Kimberlin, R.J.,
treatment of smallpox infection (
Demmler, G., Sanchez, P., Britt, W., Soong, S., Whitley, R.J., 2005.
The drug is also active against CMV and other herpesviruses.
Detection of cytomegalovirus (CMV) DNA by polymerase chain reaction
The evaluation of HDP-CDV in symptomatic congenital CMV
is associated with hearing loss in newborns with symptomatic congenital
infections is under consideration.
CMV infection involving the central nervous system. J. Infect. Dis. 191,227–233.
Buerger, I., Reefschlaeger, J., Bender, W., Eckenberg, P., Popp, A.,
8. Summary
Weber, O., Graeper, S., Klenk, H.D., Ruebsamen-Waigmann, H., Hal-lenberger, S., 2001. A novel non-nucleoside inhibitor specifically targets
Cytomegalovirus has been referred to as the "troll of trans-
cytomegalovirus DNA maturation via the UL89 and UL56 gene products.
plantation" (an apt description for an opportunis-
J. Virol. 75, 9077–9086.
tic pathogen that can produce such direct and indirect damage
Cesaro, S., Zhou, X., Manzardo, C., Buonfrate, D., Cusinato, R., Tridello, G.,
Mengoli, C., Palu, G., Messina, C., 2005. Cidofovir for cytomegalovirus
in the transplant setting. Significant progress has been made
reactivation in pediatric patients after hematopoietic stem cell transplan-
in the control of CMV infections in transplant patients using
tation. J. Clin. Virol. 34, 129–132.
the current arsenal of antiviral therapies, notably ganciclovir
Chou, S., Marousek, G.I., Senters, A.E., Davis, M.G., Biron, K.K., 2004.
and its prodrug, valganciclovir. Numerous clinical studies have
Mutations in the human cytomegalovirus UL27 gene that confer resistance
explored the optimal use of the five approved agents, and strate-
to maribavir. J. Virol. 78, 7124–7130.
Ciesla, S.L., Trahan, J., Wan, W.B., Beadle, J.R., Aldern, K.A., Painter,
gies for intervention have been shaped through a better definition
G.R., Hostetler, K.Y., 2003. Esterification of cidofovir with alkoxyalka-
of viral replication dynamics and the cor-
nols increases oral bioavailability and diminishes drug accumulation in
relation of viral load with disease progression. Broader use of
kidney. Antiviral Res. 59, 163–171.
prophylactic regimens has led to a greater occurence of late
Cihlar, T., Fuller, M.D., Cherrington, J.M., 1998. Characterization of drug
onset CMV disease, and the longer duration of drug treatment
resistance-associated mutations in the human cytomegalovirus DNA poly-merase gene by using recombinant mutant viruses generated from over-
in the face of potent immunosuppression has allowed resis-
lapping DNA fragments. J. Virol. 72, 5927–5936.
tant virus to emerge (
Cope, A.V., Sweny, P., Sabin, C., Rees, L., Griffiths, P.D., Emery, V.C.,
JID). On the other hand, prophylaxis with ganciclovir or its pro-
1997. Quantity of cytomegalovirus viruria is a major risk factor for
K.K. Biron / Antiviral Research 71 (2006) 154–163
cytomegalovirus disease after renal transplantation. J. Med. Virol. 52,
Lalezari, J.P., Aberg, J.A., Wang, L.H., Wire, M.B., Miner, R., Snow-
den, W., Talarico, C.L., Shaw, S., Jacobson, M.A., Drew, W.L., 2002.
Cvetkovic, R.S., Wellington, K., 2005. Valganciclovir: a review of its use in
Phase I dose escalation trial evaluating the pharmacokinetics, anti-human
the management of CMV infection and disease in immunocompromised
cytomegalovirus (HCMV) activity, and safety of 1263W94 in human
patients. Drugs 65, 859–878.
immunodeficiency virus-infected men with asymptomatic HCMV shed-
Cytovene-IV (ganciclovir sodium for intravenous infusion only) and Cytovene
ding. Antimicrob. Agents Chemother. 46, 2969–2976.
