Kbrb.ioz.ac.cn
The Journal of Clinical Endocrinology & Metabolism 91(5):1956 –1960
Printed in U.S.A.
Copyright 2006 by The Endocrine Society
Isolation and Cultivation of Human Testicular
Peritubular Cells: A New Model for the Investigation of
Fibrotic Processes in the Human Testis and
Male Infertility
Martin Albrecht, Romi Ra¨msch, Frank M. Ko¨hn, J. Ullrich Schwarzer, and Artur Mayerhofer
Institute of Anatomy (M.A., R.R., A.M.), Ludwig-Maximilians-University, and Department of Dermatology and Allergy(F.M.K.), Technical University, 80802 Munich, Germany; and Department of Urology (J.U.S.), Freising Hospital, 85356Freising, Germany
Context: Fibrotic remodeling, especially of the tubule wall, in testes
tissues with antibodies against markers of fibroblasts (CD90/Thy-1)
of infertile men is common, but reasons or consequences of these
and smooth muscle cells (␣-smooth muscle actin) clearly proved their
striking changes are not known. Based on cell culture and
ex vivo
origin from the peritubular region. These cells displayed morpholog-
studies, we previously suggested that mast cells via their products
ical features of myofibroblasts, and gene array analyses as well as
tryptase and histamine are involved in the development of fibrosis.
immunohistochemistry revealed the predominant expression of ex-
However, studies in a relevant human testicular model are required
tracellular matrix genes and genes coding for basement membrane
to further test this hypothesis and the mechanisms of testicular fi-
components. The cultured cells retain receptors for the major mast
brosis in general.
cell products histamine and tryptase. The addition of histamine (100
M) and the tryptase agonist peptide SLIGKV (10 M) resulted in a
Objective: The objective of the study was the isolation, culture, and
transient increase in intracellular calcium levels, confirming the func-
characterization of adult human testicular peritubular cells.
tionality of the receptors.
Patients and Interventions: Peritubular cells were obtained from
Conclusions: We conclude that human peritubular cells are a novel
biopsies of men suffering from obstructive azoospermia (n ⫽ 8) and
model for the investigation of paracrine, including mast cell initiated,
varicocele (n ⫽ 2) but displaying normal spermatogenesis.
interactions in the human testis, which will allow the study of fibrotic
processes underlying male idiopathic infertility.
(J Clin Endocrinol
Results: Explant cultures were obtained from all biopsies. Immuno-
Metab 91: 1956 –1960, 2006)
staining of the cultured cells and corresponding paraffin-embedded
INTHETESTESofinfertilepatients,fibroticthickeningof mechanisms involved in MC-peritubular cell interactions
the peritubular region is a common observation and goes
and MC involvement in tubular fibrosis and male infertility.
in parallel with increased numbers of activated testicularmast cells (MCs) in these regions (1, 2). There is evidence that
Materials and Methods
MCs via their major secretory products tryptase and hista-
Isolation and culture of primary HTPCs
mine are crucially and causally involved in these eventsclosely associated with male infertility (2– 4).
Testicular tissue was obtained by open biopsy from eight vasecto-
mized men with obstructive azoospermia but displaying normal sper-
To gain deeper insight into how MCs and their secretory
matogenesis and from two men suffering from varicocele accompanied
products contribute to the fibrotic events leading to male
by only a slight reduction of spermatogenesis. Biopsies were obtained
infertility, human studies and thus suitable cell culture mod-
from vasectomized patients as a standardized procedure before religa-
els are needed. Although several cell lines and primary cells
tion of the spermatic ducts and from the varicocele patients duringsurgical treatment. Information concerning the state of spermatogenesis
of rodent peritubular cells have been established (5– 8), stud-
was derived by histological analyses of part of the biopsy samples. Ages
ies using adult human peritubular cells have not been
of the patients were 29, 32, 32, 34, 35, 36, 40, 41, 46 and 47 yr, respectively.
