1021-1030
1021-1030 20/3/06 19:21 Page 1021
INTERNATIONAL JOURNAL OF ONCOLOGY 28: 1021-1030, 2006
Cimetidine, an unexpected anti-tumor agent, and its
potential for the treatment of glioblastoma (Review)
FLORENCE LEFRANC1, PAUL YEATON3, JACQUES BROTCHI1 and ROBERT KISS2
1Department of Neurosurgery, Erasmus University Hospital, 2Laboratory of Toxicology,
Institute of Pharmacy, Université Libre de Bruxelles, Brussels, Belgium;
3Department of Gastroenterology, University of Virginia, Charlotesville, VA, USA
Received November 2, 2005; Accepted December 29, 2005
Abstract. Cimetidine (CIM), the prototypical histamine H2
3. Cimetidine as an anti-tumor drug
receptor antagonist (H2RA), was brought to market based
4. Mechanisms of action of cimetidine in oncology
on its ability to accelerate healing of gastrointestinal ulcers
5. Cimetidine and malignant gliomas
through the inhibition of gastric acid secretion. Cimetidine,
the most studied H2RA, has been demonstrated to possess
anti-tumor activity against colon, gastric and kidney cancers,
and melanomas. This activity involves a number of different
1. Origin of cimetidine
mechanisms of action: a) CIM antagonizes tumor cell-
mediated interleukin-1-induced activation of selectins in liver
sinusoids, inhibiting tumor cell binding on liver sinusoids,
imidazol-4-yl)methyl)thio)ethyl)guanidine] is a substituted
thereby reducing the development of liver metastasis; b)
imidazole with a specific antagonistic effect on histamine H2
histamine acts as a growth factor in various tumor cell types
receptors. Briefly, cimetidine (CIM) is a weak base with a
via the activation of H2 receptors; CIM therefore may anta-
high level of water solubility which can be measured in
gonize this effect; c) CIM acts as an immunomodulator by
biological fluids including the cephalo-spinal fluid (1). CIM
enhancing the host's immune response to tumor cells. With
is metabolized in the liver by oxidative hydroxylation and
respect to malignant gliomas, CIM added to temozolomide
conjugation. Up to 80% of a single dose of CIM is excreted
was superior
in vivo when compared to temozolomide alone
in the urine (1), with up to 70% in an unchanged form (1). Its
in extending survival of nude mice with human glioblastoma
principal action is on parietal cel histamine H2 receptors, and
cells orthotopically xenografted into their brain. We review
by binding to these receptors, inhibits gastric acid secretion
the various mechanisms of action potentially associated with
stimulated by histamine, pentagastrin, acetylcholine, insulin,
the therapeutic effects of CIM in the case of experimental
food and other secretagogues (2).
glioblastomas, observations we hope will encourage clinical
investigation of CIM in the management of highly malignant
2. Initial therapeutic indications of cimetidine
CIM was the first registered histamine H2RA, its wide
acceptance was based on its clinical effectiveness in the
healing of gastrointestinal ulcers through inhibition of gastric
acid secretion (1-3). CIM was one of the most widely used
1. Origin of cimetidine
H2RA during the 1980s (3). At the time of its introduction in
2. Initial therapeutic indications of cimetidine
the late 1970s, CIM was rarely considered an agent with
clinical utility other than its primary indication (3). A primary
concern was if by virtue of their acid-inhibitory activity,
_ H2RAs increased the risk of developing gastrointestinal
malignancies (3); tiotidine, one of the earliest H2RAs
developed, was abandoned when preclinical toxicity tests
Correspondence to: Dr Robert Kiss, Laboratory of Toxicology,
demonstrated an increased incidence of gastric tumors in rats
Institute of Pharmacy, Université Libre de Bruxelles, Campus de la
(4). CIM inhibits several isozymes of the cytochrome P450
Plaine, Boulevard du Triomphe, 1050 Brussels, Belgium
E-mail:
[email protected]
enzyme system, including CYP1A2, CYP2C9, CYP2C19,
CYP2D6, CYP2E1, and CYP3A4. This inhibition forms the
Abbreviations: CIM, cimetidine; H2RAs, histamine H2 receptor
basis of the numerous drug interactions. While CIM proved
to be a safe medication, its use in peptic ulcer disease was
supplanted by the development of longer-acting H2RAs with
Key words: cimetidine, H2RAs, malignant gliomas, cancer
reduced adverse effects and the introduction of highly specific
proton pump inhibitors (2).
1021-1030 20/3/06 19:21 Page 1022
LEFRANC
et al: CIMETIDINE AND MALIGNANT GLIOMAS
Table I. Description of the various clinical trials using cimetidine in oncology.
(patient survival)
Post-operative 800 mg/d
Significant increase
Tonnesen
et al (6)
Significant increase
Burtin
et al (5)
Colorectal cancer
5 d pre-/2 d post-operative 800 mg/d
Significant increase
Adams and Morris (7)
Colorectal cancer
5FU+/-post-operative 800 mg/d, 1 y
Significant increase
Colorectal cancer
Post-operative 400 mg twice/d, 2 y
45 (Dukes C) Significant increase
Svendsen
et al (9)
Colorectal cancer
Pre-operative, 7 d
3-y survival benefit
Adams and Morris (10)
Colorectal cancer
Pre-operative 800 mg twice/d, 5 d
Kelly
et al (11)
Colorectal cancer
Non-randomized 5FU+/-post-operative 800 mg/d, 1 y
10-y survival benefit
Matsumoto
et al (13)
Advanced melanoma Phase II
Morton
et al (16)
Advanced melanoma Phase II
Creagan
et al (15)
Non-randomized Coumarin + 300 mg, 4x/d upd
Marshall
et al (17)
Coumarin + 300 mg 4x/d
Dexeus
et al (18)
Non-randomized 600 mg/d upd
Inhorn
et al (19)
INF +/- (coumarin + 400 mg 3x/d)
No significant increase Sagaster
et al (20)
upd, until progression of disease; d, day; y, year; CR, complete response; PR, partial response; INF, interferon; RCC, renal cell carcinoma.
3. Cimetidine as an anti-tumor drug
The use of CIM also has intriguing implications in the
management of advanced malignant melanomas (14-16) and
The first reports suggesting CIM exhibited a clinical onco-
metastatic renal cell carcinomas (17-20) (Table I).
logic effect appeared in 1988 in the context of gastric cancer
Our group (21) has demonstrated that CIM complements
(5,6). In a randomized study including 65 patients selected
the cytotoxic agent temozolomide in experimental glio-
because their condition contraindicated all other forms of
blastomas, a point detailed in the section entitled Cimetidine
treatment, Burtin
et al (5) found that a course of CIM (1-1.2 g/
and malignant gliomas.
day) or ranitidine (450-900 mg/day) significantly improved
the patients' survival rates. These patients survived six times
4. Mechanisms of action of cimetidine in oncology
longer than others receiving pal iative treatment with analgesics
(5). Another multicenter, randomized, double-blind, placebo-
Studies of the anti-tumor effects of CIM indicate multiple
controlled study carried out by Tonnesen
et al (6) on 181
potential mechanisms of action, characterized by three overal
patients showed that a post-operative course of CIM at a
characteristics: a) a direct inhibitory effect on tumor growth
normal therapeutic dosage (800 mg/day) significantly
by blocking the cell growth-promoting activity of histamine
prolonged the survival of gastric cancer patients.
(22-24) (Fig. 1) and an indirect effect by inhibiting tumor-
In colorectal cancer patients, Adams and Morris (7) were
associated angiogenesis (Fig. 2) (25); b) a cell-mediated
the first to demonstrate the beneficial effect of a short-course
immunomodulation by enhancing the host's immune response
perioperative treatment with CIM on surgically-induced
to tumor cells (Fig. 1) (26-28); c) an inhibition of cancer cell
immunosuppression. Their randomized study involving 34
migration (21) and adhesion to endothelial cells (29) and
patients showed a strong trend towards enhanced survival in
therefore an inhibition of tumor neo-angiogenesis (25) (Fig. 2)
the patients treated with CIM (800 mg/day) when compared
and metastasis development (29) (Fig. 3).
to controls, a finding correlated with an increase of lymphocyte
infiltration into the tumors (7).
