Joelkostka.net
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Oct. 2001, p. 4566–4572
Copyright 2001, American Society for Microbiology. All Rights Reserved.
Growth and Phylogenetic Properties of Novel Bacteria Belonging to
the Epsilon Subdivision of the
Proteobacteria Enriched from
Alvinella pompejana and Deep-Sea Hydrothermal Vents
BARBARA J. CAMPBELL,1 CHRISTIAN JEANTHON,2 JOEL E. KOSTKA,3
GEORGE W. LUTHER III,1 AND S. CRAIG CARY1*
College of Marine Studies, University of Delaware, Lewes, Delaware 199581
; UMR6539, Centre National de la
Recherche Scientifique and Universite´ de Bretagne Occidentale, 29280 Plouzane´, France2
; and
Department of Oceanography, Florida State University, Tallahassee, Florida 323063
Received 20 March 2001/Accepted 4 July 2001
Recent molecular characterizations of microbial communities from deep-sea hydrothermal sites indicate the
predominance of bacteria belonging to the epsilon subdivision of Proteobacteria (epsilon Proteobacteria). Here,
we report the first enrichments and characterizations of four epsilon Proteobacteria that are directly associated
with Alvinella pompejana, a deep sea hydrothermal vent polychete, or with hydrothermal vent chimney samples.
These novel bacteria were moderately thermophilic sulfur-reducing heterotrophs growing on formate as the
energy and carbon source. In addition, two of them (Am-H and Ex-18.2) could grow on sulfur lithoautro-
trophically using hydrogen as the electron donor. Optimal growth temperatures of the bacteria ranged from 41
to 45°C. Phylogenetic analysis of the small-subunit ribosomal gene of the two heterotrophic bacteria demon-
strated 95% similarity to Sulfurospirillum arcachonense, an epsilon Proteobacteria isolated from an oxidized
marine surface sediment. The autotrophic bacteria grouped within a deeply branching clade of the epsilon Pro-
teobacteria, to date composed only of uncultured bacteria detected in a sample from a hydrothermal vent along
the mid-Atlantic ridge. A molecular survey of various hydrothermal vent environments demonstrated the pres-
ence of two of these bacteria (Am-N and Am-H) in more than one geographic location and habitat. These re-
sults suggest that certain epsilon Proteobacteria likely fill important niches in the environmental habitats of
deep-sea hydrothermal vents, where they contribute to overall carbon and sulfur cycling at moderate thermo-
Several recent molecular studies have demonstrated the
report that suggests that an epsilon
Proteobacteria belonging to
presence and dominance of bacteria belonging to the epsilon
the
Arcobacter group is involved in filamentous sulfur produc-
subdivision of
Proteobacteria (epsilon
Proteobacteria) that are
tion at hydrothermal vents, although it has not been isolated or
both free-living and found in association with metazoans at
phylogenetically characterized (44). Similar filamentous pro-
deep-sea hydrothermal vents (4, 5, 14, 25, 29, 35; Campbell et
duction of sulfur occurred in continuous-flow H2S reactors
al., unpublished data). Epsilon
Proteobacteria have also been
with an
Arcobacter sp. isolated from shallow coastal marine
detected and/or isolated from deep subsurface sediments, oil
waters (43).
fields, activated sludge, and marine snow (13, 20, 34, 40, 45).
Our laboratory has been investigating the symbiotic relation-
Until now, however, epsilon
Proteobacteria have not been cul-
ship between
Alvinella pompejana, a deep-sea hydrothermal
tured from hydrothermal vent environments. All epsilon
Pro-
vent polychete, and the morphologically and phylogenetically
teobacteria isolated to date are involved in the sulfur cycle by
diverse episymbiont community that is integrated into its dor-
either reducing elemental sulfur to sulfide or oxidizing sulfide
sal epithelium (4, 5, 7, 14). We have demonstrated through a
to sulfur. In many cases, a single bacterium is able to do both
variety of molecular techniques that members of a single clade
(24, 37). A hallmark of the epsilon
Proteobacteria is their ability
of the epsilon
Proteobacteria dominate the microbial commu-
to utilize a variety of electron acceptors, including oxygen
nity (4, 5, 14). Several attempts have been made in the past to
(under microaerophilic conditions), nitrate, several sulfur spe-
isolate
A. pompejana epibionts under mesophilic, aerobic, and
cies, and, in some cases, arsenate, selenate, manganese, and
heterotrophic conditions (16, 31–33). In a recent study, we
Fe(III) (12, 18, 27, 28, 42). Because of these capabilities, it is
confirmed that these attempts did not isolate any epsilon
Pro-
not surprising that they flourish at hydrothermal vents, where
teobacteria and that members of the
A. pompejana episymbiont
there are high levels of many sulfur species as well as an
community identified previously by molecular studies (14) are
abundance of heavy metals (10, 17, 22, 23, 36).
not present in these extensive culture collections (Campbell et
Although inferences can be drawn about the biochemistry of
al., unpublished data).
hydrothermal vent epsilon
Proteobacteria, little is actually
The goal in this study was to isolate epsilon
Proteobacteria
known about the chemical and thermal conditions needed for
from the dorsal epithelium of
A. pompejana by enrichment
the growth of this dominant bacterial group. There is one
culture techniques. Positive enrichments would likely further
our understanding of the biochemical conditions necessary for
both epibiont and free-living bacterial growth. In addition, we
* Corresponding author. Mailing address: College of Marine Stud-
ies, University of Delaware, Lewes, DE 19958. Phone: (302) 645-4078.
extended our epsilon
Proteobacteria isolation attempts to sul-
Fax: (302) 645-4007, E-mail:
[email protected].
