Labex-csc.unistra.fr
JSPS Bilateral Joint Research Seminar
Interdisciplinary Seminar for
Innovative Organic Chemistry
Dec. 1, 2014 Dec. 3, 2014
The University of Strasbourg
Institut de Science et d'Ingénierie Supramoléculaires
1:15 - 2:00 PM Registration and Poster installation
2:00 - 2:10 PM Opening Remarks: Mir Wais Hosseini and Atsuko Hisada
Session 1:
Transition Metal Catalysis
Chair – Seiji Suga
2:10 - 3:00 PM Pierre Braunstein (Strasbourg)
3:05 - 3:55 PM Dominique Armspach (Strasbourg)
3:55 - 4:15 PM Coffee Break
Chair – Jean-Marc Planeix
4:15 - 5:05 PM Yasushi Nishihara (Okayama)
5:10 - 6:00 PM Anny Jutand (ENS Paris)
6:00 - 7:40 PM Poster Session
Session 2:
Organocatalysis
Chair – Yutaka Takaguchi
1:45 - 2:35 PM Joseph Moran (Strasbourg)
2:40 - 3:30 PM Hiroki Mandai (Okayama)
3:30 - 3:50 PM Coffee Break
Chair – Mir Wais Hosseini
3:50 - 4:40 PM Stéphane Bellemin-Laponnaz (Strasbourg)
4:45 - 5:35 PM Seiji Suga (Okayama)
Dec. 3, Wednesday
Session 3:
Supramolecular & Material Chemistry
Chair – Joseph Moran
1:45 - 2:35 PM Tomoyuki Tajima (Okayama)
2:40 - 3:30 PM Wais Hosseini (Strasbourg)
3:30 - 3:50 PM Coffee Break
Chair – Yasushi Nishihara
3:50 - 4:40 PM Yutaka Takaguchi (Okayama)
4:45 - 5:35 PM Eric Monflier (Artois)
5:35 - 5:40 PM Closing Remarks Seiji Suga
Palladium-catalyzed cascade cyclizations : An original access to Sulfur
Heterocycles
Thomas Castanheiro, Morgan Donnard, Mihaela Gulea and Jean Suffert
Laboratoire d'Innovation Thérapeutique, UMR 7200 CNRS-Unistra, Faculté
de Pharmacie, Université de Strasbourg, 74 Route du Rhin, Illkirch, France
Enantioselective Steglich Rearrangement of Oxindole by a Chiral DMAP
Derivatives: Hydrogen Bonding Strongly Affects Activity and Selectivity
Fujii,
a Hiroki Mandai*
a Toshinobu Korenaga
b and Seiji Suga*
a
aDivision of Chemistry and Biochemistry, Graduate School of Natural Science
and Technology, Okayama University, bDepartment of Chemistry and
Bioengineering, Faculty of Engineering, Iwate University
Ammonium Complexation by 18C6 in Heterogeneous Solutions: a
Simulation
Gael Benay and Georges Wipff
Laboratoire MSM, UMR7177,1 rue B. Pascal, 67 000 Strasbourg France
Kinetic Resolution of Amines by the Asymmetric Counteranion-Directed
Catalysis
Kengo Goto, Hiroki Mandai*, and Seiji Suga*
Division of Chemistry and Biochemistry, Graduate School of Natural Science
and Technology,Okayama University
Molecular tectonics: from crystals to "crystals of crystals"
Adolf,a Sylvie Ferlay,a Nathalie Kyritsakas a and Mir Wais Hosseini a
a
Molecular Tectonic Laboratory, UMR UDS-CNRS 7140, Université de
Strasbourg, Institut Le Bel, 4, rue Blaise Pascal, F-67000 Strasbourg, France.
Preparation of [4]CPDT via a Square-Shaped Tetranuclear Platinum
Yasuhiro Okuda, and Yasushi Nishihara*
Division of Earth, Life, and Molecular Sciences, Graduate School of Natural
Technology, Okayama University
Nitro-Assisted Brønsted Acid Catalysis: Application to a Challenging
Catalytic
Azidation
Marian Dryzhakov Malik Hellal, Eléna Wolf and Joseph Moran*
Laboratory of Chemical Catalysis, ISIS& icFRC, Université de Strasbourg &
8 allée Gaspard Monge, 67000 Strasbourg, France
Synthesis and Self-Assembly of a New [60]Fullerene-Pentacene
Monoadduct
Takuya Nishihama, Tomoyuki Tajima, and Yutaka Takaguchi*
Graduate School of Environmental and Life Science, Okayama University
Supramolecular Luminescent Lanthanide Dimers for Fluoride
Sequestering and Sensing
Tao Liu, Aline Nonat, Franck Camerel, Raphael Tripier, Carlos Platas-Iglesias,
and Loïc J. Charbonnière*
Laboratory of Molecular Engineering Applied to Analysis, CNRS, IPHC,
Synthesis, Self-Assembly, and Semiconducting Property
of Soluble Hexathiopentacene Derivative
Hitoshi Shirai, Tomoyuki Tajima, and Yutaka Takaguchi*
Division
Sustainability of Resources, Graduate School of Environmental and
Life Science, Okayama University
Rational design for "grid of grids"
Jan Holub, Adrian-Mihail Stadler, Jean-Marie Lehn
ISIS, 8 allée Gaspard Monge, 67083 Strasbourg, France
Organocatalyzed and Uncatalyzed C=C/C=C and C=C/C=N Exchange
Processes between Knoevenagel and Imine Compounds in Dynamic
Covalent
Chemistry
Sirinan Kulchat, Kamel Meguellati, Jean-Marie Lehn
ISIS, 8 allée Gaspard Monge, 67083 Strasbourg, France
An In Situ Combinatorial Approach to Boron Lewis Acid Catalysis
Eléna Wolf, Florian C. Falk, Malik Hellal and Joseph Moran*
Laboratory of Chemical Catalysis, ISIS, University of Strasbourg
Magnetic field-induced supramolecular self-assembly
Marichez1, A. Sato1, I. de Feijter2, P. Besenius2, E. W. Meijer2, T. M.