Inhibitory effects on tumor growth. While the mechanisms
Matsumoto (8) performed a multicenter randomized
involved are incompletely understood, CIM is known to inhibit
controlled study in 64 colorectal cancer patients receiving
the growth of several types of tumors, including gastro-
postoperative 5-fluorouracil. Post-operative treatment with
intestinal cancers, both
in vitro and
in vivo in animal models
CIM (800 mg/day) and 5-fluorouracil (150 mg/day) for about
(23,24). An active role is strongly suggested for histamine of
a year was efficacious, increasing the disease-free period and
autocrine or paracrine origins in malignant cell proliferation
survival when compared to the treatment with 5-fluoro-
(Fig. 1) (12).
uracil alone (8).
Histamine is a receptor-dependent growth factor in some,
Several subsequent studies, summarized in Table I, have but not all, human colon cancer cell lines, as well as in some
been published showing considerably enhanced survival rates
gastric, breast and melanoma cell lines (23,24,30,31). In a
in gastric and colorectal cancer patients treated with CIM (9-13).
culture study of four different colorectal tumor cell lines
1021-1030 20/3/06 19:21 Page 1023
INTERNATIONAL JOURNAL OF ONCOLOGY 28: 1021-1030, 2006
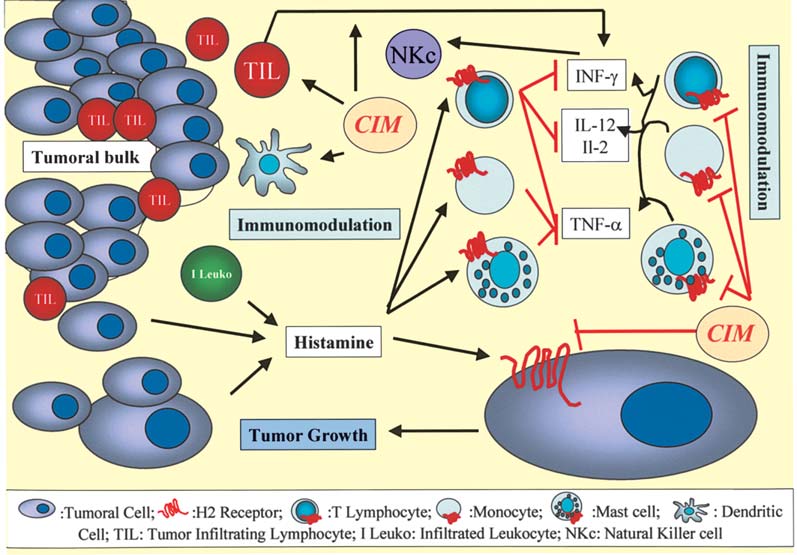
Figure 1. CIM inhibitory effect on tumor growth and CIM-mediated immunomodulation. CIM blocks the cell growth-promoting activity of histamine. The
mechanisms proposed for the cell-mediated immunomodulation of CIM include the inhibition of suppressor T lymphocyte activity, the stimulation of natural
killer cell (NKc) activity, an increase in interleukin-2 (IL-2) and interleukin-12 (IL-12) production in helper T lymphocytes, an increase in tumor inhibitory
cytokines and the enhancement of the host's anti-tumor cell-mediated immunity.
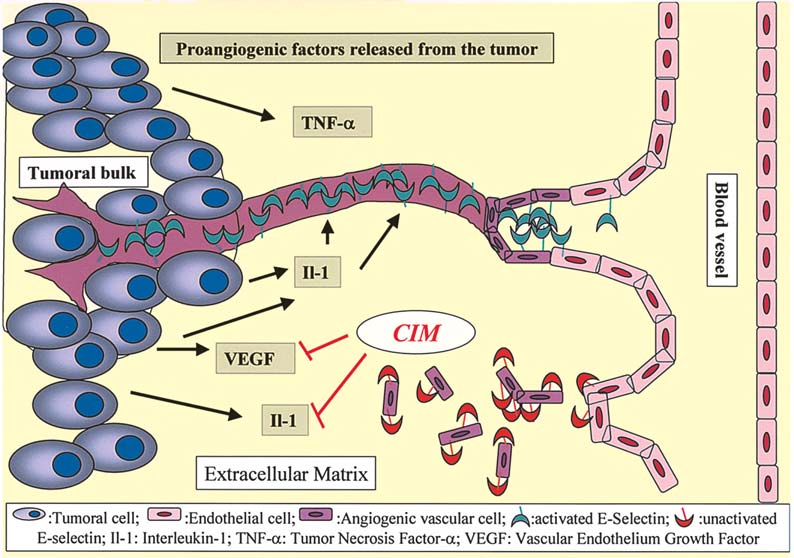
Figure 2. CIM-mediated neo-angiogenesis inhibition. CIM induces a significant decrease in VEGF expression levels and the vascular-like tube formation by
endothelial cells is significantly impaired.
(C170, Lovo, LIM2412 and LIM2405) histamine was found
absence (23). When the C170 cell line was grown in nude
to stimulate cell proliferation in two of them (C170 and
mice as a subcutaneous xenograft, CIM had a significant
LIM2412) in a dose-dependent manner (23). This effect was
dose-dependent growth-inhibitoring effect leveling out at a
reversed by CIM in the presence of histamine, but not in its
dose of 50 mg/kg/day (23).
1021-1030 20/3/06 19:21 Page 1024
LEFRANC et al: CIMETIDINE AND MALIGNANT GLIOMAS
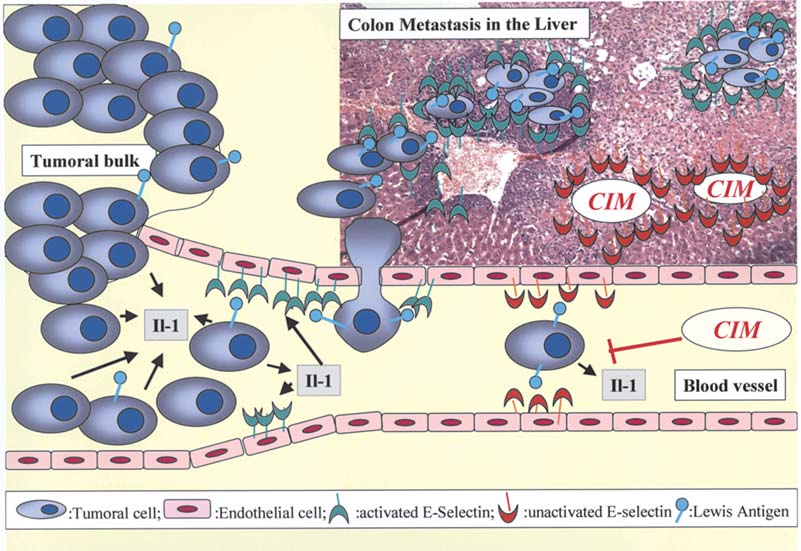
Figure 3. CIM-mediated inhibition of cancer cel migration and the development of liver metastasis. Epithelial cel s detaching themselves from primary epithelial
tumors (tumoral bulk) and migrating through the lymphatic or the blood vessels eventual y colonize the liver because epithelial cancer cel s exhibiting Lewis
antigens on their surfaces are able to adhere to endothelial cells in liver sinusoids due to the presence of selectins (the ligands for Lewis antigens) in these
endothelial liver cells. CIM prevents liver metastasis of colon cancer cells by blocking E-selectin activation by means of the inhibition of interleukin-1 (IL-1)
secretion by the tumor cells.