fidic chimney samples from hydrothermal vents at 13°N lati-
NOVEL HYDROTHERMAL VENT ε PROTEOBACTERIA
TABLE 1. Details of samples used for enrichments and PCR analysis
Vent (latitude, longitude)
Grandbonum (12°48⬘7⬙N, 103°56⬘4⬙W)
PP55 (12°49⬘84⬙N⬘, 103°56⬘8⬙W)
PP57 (12°50⬘32⬙N, 103°56⬘80⬙W)
PPHot14 (12°48⬘18⬙N, 103°56⬘39⬙W)
Grandbonum (12°48⬘7⬙N, 103°56⬘4⬙W)
PPHot14 (12°48⬘18⬙N, 103°56⬘39⬙W)
PP57 (12°50⬘32⬙N, 103°56⬘80⬙W)
Q vent (9°50⬘727⬙N, 104°17⬘58⬙W)
M vent (9°50⬘83⬙N, 104°17⬘58⬙W)
K2 (27°00⬘84⬙N, 111°24⬘48⬙W)
Robin's Roost (27°00⬘88⬙N, 111°24⬘63⬙W)
Robin's Roost (27°00⬘88⬙N, 111°24⬘63⬙W)
K2 (27°00⬘84⬙N, 111°24⬘48⬙W)
Kristin's Summit (27°00⬘83⬙N, 111°24⬘68⬙W)
Rebecca's Roost (27°00⬘66⬙N, 111°24⬘42⬙W)
a Growth with acetate, pyruvate, formate, and sulfur at 50°C for the 13°N samples and acetate, formate and sulfur at 45°C for the Guaymas samples. Based on
morphologic and identical migrations on DGGE. ND, not done.
tude along the East Pacific Rise (EPR) and at the Guaymas
Positive enrichments were subcultured five times on board ship and/or in the
basin. A molecular survey of various hydrothermal vent sites
laboratory until stable cultures (cultures that did not change, based on micro-
was also performed to investigate the ecology of two novel
scopic or molecular analysis) were obtained. These subcultures were consideredpure when microscopic and molecular evidence indicated only one type of bac-
epsilon
Proteobacteria that were enriched from the 13°N
A.
terium per culture. DNA was extracted from all the positive enrichments and
pomejana epibiont community.
stable cultures and subjected to DNA fingerprinting analysis (denaturing gradi-ent gel electrophoresis [DGGE]) as described previously (4, 38). Universal prim-ers for both bacteria and archaea were used in the DGGE analysis to assess the
MATERIALS AND METHODS
purity of the cultures. In addition purity of the cultures was assessed by micro-
Sampling and enrichment conditions. Initial enrichments were from
A. pom-
scopic analysis.
pejana worms sampled from various hydrothermal vents along the EPR during
Growth characterizations. Two of the epsilon
Proteobacteria cultures that were
the Amistad cruise, May to June 1999, to 13°N (12°49⬘N, 103°56⬘W) at a depth
considered pure (Am-H and Am-N) were subjected to limited physiological
of approximately 2,500 m (Table 1).
A. pompejana specimens (in their associated
assessment. Initial cultures were grown in the sulfur medium described above
tubes) were collected and transported to the surface via the deep-submergence
with the addition of formate and acetate as potential electron donor and carbon
vehicle (DSV) Nautile in an enclosed container. Once on board,
A. pompejana
worms were removed from their tubes and associated chimneys, and washed
The temperature range tested was from 30 to 65°C. Other carbon sources
three times in sterile 0.22-m-filtered seawater, and the epibiont community
(CO2, pyruvate [20 mM], fumarate [0.2%], 0.2% peptone, formate [10 mM], and
(found mainly on the hair-like projections) were scraped into a sterile 50-ml tube.
acetate [10 mM]), electron donors (H2 and formate), and electron acceptors
The hairs were slightly homogenized with a 20-gauges needle in a final volume of
[sulfite (5 mM), thiosulfate (10 mM), and Fe(III)] were evaluated. In addition,
10 ml containing sterile seawater and aseptically transferred to anaerobic me-
growth with various gas mixtures (H2-CO2, 90:10, 150 kPa, and N2, 100%, 150
dium (described below). A portion of the homogenate was saved for DNA
kPa) was tested. Growth using Fe(III)-oxyhydroxide was evaluated on media
extraction and preservation in glycerol at ⫺80°C.
prepared and manipulated as above (47).
For the enrichments from chimney samples, sulfides were collected from the
We modified the medium for Fe reducers by adding 2 mM ferrous chloride as
Guaymas basin, a sediment- and hydrocarbon-rich hydrothermal site in the Gulf
a reductant in place of dissolved sulfide. Amorphous Fe(III)-oxyhydroxide was
of California (27°00⬘N, 111°24⬘W) at a depth of approximately 2,000 m, during
added to the autoclaved medium as the sole electron acceptor to a final concen-
the Extreme 2000 cruise, January 2000 (Table 1). The outsides of chimneys were
tration of 50 mM. The Fe(III) oxide was prepared by neutralizing a solution of
scraped aseptically into sterile tubes, and anaerobic sterile seawater was added
FeCl3, rinsing, and autoclaving as described previously (21). All medium prep-
(10⫻, vol/vol).
aration and manipulations were carried out under strictly anoxic conditions
Approximately 1 ml of diluted sample (hairs or chimney) was used for each
unless otherwise specified. Sterile medium components for the Fe(III) medium
were combined, and the medium was dispensed into serum bottles which were
Medium used for enrichments was modified from that of Widdel and Bak (47)
sealed with butyl rubber stoppers under a gas stream of 90% N2 and 10% CO2
and contained (per liter): 20 g of NaCl, 3 g of MgCl
(100 kPa). Inocula for all the metabolic characterizations were 1/20th volume.
2 䡠 6H2O, 0.15 g of CaCl2 䡠
Positive cultures were subcultured an additional time to confirm growth in the
2O, 0.5 g of KCl, 0.25 g of NH4Cl, 0.2 g of KH2PO4, 1 ml of trace element
solution (46), 1 ml of selenite-tungstate solution, 0.015 g of resazurin, 30 ml of
tested medium (and not growth from the original inoculum). Negative cultures
were tested from the source inoculum at least twice. Additional negative controls
3, 1 ml of vitamin mixture solution, 1 ml of vitamin B12 solution, 1 ml
of thiamine solution, and 5 ml of 0.2 M Na
included growth with no added substrates (other than the basal minimal medium
2S as a reductant (47). Elemental
sulfur (approximately 5 g/liter) was sterilized by heating to 100°C three times and
with and without added sulfur). Cells were counted after 3 days by epifluorescent
added aseptically after the medium was autoclaved. The final pH was adjusted to
microscopy after fixation with 3.7% formaldehyde, staining with DAPI (4⬘,6⬘-
approximately 7.0, and the headspace consisted of N2-CO2 (80:20; 150 kPa).
diamidino-2-phenylindole) (2 g/ml), and filtration onto a 0.22-m polycarbon-
A combination of three potential electron donors and carbon sources (formate
ate filter (30). Growth was scored as positive if there was a greater than fivefold
[20 mM final concentration], acetate [2 mM final concentration], and pyruvate
increase in cells compared to control tubes with no added substrates.