All participants granted written informed consent. The study was ap-
The purpose of this study was therefore to develop a
proved by the local ethics committee.
technique that will readily allow isolation and culture of
Immediately after retrieval in the operating room, the tissue was
transferred to Ham's F12 medium (PAA GmbH, Pasching, Austria)
human testicular peritubular cells (HTPCs). Such cells would
containing 20 mm HEPES (Sigma-Aldrich Chemie GmbH, Schellendorf,
be of great value for
in vitro experiments,
e.g. to explore
Germany), 0.5 g/liter NaHCO3 (Sigma-Aldrich), 15% (vol/vol) fetal calf
serum (FCS), 100 U penicillin, and 100 g/ml streptomycin (all fromPAA GmbH). Within 2–3 h, testicular tissue covered with medium was
First Published Online February 14, 2006
dissected using tweezers under sterile conditions into 1- to 2-mm3
Abbreviations: FCS, Fetal calf serum; H1, histamine-1; HTPC, human
pieces. The tissue was then placed in recalcified human plasma that was
testicular peritubular cell; MC, mast cell; PAR2, protease activated
positioned in drops of 10 –20 l onto the surface of a plastic cell culture
dish. The procedure allowed the specimens to be glued to the bottom of
JCEM is published monthly by The Endocrine Society (http://www.
the culture dish. Each biopsy sample yielded sufficient tissue for five to
endo-society.org), the foremost professional society serving the en-
six explants. The explants were incubated for 1–2 h under humidified
conditions (37 C, 5% CO2), checked for adherence, and subsequently
Albrecht
et al. • Isolation and Culturing of Human Peritubular Cells
J Clin Endocrinol Metab, May 2006, 91(5):1956 –1960
cultured in Ham's F12 medium composed as described above. Cells
Immunofluorescence methods were used as described elsewhere (12)
started to grow out of the biopsies after about 1 wk. When the cells
using the monoclonal antibodies mentioned above. The ␣-smooth mus-
covered an area of approximately 1 cm2, which took 2–3 wk, the remnant
cle actin antibody was diluted 1:200, whereas the CD90/Thy-1 antibody
explant was carefully removed, and cells were allowed to grow for
was used at a 1:50 dilution.
another week before they were trypsinized and subcultured. Subcul-
Staining for FSH and LH receptors, indicative of the occurrence of
tured cells were grown in DMEM ⫹ 10% FCS (both from PAA GmbH)
Sertoli cells and Leydig cells in the HTPC cultures, was performed using
without antibiotics. For all experiments, cells from passages 3–7 were
a polyclonal FSH receptor antibody (Acris GmbH, Hiddenhausen, Ger-
used. In addition, we found that cells can be grown for at least nine
many), diluted 1:500, and a polyclonal LH receptor antibody (Acris
passages and cryopreserved in DMEM containing 10% FCS and 5%
GmbH) at the same dilution. Controls consisted of nonimmune mouse/
dimethylsulfoxide. Thawed and recultured cells show a viability of more
rabbit normal serum (1:5000) or omission of the primary antibody.
Reverse transcription and PCR analysis
Transmission electron microscopy
RNA extraction was performed using the RNeasy minikit (QIAGEN
For ultrastructural studies, HTPCs were cultivated as described, fixed
GmbH, Hilden, Germany), followed by reverse transcription using
with 4% paraformaldehyde/0.5% glutaraldehyde, and postfixed with
15 or random hexamer primers and PCR amplification (13). The
4/potassium hexacyanoferrate (II). After embedding in Epon,
following primers were used: histamine-1 (H1) receptor, 5⬘-CTACAAG-
semithin and ultrathin sections were cut, contrasted with uranylacetate
GCCGTACGACA-3⬘ and 5⬘-CCTGCTCATCTGTCTTGA-3⬘, yielding a
(2%)/lead citrate (2.7%) as described (9, 10), and examined with an EM10
371-bp fragment; histamine-2 receptor, 5⬘-TCTACCGCATGCAA-
electron microscope (Zeiss, Jena, Germany).