Rajendra et al (32) demonstrated that CIM at 10 µM
of the H2-mediated actions of endogeneous histamine.
inhibited the in vitro proliferation of the Caco-2 colorectal
Curiously, ranitine did not seem to exert most of the in vitro
cancer cell line in the presence of histamine by causing
and in vivo effects mentioned, an observation which would
apoptotic cell death. In the human gastric tumor cell lines
argue against H2 receptors playing a role in the effects of
MKN45 and MKN45G, CIM (10 µM) reversed the
CIM, since ranitine is marginally more potent as an H2
histamine-stimulated proliferation (30). CIM also inhibited
receptor antagonist (36). In fact, in a prospective randomized
the proliferation of MKN45 subcutaneous xenografts in nude
controlled study, the use of ranitidine in patients with gastric
mice (100 mg/kg/day, given in the drinking water) (30). In
cancer did not show any significant increase in their survival
another in vitro study, histamine significantly stimulated
rates (37). In contrast, roxatidine significantly decreased the
cells proliferating in a dose-dependent manner on the gastric
in vivo growth of colon 38 implants in mice (38). In their
cancer cell lines KATO-III and AGS, with the maximum
study, Tomita et al (38) showed that in vitro, histamine,
effect again occurring around a 10 µM concentration (31).
roxatidine, and CIM failed to achieve any growth-promotive
CIM reversed the histamine-stimulated cell proliferation,
or suppressive effects in the case of the colon 38 cell line, a
with the maximum effect at concentrations above 10 µM
cell line that lacks H2 receptors, although roxatidine and CIM
(31). Ranitidine and famotidine did not show such an effect
suppressed the in vivo growth of the tumor tissue implants.
(31). Histamine significantly stimulated growth in two of
Such a finding suggests that in this case, the tumor-suppressive
four human melanoma cell lines, and this effect was inhibited
effects of H2 receptor antagonists do not constitute the product
by CIM in a dose-dependent manner, and also by ranitidine
of any direct action on tumor cel s. Szincsak et al (39) have
and famotidine (24). CIM also inhibited tumor growth of
shown that in vivo tumor proliferation in immunodeficient
human pancreatic cancer xenografts in immunodeficient mice mice xenotransplanted with a human melanoma cell line was
diminished by CIM (50 mg/kg/day), if combined with a
Adams et al (23) suggested a role for H2 receptors located
tamoxifen derivate acting on cytochrome P450 molecules.
either on the tumor cells themselves, on immunocompetent
This suggests again that the effect of CIM cannot be restricted
cells in the host, or both. Using L-histidine decarboxylase
to an H2 receptor blocker alone. The anticancer actions of
(HDC)-deficient mice with undetectable levels of endo-
CIM might not be mediated via histamine antagonist only.
genous histamine, Takahashi et al (34,35) have shown that
Therefore, the mechanisms of action by which CIM prolongs
the daily administration of CIM (0.12 mg/kg/day) failed to
the survival of patients with various forms of cancer remain
suppress the growth of a syngeneic colon adenocarcinoma
to be clarified and are probably multifactorial. The inhibitory
despite the fact that an identical dose of CIM suppressed
effect of CIM on tumor-associated angiogenesis (25,38) is
tumor growth in wild-type mice, as the result of the inhibition developed below.
1021-1030 20/3/06 19:21 Page 1025
INTERNATIONAL JOURNAL OF ONCOLOGY 28: 1021-1030, 2006
Cell-mediated immunomodulation. Many tumors, and par-
remains unclear whether or not H2 receptors are expressed
ticularly colorectal and breast cancer, secrete histamine, a
on dendritic cells, the effect of CIM on the antigen presenting
process that results in high histamine levels within the tumors
ability of dendritic cells appears to increase because of CIM-
(13,40). Moreover, histamine is also frequently secreted in
specific actions (Fig. 1) (44). It also remains unclear whether
response to the surgical resection of colorectal cancers (40). Al
or not the modulating effects of CIM on the dendritic cell
these factors working together create an immunosuppressive
function observed in vitro by Kubota and colleagues (44)
environment both in the area of tumor growth and in the
have any clinical y substantial meaning: the clinical effective-
whole body, and in so doing they facilitate tumor growth. A
ness of CIM against gastrointestinal malignancies is considered
number of clinical studies have shown that the administration
to be due to the combined total of immunological and non-
of CIM may help in reducing the immunosuppression due to
increased histamine levels in a tumor's environment (11,41).
CIM has been reported as having better cell-mediated
Adams and Morris (7) first desribed that pre-operative
immunomodulation than other H2RAs such as famotidine
treatment with CIM (800 mg/day) significantly increased the
and ranitidine, and the differences between CIM and other
proportion of colorectal cancers that elicited a lymphocyte
H2RAs might be due to their structures and/or affinities to
response, and that the presence of tumor-infiltrating lympho-
H2 receptors (22,36).
cytes was associated with a survival advantage. In a pilot
Immunologically based therapies for various types of
study, they showed that CIM enhanced the lymphocyte
cancers are improved by adjuvant CIM therapy (47).
infiltration of human colorectal carcinomas (10). Forty-two
Interestingly enough, one study has reported that a small
patients scheduled for the elective resection of colorectal
number of patients with metastatic renal cell carcinomas
carcinomas were randomized either to receive CIM for one
(5%) responded with long-term remission to CIM mono-
week preoperatively, or to act as control (10). A positive
therapy (19). But, immunologically based therapies for renal
lymphocyte response was observed in 10 of 18 CIM-treated
cell carcinomas or disseminated malignant melanomas have
carcinoma patients compared with only 5 of the 24 control
usually been combined with CIM and the contributions of
patients (p=0.03) (10). Moreover, the presence of a lympho-
CIM have not been adequately controlled (17,20,48,49).
cyte response correlated with improved survival (10). Gastric
cancer patients also have higher levels of suppressor lympho-
Inhibition of cancer cell migration and the development of
cyte activity when compared to normal controls, and these
liver metastasis. In vitro studies have demonstrated that CIM
levels are restored to normal with CIM treatment (42). In a
inhibits the adhesion of some breast (50) and colon (29)
controlled randomized clinical trial, Lin et al (43) recently
cancer cells to human umbilical cord cells, a process that is
showed that pre-operative CIM administration at the dose of
a crucial biological step in tumor neo-angiogenesis and,
400 mg/day promoted peripheral blood lymphocytes and tumor consequently, in tumor progression and metastasis. Tomita et al
infiltrating lymphocytes in patients with gastrointestinal cancer.
(38) have shown that CIM-induced angiogenesis inhibition
The mechanisms proposed for the cell-mediated immuno-
suppresses the growth of colon cancer implants in syngeneic
modulation of CIM (Fig. 1) include the inhibition of suppressor
mice and is associated with a significant decrease in VEGF
T lymphocyte activity (26), stimulation of natural killer (NK)
expression levels in tumor tissue and the serum of colon
cel activity (27), an increase in interleukin-2 (IL-2) production 38-bearing mice (Fig. 2). In the syngeneic murine colon cancer
in helper T lymphocytes (28), an increase in tumor inhibitory
CMT93 model, CIM also significantly reduced the growth of
cytokines (35) and the enhancement of the host's anti-tumor
the subcutaneously grafted tumor and neovascularization in
cel -mediated immunity by improving the suppressed dendritic the tumor (25). CIM at this dose had no effect on the in vitro
cell function in advanced cancer patients (44).