[20 mM final concentration]) was added separately from sterile, anoxic stocks to
Growth curves of the stable subcultures were performed four times at their
individual tubes before inoculation for the 13°N enrichments. Enrichments from
optimal temperatures in minimal enrichment medium with added sulfur and
the chimneys collected at Guaymas basin were performed in the sulfur medium
formate (20 mM) under an N2-CO2 gas headspace. Growth was also measured in
with added formate and acetate. The
A. pompejana enrichments were incubated
sulfur medium without formate. Cells were counted by epifluorescent microscopy
at 30, 50, and 65°C, while the chimney enrichments were incubated at 45 and
as described above.
60°C. Growth was monitored microscopically.
Hydrogen sulfide production was measured using the Cline method (6). Light
CAMPBELL ET AL.
APPL. ENVIRON. MICROBIOL.
photomicrographs were obtained after staining with DAPI (2 g/ml) as de-
TABLE 2. Characteristics of Am-N and Am-H enriched
scribed above. The lengths and widths of the bacteria were measured on an
from
A. pompejana episymbiont biomass
Olympus Provis AX70 microscope using a 100⫻ objective with a Chroma 31000band pass filter set. Lengths and widths of the bacteria were estimated from a
frequency plot of the values for approximately 100 individual bacteria.
Slightly curved rods
Phylogenetic assessment. The 16S ribosomal DNAs (rDNAs) of the bacteria
were amplified from extracted DNA using the 21F and 1518R primers as de-
scribed previously (14) and cloned into a Topo-TA vector (Invitrogen, Carlsbad,
Calif.) according to the manufacturer's instructions. The resulting 16S rDNA
clones were bidirectionally sequenced on an ABI 310 sequencer (Applied Bio-
Temp optimum (°C)
systems, Inc. [ABI], Foster City, Calif.) using the TA vector-specific primers
Growth
a on carbon source
M13F and M13R (Invitrogen) as well as 519F, 519R, 1100F, and 1100R (1).
(in presence of S0)
DNA sequences were assembled using the ABI Autoassembler program (ABI)
and aligned to other 16S rDNA sequences using Genetic Data Environment
(GDE) (39) as described previously (14). DNA distance similarities were deter-
mined by the method of Olsen (26). Neighbor-joining and parsimony trees were
Autotrophic growth (with H2 in
obtained in GDE as previously described (14).
Presence of bacteria in the environment. DNA was extracted from the
A.
Electron donor (with S0)
pompejana epibiont samples listed in Table 1 using an Isoquick DNA extraction
kit (ORCA Research, Bothwell, Wash.) as described previously (4). Several
DNA extraction protocols were used on various chimney-flange samples (Table
1) to evaluate extraction efficiencies and potential PCR inhibition effects.
DNA was initially extracted from approximately 500 l of ground chimney
samples (slurries) from 9°N and 13°N with acetyltrimethylammonium bromide–
Pyruvate, peptone
polyvinylpyrrolidone–-mercaptoethanol (CTAB/PVP/-ME) method as de-
scribed previously (8) and resuspended in 50 l of sterile H2O. We found better
Thiosulfate, sulfite
yields and less inhibition when extracting from an equal amount of chimney
slurry with the QIAamp DNA stool mini kit (Qiagen, Valencia, Calif.). DNA wasextracted from the Guaymas chimney-flange samples using this kit and resus-
a As measured by turbidity, microscopic counts, and H2S production.
pended in 50 l of sterile H
Positive growth on sulfur granules only (microscopic evaluation and H
2O. From 1 to 10 ng of DNA was used in PCR for
DGGE analysis as described previously (4, 38). The universal forward primer
production; cultures were not turbid).
c In the presence of formate and acetate.
338F (with a GC clamp) was also used in combination with two strain-specific16S rDNA primers (for Am-H, H607R [5⬘- CTCCCGAACTCTAGTCTGA],and for Am-N, N601R [5⬘- CTAGATAAACAGTTTCAAGA], based on
Esch-
cultures in the same medium as above, it was also confirmed to
erichia coli numbering [3]) in PCR amplifications for DGGE to determine the
be a single bacterium by both microscopy and DGGE analysis
presence of strain Am-H or Am-N in the indicated samples.
(data not shown). Compared to the first bacterium, it grew fast-
Nucleotide sequence accession numbers. The 16S rDNA sequences for Am-H,
Am-N, Ex-18.1, and Ex-18.2 were deposited in GenBank and assigned accession
er at 50°C, was smaller in size, and was also motile (Table 2).
numbers AF357197, AF357198, AF357199, and AF357196, respectively.
Two other epsilon
Proteobacteria, designated Ex-18.1 and
Ex-18.2, were enriched from several chimney samples collected
from hydrothermal vents in the Guaymas basin using similar
conditions as above, except the initial incubation temperature
Enrichments. Enrichments from
A. pompejana samples col-
was reduced to 45°C and the sulfur medium contained only
lected from 13°N that were grown at 30°C yielded bacteria that
formate and acetate. The first bacterium, Ex-18.1, was mor-
varied widely in their morphologies, while little to no growth
phologically and phylogenetically similar to Am-N. Morpho-
occurred at 65°C. Successful enrichments of two morphologi-
logic and DGGE analysis of enrichments from K2 and Robin's
cally different populations of bacteria that were grown at 50°C
Roost indicated that bacteria identical to Ex-18.1 were also
were obtained from
A. pompejana samples collected from two
found at these vent sites (Table 1 and data not shown). The
separate hydrothermal vent sites (Table 1). Initially, both en-
types of samples used in the enrichments were somewhat dif-
richments contained a dominant bacterial morphotype, with
ferent; the sample from K2 was a flange outcropping, while the
several minor morphotypes. After subculturing, only the dom-
sample from Robin's Roost was a sulfidic chimney. The second
inant bacterium in each enrichment was detected by DGGE
chimney bacterium, Ex-18.2, was enriched from three other
and microscopy. The first bacterial morphotype recovered
samples collected from Guaymas: Robin's Roost flange, an-
from three separate
A. pompejana specimens (A, N, and X)
other K2 flange, and a flange collected from Kristin's Summit
grew on acetate-formate-sulfur medium (Table 1). These cul-
(Table 1 and data not shown). According to morphologic and
tures consisted of slow-growing (doubling time, ⬎24 h at 50°C)
DGGE analysis, it was morphologically and phylogenetically
motile vibrioid cells (Table 2). Stable subcultures of the dom-
similar to Am-H.