1Laboratoire des systèmes complexes hors-équilibre (ISIS), Strasbourg
2Institute for complex molecular systems (TU/e), Eindhoven
LbL multi-particle coating: Toward smart textiles
M. Twardoch, D. Martel, O. Felix, G. Decher, N. Keller, M. Motay, V. Keller
Centre National de la Recherche Scientifique, Institut Charles Sadron

Pierre Braunstein
Institute of Chemistry (UMR 7177 CNRS) - University of Strasbourg
[email protected]
Pierre Braunstein obtained his Dr. Ing. Degree and « State Doctorate » from the
Université Louis Pasteur in Strasbourg. After post-doctoral stays at University College
London with R. S. Nyholm and R. J. H. Clark and at the TU Munich (Germany) as a
Humboldt Fellow with E. O. Fischer (Nobel Laureate), he returned to Strasbourg where
he became Director of Research with the CNRS and Head of the Coordination
Chemistry Laboratory (Institute of Chemistry, UMR 7177 CNRS) of the University of
Strasbourg. His main research interests deal with the inorganic and organometallic
chemistry of the transition and main group elements where he has (co)authored over
500 scientific publications and review articles. He has received numerous national and
international awards (including the International Award of the Japan Society of
Coordination Chemistry in 2013) and is member i.a. of the french Académie of Sciences
and the German National Academy of Sciences Leopoldina. He has been recently
featured in Angewandte Chemie
Selected Reviews
1. (a) Zhang, S.; Pattacini, R.; Braunstein, P. in Organometallic Chemistry: Recent
Advances, A. J. L. Pombeiro (Ed.), Elsevier, Chapter 14, pp. 185-198, 2014. (b) Fliedel,
C.; Braunstein, P. J. Organomet. Chem. 2014, 751, 286. (c) Boudier, A. ; Breuil, P.-A.
R. ; Magna, L.; Olivier-Bourbigou, H.; Braunstein, P. Chem. Commun., 2014, 50, 1398
(d) Braunstein, P. Chem. Rev. 2006, 106, 134. (e) Speiser, F.; Braunstein, P.; Saussine,
L. Acc. Chem. Res. 2005, 38, 784.
Reactivity Resulting from Heterodonor Ligands in Mono- and Polynuclear Complexes
Pierre BRAUNSTEIN
Institut de Chimie (UMR 7177 CNRS), University of Strasbourg,
4 rue Blaise Pascal, 67081 Strasbourg, France
E-mail: [email protected]
Combining chemically-different donor groups within the same ligand allows
access to metal complexes of diverse nuclearities endowed with structural or chemical
properties often very different from those of complexes containing identical donor
groups.[1] Such systems are ideal candidates for the study of the chemoselectivity of
their coordination to metal centres. We will examine the impact of ligands containing a
phosphorous or a NHC donor group associated with another function on the synthesis
and structure of organometallic/coordination complexes and the reactivity and catalytic
applications of the latter.[2-11]
[1] For recent reviews, see e.g. (a) Speiser, F.; Braunstein, P.; Saussine, L. Acc. Chem.
Res. 2005, 38, 784. (b) Braunstein, P. Chem. Rev. 2006, 106, 134. (c) Fliedel, C.;
Braunstein, P. J. Organomet. Chem. 2014, 751, 286.
[2] Liu, X.; Braunstein, P. Inorg. Chem. 2013, 52, 7367.
[3] Rosa, V. ; Fliedel, C. ; Ghisolfi, A.; Pattacini, R.; Aviles, T.; Braunstein, P.
Dalton Trans. 2013, 42, 12019.
[4] Ghisolfi, A.; Fliedel, C.; Rosa, V.; Pattacini, R.; Thibon, A.; Monakhov, K. Yu.;
Braunstein, P. Chem. Asian J. 2013, 8, 1795.
[5] Liu, P.; Wesolek, M.; Danopoulos, A. A.; Braunstein, P. Organometallics, 2013,
32, 6286.
[6] Ai, P. ; Danopoulos, A. A.; Braunstein, P. ; Monakhov, K. Yu. Chem. Commun.
2014, 50, 103.
[7] Massard, A.; Rogez, G.; Braunstein, P. Dalton Trans. 2014, 43, 42.
[8] Ai, P.; Danopoulos, A. A.; Braunstein, P. Dalton Trans. 2014, 43, 1957.
[9] Fliedel, C.; Faramarzi, V.; Rosa, V.; Doudin, B.; Braunstein, P. Chem. Eur. J.
2014, 20, 1263.
[10] Hameury, S.; de Frémont, P.; Breuil, P.-A. R.; Olivier-Bourbigou, H.; Braunstein,
P., Dalton Trans. 2014, 43, 4700; Inorg. Chem. 2014, 53, 5189.

Dominique Armspach
Laboratoire de Chimie Organique Moléculaire et Catalyse
Institut de Chimie de Strasbourg (UMR 7177), Université de Strasbourg
Phone: +33(0)368851621
[email protected]
Dominique Armspach was born in Mulhouse (France) in 1965. As a graduate of the Ecole
Nationale Supérieure de Chimie de Mulhouse, he joined Professor J. F. Stoddart's group
at the University of Birmingham (GB) in 1990 where he completed his Ph.D. in 1994. He
then spent three years at the University of Basle (Switzerland) as a postdoctoral fellow
and teaching assistant in Professor E. C. Constable's laboratory before becoming
Lecturer in Organic Chemistry in Strasbourg in 1996. In 2003, he was habilitated to
conduct independent research (HDR) by the Université Louis Pasteur in Strasbourg and
was promoted Professor of Organic Chemistry four years later at the University of
Strasbourg. His present interests focus on synthetic methodology, supramolecular
chemistry, organometallic chemistry and catalysis associated with molecular receptors.
Selected Reviews and Papers
1. A Cavity-Shaped Diphosphane Displaying "Oschelating" Behavior. R. Gramage-Doria, D. Armspach,
Dominique Matt, L. Toupet, Angew. Chem. Int. Ed. 2011, 50, 1554-1559.
2. TRANSDIP: A trans-Chelating Ligand Tailor-Made for Probing Unusual Pd0 and PdII Intermediates. R.
Gramage-Doria, D. Armspach, D. Matt, L. Toupet, Chem. Eur. J. 2012, 10813-10816.
3. Confining Phosphanes Derived from Cyclodextrins for Efficient Regio and Enantioselective
Hydroformylation. M. Jouffroy, R. Gramage-Doria, D. Armspach, D. Sémeril, W. Oberhauser, D.
Matt, L. Toupet, Angew. Chem. Int. Ed. 2014, 53, 3937-3940.
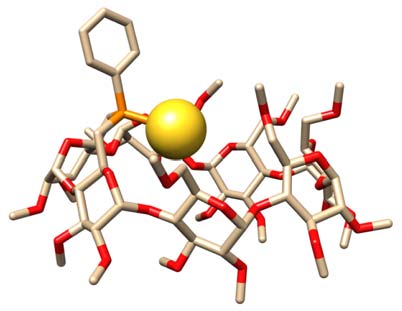
Phosphinocyclodextrins as confinement ligands for late transition metal centres
Dominique ARMSPACH
Laboratoire de Chimie Organique Moléculaire et Catalyse
Institut de Chimie de Strasbourg (UMR 7177), Université de Strasbourg
1, rue Blaise Pascal
67008 STRASBOURG Cedex
Phone: +33(0)368851621
[email protected]
Confinement is one of the most defining features in enzymes, Nature's own
catalysts. By combining P(III) coordinating units with cyclodextrin-based receptors, we
were able to synthesise ligands that force late transition metals to stay within a
cyclodextrin cavity so as to mimic the confinement of the active site that is observed in
metalloenzymes.1 The chiral environment that surrounds the metal centre is responsible
for unusual coordination chemistry, unique supramolecular interactions and highly
selective catalytic reactions of industrial importance taking place within the
cyclodextrin hollow.