(ganciclovir capsules for oral administration only) package insert. Roche
Li, C.R., Greenberg, P.D., Gilbert, M.J., Goodrich, J.M., Riddell, S.R.,
Laboratories Inc., 2000.
1994. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell
Deayton, J.R., Sabin, C.A., Johnson, M.A., Emery, V.C., Wilson, P., Griffiths,
responses after allogeneic bone marrow transplant: correlation with CMV
P.D., 2004. Importance of cytomegalovirus viraemia in risk of disease
disease and effect of ganciclovir prophylaxis. Blood 83, 1971–1979.
progression and death in HIV-infected patients receiving highly active
Limaye, A.P., Corey, L., Koelle, D.M., Davis, C.L., Boeckh, M., 2000. Emer-
antiretroviral therapy. Lancet 363, 2116–2121.
gence of ganciclovir-resistant cytomegalovirus disease among recipients
De Clercq, E., Field, H.J., 2006. Antiviral prodrugs—the development of
of solid-organ transplants. Lancet 356, 645–649.
successful prodrug strategies for antiviral chemotherapy. Br. J. Pharmacol.
Ljungman, P., Deliliers, G.L., Platzbecker, U., Matthes-Martin, S., Baci-
147, 1–11.
galupo, A., Einsele, H., Ullmann, J., Musso, M., Trenschel, R., Ribaud,
De Clercq, E., Holy, A., 2005. Acyclic nucleoside phosphonates: a key class
P., Bornhauser, M., Cesaro, S., Crooks, B., Dekker, A., Gratecos, N.,
of antiviral drugs. Nat. Rev. Drug Discov. 4, 928–940.
Klingebiel, T., Tagliaferri, E., Ullmann, A.J., Wacker, P., Cordonnier, C.,
Elion, G.B., Furman, P.A., Fyfe, J.A., de Miranda, P., Beauchamp, L.,
2001. Cidofovir for cytomegalovirus infection and disease in allogeneic
Schaeffer, H.J., 1977. Selectivity of action of an antiherpetic agent,
stem cell transplant recipients. The Infectious Diseases Working Party of
9-(2-hydroxyethoxymethyl) guanine. Proc. Natl. Acad. Sci. U.S.A. 74,
the European Group for Blood and Marrow Transplantation. Blood 97,
Fiddian, P., Sabin, C.A., Griffiths, P.D., 2002. Valacyclovir provides opti-
Lowance, D., Neumayer, H.H., Legendre, C.M., Squifflet, J.P., Kovarik,
mum acyclovir exposure for prevention of cytomegalovirus and related
J., Brennan, P.J., Norman, D., Mendez, R., Keating, M.R., Coggon,
outcomes after organ transplantation. J. Infect. Dis. 186 (Suppl. 1),
G.L., Crisp, A., Lee, I.C., 1999. Valacyclovir for the prevention of
cytomegalovirus disease after renal transplantation. International Valacy-
Gandhi, M.K., Khanna, R., 2004. Human cytomegalovirus: clinical aspects,
clovir Cytomegalovirus Prophylaxis Transplantation Study Group. 1. N.
immune regulation, and emerging treatments. Lancet Infect. Dis. 4,
Engl. J. Med. 340, 1462–1470.
Marschall, M., Freitag, M., Suchy, P., Romaker, D., Kupfer, R., Hanke,
Gilbert, C., Boivin, G., 2005. Human cytomegalovirus resistance to antiviral
M., Stamminger, T., 2003. The protein kinase pUL97 of human
drugs. Antimicrob. Agents Chemother. 49, 873–883.
cytomegalovirus interacts with and phosphorylates the DNA polymerase
Goodrich, J.M., Bowden, R.A., Fisher, L., Keller, C., Schoch, G., Meyers,
processivity factor pUL44. Virology 311, 60–71.
J.D., 1993. Ganciclovir prophylaxis to prevent cytomegalovirus disease
Martin, D.F., Kuppermann, B.D., Wolitz, R.A., Palestine, A.G., Li, H.,
after allogeneic marrow transplant. Ann. Intern. Med. 118, 173–178.