inant morphotypes were obtained after decreasing the incuba-
Preliminary characterization of bacteria and growth rates.
tion temperature to 45°C. DGGE analysis of the three subcul-
Two of the isolates, Am-H and Am-N, were chosen for further
tures demonstrated three identically migrating bands that were
characterization. Am-H and Am-N are slightly curved rods
indistinguishable by sequence analysis of 110 bp (data not
with widths of 0.3 and 0.3 m and lengths of 0.4 and 0.8 m,
shown). These strains were considered similar. Therefore, a
respectively (Table 2). Am-H is highly motile, while Am-N is
subculture from the N enrichment was chosen to be charac-
less motile. As shown in Table 2, the temperature growth
terized; it was designated Am-N.
ranges of these organisms varied slightly; Am-H generally grew
The second bacterial morphotype (Am-H) was enriched
at higher temperatures (up to 55°C but not above) and had a
from a single
A. pompejana specimen collected from PP55, also
higher temperature optimum (45°C) than Am-N (50 and 41°C,
located along the EPR at 13°N (Table 1). After several sub-
respectively). Growth curves for the two bacteria were per-
NOVEL HYDROTHERMAL VENT ε PROTEOBACTERIA
Phylogenetic affiliations. According to their 16S rDNA se-
quences, Am-N and Am-H and their close relatives from the
Guaymas basin (Ex-18.1 and Ex-18.2) grouped into the epsilon
subdivision of the
Proteobacteria (Fig. 2). Am-N showed 99.2%
identity with Ex-18.1 and 96.5 and 94.5% identity with
Sulfu-
rospirillum arcachonense and
Sulfurospirillum barnesii, respec-
tively, by DNA distance analysis. Am-H and Ex-18.2 (Fig. 2)
were much more distantly related to the
Sulfurospirillum,
grouping into a previously described deeply branching epsilon
clade that contains uncultured 16S rDNA clones from a hy-
drothermal vent cap deployed at the Snake Pit vent along the
mid-Atlantic ridge (35). Am-H was 99% identical to Ex-18.2
and showed 95.4 and 87.5% identity to VC2.1 Bac43 and
VC2.1 Bac30, respectively.
Ecological significance of isolates. Nine
A. pompejana sam-
ples and nine chimney or flange samples (samples designated
by letters and numbers in Table 1) were tested by PCR with
strain-specific primers, followed by DGGE analysis for the
presence of bacteria with migration patterns identical to those
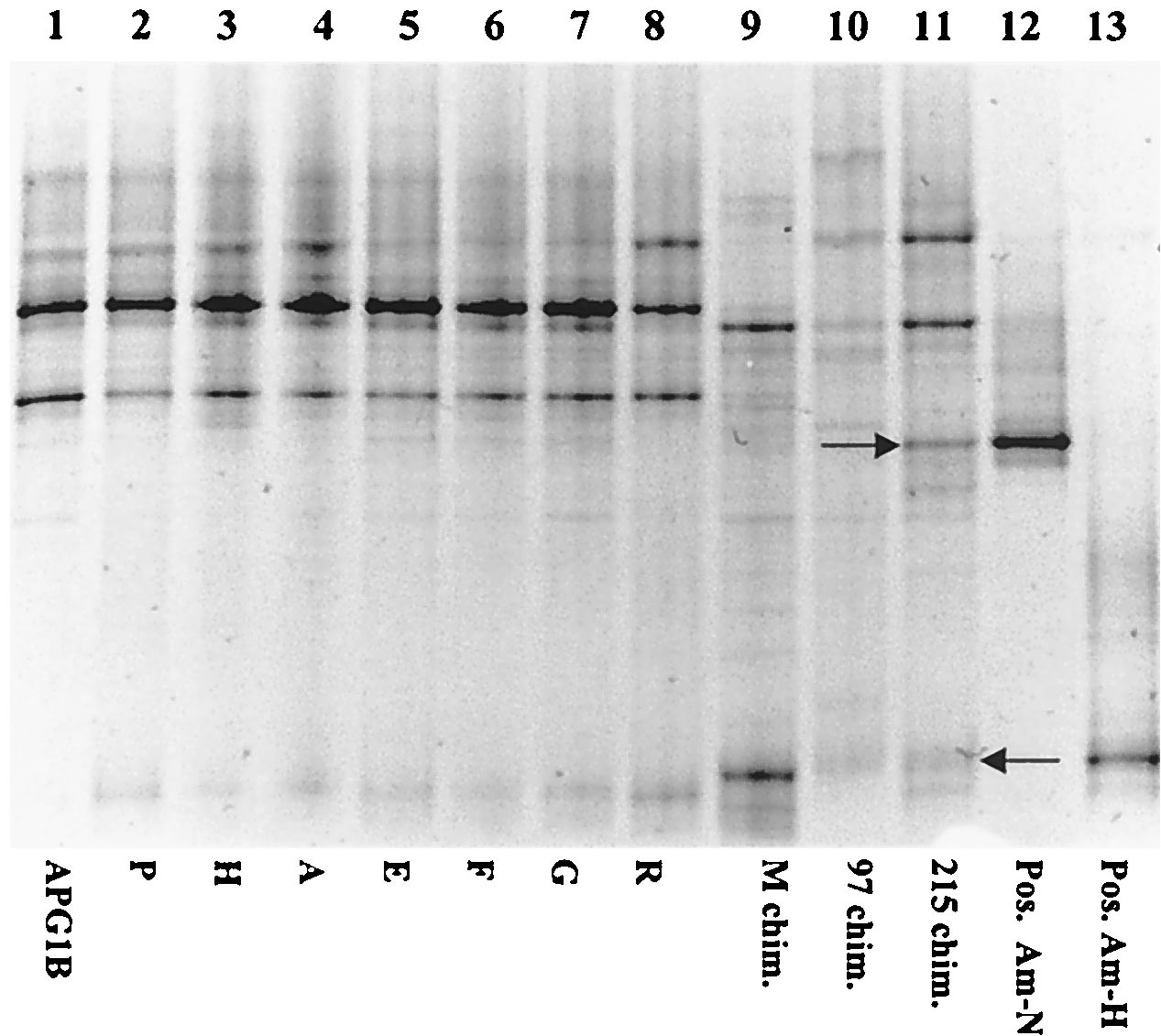
of either Am-N or Am-H (Fig. 3). Positive PCRs which mi-
grated identically to Am-N on a DGGE gel were obtained
from all
A. pompejana specimens tested from the 13°N latitude
with the primer designed to specifically amplify Am-N (repre-
sentative amplifications are shown in Fig. 3A). Bands migrat-
ing identically to Am-N were also amplified from DNA ex-
tracted from two chimney samples, one from 13°N (M chim.)