Metal confinement
M = late transition metal
1. Metallated Cavitands (Calixarenes, Resorcinarenes, Cyclodextrins) with Internal Coordination Sites.
R. Gramage-Doria, D. Armspach, D. Matt, Coord. Chem. Rev. 2013, 257, 776-816.

Yasushi Nishihara
Division of Earth, Life, and Molecular Sciences, Graduate School of Natural Science
and Technology, 3-1-1 Tsushimanaka, Okayama 700-8530, Japan
Education B Sc: 1992, Faculty of Science, Hiroshima University M Sc: 1994, Graduate School of Science, Hiroshima University Ph D: 1997, The Graduate University for Advanced Studies
Professional Experience
1994.10-2004.3
Assistant Professor, Chemical Resources Laboratory, Tokyo
Institute of Technology 2004.3-2010.3
Associate Professor, Okayama University
Professor, Okayama University
Awards 1. Young Top Researcher, Okayama University (2007) 2. Chemical Society of Japan Presentation Award 2008 for Industries (2008) 3. Incentive Award in Synthetic Organic Chemistry, Japan (2009) 4. Incentive Culture Award in Okayama Prefecture, Japan (2010) Selected Reviews and Papers 1. Nishihara, Y.; Okada, Y.; Jiao, J.; Suetsugu, M.; Lan, M.-T.; Kinoshita, M.;
Iwasaki, M.; Takagi, K. Angew. Chem. Int. Ed. 2011, 50, 8660.
2. Nishihara, Y.; Miyasaka, M.; Okamoto, M.; Takahashi, H.; Inoue, E.; Tanemura,
K.; Takagi, K. J. Am. Chem. Soc. 2007, 129, 12634.
Synthesis of Substituted Picenes and Their Derivatives and
Their Application to Field-Effect Transistor Devices
Yasushi Nishihara
Graduate School of Natural Science and Technology,
Okayama University, Okayama 700-8530, Japan
Japan Science and Technology Agency (JST), ACT-C,
Kawaguchi, Saitama 332-0012, Japan
Organic thin-film transistors (OTFTs) with [n]phenacenes have attracted much
attention because of their superior organic field-effect transistors (OFETs) characteristics. The [5]phenacene (picene) and [6]phenacene (fulminene)-based OTFT have been fabricated and the field-effect mobility, μFET, reached the value as high as 3.2 and 3.7 cm2/Vs under O2 atmosphere, respectively. From the synthetic point of view, there are several critical drawbacks in the reported synthetic methods for [n]phenacenes. Therefore, a simple and convenient strategy for the synthesis of various substituted [n]phenacenes is highly desirable in order to promote further investigations into the ideal OFETs, which may be solution-processed and exhibit high carrier mobility and solubility in common organic solvents.
Previously, we have reported the synthesis of picene,1,2 fulminene,3 and
phenanthro[1,2-b:8,7-b']dithiophene (PDT)4 through cross-coupling reaction of polyhalobenzene with (Z)-alkenylboron compounds and sequential double cyclization via C-H bond activation (Figure 1). However, this synthetic method is not suitable for a large-scale synthesis due to a tedious isolation of stereoisomers of precursor and a low overall yield.
Chart 1. Structures of [5]- and [6]phenacenes and PDT
In this presentation, we disclose a new synthetic route to PDF and
structure-property relationships in Cn-PDT-based OFETs.5
First, PDT 6 was synthesized through the palladium-catalyzed Suzuki–Miyaura
or Negishi couplings of 2-thienylboronic acid 1 or the corresponding zinc compound 4
with 1,4-dibromobenzene 2 followed by epoxidation/Lewis-acid-catalyzed
Friedel-Crafts-type intramolecular cyclization sequences. Furthermore, dibromination
of 6 and sequential Suzuki–Miyaura coupling with alkylboranes, derived from 9-BBN
and terminal alkenes, gave Cn-PDTs 8a-8f in 69-80% yields (Scheme 1).
Scheme 1. Synthetic procedure of PDT and Cn-PDTs
Next, OFET devices were fabricated by using 8a-8f on Si/SiO2 substrate, in which
thin films were formed by thermal deposition. As a result, the
2,9-didodecylphenanthro[1,2-b:8,7-b']dithiophene (C12-PDT) thin-film FET displays
superior properties, with μ's as high as 1.4 cm2 V–1 s–1 for the SiO2 gate dielectric and 2.2
cm2 V–1 s–1 for the HfO2 gate dielectric. The average μ values, <μ>'s, reach 1.1(5) and
1.8(6) cm2 V–1 s–1, respectively, for the SiO2 and ZrO2 gate dielectrics.
References
1. Chang, N.; Chen, X.; Nonobe, H.; Okuda, Y.; Mori, H.; Nakajima, K.; Nishihara, Y.
Org. Lett. 2013, 15, 3558.
2. Mori, H.; Chen, X.; Chang, N.; Hamao, S.; Kubozono, Y.; Nakajima, K.; Nishihara,
Y. J. Org. Chem. 2014, 79, 4973.
3. Chang, N.; Mori, H.; Chen, X.; Okuda, Y.; Okamoto, T.; Nishihara, Y. Chem. Lett.
2013, 42, 1257.
4. Nishihara, Y.; Kinoshita, M.; Hyodo, K.; Okuda, Y.; Eguchi, R.; Goto, H.; Hamao,
S.; Takabayashi, Y.; Kubozono, Y. RSC Adv. 2013, 3, 19341.
5. Hyodo, K.; Nonobe, H.; Nishinaga, S.; Nishihara, Y. Tetrahedron Lett. 2014, 55,
Anny Jutand
Ecole Normale Supérieure, Département de Chimie
24 Rue Lhomond, F-75231 Paris Cedex 5, France
Phone: +33144323872
[email protected]
Anny Jutand obtained her Master Degree in 1971 at the Ecole Nationale Supérieure de
Chimie, Paris VI and the PhD in Chemistry in 1980 at the University Paris XIII
(advisor: Professor J. F. Fauvarque), developing palladium/nickel-catalyzed arylation of
Grignard reagents and zinc enolates. In 1980-1981, she was a Post-doctoral fellow at the
Royal Institute of Technology in Stockholm, Sweden (advisor: Professor B. Åkermark)
working on nucleophilic attack of enolates on -allyl palladium and coupling of
nucleophiles with cuprates via electrochemical oxidation. In1981-1985, she went back
to University Paris XIII as Chargé de Recherche (equivalent to Associate Professor) at
CNRS (Centre National de la Recherche Scientifique) where she developed
nickel-catalyzed electrosynthesis of anti-inflammatory agents (ibuprofen, naproxen…).