GATC-3⬘ and 5⬘-CGAGGCTGATCATGAAGA-3⬘ in combination withthe following nested primers: 5⬘-TCATCCTCATCACCGTTG-3⬘ and 5⬘-
Gene arrays
TGGTAGATGGCAGAGAAG-3⬘, yielding a 155-bp fragment; hista-
Gene expression profiles were evaluated, using commercial chemi-
mine-3 receptor, 5⬘-ATGTACCCTACGTGCTGA-3⬘ and 5⬘-GTGAT-
luminescent human extracellular matrix gene array kits (SuperArray;
GAGGAAGTACCAG-3⬘ in combination with the following nested
Biomol GmbH, Hamburg, Germany) as described elsewhere (4). Gene
primers: 5⬘-CAACATCGTGCTCATCAG-3⬘ and 5⬘-TACTCCCAGCT-
arrays were performed in duplicates.
CAGGATG-3⬘, yielding a 158-bp fragment; histamine-4 receptor, 5⬘-TCTCAGTAGGTGCCAAAG-3⬘ and 5⬘-AGAATGGCCAGTGACTTG-3⬘in combination with the following nested primers: 5⬘-GAGACAGAG-
GAGAAAGAG-3⬘ and 5⬘-GGCTCTAAGCAGTTCAAC-3⬘, yielding a
After dissecting the testicular material into small pieces, one part was
142-bp fragment; and protease activated receptor-2 (PAR2), 5⬘-CATC-
explanted as described above, whereas a remaining part was fixed in
CTGCTAGCAGCCTC-3⬘ and 5⬘-ACCTCTGCACACTGAGGC-3⬘, yield-
Bouin's solution, embedded in paraffin, and sectioned. In preparation
ing a 480-bp fragment. Tissue library cDNA (CLONTECH, Palo Alto,
for immunohistochemistry, as previously reported (11), deparaffinized
CA) was used as positive control in all PCR experiments. Negative
tissue sections of human testes were treated with 3% H
controls were performed by omitting the respective input cDNA. The
for 20 min to block endogenous peroxidase activity and then incubated
identity of PCR products was verified by commercial sequencing (13).
with 5% normal goat serum for 30 min to reduce nonspecific antibodybinding. The sections were kept overnight at 4 C with a monoclonal
antihuman ␣-smooth muscle actin antibody (dilution 1:2000, clone 1A4;Sigma-Aldrich) and a monoclonal antihuman CD90/Thy-1 antibody
For calcium measurements, HTPCs were grown on glass coverslips
(dilution 1:50, clone AS02; Dianova, Hamburg, Germany) and probed
in DMEM supplemented with 10% FCS. The cells were loaded with 5 m
with a biotin-coupled goat antimouse antibody (1:500). The sites of
fluo-4, AM (Molecular Probes, Eugene, OR) in FCS-free DMEM for 30
immunoreaction were visualized by the ABC method (Vectastain elite
min at 37 C and 5% CO2 (for details, see Ref. 4). Finally, the cells were
kit, Vector Laboratories, Burlingame, CA) and addition of 3,3⬘-diami-
transferred into a recording chamber mounted on a TCS SP2 confocal
nobenzidine tetrahydrochloride solution containing H2O2. Controls con-
microscope (Leica Microsystems, Wetzlar, Germany). Fluorescence was
sisted of nonimmune mouse normal serum (1:5000) or omission of the
monitored at 500 –540 nm ( ⫽
488 nm) every 2 sec, and the intensity
primary antibody.
was quantified over single cells. Real-time changes of intracellular cal-
FIG. 1. Explant cultures of HTPCs. Small pieces of tes-ticular biopsies were seeded onto culture dishes (A). Be-tween wk 1 (B) and 2 (C), cells start growing out of thewalls of the seminiferous tubules (depicted with a T).