and one from 9°N (97 chim.). No positive PCRs were obtained
with the Am-N-specific primer with DNA extracted from one
chimney sample from 9°N (215 chim.) or from any of the
samples collected from the Guaymas basin. Similar results
FIG. 1. Representative growth curves of strains Am-H (A, }) and
were obtained with an Am-H-specific primer. However, two
A.
Am-N (B, ■). Cell densities (solid lines) and hydrogen sulfide produc-
tion (dashed lines) were measured with formate as the electron donor
pompejana specimens were negative (Am-G and Am-N), while
and elemental sulfur as the electron acceptor at their individual tem-
all the chimney samples from 9°N and 13°N were positive (Fig.
perature optimums under anaerobic conditions (N2-CO2 gas phase).
3B). Very weak amplification products were obtained with an
Controls were inoculated tubes without added formate (F).
Am-H-specific primer on two samples collected from the
Guaymas basin, and three others were negative.
The
A. pompejana worms and the three chimney samples
formed at least four separate times in a basal minimal medium
from 9°N and 13°N were also tested for the presence of the
with added elemental sulfur and formate. Representative
isolates Am-H and Am-N by DGGE analysis with universal
primers to detect all bacteria present in the samples (Fig. 4).
curves are illustrated in Fig. 1. Am-N had a slightly longer
The
A. pompejana bacterial communities from 13°N contained
doubling time than Am-H (9 h versus 6 h, respectively), as
very similar members, as indicated by the number of identically
calculated by the slope of the growth curves during the linear
migrating bands. However, no bands corresponding to Am-H
phase of growth (Fig. 1). Based on the graphic comparison of
or Am-N were observed on the gel, suggesting that these bac-
the number of cells per mole of H2S produced, growth yields of
teria were minor members of the communities tested. This was
Am-H were approximately twice that of Am-N (data not
also the case for two of the chimney samples (M and 97).
Chimney 215 did have observable bands migrating similarly to
Growth of Am-N and Am-H was tested with a limited series
PCR amplicons from the bacterial cultures Am-H and Am-N
of gas mixtures, electron acceptors, and carbon sources (Table
(indicated by the arrows in Fig. 4). After sequencing these
2). Both Am-N and Am-H grew heterotrophically using for-
highly visible bands, it was determined that they were not
mate as a carbon source and sulfur as the electron acceptor.
identical to either Am-H or Am-N, confirming the previous
They were not able to use thiosulfate, sulfite, and Fe(III)-
negative PCR results with the Am-N-specific primers on the
oxyhydroxide as alternative electron acceptors in the presence
sample from chimney 215.
of formate. None used acetate as an energy and carbon source.
With sulfur, Am-H was able to grow lithoautotrophcally using
hydrogen and formate as electron donors and heterotrophi-cally in the presence of pyruvate. Fermentation of fumarate
This is the first report of the isolation of
Proteobacteria belong-
was performed by Am-N. The ability of Am-N and Am-H to
ing to the epsilon subdivision from deep-sea hydrothermal vents.
grow under low levels of oxygen and with nitrate as electron
Our and previous PCR-based experiments demonstrated that in
acceptors was not tested.
hydrothermal vent environments, epsilon
Proteobacteria dominate
CAMPBELL ET AL.
APPL. ENVIRON. MICROBIOL.
FIG. 2. Phylogenetic tree showing the relationships between isolated strains with other members of the epsilon subdivision of the
Proteobacteria.
The trees are based on alignments of approximately 1,500 bp from the 16S rDNA gene minus insertions, deletions, and ambiguous bases.
E. coli
was used as the outgroup. Bootstrap values from 100 resamplings are indicated prior to the branch points of the tree. Sequences from the isolates
are marked in boldface type. The scale bar represents the calculated number of changes per nucleotide position.
the free-living organisms on the outer surfaces of chimneys and/or
not unique to the
Proteobacteria, but has only been described
are closely associated with invertebrate hosts (4, 5, 14, 25, 29).
in one other previously identified epsilon
Proteobacteria (an
Large percentages of diverse epsilon
Proteobacteria have also
Arcobacter sp.) isolated from oil field brine (13). Until this
been detected from a vent cap deployment along the mid-Atlantic
report, all anaerobic elemental sulfur-reducing chemolithoau-
ridge (35). These reports suggest that the epsilon subdivision of
totrophic bacteria described from hydrothermal vents were
Proteobacteria plays a major role in the bacterial communities at
thermophiles and hyperthermophiles (2, 19, 41). Many sulfur-
hydrothermal vents. One of the strains described here (Am-H)
oxidizing chemolithoautotrophs have been described from ma-
has some properties similar to other cultured epsilon
Proteobac-
rine environments, including hydrothermal vents, but these
teria, such as growth with fumarate. However, both strains are
microorganisms oxidize sulfides in the presence of O2 (9, 15).
novel in that they grow at moderate thermophilic temperatures.
Alternate chemolithoautotrophic metabolisms involving the
While we have no direct evidence for carbon fixation by Am-H,
disproportionation of elemental sulfur have been described in
the deeply branching epsilon
Proteobacteria are able to au-
marine environments (11), but have not been described from
totrophically use elemental sulfur, hydrogen, and CO2 for growth
bacteria isolated at deep-sea hydrothermal vents or by epsilon
under anaerobic conditions.