In 1985, she joined Dr. C. Amatore' s group at the Ecole Normale Superieure in Paris.
She became Research Director 2nd class at CNRS (a position equivalent to full
Professor) in 1992 and then Director of Research 1st class at CNRS in 2005. She is
Emeritus since Oct 2013.
Current Research Interests since 1985 :
* Mechanistic studies on transition metal-catalyzed reactions (Pd, Ni, Cu, Fe, Ru, Rh)
* Activation of organic molecules by transition metal complexes and by electron
transfer. Synthetic development and mechanism
Author of 169 publications in international journals, 7 industrial patents, 11 articles or
chapters in collective books, 115 conferences or seminars - H factor: 48
1. Award 2003 of the Organic Chemistry Division of the French Chemical Society
2. Grand Prix d'Etat of the French Academy of Sciences, 2008
3. Prix Achille Le Bel (French Chemical Society) 2013
Selected Reviews and Papers
1. Acc. Chem. Res. 2000, 33, 314-321.
2. Eur. J. Inorg. Chem. 2003, 2017-2040.
3. Chem. Rev. 2008, 108, 2300-2347.
Recent insights into the mechanism of transition metal-catalyzed reactions
Anny Jutand
Ecole Normale Supérieure, Département de Chimie
24 Rue Lhomond, F-75231 Paris Cedex 5, France
[email protected]
Transition metal catalyzed reactions proceed via catalytic cycles which are a
succession of chemical steps involving catalytic species whose metal exhibits different
oxidation states. Most organometallic complexes are electroactive. Consequently, they
can be detected and characterized by their reduction (or oxidation) potential by means
of electrochemical techniques. Moreover, since their reduction (or oxidation) currents
are proportional to their concentration, the reactivity of organometallic species with
organic substrates can be monitored by electrochemistry and the rate constants (or
equilibrium constants) determined.1
It is thus possible to investigate the
mechanism of all steps of a catalytic cycle, to
determine factors that control the efficiency
of a catalytic reaction, to understand how and
why a catalytic reaction works, so that to
increase its efficiency in terms of turn-over
and selectivity.
The mechanisms of palladium2, copper3 and
ruthenium4–catalyzed reactions will be
References
1. A. Jutand, Chem. Rev. 108, 2008, 2300.
2. C. Amatore, A. Jutand, G. Le Duc, Chem. Eur. J. 2011, 17, 2492; Angew. Chem. Int.
Ed. 2012, 51, 1379; Chem. Eur. J. 2012, 18, 6616. Chem. Eur. J. 2013, 19, 10082.
3. G. Franc, Q. Cacciuttolo, G. Lefèvre, C. Adamo, I. Ciofini, A. Jutand, ChemCatChem
2011, 3, 305; Organometallics, 2012, 31, 914.
4. E. Ferrer Flegeau, C. Bruneau, P. H. Dixneuf, A. Jutand, J. Am. Chem. Soc. 2011,
133, 10161. I. Fabre, N. von Wolff, G. Le Duc, E. Ferrer Flegeau, C. Bruneau, P. H.
Dixneuf, A. Jutand, Chem. Eur. J. 2013, 19, 7595.

Joseph Moran
ISIS & icFRC, Université de Strasbourg & CNRS,
8 allée Gaspard Monge, 67000 Strasbourg, France
Phone: +33-0368855202
[email protected]
Joseph Moran is an Assistant Professor of Chemistry at the University of Strasbourg's
Institute of Supramolecular Science and Engineering (ISIS) and is the director of the
Laboratory of Chemical Catalysis since September 2012. He received his Ph.D. in 2009
from the University of Ottawa (Canada) under the direction of Prof. André M.
Beauchemin. After a brief stay as a visiting scientist at the National Research Council of
Canada under the direction of John P. Pezacki, in 2010 he moved to the University of
Texas at Austin to take up an NSERC Postdoctoral Fellowship under the direction of
Prof. Michael J. Krische from 2010.
Marie Curie CIG (2013), Thieme Chemistry Journal Award (2013), NSERC
Postdoctoral Fellowship (2010), Boehringer Ingelheim Graduate Research Award in
Organic Chemistry (2008), NSERC Doctoral Scholarship (2006).
Selected Reviews and Papers
1. Review: Pure Appl. Chem. 2012, 84, 1729-1739.
2. J. Am. Chem. Soc. 2011, 133, 20100-20103.
3. J. Am. Chem. Soc. 2011, 133, 18618-18621.
4. Nature Chem. 2011, 3, 287-290.
Harnessing Complexity in Catalysis: From Supramolecular
Preorganization to Combinatorial Strategies
Joseph Moran
ISIS & icFRC, Université de Strasbourg & CNRS
8 allée Gaspard Monge, 67000 Strasbourg, France
[email protected].
The spatial pre-organization of multiple hydrogen bond donor sites within the
molecular framework of enzymes is a major reason why biological catalysts produce
dramatic rate acceleration with impressive selectivity. Supramolecular approaches to the
pre-organization of multiple hydrogen bond catalysts can also lead to much higher
reaction rates while providing a modular and easily tunable catalyst system without the
need for intensive synthetic efforts. The first half of the talk will describe our efforts to
develop and exploit template co-catalysts for the supramolecular preorganization of
multiple hydrogen bond catalysts.
Catalytic reaction discovery and development is a multidimensional problem
that often requires extensive experimentation to obtain a lead result. In a representative
scenario, metal, ligand, solvent, acid/base additive and temperature may all be critical to
a desired reaction, making catalytic reaction development a bit like finding a needle in a
haystack. The second half of the talk will describe our efforts to develop a
multidimensional combinatorial approach to the discovery of catalyst systems that are
generated in situ from complex mixtures, a technique that we hope can dramatically
reduce the number of reactions required to obtain a lead result.
1. Org. Biomol. Chem. 2014, 12, 5990-5994.

Hiroki Mandai
Division of Chemistry and Biotechnology
Graduate School of Natural Science and Technology, Okayama University
Phone: +81-86-251-8604, FAX: +81-86-251-8082
Hiroki Mandai obtained his Ph. D degree in 2006 from Tokyo University of Science
under the direction of Professor Teruaki Mukaiyama, focusing on the development of
stereoselective glycosylation reaction. He then joined the group of Amir H. Hoveyda at
Boston College as postdoctoral fellow from 2006 to 2008. He became assistant
professor at Okayama University from October 2008. His current research interests
include development of enantioselective reactions with chiral nucleophilic catalyst, and
synthesis of biologically important molecules.