Robinson, C.A., 1999. Oral ganciclovir for patients with cytomegalovirus
Griffiths, P.D., Walter, S., 2005. Cytomegalovirus. Curr. Opin. Infect. Dis.
retinitis treated with a ganciclovir implant. N. Engl. J. Med. 340,
18, 241–245.
Ho, E.S., Lin, D.C., Mendel, D.B., Cihlar, T., 2000. Cytotoxicity of antiviral
Mattes, F.M., Hainsworth, E.G., Geretti, A.M., Nebbia, G., Prentice, G., Pot-
nucleotides adefovir and cidofovir is induced by the expression of human
ter, M., Burroughs, A.K., Sweny, P., Hassan-Walker, A.F., Okwuadi, S.,
renal organic anion transporter 1. J. Am. Soc. Nephrol. 11, 383–393.
Sabin, C., Amooty, G., Brown, V.S., Grace, S.C., Emery, V.C., Grif-
Hodson, E.M., Jones, C.A., Webster, A.C., Strippoli, G.F., Barclay, P.G.,
fiths, P.D., 2004. A randomized, controlled trial comparing ganciclovir
Kable, K., Vimalachandra, D., Craig, J.C., 2005. Antiviral medications
to ganciclovir plus foscarnet (each at half dose) for preemptive therapy
to prevent cytomegalovirus disease and early death in recipients of solid-
of cytomegalovirus infection in transplant recipients. J. Infect. Dis. 189,
organ transplants: a systematic review of randomised controlled trials.
Lancet 365, 2105–2115.
McSharry, J.J., McDonough, A., Olson, B., Hallenberger, S., Reef-
Kalil, A.C., Levitsky, J., Lyden, E., Stoner, J., Freifeld, A.G., 2005.
schlaeger, J., Bender, W., Drusano, G.L., 2001. Susceptibilities of human
Meta-analysis: the efficacy of strategies to prevent organ disease by
cytomegalovirus clinical isolates to BAY 38-4766, BAY 43-9695, and
cytomegalovirus in solid organ transplant recipients. Ann. Intern. Med.
ganciclovir. Antimicrob. Agents Chemother. 45, 2925–2927.
143, 870–881.
Merigan, T.C., Renlund, D.G., Keay, S., Bristow, M.R., Starnes, V.,
Kimberlin, D.W., Lin, C.Y., Sanchez, P.J., Demmler, G.J., Dankner, W., Shel-
O'Connell, J.B., Resta, S., Dunn, D., Gamberg, P., Ratkovec, R.M., 1992.
ton, M., Jacobs, R.F., Vaudry, W., Pass, R.F., Kiell, J.M., Soong, S.J.,
A controlled trial of ganciclovir to prevent cytomegalovirus disease after
Whitley, R.J., National Institute of Allergy and Infectious Diseases Col-
heart transplantation. N. Engl. J. Med. 326, 1182–1186.
laborative Antiviral Study Group, 2003. Effect of ganciclovir therapy on
Musch, D.C., Martin, D.F., Gordon, J.F., Davis, M.D., Kuppermann, B.D.,
hearing in symptomatic congenital cytomegalovirus disease involving the
1997. Treatment of cytomegalovirus retinitis with a sustained-release gan-
central nervous system: a randomized, controlled trial. J. Pediatr. 143,
ciclovir implant. The Ganciclovir Implant Study Group. N. Engl. J. Med.
337, 83–90.
Komazin, G., Ptak, R.G., Emmer, B.T., Townsend, L.B., Drach, J.C.,
Painter, G.R., Hostetler, K.Y., 2004. Design and development of oral drugs for
2003. Resistance of human cytomegalovirus to the benzimidazole l-
the prophylaxis and treatment of smallpox infection. Trends Biotechnol.
ribonucleoside maribavir maps to UL27. J. Virol. 77, 11499–11506.
22, 423–427.
Krosky, P.M., Baek, M.C., Coen, D.M., 2003a. The human cytomegalovirus
Pass, R.F., 1985. Epidemiology and transmission of cytomegalovirus. J.
UL97 protein kinase, an antiviral drug target, is required at the stage of
Infect. Dis. 152, 10–16.
nuclear egress. J. Virol. 77, 905–914.