Cells subcultivated after 4 wk display an elongated phe-notype (D).
Bars, 50 m.
J Clin Endocrinol Metab, May 2006, 91(5):1956 –1960
Albrecht
et al. • Isolation and Culturing of Human Peritubular Cells
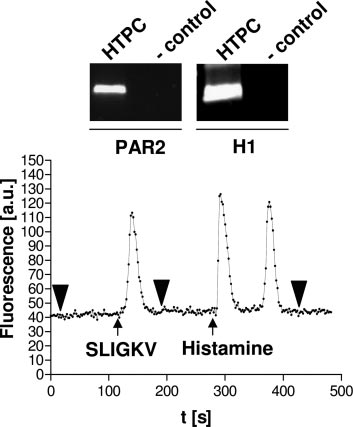
cium levels were recorded during application of 100 m histamine(Sigma-Aldrich), 100 m pyrilamine (RBI, Natick, MA), and 10 m of aPAR2 agonist peptide SLIGKV (SLIGKV-amide; NeoMPS, Strasbourg,France).
Morphology and characterization of the explant cultures
Explants of human testicular tissue (Fig. 1A) were cultured
as described in
Materials and Methods. Elongated cells becamevisible extending from the tubules between wk 1 and 2 (Fig.
1, B and C). These HTPC cultures were trypsinized andsubcultured after 4 wk (Fig. 1D) and showed a morphologythat was characterized by mainly elongated cells with fibro-blast/smooth muscle cell appearance (Fig. 1D). This myofi-broblastic morphology was retained up to at least nine pas-sages (data not shown).
HTPCs were immunonegative for FSH and LH receptors
(data not shown), thus excluding contaminations with Sertolior Leydig cells but stained specifically with antibodies di-rected against smooth muscle cell and fibroblast markers(␣-smooth muscle actin and CD90/Thy-1; Fig. 2, A and B).
Immunostaining of the corresponding paraffin-embeddedtesticular tissue (from which the explant cultures were de-rived) using the same antibodies resulted in a selective stain-ing of peritubular cells, proving clearly the peritubular originof HTPCs (Fig. 2, E and F).
Using electron microscopy experiments, electron dense
vesicles were detected in the cytoplasm of HTPCs (Fig. 3A).
Several authors (14, 15) previously reported the occurrenceof so-called intracellular collagen fibers in proliferating myo-fibroblasts; the vesicles found in HTPCs may represent or berelated to intracellular collagen fibers, although the finalproof for this is still missing.
HTPCs express several genes coding for proteins of the
extracellular matrix and basement membrane, such as col-lagen-I, collagen-IV, collagen-XVIII, fibronectin, secreted
FIG. 2. HTPCs show characteristics of smooth muscle cells and fi-
protein acidic and rich in cystein (SPARC), and laminin (Fig.
broblasts and originate from the peritubular region. HTPCs are pos-
3B). The synthesis of the proteins collagen-I and fibronectin
itive for smooth muscle cell and fibroblast markers [␣-smooth muscle
was shown by immunohistochemistry (Fig. 3, C and D).
actin (A) and CD90/Thy-1 (B)]. Sections of the corresponding testic-ular tissue from which the explant cultures were derived contain
HTPCs as model to investigate effects of MC products on
␣-smooth muscle actin-positive and CD90/Thy-1-positive cells in the
human peritubular cells
peritubular region (E and F). In addition to peritubular cells, smoothmuscle cells of small blood vessels are also positive for ␣-smooth
To examine the suitability of the established cell culture
muscle actin (E,
arrowhead). Respective negative controls performed
model of human peritubular cells as a system for the inves-
by omitting the primary antibody are shown in C, D, G, and H.