Awards
Lectureship Award of the 89th Annual Meeting of the Chemical Society of Japan
(2009), Science and Technology Award of Okayama Foundation of Science and
Technology (2009), KANEKA CORPORATION Award in Synthetic Organic
Chemistry, Japan (2009).
Selected Reviews and Papers
1. Mandai, H.; Murota, K.; Mitsudo, K.; Suga, S. Org. Lett. 2012, 14, 3486.
2. Mandai, H.; Shimowaki, K.; Mitsudo, K.; Suga, S. Asian J. Org. Chem. 2014, 3, 437.
3. Mandai, H.; Omori, K.; Yamamoto, D.; Tsumura, T.; Murota, K.; Yamamoto, S.; Mitsudo,
K.; Ibaragi, S.; Sasaki, A.; Maeda, H.; Takashiba, S.; Suga, S. Biorg. Med. Chem. 2014, 22,
5338.
Development of Chiral Nucleophilic Catalysts
Hiroki Mandai*
Division of Chemistry and Biotechnology
Graduate School of Natural Science and Technology, Okayama University
3-1-1 Tsushima-naka, Kita-ku, Okayama 700-8530, Japan
Over the past two decades, the development of chiral nucleophilic catalysts has
become one of the most important fields in the enantioselective catalysis.1 Chiral catalysts with N,N-4-dimethylaminopyridine (DMAP) or 4-pyrrolidinopyridine (PPY) scaffolds have been widely studied in response to the pioneering studies by Vedejs2 and Fu,3 and have been used in various enantioselective transformations, such as the kinetic resolution of racemic alcohols or amines, desymmetrization of meso-compounds, Steglich rearrangements, and many others. Although considerable effort has been made to explore a variety of chiral DMAP and PPY derivatives, these catalysts often required the optical resolution of a racemic intermediate or catalyst during catalyst synthesis. Thus, highly active and enantioselective chiral nucleophilic catalysts, which can be synthesized from both readily available enantiomers of a chiral source and which do not require optical resolution during catalyst synthesis, are strongly desired. Against this background, we designed and synthesize a new class of chiral DMAP derivatives based on two strategies: (1) the use of diastereoselective Ugi reaction,4 and (2) the use of (S)-1,1́-bi-2-naphtol (BINOL) as chiral source. This lecture will be focused on the development of chiral nucleophilic catalysts and application to enantioselective transformations. References 1.
(a) Wurz, R. P. Chem. Rev. 2007, 107, 5570; (b) Müller, C. E.; Schreiner, P. R. Angew.
Chem. Int. Ed. 2011, 50, 6012; (c) Pellissier, H. Adv. Synth. Catal. 2011, 353, 1613.
Vedejs, E.; Chen, X. J. Am. Chem. Soc. 1996, 118, 1809.
Fu, G. C. Acc. Chem. Res. 2000, 33, 412.
(a) Mandai, H.; Irie, S.; Mitsudo, K.; Suga, S. Molecules 2011, 16, 8815; (b) Mandai,
H.; Irie, S.; Akehi, M.; Yuri, K.; Yoden, M.; Mitsudo, K.; Suga, S. Heterocycles 2013,


Stéphane Bellemin-Laponnaz
Insititut de Physique et Chimie des Matériaux de Strasbourg
CNRS-Université de Strasbourg
Phone +33-(0)388-107-166 [email protected]
Stéphane Bellemin-Laponnaz studied chemistry at the Université Joseph Fourier
(Grenoble) and the Université Louis Pasteur (Strasbourg). He obtained his doctorate in
1998 under the direction of Prof. John A. Osborn and Dr J. P. Le Ny working in the
field of homogeneous catalysis. He then joined the group of Prof. Gregory C. Fu at the
Massachusetts Institute of Technology (Cambridge, MA) as a post-doctoral fellow
working on asymmetric catalysis. In late 2000, he joined the group of Prof. Lutz H.
Gade at the Université de Strasbourg as an associate researcher CNRS and moved in
2010 to the Institut de Physique et Chimie des Matériaux de Strasbourg (IPCMS) as
Directeur de recherche CNRS. His research interests are in the fields of organometallic
chemistry, coordination chemistry, catalysis and medicinal chemistry.
1. CNRS, Bronze Medal 2005
2. French Chemical Society, Coordination Chemistry Division Award 2009
3. Swiss Chemical Society, Sandmeyer Award 2013
4. USIAS, Fellow 2013
1. Angew. Chem. Int. Ed. 2008, 47, 4546; 2009, 48, 1609; 2010, 49, 2198.
2. Chem. Commun. 2011, 47, 5864; 2012, 2213.
3. Organometallics 2010, 29, 1191; 2012, 31, 7618; 2013, 32, 2736; 2014, 33,
4. Chem. Rev. 2011, 111, 2705; 2014, 114, 8747.
Catalytic Performance And Recycling of Oxazoline-Based Catalysts
Maria Torres,1 Manuela Gaab,1,2 Carole Foltz-César,1,2 Lutz H. Gade,2 Stéphane
Bellemin-Laponnaz*,1
1IPCMS CNRS-Université de Strasbourg, STRASBOURG, France,
2 Anorganisch-Chemisches Institut, Universität Heidelberg, HEIDELBERG, Germany
Asymmetric catalysis constitutes a privileged approach for the production of
enantiopure compounds. Today a large number of chiral catalytic processes may deliver
products with very high enantiomeric excesses (and yields) and some systems have
been applied on an industrial scale.
However, homogeneous asymmetric catalytic systems frequently exhibit
relatively low activity for a large-scale application and they also suffer from two
drawbacks: (i) possible product contamination: in particular, metal contamination in
active pharmaceutical ingredients or fine chemicals is a serious concern and the
remaining metal traces must be reduced to ppm amount in the final products and, (ii)
inability to reuse the homogeneous catalyst.
Due to the high cost of both the chiral ligand and the metal, it is highly desirable
to develop catalytic systems that are active at a very low catalytic level and/or that allow
an easy separation from reaction mixture and efficient recycling.
In the first part of this lecture, highly symmetric oxazoline-based catalysts will
be described. In particular, we will present how the concept of stereoelectronic
hemilabillity allows a decrease of the catalyst loading.1
In the second part of this lecture, recent progress in the development of reusable
asymmetric catalysts will be discussed. Two strategies have been investigated: (i)
covalent attachment to carbosilane dendrimers2 and (ii) conception of polytopic ligands
for the development self-supported systems.3
1. a) Gade, L. H.; Bellemin-Laponnaz, S. Chem. Eur. J. 2008, 14, 4152; b) Foltz, C.;
Stecker, B.; Marconi, G.; Bellemin-Laponnaz, S.; Wadepohl, H.; Gade L. H. Chem.
Eur. J. 2007, 13, 9912; c) Foltz, C.; Enders, M; Bellemin-Laponnaz, S.; Wadepohl,
H.; Gade, L. H. Chem. Eur. J. 2007, 13, 5994.