Paya, C., Humar, A., Dominguez, E., Washburn, K., Blumberg, E., Alexander,
Krosky, P.M., Baek, M.C., Jahng, W.J., Barrera, I., Harvey, R.J., Biron, K.K.,
B., Freeman, R., Heaton, N., Pescovitz, M.D., Valganciclovir Solid Organ
Coen, D.M., Sethna, P.B., 2003b. The human cytomegalovirus UL44 pro-
Transplant Study Group, 2004. Efficacy and safety of valganciclovir vs.
tein is a substrate for the UL97 protein kinase. J. Virol. 77, 7720–7727.
oral ganciclovir for prevention of cytomegalovirus disease in solid organ
Krosky, P.M., Underwood, M.R., Turk, S.R., Feng, K.W., Jain, R.K., Ptak,
transplant recipients. Am. J. Transplant. 4, 611–620.
R.G., Westerman, A.C., Biron, K.K., Townsend, L.B., Drach, J.C., 1998.
Razonable, R.R., Brown, R.A., Humar, A., Covington, E., Alecock, E., Paya,
Resistance of human cytomegalovirus to benzimidazole ribonucleosides
C.V., PV16000 Study Group, 2005. Herpesvirus infections in solid organ
maps to two open reading frames: UL89 and UL56. J. Virol. 72,
transplant patients at high risk of primary cytomegalovirus disease. J.
Infect. Dis. 192, 1331–1339.
K.K. Biron / Antiviral Research 71 (2006) 154–163
Razonable, R.R., Emery, V.C., 2004. Management of CMV infection and
cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is
disease in transplant patients [consensus article—IHMF® management
mediated through the UL89 gene product. J. Virol. 72, 717–725.
recommendations]. Herpes 11, 77–86.
Valcyte (valgancyclovir hydrochloride tablets) package insert. Roche Labo-
Reefschlaeger, J., Bender, W., Hallenberger, S., Weber, O., Eckenberg, P.,
ratories Inc., 2003.
Goldmann, S., Haerter, M., Buerger, I., Trappe, J., Herrington, J.A.,
Valtrex (valacyclovir hydrochloride caplets) package insert. GlaxoSmithKline
Haebich, D., Ruebsamen-Waigmann, H., 2001. Novel non-nucleoside
inhibitors of cytomegaloviruses (BAY 38-4766): in vitro and in vivo
ViroPharma Inc., 2005a. ViroPharma announces presentation of Phase 1 clin-
antiviral activity and mechanism of action. J. Antimicrob. Chemother.
ical data for maribavir. Available: (accessed
48, 757–767.
January 31, 2006).
Reusser, P., Einsele, H., Lee, J., Volin, L., Rovira, M., Engelhard, D., Finke,
ViroPharma Inc., 2005b. ViroPharma Completes Enrollment in Phase 2 clin-
J., Cordonnier, C., Link, H., Ljungman, P., 2002. Randomized multi-
ical study of maribavir in bone marrow transplant patients. Available:
center trial of foscarnet versus ganciclovir for preemptive therapy of
(accessed January 31, 2006).
cytomegalovirus infection after allogeneic stem cell transplantation. Blood
Vistide package insert, 1996. Vistide (cidofovir injection) package insert.
99, 1159–1164.
Gilead Sciences Inc., 1996.
Ross, S.A., Boppana, S.B., 2004. Congenital cytomegalovirus infection: out-
Wang, L.H., Peck, R.W., Yin, Y., Allanson, J., Wiggs, R., Wire, M.B., 2003.
come and diagnosis. Semin. Pediatr. Infect. Dis. 16, 44–49.
Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti-
Schleiss, M.R., Bernstein, D.I., McVoy, M.A., Stroup, G., Bravo, F., Creasy,
human cytomegalovirus agent, in healthy and human immunodeficiency
B., McGregor, A., Henninger, K., Hallenberger, S., 2005. The non-
virus-infected subjects. Antimicrob. Agents Chemother. 47, 1334–1342.
nucleoside antiviral, BAY 38-4766, protects against cytomegalovirus
Weber, O., Bender, W., Eckenberg, P., Goldmann, S., Haerter, M., Hallen-
(CMV) disease and mortality in immunocompromised guinea pigs. Antivi-
berger, S., Henninger, K., Reefschlager, J., Trappe, J., Witt-Laido, A.,
ral Res. 65, 35–43.