Proteobacteria. It seems likely that the deeply branching epsi-
Autotrophy involving the reduction of elemental sulfur is
lon
Proteobacteria described here (Am-H), and possibly other
NOVEL HYDROTHERMAL VENT ε PROTEOBACTERIA
The epsilon Proteobacteria described in this paper were en-
riched from both A. pompejana samples and geographically dis-
tinct chimney samples from deep-sea hydrothermal vents from
13°N and the Guaymas basin. Because of the presumed and
measured chemical differences in the samples from the sediment-
starved EPR and the hydrocarbon and sediment-rich Guaymas
basin (10, 22, 23; Luther et al., unpublished data), we were ini-
tially surprised by our ability to enrich for such phylogenetically
similar bacteria (99%) from these two areas. We therefore believe
that the physiological abilities of the cultured epsilon Proteobac-
teria reported in this paper are possibly far more diverse than we
have described. The molecular survey that demonstrated these
isolates at geographically and chemically distinct hydrothermal
vent sites (9°N, 13°N, and the Guaymas basin) supports the
FIG. 3. DGGE of positive PCR products obtained after amplifica-
hypothesis that these epsilon Proteobacteria potentially have
tion using strain-specific primers for Am-N (A) and Am-H (B). Lanes
wide physiological abilities.
1 to 8, separated amplification products obtained from samples of
various A. pompejana epibiont biomass and hydrothermal chimney
While we were able to cultivate hydrothermal vent epsilon
samples as listed in Table 1. Lane 9, positive controls (Am-N in A,
Proteobacteria from the A. pompejana episymbiont community as
Am-H in B). A slight frown occurred in the gel shown in panel B.
well as from chimney samples, we were unsuccessful in enriching
for the dominant filamentous epsilon Proteobacteria phylotypes
phylogenetically similar bacteria (35), fill an important niche in
found integrated into the hair-like projections on the worm's
the environmental habitat of deep-sea hydrothermal vents,
dorsal epithelium (5, 14). We found, during the course of this
where they may contribute both to an increase in biomass and
investigation, that the medium designed for cultivation of epsilons
to overall carbon production.
was limited and selected for specific growth of two types of epsi-
Two of the epsilons (Am-N and Ex-18.1) described in this
lon Proteobacteria. The culturing conditions were restricted by
report phylogenetically group with the Sulfurospirillum spp., a
temperature range, carbon source used, electron donor-acceptor
distinct clade within the epsilon subdivision of Proteobacteria (12,
pairs tested, and pH. Any one or a combination of these factors
37, 42). Other members of the Sulfurospirillum group are not able
will need to be tested further for potential growth of the free-
to grow at 42°C but have pH requirements similar to that of
living counterparts of the dominant episymbionts that were de-
Am-N. Sulfurospirillum spp. also use a variety of electron
tected in chimney samples during our previous investigation (5).
donors and are able to ferment fumarate (42). S. arca-
Furthermore, as determined by their relative band intensities by
chonense, the closest phylogenetic representative to Am-N,
DGGE analysis, neither Am-N nor Am-H was numerically dom-
also seems very close metabolically since, like Am-N, it does
inant in any of the 13°N A. pompejana samples or chimney sam-
not reduce thiosulfate, sulfite, and Fe(III). However, another
ples from 9°N. However, a bacterium phylogenetically identical to
species of this genus, S. barnesii, is able to use a diverse spec-
Am-H was detected by DGGE analysis in a 10⫺7 dilution of a
trum of electron acceptors, including arsenate, selenate, and
hydrothermal vent chimney enrichment for Fe(III) reducers from
Fe(III) (18, 27, 42), indicating the potential of diverse physio-
the same cruise at 13°N (38). Additionally, phylogenetically sim-
logical abilities of these bacteria, an adaptation certainly ap-
ilar deeply branching bacteria have been observed at other hy-
propriate for organisms thriving in deep-sea hydrothermal vent
drothermal vent sites devoid of A. pompejana specimens (35). It
seems likely, then, that Am-H (or phylogenetically similar bacte-
ria) exists in higher numbers in the chimney samples than on A.
pompejana specimens from 13°N EPR.
Bacteria belonging to the epsilon subdivision of the Proteo-
bacteria are clearly important in the ecology of hydrothermal
vents, as indicated by their dominance in several molecular
surveys (4, 14, 25, 35). The enrichment of autotrophic and
heterotrophic epsilon Proteobacteria contributes to our under-
standing of carbon and sulfur cycling in hydrothermal vent
environments. Our successful culturing of four phylogeneti-
cally distinct epsilon Proteobacteria from different hydrother-
mal vent environments paves the way for more biochemical
testing of these isolates and further attempts to culture addi-
tional epsilons from these extreme environments.
FIG. 4. DGGE of positive PCR products obtained after amplifica-
This research was supported by grants to S.C.C. from the LEXEN
tion using universal primers. Lanes 1 to 11, separated amplification
initiative (OCE-9907666) and the Delaware Sea Grant Program (R/
products obtained from samples of various A. pompejana epibiont
B37) as well as a LEXEN initiative grant to G. Luther and S.C.C.
biomass and hydrothermal chimney samples as listed in Table 1. Lanes
(OCE-9729784). The Amistad cruise was organized by the Centre
12 and 13, positive controls. Arrows indicate bands that were reampli-
National de la Recherche Scientifique.
fied for DNA sequencing.
We gratefully acknowledge the following people for their technical
CAMPBELL ET AL.
APPL. ENVIRON. MICROBIOL.
assistance: L. Waidner, S. L'Haridon, D. Dalton, and M. Cottrell. We
hydrothermal vent waters. J. Environ. Manage. 62:61–66.
thank K. Coyne, C. DiMeo, and two anonymous reviewers for critically
23. Luther, G. W., T. F. Rozan, M. Taillefert, D. B. Nuzzio, C. Di Meo, T. M.
reviewing the manuscript. We thank the captains and crews of the R/Vs
Shank, R. A. Lutz, and S. C. Cary. 2001. Chemical speciation drives hydro-
Atlantis and L'Atalante and especially the pilots of the DSVs Alvin and
thermal vent ecology. Nature 410:813–816.
Nautile for their essential roles in the collection of specimens.
24. Macy, J. M., I. Schroder, R. K. Thauer, and A. Kroger. 1986. Growth the
Wolinella succinogenes on H2S plus fumarate and on formate plus sulfur as
energy-sources. Arch. Microbiol. 144:147–150.