2. Gaab, M.; Bellemin-Laponnaz, S.; Gade, L. H. Chem. Eur. J. 2009, 15, 5450.
3. a) Torres, M.; Heinrich, B.; Miqueu, K.; Bellemin-Laponnaz, S.; Eur. J. Inorg. Chem.
2012, 3384; b) Torres, M.; Maisse-François, A.; Bellemin-Laponnaz, S.
ChemCatChem 2013, 5, 3078; c) Torres, M.; Nano, A.; Maisse-François, A.;
Bellemin-Laponnaz, S. New J. Chem. 2014 in press.
Seiji Suga
Division of Chemistry and Biotechnology
Graduate School of Natural Science and Technology, Okayama University
Phone: +81-86-251-8081 FAX: +81-86-251-8081
[email protected]
Prof. Seiji Suga educated in organic chemistry at Nagoya University under the direction
of Prof. Ryoji Noyori. After earning his PhD degree in 1995, he became a postdoctoral
fellow (JSPS Postdoctoral Fellowships for Research Abroad) with Prof. Sir Jack E.
Baldwin at Oxford University. Then he joined the group of Jun-ichi Yoshida at Kyoto
University as Instructor in 1996, and was promoted to Lecture (1999) and Associate
Professor (2004). In 2008 he was appointed Professor of Okayama University. His
research interest has been focused on electron-transfer reactions of organic compounds
and reaction processes development.
Fujisawa Pharmaceutical Co. Award in Synthetic Organic Chemistry, Japan (1999),
Incentive Award in Synthetic Organic Chemistry, Japan (2004), Incentive Award in
Organic Electron-transfer Chemistry, Japan (2004), BCSJ Award (2005), Nagase
Foundation Award (2012)
1. Suga, S.; Nishida, T.; Yamada, D.; Nagaki, A.; Yoshida, J. J. Am. Chem. Soc. 2004, 126, 14338.
2. Matsumoto, K.; Fujie, S.; Ueoka, K.; Suga, S.; Yoshida, J. Angew. Chem. Int. Ed. 2008, 47,
3. Suga, S.; Yamada, D.; Yoshida, J. Chem. Lett. 2010, 39, 404.
4. Mitsudo, K.; Shimohara, S.; Mizoguchi, J.; Mandai, H.; Suga, S. Org.Lett. 2012, 14, 2702.
Electrochemically Generated Carbocations
for Stereoselective Synthesis and Catalytic Reactions
Seiji Suga*
Division of Chemistry and Biotechnology
Graduate School of Natural Science and Technology, Okayama University
3-1-1 Tsushima-naka, Kita-ku, Okayama 700-8530, Japan
[email protected]
Electric process in organic synthesis is advantageous because of its versatility
and environmental benefit. It is also noteworthy that highly reactive species such as
synthetically useful carbocations can easily be generated by the simple electron-transfer
The first topic will be diastereoselective synthesis of di-substituted piperidine
derivatives accomplished by the reaction of N-acyliminium ions prepared by the
Indirect Cation Pool Method.2 The highly reactive N-acyliminium ions having a
piperidine skeleton with a substituent can be generated and accumulated from the
corresponding thioaminals by the treatment of electrochemically generated
ArS(ArSSAr)+ pool. The nucleophilic addition of carbon nucleophiles gave rise to the
formation of the corresponding of di-substituted piperidine derivatives in a
highly-stereoselective manner.
Organocations such as trityl cation effectively promote Mukaiyama-Aldol
reactions.3 The interesting reactions involving carbocation chemistry prompted us to
develop a redox-switchable reaction involving entirely organic compounds, because the
redox-switchable catalysis is an emerging research field that seeks to regulate chemical
reactions by the simple reduction-oxidation manipulation. The second topic in this
presentation will be the catalytic reaction promoted by the electrochemically generated
carbocations. When the solution of an aldehyde, enol silyl ether and a precursor of
organo-dication4 were subjected to the electrochemical oxidation, the aldol reaction
smoothly started. Subsequent electrochemical reduction of the solution brought the
reaction to a stop.
(1) J. Yoshida, S. Suga, Chem. Eur. J. 2002, 8, 2650. (2) S. Suga, K. Matsumoto, K. Ueoka, J.
Yoshida, J. Am. Chem. Soc. 2006, 128, 7710. (3) T. Mukaiyama, S. Kobayashi, M. Murakami, Chem.
Lett. 1984, 1759. J. Bah, J. Franzén, Chem.-Eur. J. 2014, 20, 1066 (4) M. Okajima, S. Suga, K. Itami,
J. Yoshida, J. Am. Chem. Soc. 2005, 127, 6930.
Tomoyuki Tajima
Graduate School of Environmental and Life Science, Okayama University
Phone & Fax: +81-86-251-8898
Tomoyuki Tajima received his Ph. D. (2005) degree from Kyoto University under the
supervision of Prof. Norihiro Tokitoh. He was granted a Fellowship of the Japan
Society for the Promotion of Science (JSPS) for Young Scientists (2004-2005). In 2006,
he moved to Saitama University of Science as a research associate of Prof. Masaichi
Saito group. In 2007, he joined the Research Center for Materials Science (RCMS),
Prof. Kazuyuki Tatsumi group, at Nagoya University as a researcher. He moved back to
Kyoto University as an assistant professor in 2008. Then, he appointed as a senior
assistant professor at Okayama University in 2009.
Selected Reviews and Paper
1. Photosensitized hydrogen evolution from water using single-walled carbon
nanotube/fullerodendron/Pt(II) coaxial nanohybrids, Y. Sasada, T. Tajima, T. Wada, T.
Uchida, M. Nishi, T. Ohkubo and Y. Takaguchi, New Journal of Chemistry 2013, 37,
4214-4219.
2. Fabrication of novel core-shell microspheres consisting of single-walled carbon
nanotubes and CaCO3 through biomimetic mineralization, T. Tajima, A. Tsutsui, T.
Fujii, J. Takada and Y. Takaguchi, Polymer Journal 2012, 44, 620-624.
3. Synthesis and characterization of 2,3,9,10-tetradendronized pentacene, T. Tajima,
A. Yamakawa, K. Fukuda, Y. Hayashi, M. Nakano, and Y. Takaguchi, Chemistry
Letters 2012, 41, 1622-1624.
4. Photoreactive molecule incorporated within dendritic architecture, Y. Takaguchi
and T. Tajima, Journal of Synthetic Organic Chemistry Japan 2011, 69, 705-714.