Bars,40 m.
tigation of paracrine, in particular, MC-mediated effects, thecapacity of HTPCs to react to the two major MC secretory
These cells are involved in the development of tubular fi-
products histamine and tryptase was investigated. Receptors
brosis, a hallmark of infertility in men, which is based on
for tryptase (PAR2) and histamine (H1) were shown to be
fibrotic remodeling and thickening of the peritubular region
expressed in HTPCs by RT-PCR (Fig. 4). Changes in intra-
due to increased cell proliferation and extracellular matrix
cellular calcium levels were evaluated after stimulation with
production (16, 17).
histamine and the PAR2 agonist SLIGKV. Both substances
Numbers of testicular MCs (1, 17, 18) and other immune
induced a reversible increase in intracellular calcium levels,
cells such as macrophages (13) and lymphocytes (17) are
proving the response of HTPCs to the two major MC prod-
increased in infertile patients, but their involvement in tu-
ucts (Fig. 4). The effect of histamine was mediated via H1
bular fibrosis is largely unknown. We and others (2– 4, 17)
because the specific H1 blocker pyrilamine was able to inhibit
recently provided evidence that activated testicular MCs are
histamine-induced calcium fluxes (data not shown).
promoting these fibrotic events and may therefore play acentral role in male idiopathic infertility. This hypothesis is
supported by studies showing that MC blockers are able to
This paper describes a rapid and economical method for
increase fertility in subfertile patients (19 –21).
the isolation and cultivation of human peritubular cells.
Although the proposal of an altered MC-fibroblast inter-
Albrecht et al. • Isolation and Culturing of Human Peritubular Cells
J Clin Endocrinol Metab, May 2006, 91(5):1956 –1960
FIG. 4. HTPCs possess functional tryptase and histamine receptorslinked to signal transduction events involving calcium. HTPCs ex-press receptors for tryptase (PAR2) and histamine (H1). IncubatingHTPCs with the PAR2 agonist SLIGKV (10 M) or histamine (100 M)results in an immediate and reversible increase in intracellular cal-cium levels. Note that histamine induces a biphasic signal. One rep-resentative experiment of a single-cell measurement of changes in
FIG. 3. HTPCs display a characteristic ultrastructure and express
intracellular calcium levels is shown. All experiments were indepen-
extracellular matrix and basement membrane genes. HTPCs are
dently carried out five times, with at least 10 single-cell determina-
characterized by an elongated phenotype containing various electron-
tions per experiment. Bold arrowheads denote the addition of buffer.
dense intracytoplasmic vesicles of unknown composition (A, arrows).
Gene array analyses reveal the expression of several molecules of theextracellular matrix and basement membrane (B). Collagen-I (C) and
we cannot completely exclude the possibility that HTPC
fibronectin (D) are two of the proteins typically produced by HTPCs.
cultures contain some vascular smooth muscle cells. Never-
E and F show respective negative controls performed by omitting theprimary antibody. Bars, 5 m (A) and 35 m (C–F). Asterisks (B)
theless, because the explants consisted mainly of seminifer-
denote basement membrane proteins. One representative array is
ous tubules with very few testicular stroma attached (Fig.
1A) and because the cells grew out of the tubule walls (Fig.
1B), we expect at most a very small fraction of vascular
action in the development of male infertility is very tempting
smooth muscle cells within the HTPC population.
and also supported by one of our previous studies evaluating
Gene arrays and immunohistochemistry revealed the ex-
the effects of MC products on human fetal foreskin fibro-
pression of several genes and proteins coding for basement
blasts (3), the final proof of such an interaction is missing and
membrane components (e.g. collagen-IV, fibronectin, lami-
requires the investigation of human peritubular cell systems.
nin, and secreted protein acidic and rich in cystein). Base-
Regarding the existence of human peritubular cell models,
ment membrane proteins have been shown to be expressed
we are aware of only one approach. Cigorraga et al. (22)
by peritubular cells of the rat testis (23, 24), and Pollanen et
described a method for the isolation and culture of human
al. (25) described the presence of laminin and type IV col-
peritubular cells from prepubertal patients. However, to the
lagen in the myoid cell layers of human seminiferous tubules.
best of our knowledge, there is no in vitro cell culture system
Therefore, these results further substantiate the peritubular
of adult human peritubular cells available so far.
characteristics of HTPCs.