proliferation of this cel line (25). The cancer cel s' production
Takahashi et al (35) have demonstrated that: a) a daily
of the vascular endothelial growth factor was not affected by
injection of CIM suppressed tumor progression in mice after
CIM, whereas the vascular-like tube formation by endothelial
the syngeneic transplantation of CT-26 cells (a colon adeno-
cells in vitro was significantly impaired in the presence of
carcinoma cell line); and b) decreased expression of TNF-·
CIM (Fig. 2) (25). Their findings suggest that CIM suppresses
and INF-Á associated with the tumor development was restored tumor growth, at least in part by inhibiting tumor-associated
following treatment with CIM. CIM dramatically increased
angiogenesis. One of the major classes of adhesion molecules
IFN-Á production by human lymphocytes (Fig. 1) via a possibly present on the surface of endothelial cells includes selectins
histamine-independent (non-histamine receptor mediated)
(51). The direct implication of P-selectin in endothelial cell
pathway, most likely through cytochrome P450 moieties (45). migration has been reported previously (52) and we recently
High concentrations of INF-Á resulted in the inhibition of cell
suggested a direct implication of E-selectin in human endo-
proliferation by the direct stimulation of natural killer cells
thelial cell migration during tubulogenesis (53). Both E- and
(Fig. 1) (45). The use of CIM also retarded the growth of
P-selectins are induced in endothelial cells by proangiogenic
human melanomas in a nude mouse model and prolonged the
cytokines such as the tumor necrosis factor (TNF)-· or IL-1ß
survival of the tumor-bearing mice by directly inhibiting the
(51). Since Kobayashi et al (29) have shown that CIM
proliferation of tumor cells and indirectly promoting the
prevented liver metastasis of colon cancer cells in nude mice
infiltration of activated macrophages into the tumor site (39).
by blocking the E-selectin expression on the endothelial
It is also reported that H2RAs such as CIM can reverse the
cells, the anti-angiogenic effect of CIM could also be related
inhibition of the secretion of human interleukin-12 (IL-12)
to the decrease in E-selectin expression on endothelial cells
induced by histamine via H2 receptors expressed on mono-
and therefore to its anti-metastatic effect against carcinoma
cytes (the precursors of dendritic cells) (Fig. 1) (46). While it
cells invading the liver (Figs. 2 and 3).
1021-1030 20/3/06 19:21 Page 1026
LEFRANC et al: CIMETIDINE AND MALIGNANT GLIOMAS
Kobayashi et al (29) have also shown that CIM (daily doses
gliomas (58,68). Because experimental y decreasing migration
of 200 mg/kg) prevented liver metastasis of colon cancer
in apoptosis-resistant migrating tumor astrocytes restores
cells in nude mice by blocking E-selectin expression on the
sensitivity to apoptosis (58,68) and thus to pro-apoptotic drugs,
endothelial cells, a ligand for sialyl Lewis antigens on tumor
it would be interesting to elaborate new therapeutic strategies
cells (Fig. 3). Epithelial cells detaching themselves from
targeting migrating glioma cel s. Cel migration includes very
primary epithelial tumors (carcinomas) and migrating through
complex cellular and molecular processes in which at least
the lymphatic or the blood vessels (Fig. 3) eventual y colonize three independent but highly coordinated biological steps are
the liver due to the fact that epithelial cancer cells exhibiting
involved, i.e.: a) cell adhesion to specific components of the
Lewis antigens [involving CD15 with fucose moieties, i.e.
extracellular matrix (ECM) (72-74); b) modifications to the
fucosyl-N-acetyl-lactosamine (fucosyl-LacNAc)] on their
organization of the actin cytoskeleton (75-77); and c) the
surface are able to adhere to endothelial cel s in liver sinusoids secretion of proteases (78). Gene-expression profiling has
because of the presence of selectins (the ligands for Lewis
implicated numerous genes involved in glioma cel migration,
antigens) in these endothelial liver cells (Fig. 3) (13,54-56).
and many of these genes relate to cell adhesion molecules
Kaji et al (54) and Khatib et al (55) showed that upon entry
that directly interact with specific ECM components (79-84).
into the hepatic circulation, epithelial tumor cells can rapidly
Gladson has detailed the molecular nature of ECM in gliomas
trigger a molecular cascade (involving interleukin-1 secretion (85), the crucial roles of which have been emphasized for the
by tumor cel s) leading to the induction of E-selectin expression first time by Rutka and colleagues (86,87) with respect to
on the sinusoidal endothelium (Fig. 3). Khatib et al (55) thus
gliomas. Apart from integrins (85,88,89), galectins (75,90-92)
suggested that E-selectin induction in liver sinusoids by
also play a number of crucial roles in glioma cell migration.
carcinoma cells contributes to the liver-colonizing potential
While integrins employ protein-protein interactions with ECM
of carcinoma cells (Fig. 3). Again, these actions of CIM
components, galectins use protein-carbohydrate interactions
probably do not occur via the blocking of the histamine
between themselves and ECM glycoproteins, with the core of
receptor because famotidine and ranitidine did not show any
carbohydrate ligands for the galectins being represented by
similar effect. CIM treatment was particularly effective in
LacNAc moieties, i.e. Lewis antigens without fucosylation
colorectal cancer patients with tumors expressing higher levels (58). We have shown that the interactions between the oligo-
of sialyl Lewis-X and sialyl Lewis-A epitopes which are
saccharide moieties present in the glioma ECM and cell
involved in E-selectin mediated cell adhesion with endo-
adhesion molecules present on the surface of glioma cel s play
thelial cells (13).
a number of major roles in glioma cel migration (75,90-93).
Among these oligosaccharide moieties that play a number
5. Cimetidine and malignant gliomas
of major roles in glioma cell migration are fucose and lactose
Malignant gliomas are the most frequently encountered
One major target in the fight against glioma cel migration
primary brain tumors in adults and children (57,58); these
is connected with the successful decrease in protease
malignant gliomas include neoplasms of astrocytic (anaplastic expression by glioma cel s (78). Another major target involves
astrocytomas and glioblastomas) and oligodendroglial (ana-
adhesion molecules and their ligand in the extracellular
plastic oligodendrogliomas) lineages (59). The standard
matrix. By example, tenascin, an integrin ligand, is over-
treatment for these malignant gliomas is typically surgery,
expressed in the extracellular matrix of malignant gliomas
followed by radiotherapy and chemotherapy (58,60-63).
when compared to low-grade gliomas and normal brain
However, only those malignant gliomas that exhibit a loss of
parenchyma (85), and clinical applications serve to specifical y
heterozygosity (LOH) of chromosomes 1p and 19q are
combat this particular feature of glioma cell migration (95).
chemoresponsive (64,65). Unfortunately, gliomas exhibiting
Complementary to conventional chemotherapy, CIM has
1p/19q LOH are mainly malignant oligodendrogliomas, i.e.
been used successfully to inhibit cancer cell migration of
a minor proportion of malignant gliomas (59,66). In other
epithelial origins (carcinomas) towards the liver (13,29). It
words, most malignant gliomas are of astrocytic origin,
should be remembered that metastatic implantation of epi-
without 1p/19q LOH, and are therefore weakly sensitive to
thelial cancers in the liver involves cancer cel -mediated oligo-
any type of chemotherapy if at all (58). Malignant gliomas
saccharide moiety (the fucose moiety present on Lewis
are biologically heterogeneous and include sub-populations
antigens) interactions with cel adhesion molecules (selectins)
of proliferating and migrating cells (58,67,68). While certain
present in liver microvasculatures (13,29,56). In view of the
intracellular signaling pathways specifically control cell
fact that levels of expression of fucose binding activities in
proliferation and/or apoptosis, other intracellular signaling
malignant gliomas differ in relation to the levels of malignancy
pathways control cell migration (58,68-71). For example, the
(91) and that these receptor types could influence the levels
CAS/Crk assembly serves as a ‘molecular switch' for the
of proliferation of human glioma cel s (93), we postulated that
induction of cell migration and appears to contribute to the
addition of CIM to temozolomide treatment would improve
invasive property of tumors (70). Moreover, accumulating
survival of human glioblastoma orthotopic xenograft-bearing
evidence suggests invasive glioma cells associated with high
immunodeficient mice when compared to temozolomide
levels of migration display a decreased proliferation rate and
therapy alone. We chose the human U373 model because it
a relative resistance to apoptosis (57,58,68,70,71), a feature
is of astrocytic origin, devoid of 1p/19q LOH and weakly
that may contribute to chemotherapy and radiotherapy
sensitive to temozolomide (96), and the rat 9L sarcoma model
resistance (71). It is these migrating glioma cells that renders
because of its diffuse invasive abilities with respect to the
dismal the prognosis associated with high-grade malignant
brain parenchyma (97). We observed that combining CIM
1021-1030 20/3/06 19:21 Page 1027
INTERNATIONAL JOURNAL OF ONCOLOGY 28: 1021-1030, 2006
with temozolomide improved survival of the U373 orthotopic
cimetidine has been proven to be a useful adjunct in colon
xenograft-bearing nude mice (21). However, human glio-
cancer chemotherapy because it delays the formation of liver
blastoma U373 cells do not express H2 receptors (98), an
metastasis. Cimetidine also displays anti-tumor effects in
observation which again argues against the possibility of H2
gastric and renal carcinomas, and in melanomas. Cimetidine
receptors on tumor cells playing a role in the CIM-induced
can also act as an immunomodulator by enhancing the host's
immune response to tumor cells. We have recently shown
In vitro colorimetric MTT-based assay have revealed that
that combining CIM with temozolomide improved survival
cimetidine significantly decreased growth of both human U373 when compared to temozolomide alone in human glioblastoma
glioblastoma and rat 9L gliosarcoma cells at concentrations
orthotopic xenograft-bearing nude mice. As reviewed in the
≥100 µM (21). Van der Ven and col eagues (99) and Finn and
present report, various mechanisms of action can be associated
col eagues (100) had previously tested the growth-modulating with the beneficial therapeutic effects contributed by cimetidine
effects of CIM on glioma cultures derived from human brain
in the case of experimental glioblastomas, a fact that should
tumors. They observed that high dose (1 mM) CIM induced
encourage clinical investigators to enter highly malignant
inhibition of in vitro proliferation of gliomas, while lower
gliomas to cimetidine-related clinical trials.