25. Moyer, C. L., F. C. Dobbs, and D. M. Karl. 1995. Phylogenetic diversity of
1. Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identifi-
the bacterial community from a microbial mat at an active, hydrothermal
cation and in situ detection of individual microbial cells without cultivation.
vent system, Loihi Seamount, Hawaii. Appl. Environ. Microbiol. 61:1555–1562.
Microbiol. Rev. 59:143–169.
26. Olsen, G. J. 1988. Phylogenetic analysis using ribosomal RNA. Methods
2. Blochl, E., R. Rachel, S. Burggraf, D. Hafenbradl, H. W. Jannasch, and K. O.
Stetter. 1997. Pyrolobus fumarii, gen. and sp. nov., represents a novel group
27. Oremland, R. S., J. S. Blum, C. W. Culbertson, P. T. Visscher, L. G. Miller,
of archaea, extending the upper temperature limit for life to 113 degrees C.
P. Dowdle, and F. E. Strohmaier. 1994. Isolation, growth, and metabolism of
an obligately anaerobic, selenate-respiring bacterium, strain SES-3. Appl.
3. Brosius, J., T. J. Dull, D. D. Sleeter, and H. Noller. 1981. Gene organization
Environ. Microbiol. 60:3011–3019.
and primary structure of a ribosomal RNA operon from Escherichia coli. J.
28. Pfenning, N., and H. Biebl. 1981. The dissimilatory sulfur-reducing bacteria,
Mol. Biol. 148:107–127.
p. 941–947. In M. P. Starr, H. Stolp, H. G. Truper, A. Balows, and H. G.
4. Campbell, B. J., and S. C. Cary. 2001. Characterization of a novel spirochete
Schlegel (ed.), The prokaryotes, vol. 1, Springer-Verlag, New York, N.Y.
associated with the hydrothermal vent polychaete annelid Alvinella pompe-
29. Polz, M. F., and C. M. Cavanaugh. 1995. Dominance of one bacterial phy-
jana. Appl. Environ. Microbiol. 67:110–117.
lotype at a mid-Atlantic Ridge hydrothermal vent site. Proc. Natl. Acad. Sci.
5. Cary, S. C., M. T. Cottrell, J. L. Stein, F. Camacho, and D. Desbruye res.
1997. Molecular identification and localization of a filamentous symbiotic
30. Porter, K. G., and Y. S. Feig. 1980. The use of DAPI for identifying and
bacteria associated with the hydrothermal vent annelid Alvinella pompejana.
counting aquatic microflora. Limnol. Oceangr. 25:943–948.
Appl. Environ. Microbiol. 63:1124–1130.
31. Prieur, D., and C. Jeanthon. 1987. Preliminary study of heterotrophic bac-
6. Cline, J. D. 1969. Spectrophotometric determination of hydrogen sulfide in
teria isolated from deep sea hydrothermal vent invertebrates: Alvinella pompe-
naturals waters. Limnol. Oceanogr. 14:454–458.
jana (Polychaete) and Bathymodiolus thermophilus (Bivalve). Symbiosis 4:87–98.
7. Cottrell, M. T., and S. C. Cary. 1999. Diversity of dissimilatory bisulfite re-
32. Prieur, D., S. Chamroux, P. Durand, G. Erauso, P. Fera, C. Jeanthon, L. Le
ductase genes of bacteria associated with the deep-sea hydrothermal vent poly-
borgne, G. Me´vel, and P. Vincent. 1990. Metabolic diversity in epibiotic flora
chaete annelid Alvinella pompejana. Appl. Environ. Microbiol. 65:1127–1132.
associated with the pompeii worms, Alvinella pompejana and Alvinella cau-
8. Dempster, E. L., K. V. Pryor, D. Francis, J. E. Young, and H. J. Rogers. 1999.
data (Polychaeta: Annelida) from deep-sea hydrothermal vents. Mar. Biol.
Rapid DNA extraction from ferns for PCR-based analyses. Biotechniques
33. Raguenes, G., P. Pignet, G. Gauthier, A. Peres, R. Christen, H. Rougeaux, G.
9. Durand, P., A. L. Reysenbach, D. Prieur, and N. Pace. 1993. Isolation and
Barbier, and J. Guezennec. 1996. Description of a new polymer-secreting
characterization of Thiobacillus hydrothermalis sp. nov., a mesophilic obli-
bacterium from a deep-sea hydrothermal vent, Alteromonas macleodii subsp.
gately chemolithotrophic bacterium isolated from a deep-sea hydrothermal
fijiensis, and preliminary characterization of the polymer. Appl. Environ.
vent in Fiji Basin. Arch. Microbiol. 159:39–44.
10. Edmond, J. M., and K. L. Von Damm. 1985. Chemisty of ridge crest hot
34. Rath, J., K. Y. Wu, G. J. Herndl, and E. F. DeLong. 1998. High phylogenetic
springs. Biol. Soc. Wash. Bull. 6:43–47.
diversity in a marine-snow-associated bacterial assemblage. Aquat. Microb.
11. Finster, K., W. Liesack, and B. Thamdrup. 1998. Elemental sulfur and
thiosulfate disproportionation by Desulfocapsa sulfoexigens sp. nov., a new
35. Reysenbach, A. L., K. Longnecker, and J. Kirshtein. 2000. Novel bacterial
anaerobic bacterium isolated from marine surface sediment. Appl. Environ.
and archaeal lineages from an in situ growth chamber deployed at a mid-
Atlantic Ridge hydrothermal vent. Appl. Environ. Microbiol. 66:3798–3806.
12. Finster, K., W. Liesack, and B. J. Tindall. 1997. Sulfurospirillum arca-
36. Rozan, T. F., S. M. Theberge, and G. Luther. 2000. Quantifying elemental
chonense sp. nov., a new-microaerophilic sulfur-reducing bacterium. Int. J.
sulfur (S0), bisulfide (HS⫺) and polysulfides (S 2⫺
) using a voltammetric
Syst. Bacteriol. 47:1212–1217.
method. Anal. Chim. Acta 415:175–184.
13. Gevertz, D., A. J. Telang, G. Voordouw, and G. E. Jenneman. 2000. Isolation
37. Schumacher, W., P. M. H. Kroneck, and N. Pfennig. 1992. Comparative
and characterization of strains CVO and FWKOB, two novel nitrate-reduc-
systematic study on "Spirillum" 5175, Campylobacter, and Wolinella spe-
ing, sulfide-oxidizing bacteria isolated from oil field brine. Appl. Environ.
cies—description of "Spirillum" 5175 as Sulfurospirillum deleyianum gen.,
nov. spec. nov. Arch. Microbiol. 158:287–293.