In the study presented, we address the lack of human
Tryptase and histamine are typical mediators of activated
peritubular cell models and describe a method to obtain
testicular MCs and are believed to exert various effects on
HTPCs for in vitro studies using testicular biopsy explants.
nearby cells in the human testis (1, 2, 16, 17). To examine the
Cells growing out of the explants displayed an elongated
suitability of the established cell culture model of human
phenotype. Immunostaining of the cells and sections of the
peritubular cells as a system for the investigation of MC-
corresponding testicular tissue for smooth muscle cell- and
mediated effects, the ability of HTPCs to react to the two
fibroblast-specific markers revealed the origin of the cells
major MC secretory products, histamine and tryptase, was
from the peritubular region. In tissue sections, besides the
investigated. Both the tryptase analog SLIGKV and hista-
peritubular cells, smooth muscle cells of small blood vessels
mine induced a reversible increase of intracellular calcium
also stained positive with the anti-␣-smooth muscle actin
levels. Because SLIGKV is specific for the PAR2 receptor (26,
antibody, confirming the specificity of the used marker. A
27) and because histamine-induced calcium fluxes in fibro-
similar staining pattern was observed, with the fibroblast-
blasts are primarily mediated via the H1 receptor (28), we
specific antibody (CD90/Thy-1) and peritubular cells as well
suggest that these receptors are mainly responsible for the
as vascular smooth muscle cells (data not shown). Therefore,
induction of calcium signaling events in HTPCs.
J Clin Endocrinol Metab, May 2006, 91(5):1956 –1960
Albrecht et al. • Isolation and Culturing of Human Peritubular Cells
In summary, testicular biopsy material was derived from
for human and bovine growth hormones on age-related changes in ovarian
vasectomized patients displaying normal spermatogenesis.
morphology in mice. Anat Rec 227:175–186
10. Mayerhofer A, Hohne-Zell B, Gamel-Didelon K, Jung H, Redecker P, Grube
Although we propose that HTPC cultures represent cells of
D, Urbanski HF, Gasnier B, Fritschy JM, Gratzl M 2001 ␥-Aminobutyric acid
the normal testis, the majority of vasectomized men develop
(GABA): a para- and/or autocrine hormone in the pituitary. FASEB J 15:1089 –
sperm antibodies, which may have an impact on sperm func-
11. Fritz S, Fohr KJ, Boddien S, Berg U, Brucker C, Mayerhofer A 1999 Functional
tion and fertility (29).
and molecular characterization of a muscarinic receptor type and evidence for
We conclude that isolated HTPCs are a readily available
expression of choline-acetyltransferase and vesicular acetylcholine transporterin human granulosa-luteal cells. J Clin Endocrinol Metab 84:1744 –1750
model for adult human peritubular cells because they can be
12. Mayerhofer A, Hemmings Jr HC, Snyder GL, Greengard P, Boddien S, Berg
obtained from small amounts of testicular tissue. The estab-
U, Brucker C 1999 Functional dopamine-1 receptors and DARPP-32 are ex-
lished culture system will lead to a better understanding of
pressed in human ovary and granulosa luteal cells in vitro. J Clin EndocrinolMetab 84:257–264
the pathogenic determinants (30) and significance of fibrotic
13. Frungieri MB, Calandra RS, Lustig L, Meineke V, Kohn FM, Vogt HJ,
processes underlying male idiopathic infertility.