concentrations (1 µM) were less effective (99,100). We
observed that in vitro 0.1-1 µM CIM significantly decreased
migration of both U373 and 9L brain tumor cells (21). We
also demonstrated that 30 daily intraperitoneal injections of
We thank Steven Decorte, the GSK Belgium Medical
30 mg/kg CIM markedly decreased the percentage of 9L
Advisor, for his help with the bibliography. R.K. is a
tumor cel s exhibiting endogenous receptors for fucose moieties Director of Research with the Fonds National de la Recherche
and the concentration of endogenous receptors for fucose
Scientifique (FNRS, Belgium) and F.L. is a Clinical Research
moieties in 9L tumor cells (21). This CIM-mediated decrease
Fellow with the FNRS.
in endogenous receptors for fucose moieties could partly
explain the cimetidine-induced decrease in 9L (and also U373) References
tumor cel migration and, in turn, the in vivo benefit of adding
cimetidine to temozolomide.
1. Somogyi A and Gugler R: Clinical pharmacokinetics of
cimetidine. Clin Pharmacokinet 8: 463-495, 1983.
Fucose-containing glycans with potential clinical
2. Brogden RN, Heel RC, Speight TM and Avery GS: Cimetidine: a
applications are hypothesized to combat the development of
review of its pharmacological properties and therapeutic efficacy
malignant gliomas. Indeed, it has long been known that under
in peptic ulcer disease. Drugs 15: 93-131, 1978.
3. Mol er H, Lindvig K, Klefter R, Mosbech J and Mol er Jensen O:
normal circumstances, the astrocyte number is kept constant
Cancer occurrence in a cohort of patients treated with cimetidine.
in the mammalian central nervous system during adulthood
Gut 30: 1558-1562, 1989.
and old age, as a result of the balance of division promoters
4. Streett CS, Cimprich RE and Robertson JL: Pathologic findings
in the stomachs of rats treated with the H2-receptor antagonist
and division inhibitors (101). Moreover, Nieto-Sampedro (102)
tiotidine. Scand J Gastroenterol Suppl 101: 109-117, 1984.
identified the mitogen inhibitors as immunologically related
5. Burtin C, Noirot C, Scheinmann P, Galoppin L, Sabolovic D and
to blood group oligosaccharides (i.e. Lewis antigen-related
Bernard P: Clinical improvement in advanced cancer disease
after treatment combining histamine and H2-antihistaminics
structures) and to glycan epitopes of the epidermal growth
(ranitidine or cimetidine). Eur J Cancer Clin Oncol 24: 161-167,
factor receptor. On the basis of these data, Aguilera et al
(103) synthesized a family of oligosaccharides with a
6. Tonnesen H, Knigge U, Bulow S, Damm P, Fischerman K,
Hesselfeldt P, Hjortrup A, Pedersen IK, Pedersen VM,
common Lewis-X-type structure, i.e. fucosyl-LacNAc-related
Siemssen OJ, et al: Effect of cimetidine on survival after
structures, and these compounds are the source of a significant
gastric cancer. Lancet ii: 990-992, 1988.
level of antiproliferative activity against malignant glio-
7. Adams WJ and Morris DL: Short-course cimetidine and survival
with colorectal cancer. Lancet 344: 1768-1769, 1994.
blastoma cells (104). Our recent study also revealed that
8. Matsumoto S: Cimetidine and survival with colorectal cancer.
CIM significantly decreased the expression of endogenous
Lancet 346: 115, 1995.
receptors for LacNAc moieties (21), knowing that such
9. Svendsen LB, Ross C, Knigge U, Frederiksen HJ, Graversen P,
Kjaergard J, Luke M, Stimpel H and Sparso BH: Cimetidine as
endogenous ligands involve, for example, different types of
an adjuvant treatment in colorectal cancer. A double-blind,
galectins whose levels of expression can be modulated by
randomized pilot study. Dis Colon Rectum 38: 514-518, 1995.
anti-inflammatory compounds (105-107). We defined the
10. Adams WJ and Morris DL: Pilot study - cimetidine enhances
lymphocyte infiltration of human colorectal carcinoma: results
role played by galectin-1 on glioma cell migration features
of a small randomized control trial. Cancer 80: 15-21, 1997.
(75,90). Thus, this CIM-induced decrease in endogenous
11. Kelly MD, King J, Cherian M, Dwerryhouse SJ, Finlay IG,
ligands for LacNAc (and maybe galectin-1) can act syner-
Adams WJ, King DW, Lubowski DZ and Morris DL:
Randomized trial of preoperative cimetidine in patients with
gistically with the CIM-induced decrease in endogenous
colorectal carcinoma with quantitative assessment of tumor-
receptors for fucose on both 9L and U373 tumor cel migration
associated lymphocytes. Cancer 85: 1658-1663, 1999.
levels and on the benefit in vivo of adding CIM to temozo-
12. Bolton E, King J and Morris DL: H2-antagonists in the treatment
of colon and breast cancer. Semin Cancer Biol 10: 3-10, 2000.
13. Matsumoto S, Imaeda Y, Umemoto S, Kobayashi K, Suzuki H
and Okamoto T: Cimetidine increases survival of colorectal
cancer patients with high levels of sialyl Lewis-X and sialyl
Lewis-A epitope expression on tumour cells. Br J Cancer 86:
161-167, 2002.
Cimetidine is a histamine receptor-type H2 blocker whose
14. Hel strand K, Naredi P, Lindner P, Lundholm K, Rudenstam CM,
clinical usefulness was clearly demonstrated several decades
Hermodsson S, Asztely M and Hafstrom L: Histamine in immuno-
therapy of advanced melanoma: a pilot study. Cancer Immunol
ago in the treatment of peptic ulcer disease. More recently,
Immunother 39: 416-419, 1994.
1021-1030 20/3/06 19:21 Page 1028
LEFRANC et al: CIMETIDINE AND MALIGNANT GLIOMAS
15. Creagan ET, Ahmann DL, Green SJ, Long HJ, Frytak S and
37. Primrose JN, Miller GV, Preston SR, Gokhale J, Ambrose NS,
Itri LM: Phase II study of recombinant leukocyte A interferon
Ward UM, Mil s JG, Ehsanul ah RS and Darekar B: A prospective
(IFN-rA) plus cimetidine in disseminated malignant melanoma.
randomised controlled study of the use of ranitidine in patients
J Clin Oncol 3: 977-981, 1985.
with gastric cancer. Yorkshire GI Tumour Group. Gut 42: 17-19,
16. Morton RF, Creagan ET, Cullinan SA, Mailliard JA, Ebbert L,
Veeder MH and Chang M: Phase II studies of single-agent
38. Tomita K, Izumi K and Okabe S: Roxatidine- and cimetidine-
cimetidine and the combination N-phosphonacetyl-L-aspartate
induced angiogenesis inhibition suppresses growth of colon cancer
(NSC-224131) plus L-alanosine (NSC-153353) in advanced
implants in syngeneic mice. J Pharmacol Sci 93: 321-330, 2003.
malignant melanoma. J Clin Oncol 5: 1078-1082, 1987.