Synthesis and Properties of (Terthiophene)4-poly(amidoamine)-C60 pentad
Tomoyuki Tajima*, Takuya Nishihama, Shogo Miyake, Nobuhiro Takahashi, and
Yutaka Takaguchi*
Graduate School of Environmental and Life Science, Okayama University
3-1-1 Tsushima-Naka, Kita-Ku, Okayama 700-8530 JAPAN
Corresponding to Y. Takaguchi (E-mail: [email protected]) or T. Tajima
(E-mail: [email protected])
Photoinduced polymerization of thiophene is considered to be useful for the
production of electronic devices materials in thin film forms. Various strategies have
been reported to polymerize thiophene derivatives photochemically.1 On the other hand,
photoinduced electron transfer systems of poly(thiophene) derivatives and C60 are of
interest in view of organic photovoltaic devices. From this point of view, photoinduced
polymerization of terthiophene-C60 interconnected system is of great interest. Although
Murata and Komatsu reported the preparation of terthiophene-C60 dyad thin films,
which was polymerized electrochemically on an electrode surface, and its
photoelectrochemical property2 much less is known about the photopolymerization of
terthiophene-C60 dyad. Here we report the synthesis and electrochemical polymerization
of a new conjugate system based on terthiophene and C60, i.e., (terthiophene)4-
poly(amidoamine)-C60 pentad 1. Photopolymerizations of 1 and its photoelectro-
chemical properties are also investigated. Interestingly, photopolymerized film of the
(terthiophene)4-poly(amidoamine)-C60 pentad (Figure 1b) was much conductive than
pentad monomer 1 (Figure 1a).
Figure 1. Photocurrent response of a film of monomer 1 and photopolymerized film of 1.
1. M. Sangermano, F. Sordo, A. Chiolerio, Y. Yagci, Polymer 2013, 54, 2077.
2. a) Y. Murata, M. Suzuki, K. Komatsu, Org. Biomol. Chem. 2003, 1, 2624. b) T. Yamazaki, Y.
Murata, K. Komatsu, K. Furukawa, M. Morita, N. Maruyama, T. Yamao, S. Fujita, Org. Lett.
2004, 6, 4865. c) A. Han, J. Bai, Y. Murata, K. Komatsu, Heteroatom Chem. 2011, 22, 72.
Mir Wais Hosseini
Université de Strasbourg, Institut Le Bel
4, rue Blaise Pascal, CS 90032, 67081 Strasbourg, France
Phone: +33-0368851323, Fax: +33-0368851325
[email protected]
Mir Wais Hosseini is Professor of chemistry at the University of Strasbourg and Senior
Member of the Institut Universitaire de France, Chair of Molecular Tectonics and
director of the Molecular Tectonics Laboratory. He obtained a Ph. D degree in 1983
from the Univesity Louis Pasteur, Strasbourg under the supervision of Professor
Jean-Marie Lehn. After spending 10 years at the CNRS as Research Assistant and
Associate in the group of Professor Lehn, he assumed a postdoctoral position at
Berkeley in the group of Professor K. N. Raymond. In 1990 he was hired as a full
Professor. He was invited Professor at University of Geneva, Institute of Materials and
Chemical Research, Tsukuba, JSPS Invited Professor at The University of Tokyo, Invited
Professor at The Academia Sinica, Taipei, Taiwan; JSPS Invited Professor at The University of
Kyoto and invited Professor at The University Hokkaido.
1. PhD Prize, Young Researcher Prize, Coordination and Organic Divisions awards of the
French Chemical Society, Prize of "Academie Rhenane", Gheorghe Spacu Medal of the
Romanian Chemical Society, French-Italian bi-national Prize, German-French bi-national
Grignard-Wittig Prize, Silver Medal of CNRS, Alexander von Humboldt Research Award,
Izatt-Christensen award, Fellow of the Royal Society of Chemistry (FRSC), Member of The
European Academy of Sciences, Arts and Humanities, Member of Academia Europaea;
Selected Reviews and Papers
1. M. W. Hosseini, Acc. Chem. Res., 2005, 38, 313-323.
Perspectives in Molecular Tectonics
Mir Wais Hosseini
University of Strasbourg
4, rue Blaise Pascal, CS 90032, 67081 Strasbourg, France
[email protected]
The design and construction of periodic architectures in the crystalline phase are
attracting considerable interest over the last two decades. For both design and analysis of
molecular crystals, we have developed a strategy called molecular tectonics which is
based on the formation of molecular networks through the design of complementary
tectons or molecular construction units. The generation of molecular networks and
subsequently of crystals is achieved by self-assembly processes based on repetitive
molecular recognition events. This approach, combining supramolecular synthesis and
self-assembly processes in the solid state, is operational and versatile and allows the
design and construct a variety of complex purely organic or hybrid architectures. The
approach will be presented and illustrated by a variety of tectons and networks.
1. M. W. Hosseini, Acc. Chem. Res., 38, 313 (2005).
2. M. W. Hosseini, Chem. Commun., Focus Article, ,582 (2005).
3. M. W. Hosseini, CrystEngComm., 6, 318 (2004).
Yutaka TAKAGUCHI
Graduate School of Environmental and Life Science, Okayama University
3-1-1 Tsushima-Naka, Kita-ku, Okayama 700-8530, Japan
Phone & Fax: +81-86-251-8903
E-mail: [email protected]
Professor Yutaka Takaguchi was born in Kobe, Japan, in 1968. He received his Ph.D. degrees in Chemistry from the University of Tsukuba in 1996 under the supervision of Prof. Naomichi Furukawa, where he worked on the syntheses and properties of organochalcogen compounds. He then was a RIKEN Special Postdoctoral Researcher in the group of Prof. Yasuo Wakatsuki, during which period he worked on the development of metathesis reactions using ruthenium complexes. He joined the Faculty of Textile Science and Technology at Shinshu University as an assistant professor in 1996, where he worked on the photochemistry of various compounds including dendrimers, fullerenes, and chalcogen atoms. In 2002, he was appointed as an associate professor at Okayama University. His research interests include (1) chemistry of nanocarbons (fullerenes and carbon nanotubes), (2) self-assembly and photoproperties of molecular semiconductors, and (3) fabrication and properties of organic-inorganic hybrids having a hierarchical architecture. Selected Papers 1. Template-Free Fabrication of Cylindrical Macropore Array in SnO2. Y. Ozawa, T.
Tajima, M. Nishi, T. Ohkubo, Y. Takaguchi, RSC Advances 2013, 3, 22949-22952.
2. Photosensitized Hydrogen Evolution from Water Using a Single-Walled Carbon
Nanotube/Fullerodendron/SiO2 Coaxial Nanohybrid. T. Tajima, W. Sakata, T. Wada,
A. Tsutsui, S. Nishimoto, M. Miyake, Y. Takaguchi. Avd. Mater. 2011, 23,
5750-5754.