14. Haddad, M. A., F. Camacho, P. Durand, and S. C. Cary. 1995. Phylogenetic
38. Slobodkin, A., B. J. Campbell, S. C. Cary, E. Bonch-Osmolovskaya, and C. Jean-
characterization of the epibiotic bacteria associated with the hydrothermal
thon. 2001. Thermophilic Fe(III)-reducing microorganisms inhabit deep-sea
vent polychaete Alvinella pompejana. Appl. Environ. Microbiol. 61:1679–1687.
hydrothermal vents on the east Pacific rise. FEMS Microbiol. Ecol. 36:235–243.
15. Jannasch, H. W., C. O. Wirsen, D. C. Nelson, and L. A. Robertson. 1985.
39. Smith, S. W., R. Overbeek, G. Olsen, C. Woese, P. M. Gillevet, and W.
Thiomicrospira crunogena sp. nov., a colorless, sulfur-oxidizing bacterium
Gilbert. 1992. Genetic data environment and the Harvard genome database:
from a deep-sea hydrothermal vent. Int. J. Syst. Bacteriol. 35:422–424.
genome mapping and sequencing. Cold Spring Harbor Laboratory, Cold
16. Jeanthon, C., and D. Prieur. 1990. Susceptibility to heavy metals and char-
Spring Harbor, N.Y.
acterization of heterotrophic bacteria isolated from two hydrothermal vent
40. Snaidr, J., R. Amann, I. Huber, W. Ludwig, and K. H. Schleifer. 1997.
polychaetes, Alvinella pompejana and Alvinella caudata. Appl. Environ. Mi-
Phylogenetic analysis and in situ identification of bacteria in activated sludge.
Appl. Environ. Microbiol. 63:2884–2896.
17. Juniper, S. K., and J. Sarrizan. 1995. Interaction of vent biota and hydro-
41. Stetter, K. O. 1996. Hyperthermophilic procaryotes. FEMS Microbiol. Rev.
thermal deposits: present evidence and future experimentation, p. 178–193.
In S. E. Humpris, R. A. Zierenberg, L. S. Mullineaux, and R. E. Thomson
42. Stolz, J. F., D. J. Ellis, J. S. Blum, D. Ahmann, D. R. Lovley, and R. S.
(ed.), Seafloor hydrothermal systems: physical, chemical, biological, and
Oremland. 1999. Sulfurospirillum barnesii sp. nov. and Sulfurospirillum ar-
geological interactions. American Geophysical Union, Washington, D.C.
senophilum sp. nov., new members of the Sulfurospirillum clade of the epsilon
18. Laverman, A. M., J. S. Blum, J. K. Schaefer, E. J. P. Phillips, D. R. Lovley,
Proteobacteria. Int. J Syst. Bacteriol. 49:1177–1180.
and R. S. Oremland. 1995. Growth of strain SES-3 with arsenate and other
43. Taylor, C. D., and C. O. Wirsen. 1997. Microbiology and ecology of filamen-
diverse electron acceptors. Appl. Environ. Microbiol. 61:3556–3561.
tous sulfur formation. Science 277:1483–1485.
19. L'Haridon, S., V. Cilia, P. Messner, G. Raguenes, A. Gambacorta, U. B.
44. Taylor, C. D., C. O. Wirsen, and F. Gaill. 1999. Rapid microbial production
Sleytr, D. Prieur, and C. Jeanthon. 1998. Desulfurobacterium thermolithotro-
of filamentous sulfur mats at hydrothermal vents. Appl. Environ. Microbiol.
phum gen. nov., sp. nov., a novel autotrophic, sulpfur-reducing bacterium iso-
lated from a deep-sea hydrothermal vent. Int. J. Syst. Bacteriol. 48:701–711.
45. Watanabe, K., Y. Kodama, K. Syutsubo, and S. Harayama. 2000. Molecular
20. Li, L. N., C. Kato, and K. Horikoshi. 1999. Bacterial diversity in deep-sea
characterization of bacterial populations in petroleum-contaminated
sediments from different depths. Biodivers. Conserv. 8:659–677.
groundwater discharged from underground crude oil storage cavities. Appl.
21. Lovley, D. R., and E. J. P. Phillips. 1986. Organic-matter mineralization with
Environ. Microbiol. 66:4803–4809.
reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol.
46. Widdel, F. 1983. Methods for enrichment and pure culture isolation of
filamentous gliding sulfate-reducing bacteria. Arch. Microbiol. 134:282–285.
22. Luther, G. W., B. T. Glazer, L. Hohmann, J. I. Popp, M. Taillefert, T. F.
47. Widdel, F., and F. Bak. 1992. Gram-negative mesophilic sulfate-reducing
Rozan, P. J. Brendel, S. M. Theberge, and D. B. Nuzzio. 2001. Sulfur spe-
bacteria, p. 3352–3378. In A. Balows, H. G. Truper, M. Dworkin, W. Harder,
ciation monitored in situ with solid state gold amalgam voltammetric micro-
and K. H. Schleifer (ed.), The prokaryotes, vol. IV. Springer-Verlag, New
electrodes: polysulfides as a special case in sediments, microbial mats and
Source: http://www.joelkostka.net/pubs/CampbellAEM01.pdf
Anatomy 103 halts ongoing behaviours and drives exploration, will have connections (1) to the motor areas generating this pattern and (2) to that part of the hypothalamus driving pituitary output in response to environmental uncertainty. 3.2 The internal structure of the hippocampus Removal of the posterior and temporal neocortex of an animal such as the rat
International Journal of Pharmacology and Pharmaceutical Sciences 2016; Vol: 3, Issue: 3, 14-18 Research Article ISSN: 2394-613X FORMULATION AND EVALUATION OF FAST DISSOLVING TABLETS OF PERINDOPRIL USING NATURAL AND SYNTHETIC SUPER DISINTEGRANTS P. Sobhita Rani *, Srilakshmi N, T Neelima Rani, Singireddy Anandam Malla Reddy Pharmacy College, Maisammaguda, Dhulapally, Hyderabad (India) *Corresponding Author