39. Szincsak N, Hegyesi H, Hunyadi J, Falus A and Juhasz I:
17. Marshall ME, Mendelsohn L, Butler K, Riley L, Cantrell J,
Different h2 receptor antihistamines dissimilarly retard the growth
Wiseman C, Taylor R and MacDonald JS: Treatment of metastatic
of xenografted human melanoma cel s in immunodeficient mice.
renal cell carcinoma with coumarin (1,2-benzopyrone) and
Cell Biol Int 26: 833-836, 2002.
cimetidine: a pilot study. J Clin Oncol 5: 862-866, 1987.
40. Garcia-Caballero M, Nunezed X, Castro I, Kusche J and Vora-
18. Dexeus FH, Logothetis CJ, Sel a A, Fitz K, Amato R, Reuben JM
Thorbeck L: Histamine metabolism in human breast and colo-
and Dozier N: Phase II study of coumarin and cimetidine in
rectal cancer: its effects on other host tissues. Adv Biosci 89:
patients with metastatic renal cell carcinoma. J Clin Oncol 8:
273-287, 1993.
325-329, 1990.
41. Nishiguchi S, Tamori A, Shiomi S, Enomoto M, Tatsumi N,
19. Inhorn L, Williams SD, Nattam S and Stephens D: High-dose
Koh N, Habu D, Sakaguchi H, Takeda T, Seki S, et al:
cimetidine for the treatment of metastatic renal cell carcinoma.
Cimetidine reduces impairment of cellular immunity after
A Hoosier Oncology Group study. Am J Clin Oncol 15: 157-159,
transcatheter arterial embolization in patients with hepato-
cellular carcinoma. Hepatogastroenterology 50: 460-462, 2003.
20. Sagaster P, Micksche M, Flamm J and Ludwig H: Randomised
42. Hahm KB, Lee SI, Chung JP, Kim WH, Kim JH and Park IS:
study using IFN-alpha versus IFN-alpha plus coumarin and
Comparison of immunomodulative effects of histamine-2
cimetidine for treatment of advanced renal cell cancer. Ann
receptor antagonists in gastric cancer patients: focus on the
Oncol 6: 999-1003, 1995.
lymphoblastogenesis and cytotoxicity of peripheral blood mono-
21. Lefranc F, James S, Camby I, Gaussin JF, Darro F, Brotchi J,
nuclear cells. Int J Immunopharmacol 16: 985-993, 1994.
Gabius J and Kiss R: Combined cimetidine and temozolomide,
43. Lin CY, Bai DJ, Yuan HY, Wang K, Yang GL, Hu MB, Wu ZQ
compared with temozolomide alone: significant increases in
and Li Y: Perioperative cimetidine administration promotes
survival in nude mice bearing U373 human glioblastoma
peripheral blood lymphocytes and tumor infiltrating lympho-
multiforme orthotopic xenografts. J Neurosurg 102: 706-714,
cytes in patients with gastrointestinal cancer: results of a
randomized controlled clinical trial. World J Gastroenterol
22. Morris DL and Adams WJ: Cimetidine and colorectal cancer - old
10: 136-142, 2004.
drug, new use? Nat Med 1: 1243-1244, 1995.
44. Kubota T, Fujiwara H, Ueda Y, Itoh T, Yamashita T,
23. Adams WJ, Lawson JA and Morris DL: Cimetidine inhibits
Yoshimura T, Okugawa K, Yamamoto Y, Yano Y and
in vivo growth of human colon cancer and reverses histamine
Yamagishi H: Cimetidine modulates the antigen presenting
stimulated in vitro and in vivo growth. Gut 35: 1632-1636,
capacity of dendritic cells from colorectal cancer patients. Br
J Cancer 86: 1257-1261, 2002.
24. Reynolds JL, Akhter J and Morris DL: In vitro effect of histamine
45. Horvath BV, Szalai C, Mandi Y, Laszlo V, Radvany Z, Darvas Z
and histamine H1 and H2RAs on cellular proliferation of human
and Falus A: Histamine and histamine-receptor antagonists
malignant melanoma cell lines. Melanoma Res 6: 95-99, 1996.
modify gene expression and biosynthesis of interferon gamma
25. Natori T, Sata M, Nagai R and Makuuchi M: Cimetidine inhibits
in peripheral human blood mononuclear cells and in CD19-
angiogenesis and suppresses tumor growth. Biomed Pharmacother
depleted cell subsets. Immunol Lett 70: 95-99, 1999.
59: 56-60, 2005.
46. Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA,
26. Osband ME, Hamilton D, Shen YJ, Cohen E, Shlesinger M,
Chrousos GP and Wilder RL: Histamine potently suppresses
Lavin P, Brown A and McCaffrey R: Successful tumour immuno-
human IL-12 and stimulates IL-10 production via H2 receptors.
therapy with cimetidine in mice. Lancet i: 636-638, 1981.
J Immunol 161: 2586-2593, 1998.
27. Hel strand K and Hermodsson S: Histamine H2-receptor-mediated
47. Smith T: Histamine type 2-receptor antagonists and cancer
regulation of human natural killer cell activity. J Immunol 137:
immunotherapy. Compr Ther 16: 8-13, 1990.
656-660, 1986.
48. Creagan ET, Schaid DJ, Ahmann DL and Frytak S: Disseminated
28. Gifford RR and Tilberg AF: Histamine type-2 receptor antagonist
malignant melanoma and recombinant interferon: analysis of
immune modulation. II. Cimetidine and ranitidine increase
seven consecutive phase II investigations. J Invest Dermatol 95:
interleukin-2 production. Surgery 102: 242-247, 1987.
188S-192S, 1990.
29. Kobayashi K, Matsumoto S, Morishima T, Kawabe T and
49. Kinouchi T, Saiki S, Maeda O, Kuroda M, Usami M and
Okamoto T: Cimetidine inhibits cancer cell adhesion to endo-
Kotake T: Treatment of advanced renal cell carcinoma with a
thelial cells and prevents metastasis by blocking E-selectin
combination of human lymphoblastoid interferon-alpha and
expression. Cancer Res 60: 3978-3984, 2000.
cimetidine. J Urol 157: 1604-1607, 1997.
30. Watson SA, Wilkinson LJ, Robertson JF and Hardcastle JD:
50. Bobek V, Boubelik M, Kovarik J and Taltynov O: Inhibition
Effect of histamine on the growth of human gastrointestinal
of adhesion breast cancer cells by anticoagulant drugs and
tumours: reversal by cimetidine. Gut 34: 1091-1096, 1993.
cimetidine. Neoplasma 50: 148-151, 2003.
31. Hahm KB, Park IS, Kim HC, Lee KJ, Kim JH, Cho SW and
51. Vestweber D and Blanks JE: Mechanisms that regulate the
Lee SI: Comparison of antiproliferative effects of 1-histamine-2
function of the selectins and their ligands. Physiol Rev 79:
receptor antagonists, cimetidine, ranitidine and famotidine, in
181-213, 1999.
gastric cancer cells. Int J Immunopharmacol 18: 393-399, 1996.
52. Morbidelli L, Brogelli L, Crancer HJ and Ziche M: Endothelial
32. Rajendra S, Mulcahy H, Patchett S and Kumar P: The effect of
cel migration is induced by soluble P-selectin. Life Sci 62: 7-11,
H2 antagonists on proliferation and apoptosis in human colo-
rectal cancer cell lines. Dig Dis Sci 49: 1634-1640, 2004.