Mayerhofer A 2002 Number, distribution pattern, and identification of mac-
rophages in the testes of infertile men. Fertil Steril 78:298 –306
14. Dominguez-Malagon H 2004 Intracellular collagen and fibronexus in fibro-
matosis and other fibroblastic tumors. Ultrastruct Pathol 28:67–73
15. Welsh RA, Meyer AT 1967 Intracellular collagen fibers. In human mesenchy-
We thank Dr. Lars Kunz, Dr. Monica Frungieri, and Christoph Schell
mal tumors and inflammatory states. Arch Pathol 84:354 –362
for discussion and Annette Krieger, Gabriele Terfloth, and Barbara
16. Apa DD, Cayan S, Polat A, Akbay E 2002 Mast cells and fibrosis on testicular
Zschiesche for technical assistance. We especially thank Astrid Tiefen-
biopsies in male infertility. Arch Androl 48:337–344
bacher for cell culture work.
17. Hussein MR, Abou-Deif ES, Bedaiwy MA, Said TM, Mustafa MG, Nada E,
Ezat A, Agarwal A 2005 Phenotypic characterization of the immune and mast
Received September 30, 2005. Accepted February 7, 2006.
cell infiltrates in the human testis shows normal and abnormal spermatogen-
Address all correspondence and requests for reprints to: Professor
esis. Fertil Steril 83:1447–1453
18. Yamanaka K, Fujisawa M, Tanaka H, Okada H, Arakawa S, Kamidono S 2000
Artur Mayerhofer, M.D., Institute of Anatomy, Ludwig-Maximilians-
Significance of human testicular mast cells and their subtypes in male infer-
University, Biedersteiner Strasse 29, 80802 Munich, Germany. E-mail:
tility. Hum Reprod 15:1543–1547
19. Cayan S, Apa DD, Akbay E 2002 Effect of fexofenadine, a mast cell blocker,
This work was supported by the German Research Foundation (Deut-
in infertile men with significantly increased testicular mast cells. Asian J
sche Forschungsgemeinschaft) Ma 1080/16-1.
Androl 4:291–294
M.A., R.R., F.M.K., J.U.S., and A.M. have nothing to declare.
20. Hibi H, Kato K, Mitsui K, Taki T, Yamada Y, Honda N, Fukatsu H, Yamamoto
M 2001 The treatment with tranilast, a mast cell blocker, for idiopathic oli-
gozoospermia. Arch Androl 47:107–111
21. Matsuki S, Sasagawa I, Suzuki Y, Yazawa H, Tateno T, Hashimoto T, Nakada
1. Meineke V, Frungieri MB, Jessberger B, Vogt H, Mayerhofer A 2000 Human
T, Saito H, Hiroi M 2000 The use of ebastine, a mast cell blocker, for treatment
testicular mast cells contain tryptase: increased mast cell number and altered
of oligozoospermia. Arch Androl 44:129 –132
distribution in the testes of infertile men. Fertil Steril 74:239 –244
22. Cigorraga SB, Chemes H, Pellizzari E 1994 Steroidogenic and morphogenic
2. Albrecht M, Frungieri MB, Gonzalez-Calvar S, Meineke V, Kohn FM, May-
characteristics of human peritubular cells in culture. Biol Reprod 51:1193–1205
erhofer A 2005 Evidence for a histaminergic system in the human testis. Fertil
23. Konrad L, Albrecht M, Renneberg H, Ulrix W, Hoeben E, Verhoeven G,
Steril 83:1060 –1063
Aumuller G 2000 Mesenchymal entactin-1 (nidogen-1) is required for adhe-
3. Frungieri MB, Weidinger S, Meineke V, Kohn FM, Mayerhofer A 2002
sion of peritubular cells of the rat testis in vitro. Eur J Cell Biol 79:112–120
Proliferative action of mast-cell tryptase is mediated by PAR2, COX2, pros-
24. Richardson LL, Kleinman HK, Dym M 1995 Basement membrane gene ex-
taglandins, and PPAR␥: possible relevance to human fibrotic disorders. Proc