Ruebsamen-Waigmann, H., 2001. Inhibition of murine cytomegalovirus
Schleiss, M.R., Heineman, T.C., 2005. Progress toward an elusive goal: cur-
and human cytomegalovirus by a novel non-nucleosidic compound in
rent status of cytomegalovirus vaccines. Expert Rev. Vaccines 4, 381–406.
vivo. Antiviral Res. 49, 179–189.
Singh, N., 2006. Cytomegalovirus infection in solid organ transplant recip-
Whitley, R.J., Cloud, G., Gruber, W., Storch, G.A., Demmler, G.J., Jacobs,
ients: new challenges and their implications for preventive strategies. J.
R.F., Dankner, W., Spector, S.A., Starr, S., Pass, R.F., Stagno, S., Britt,
Clin. Virol. 35, 474–477.
W.J., Alford Jr., C., Soong, S., Zhou, X.J., Sherrill, L., FitzGerald, J.M.,
Sullivan, V., Talarico, C.L., Stanat, S.C., Davis, M., Coen, D.M., Biron, K.K.,
Sommadossi, J.P., 1997. Ganciclovir treatment of symptomatic congenital
1992. A protein kinase homologue controls phosphorylation of ganciclovir
cytomegalovirus infection: results of a phase II study. National Institute
in human cytomegalovirus-infected cells. Nature 358, 162–164.
of Allergy and Infectious Diseases Collaborative Antiviral Study Group.
Townsend, L.B., Devivar, R.V., Turk, S.R., Nassiri, M.R., Drach, J.C., 1995.
J. Infect. Dis. 175, 1080–1086.
Design, synthesis, and antiviral activity of certain 2,5,6-trihalo-1-(beta-d-
Wolf, D.G., Courcelle, C.T., Prichard, M.N., Mocarski, E.S., 2001. Dis-
ribofuranosyl)benzimidazoles. J. Med. Chem. 38, 4098–4105.
tinct and separate roles for herpesvirus-conserved UL97 kinase in
Underwood, M.R., Ferris, R.G., Selleseth, D.W., Davis, M.G., Drach,
cytomegalovirus DNA synthesis and encapsidation. Proc. Natl. Acad. Sci.
J.C., Townsend, L.B., Biron, K.K., Boyd, F.L., 2004. Mechanism of
U.S.A. 98, 1895–1900.
action of the ribopyranoside benzimidazole GW275175X against human
Zacny, V.L., Gershburg, E., Davis, M.G., Biron, K.K., Pagano, J.S., 1999.
cytomegalovirus. Antimicrob. Agents Chemother. 48, 1647–1651.
Inhibition of Epstein-Barr virus replication by a benzimidazole l-riboside:
Underwood, M.R., Harvey, R.J., Stanat, S.C., Hemphill, M.L., Miller, T.,
novel antiviral mechanism of 5, 6-dichloro-2-(isopropylamino)-1-beta-l-
Drach, J.C., Townsend, L.B., Biron, K.K., 1998. Inhibition of human
ribofuranosyl-1H-benzimidazole. J. Virol. 73, 7271–7277.
Source: http://www.idpublications.com/journals/pdfs/avres/avres_mostcited_1.pdf
Table of Contents Ⅰ. Overview of Training Program -------------------------------1 1. Purpose of Training Program -------------------------------1 2. Principles of Training Program ----------------------------1 3. Session Overview ------------------------------------------2 4. Training Period -------------------------------------------2 5. Weekly Schedule for In-service Training -------------------3
ECUADOR DATASHEET LAST MODIFIED 22.05.2008 1. PRESHIPMENT INSPECTION (PSI) MANDATE: Corporación Aduanera Ecuatoriana (CAE) All goods shipped until and including February 29, 2008 shall be subject to PSI. NNRFs (Avisos de No Conformidad) shall be issued in case final documents are not received within 15 working days from the