53. Lefranc F, Mijatovic T, Mathieu V, Rorive S, Decaestecker C,
33. Surucu O, Middeke M, Hoschele I, Kalder J, Hennig S, Dietz C
Debeir O, Brotchi J, van Ham P, Salmon I and Kiss R: Chara-
and Celik I: Tumour growth inhibition of human pancreatic
cterization of gastrin-induced proangiogenic effects in vivo in
cancer xenografts in SCID mice by cimetidine. Inflamm Res 53
orthotopic U373 experimental human glioblastomas and in vitro
(Suppl 1): S39-S40, 2004.
in human umbilical vein endothelial cells. Clin Cancer Res 10:
34. Takahashi K, Tanaka S, Furuta K and Ichikawa A: Histamine
8250-8265, 2004.
H(2) receptor-mediated modulation of local cytokine expression
54. Kaji M, Ishikura H, Kishimoto T, Omi M, Ishizu A, Kimura C,
in a mouse experimental tumor model. Biochem Biophys Res
Takahashi T, Kato H and Yoshiki T: E-selectin expression
Commun 297: 1205-1210, 2002.
induced by pancreas-carcinoma-derived interleukin-1 alpha
35. Takahashi K, Tanaka S and Ichikawa A: Effect of cimetidine on
results in enhanced adhesion of pancreas-carcinoma cells to
intratumoral cytokine expression in an experimental tumor.
endothelial cells. Int J Cancer 60: 712-717, 1995.
Biochem Biophys Res Commun 281: 1113-1119, 2001.
55. Khatib AM, Kontogiannea M, Fallavollita L, Jamison B,
36. Lawson JA, Adams WJ and Morris DL: Ranitidine and cimetidine
Meterissian S and Brodt P: Rapid induction of cytokine and
differ in their in vitro and in vivo effects on human colonic
E-selectin expression in the liver in response to metastatic
cancer growth. Br J Cancer 73: 872-876, 1996.
tumor cells. Cancer Res 59: 1356-1361, 1999.
1021-1030 20/3/06 19:21 Page 1029
INTERNATIONAL JOURNAL OF ONCOLOGY 28: 1021-1030, 2006
56. Weston BW, Hil er KM, Mayben JP, Manousos GA, Bendt KM,
79. Kucharczak J, Pannequin J, Camby I, Decaestecker C, Kiss R
Liu R and Cusack JC Jr: Expression of human alpha(1,3)fucosyl-
and Martinez J: Gastrin induces over-expression of genes involved
transferase antisense sequences inhibits selectin-mediated
in human U373 glioblastoma cell migration. Oncogene 20:
adhesion and liver metastasis of colon carcinoma cells. Cancer
7021-7028, 2001.
Res 59: 2127-2135, 1999.
80. Mariani L, McDonough WS, Hoelzinger DB, Beaudry C,
57. Burton EC and Prados MD: Malignant gliomas. Curr Treat
Kaczmarek E, Coons SW, Giese A, Moghaddam M, Seiler RW
Option Oncol 1: 459-468, 2000.
and Berens ME: Identification and validation of P311 as a
58. Lefranc F, Brotchi J and Kiss R: Possible future issues in the
glioblastoma invasion gene using laser capture microdissection.
treatment of glioblastomas: special emphasis on cell migration
Cancer Res 61: 4190-4196, 2001.
and the resistance of migrating glioblastoma cells to apoptosis.
81. Rickman DS, Bobek MP, Misek DE, Kuick R, Blaivas M,
J Clin Oncol 23: 2411-2422, 2005.
Kurnit DM, Taylor J and Hanash SM: Distinctive molecular
59. Kleihues P and Cavenee WK: Pathology and Genetics of
profiles of high-grade and low-grade gliomas based on oligo-
Tumours of the Nervous System. International Agency for
nucleotide microarray analysis. Cancer Res 61: 6885-6891,
Research on Cancer (IARC). WHO Health Organisation,
Oxford. IARC Press, Lyon, 2000.
82. Tatenhorst L, Senner V, Puttmann S and Paulus W: Regulators
60. Brandes AA: State-of-the-art treatment of high-grade brain
of G-protein signaling 3 and 4 (RGS3, RGS4) are associated with
tumors. Semin Oncol 30: 4-9, 2003.
glioma cell motility. J Neuropathol Exp Neurol 63: 210-222,
61. De Angelis LM: Benefits of adjuvant chemotherapy in high-
grade gliomas. Semin Oncol 30: 15-18, 2003.
83. Hoelzinger DB, Mariani L, Weis J, Woyke T, Berens TJ,
62. Laws ER, Parney IF, Huang W, Anderson F, Morris AM,
McDonough WS, Sloan A, Coons SW and Berens ME: Gene
Asher A, Lillehei KO, Bernstein M, Brem H, Sloan A, et al:
expression profile of glioblastoma multiforme invasive pheno-
Survival following surgery and prognostic factors for recently
type points new therapeutic targets. Neoplasia 1: 7-16, 2005.
diagnosed malignant glioma: data from the Glioma Outcomes
84. Paulus W, Baur I, Dours-Zimmermann MT and Zimmermann DR:
Project. J Neurosurg 99: 467-473, 2003.
Differential expression of versican isoforms in brain tumors. J
63. MacDonald DR: New frontiers in the treatment of malignant
Neuropathol Exp Neurol 55: 528-533, 1996.
glioma. Semin Oncol 30: 72-76, 2003.
85. Gladson CL: The extracellular matrix of gliomas: modulation
64. Bigner SH, Matthews MR, Rasheed BK, Wiltshire RN,
of cell function. J Neuropathol Exp Neurol 58: 1029-1040,
Friedman HS, Friedman AH, Stenzel TT, Dawes DM,
McLendon RE and Bigner DD: Molecular genetic aspects of
86. Rutka JT, Apodaca G, Stern R and Rosenblum M: The extra-
oligodendrogliomas including analysis by comparative genomic
cellular matrix of the central and peripheral nervous systems:
hybridization. Am J Pathol 155: 375-386, 1999.
structure and function. J Neurosurg 69: 155-170, 1988.
65. Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM,
87. Rutka JT, Myatt CA, Giblin JR, Davis RL and Rosenblum ML:
Hammond RR, Silver JS, Stark PC, MacDonald DR, Ino Y, et al:
Distribution of extracellular matrix proteins in primary human
Specific genetic predictors of chemotherapeutic response and
brain tumours: an immunohistochemical analysis. Can J Neurol
survival in patients with anaplastic oligodendrogliomas. J Natl
Sci 14: 25-30, 1987.
Cancer Inst 90: 1473-1479, 1998.
88. Kanamori M, van den Berg SR, Bergers G, Berger MS and
66. Nutt CL, Mani DR, Betensky RA, Tamayo P, Cairncross JG,
Pieper RO: Integrin beta3 overexpression suppresses tumor
Ladd C, Pohl U, Hartmann C, McLaughlin ME, Batchelor TT,
growth in a human model of gliomagenesis: implications for the
et al: Gene expression-based classification of malignant gliomas
role of beta3 overexpression in glioblastoma multiforme. Cancer
correlates better with survival than histological classification.
Res 64: 2751-2758, 2004.
Cancer Res 63: 1602-1607, 2003.
89. Paulus W, Baur I, Schuppan D and Roggendorf W: Chara-
67. Dunn IF and Black PM: The neurosurgeon as local oncologist:
cterization of integrin receptors in normal and neoplastic human
cel ular and molecular neurosurgery in malignant glioma therapy.
brain. Am J Pathol 143: 154-163, 1993.
Neurosurgery 52: 1411-1422, 2003.