pression by Sertoli and peritubular myoid cells in vitro in the rat. Biol Reprod
Natl Acad Sci USA 99:15072–15077
4. Frungieri MB, Albrecht M, Raemsch R, Mayerhofer A 2005 The action of the
25. Pollanen PP, Kallajoki M, Risteli L, Risteli J, Suominen JJ 1985 Laminin and
mast cell product tryptase on cyclooxygenase-2 (COX2) and subsequent fi-
type IV collagen in the human testis. Int J Androl 8:337–347
broblast proliferation involves activation of the extracellular signal-regulated
26. Kawabata A, Kuroda R 2000 Protease-activated receptor (PAR), a novel family
kinase isoforms 1 and 2 (erk1/2). Cell Signal 17:525–533
of G protein-coupled seven trans-membrane domain receptors: activation
5. Skinner MK, Takacs K, Coffey RJ 1989 Transforming growth factor-␣ gene
mechanisms and physiological roles. Br J Pharmacol 82:171–174
expression and action in the seminiferous tubule: peritubular cell-Sertoli cell
27. Berger P, Tunon-De-Lara JM, Savineau JP, Marthan R 2001 Selected contri-
interactions. Endocrinology 124:845– 854
bution: tryptase-induced PAR-2-mediated Ca(2⫹) signaling in human airway
6. Skinner MK, Fetterolf PM, Anthony CT 1988 Purification of a paracrine
smooth muscle cells. J Appl Physiol 91:995–1003
factor, P-Mod-S, produced by testicular peritubular cells that modulates Sertoli
28. Johnson CL, Johnson CG, Bazan E, Garver D, Gruenstein E, Ahluwalia M
cell function. J Biol Chem 263:2884 –2890
1990 Histamine receptors in human fibroblasts: inositol phosphates, Ca2⫹, and
7. Kierszenbaum AL, Crowell JA, Shabanowitz RB, DePhilip RM, Tres LL 1986
cell growth. Am J Physiol 258:C533–C543
Protein secretory patterns of rat Sertoli and peritubular cells are influenced by
29. McDonald SW 2000 Cellular responses to vasectomy. Int Rev Cytol 199:295–
culture conditions. Biol Reprod 35:239 –251
8. Hutson JC, Garner CW, Stocco DM 1980 Effects of serum components on the
30. Mayerhofer A, Frungieri MB, Fritz S, Bulling A, Jessberger B, Vogt HJ 1999
morphology of Sertoli cells in culture. Anat Rec 197:205–211
Evidence for catecholaminergic, neuronlike cells in the adult human testis:
9. Mayerhofer A, Weis J, Bartke A, Yun JS, Wagner TE 1990 Effects of transgenes
changes associated with testicular pathologies. J Androl 20:341–347
JCEM is published monthly by The Endocrine Society (http://www.endo-society.org), the foremost professional society serving the
Source: http://kbrb.ioz.ac.cn/paper/kbrbPdf/16478819.pdf
Nunca nos bañamos dos veces en el mismo río, proclamó un filósofo griego, pero lo que no dijo es que el cambio genera oportunidades. Bajo esta premisa, y adaptándose a las nuevas necesidades del negocio, Novartis Argentina creó el Equipo de Cuentas Corporativas, cuyo objetivo es potenciar el vínculo con clientes clave partiendo de la importancia estratégica de los pacientes.
STANDING ORDERS FOR THE MANAGEMENT OF WARFARIN Dose adjustment and INR testing frequency Applicable to: Pharmacists Issued by: Pharmacy warfarin management project medical Standing Order used for the Pharmacy based anticoagulant management Project. Dr Paul Harper, Consultant Haematologist, Palmerston North Hospital To improve the safety of Warfarin management by providing anticoagulant control through a pharmacist led service using point of care testing (CoaguChek XS Plus) and online computer decision support (INR Online Ltd).