90. Camby I, Belot N, Rorive S, Lefranc F, Maurage CA, Lahm H,
68. Giese A, Bjerkvig R, Berens ME and Westphal M: Cost of
Kaltner H, Hadari Y, Ruchoux MM, Brotchi J, et al: Galectins
migration: invasion of malignant gliomas and implications for
are differentially expressed in supratentorial pilocytic astro-
treatment. J Clin Oncol 21: 1624-1636, 2003.
cytomas, astrocytomas, anaplastic astrocytomas and glioblastomas
69. Belien AT, Paganetti PA and Schwab ME: Membrane-type 1
and significantly modulate tumor astrocyte migration. Brain
matrix metalloprotease (MT1-MMP) enables invasive migration
Pathol 11: 12-26, 2001.
of glioma cells in central nervous system white matter. J Cell
91. Camby I, Decaestecker C, Gordower L, De Decker R, Kacem Y,
Biol 144: 373-384, 1999.
Lemmers A, Siebert HC, Bovin NV, Wesseling P, Danguy A,
70. Klemke RL, Leng J, Molander R, Brooks PC, Vuori K and
et al: Distinct differences in binding capacity to saccharide
Cheresh DA: CAS/Crk coupling serves as a ‘molecular switch'
epitopes in supratentorial pilocytic astrocytomas, astrocytomas,
for induction of cell migration. J Cell Biol 140: 961-972,
anaplastic astrocytomas and glioblastomas. J Neuropathol Exp
Neurol 60: 75-84, 2001.
71. Puchner MJ and Giese A: Tamoxifen-resistant glioma-cell sub-
92. Camby I, Decaestecker C, Lefranc F, Kaltner H, Gabius HJ and
populations are characterized by increased migration and
Kiss R: Galectin-1 knocking down in human U87 glioblastoma
proliferation. Int J Cancer 86: 468-473, 2000.
cells alters their gene expression pattern. Biochem Biophys Res
72. Giancotti FG and Ruoslahti E: Integrin signaling. Science 285:
Commun 335: 27-35, 2005.
1028-1032, 1999.
93. Camby I, Salmon I, De Decker R, Pasteels JL, Brotchi J,
73. Hood JD and Cheresh DA: Role of integrins in cell invasion and
Danguy A and Kiss R: Lectin histochemistry of astrocytic tumors
migration. Nat Rev Cancer 2: 91-100, 2002.
and in vitro characterization of lectin-induced modifications on
74. Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA and
the proliferation of the SW1088, U373 and U87 human astro-
Horwitz AF: Integrin-ligand binding properties govern cell
cytic cell lines. J Neurooncol 34: 111-122, 1997.
migration speed through cell-substratum adhesiveness. Nature
94. Yates AJ, Comas T, Scheithauer BW, Burger PC and Pearl DK:
385: 537-540, 1997.
Glycolipid markers of astrocytomas and oligodendrogliomas.
75. Camby I, Belot N, Lefranc F, Sadeghi N, De Launoit Y,
J Neuropathol Exp Neurol 58: 1250-1262, 1999.
Kaltner H, Musette S, Darro F, Danguy A, Salmon I, et al:
95. Reardon DA, Akabani G, Coleman RE, Friedman AH,
Galectin-1 modulates human glioblastoma cell migration into
Friedman HS, Herndon JE II, Cokgor I, McLendon RE,
the brain through modifications to the actin cytoskeleton and
Pegram CN, Provenzale JM, et al: Phase II trial of murine
levels of expression of smal GTPases. J Neuropathol Exp Neurol
(131)I-labeled antitenascin monoclonal antibody 81C6 admi-
61: 585-596, 2002.
nistered into surgically created resection cavities of patients
76. Lefranc F, Camby I, Belot N, Bruyneel E, Chaboteaux C,
with newly diagnosed malignant gliomas. J Clin Oncol 20:
Brotchi J, Mareel M, Salmon I and Kiss R: Gastrin significantly
1389-1397, 2002.
modifies the migratory abilities of experimental glioma cells.
96. Branle F, Lefranc F, Camby I, Jeuken J, Geurts-Moespot A,
Lab Invest 82: 1241-1252, 2002.
Sprenger S, Sweep F, Kiss R and Salmon I: Evaluation of the
77. Raftopoulou M and Hall A: Cell migration: Rho GTPases lead
efficiency of chemotherapy in in vivo orthotopic models of
the way. Dev Biol 265: 23-32, 2004.
human glioma cells with and without 1p19q deletions and in C6
78. Rao JS: Molecular mechanisms of glioma invasiveness: the role
rat orthotopic allografts serving for the evaluation of surgery
of proteases. Nat Rev Cancer 3: 489-501, 2003.
combined with chemotherapy. Cancer 95: 641-655, 2002.
1021-1030 20/3/06 19:21 Page 1030
LEFRANC et al: CIMETIDINE AND MALIGNANT GLIOMAS
97. Lefranc F, Sadeghi N, Metens T, Brotchi J, Salmon I and Kiss R:
103. Aguilera B, Romero-Ramirez L, Abad-Rodriguez J, Corrales G,
Characterization of gastrin-induced cytostatic effect on cell
Nieto-Sampedro M and Fernandez-Mayoralas A: Novel
proliferation in experimental malignant gliomas. Neurosurgery
disaccharide inhibitors of human glioma cell division. J Med
52: 881-890, 2003.
Chem 41: 4599-4606, 1998.
98. Hernandez-Angeles A, Soria-Jasso LE, Ortega A and Arias-
104. Nieto-Sampedro M, Bailon C, Fernandez-Mayoralas A,
Montano JA: Histamine H1 receptor activation stimulates mito-
Martin-Lomas M, Mellstrom B and Naranjo JR: Experimental
genesis in human astrocytoma U373 MG cells. J Neurooncol
brain glioma: growth arrest and destruction by a blood-group-
55: 81-89, 2001.
related tetrasaccharide. J Neuropathol Exp Neurol 55: 169-177,
99. Van der Ven LT, Prinsen IM, Jansen GH, Rohol PJ, Defferrari R,
Slater R and den Otter W: Growth of cultured human glioma
105. Chiariotti L, Salvatore P, Frunzio R and Bruni CB: Galectin
tumour cells can be regulated with histamine and histamine
genes: regulation of expression. Glycoconj J 19: 441-449,
antagonists. Br J Cancer 68: 475-483, 1993.
100. Finn PE, Purnell P and Pilkington GJ: Effect of histamine and
106. Delbrouck C, Doyen I, Belot N, Decaestecker C, Ghanooni R,
the H2 antagonist cimetidine on the growth and migration of
De Lavareille A, Kaltner H, Choufani G, Danguy A, van den
human neoplastic glia. Neuropathol Appl Neurobiol 22: 317-324,
Hoven G, et al: Galectin-1 is overexpressed in nasal polyps
under budesonide and inhibits eosinophil migration. Lab Invest
101. Korr H: Proliferation and cell cycle parameters of astrocytes.
82: 147-158, 2002.
In: Astrocytes. Vol. 3. Fedoroff S and Vernadakis A (eds).
107. Git MA and Barondes SH: Genomic sequence and organization
Academic Press Inc. Ltd., London, pp77-127, 1986.
of two members of a human lectin gene family. Biochemistry
102. Nieto-Sampedro M: Astrocyte mitogen inhibitor related to
30: 82-89, 1991.
epidermal growth factor receptor. Science 240: 1784-1785, 1988.
Source: http://www.integratedhealthclinic.com/assets/byCancerType/Primary%20Brain/5-Cimetidine%20and%20GBM.pdf
VOLUME 2 ISSUE 9 SEPTEMBER 2014 Regional medical practice of concomitant medication CDSCO - REGULATORY MATTERS 1. CONSIDERATION OF ETHNICITY FOR APPROVAL OF NEW DRUGS Severity distribution of eligible subjects. Similarity of dose and dosage regimen. Based on the recommendation of the expert committee constituted by the MINISTRY OF HEALTH AND FAMILY
User manual v1.2EWK2 compatible with: • ESIM264 v7.14.00 and up + EWT1 v16.18 and up. • ESIM364 v02.04.10 and up. • EPIR2 v01.01.08 and up. • EPIR3 all versions EWK2A compatible with: • ESIM364 v02.10.03 and up. • EPIR2 v01.02.00 and up. • EPIR3 v01.03.00 and up. Main features:• Alarm system arming & disarming;