Fabrication and Photosensitizing Properties of Coaxial Nanohybrids
Based on Single-Walled Carbon Nanotube
Yutaka Takaguchi*
Graduate School of Environmental and Life Science, Okayama University
3-1-1 Tsushima-Naka, Kita-ku, Okayama 700-8530, Japan
Phone & Fax: +81-86-251-8903; E-mail: [email protected]
The construction of well-organized nano- and meso-structures via biomimetic processes is of interest from the point of view of organic-inorganic hybrid materials. In particular, hybrid materials based on nanocarbons have attracted many attentions because of the potential applications for photofunctional materials. In this paper, we will describe our approach to fabricate the co-axial nanowire structure having single-walled carbon nanotube (SWCNT) core and other hierarchical architectures.1-5 Furthermore, their photosensitizing property to produce hydrogen from water will be discussed. For example, SWCNT/fullerodendron/Pt(II) complex was prepared via stepwise self-organization processes and used for the photosensitizer of hydrogen evolution from water (Fig. 1).
h
Dendron + Pt(II) complex layer
Fig. 1 (a) Schematic illustration and a TEM image of the coaxial structure of SWCNT/fullerodendron/Pt(II) complex
and (b) energy diagram for hydrogen evolution from water (path A: an ordinary three component system and path B:
the interconnected system).
1. H. Suzuki, Y. Iizumi, M. Tange, S.-K. Joung, A. Furube, T. Wada, T. Tajima, Y. Takaguchi,
T. Okazaki, Fullerenes, Nanotubes and Carbon Nanostructures 2014, 22, 44-56.
2. Y. Sasada, T. Tajima, T. Wada, T. Uchida, M. Nishi, T. Ohkubo, Y. Takaguchi, New J.
Chem. 2013, 37, 4214-4219.
3. T. Tajima, A. Tsutsui, T. Fujii, J. Takada, Y. Takaguchi. Polym. J. 2012, 44, 620-624.
4. T. Tajima, W. Sakata, T. Wada, A. Tsutsui, S. Nishimoto, M. Miyake, Y. Takaguchi, Avd.
Mater. 2011, 23, 5750-5754.
5. A. S. D. Sandanayaka, Y. Takaguchi, Y. Sako, M. Tamura, O. Ito, Adv. Sci. Lett. 2010, 3,
Eric Monflier
Université d'Artois, UCCS UMR 8181
Faculté des Sciences Jean Perrin, Rue Jean Souvraz, SP 18-62307 Lens Cédex, France
Phone: (+33) 3 21 79 17 72
Eric Monflier graduated from the Ecole Nationale Supérieure de Chimie de Lille
(ENSCL) in 1989 and received his Ph.D. degree from the University of Lille in 1992
under the supervision of Professor Francis Petit in the field of organometallic chemistry
and homogeneous catalysis. In 1992, he became Associate Professor at the University
of Artois where he set up an independent research group working on aqueous
organometallic catalysis. He was promoted to Full Professor in 1996. His current
research interests are mainly in the field of supramolecular catalysis and catalysis in
multiphase systems. He has played a key role in the development of efficient
supramolecular mass transfer promoters for aqueous organometallic catalysis. He is
currently head of the research group "Catalysis and Supramolecular Chemistry" and has
authored more than 180 international scientific publications, 21 book chapters, and 12
Eric Monflier received the « Prix de la Division Catalyse » from French Chemical
Society in 1996, the « Prix des Techniques Innovantes pour l'Environnement » from
French agency for environment and energy management in 2004, and the « Prix de
l'Innovation et de la Valorisation de la Recherche » from OSEO - French innovation
Transition metal catalysis in water assisted by cyclodextrins
Eric Monflier
Université d'Artois, UCCS UMR 8181,
Faculté des Sciences Jean Perrin, Rue Jean Souvraz, SP 18-62307 Lens Cédex, France.
During the past decade, ecological requirements have pressed chemists to
develop clean catalytic processes and technologies. In this context, the immobilization
of homogeneous or heterogeneous transition metal catalysts in an aqueous phase
appears as an eco-friendly technique to produce organic compounds. Indeed, the
catalyst can be easily recovered in an active form at the end of reaction by decantation
of the aqueous and organic phases and the production costs are significantly lower.
However, the scope of aqueous catalysis is greatly reduced by the low solubility of most
organic substrates in water and by the need to synthesize water-soluble ligands or
stabilizing agents to immobilize the catalyst in water.
In this lecture, we will demonstrate that cyclodextrins are very useful
compounds to develop catalytic processes in water.1 These cyclic oligosaccharides can
be used as mass transfer promoters, ligands platforms or dispersing and stabilizing
agents of metallic nanoparticles or supported metals. The possibility to use the
cyclodextrins in other green solvents will also be briefly discussed.2
1. Recent review: F. Hapiot, A. Ponchel, S. Tilloy, E. Monflier, C. R. Chimie, 2011, 14,
149; F. Hapiot, H. Bricout, S. Tilloy, E. Monflier, Eur. J. Inorg. Chem. 2012,
1571-1578; S. Noël, B. Léger, A. Ponchel, K. Philippot, A. Denicourt-Nowicki, A.
Roucoux, E. Monflier, Catal. Today, 2014, 235, 20-32; F. Hapiot, H. Bricout, S.
Menuel, S. Tilloy, E. Monflier, Catal. Sci. Technol., 2014, 4, 1899-1908.
2. C. Tortosa Estorach, M. Giménez-Pedrós, A.M. Masdeu-Bultó, A.D. Sayede, E.
Monflier, Eur. J. Inorg. Chem. 2008, 2659; F. Wyrwalski, B. Léger, C. Lancelot, A.
Roucoux, E. Monflier, A. Ponchel, Appl. Catal. A Gen. 2011, 391, 334-341; F.
Jerome, M. Ferreira, H. Bricout, S. Menuel, E. Monflier, S. Tilloy, Green Chem.,
Source: http://labex-csc.unistra.fr/uploads/media/SeminarProgramJSPS.pdf
Doing Business in Cameroon: 2013 Country Commercial Guide for U.S. Companies INTERNATIONAL COPYRIGHT, U.S. & FOREIGN COMMERCIAL SERVICE AND U.S. DEPARTMENT OF STATE, 2010. ALL RIGHTS RESERVED OUTSIDE OF THE UNITED STATES. Chapter 1: Doing Business In Cameroon M arket Overview • Cameroon is the largest economy in the six-nation Central African Economic and Monetary Community (CEMAC). With a population of over 20 million people, the IMF estimated Cameroon's GDP (at purchasing power parity) for 2012 to be over $50 billion. The IMF projects 5% growth in 2013 and notes that Cameroon boasts one of the highest per capita GDPs (by purchasing power parity) in sub-Saharan Africa, at $2,366. • Cameroon has a wealth of natural resources, including rich potential in the agricultural, forestry, and mining sectors, an ample labor force, and an enviable location between markets in Nigeria to the west and Central Africa, Chad, Republic of Congo, Gabon, and Equatorial Guinea to the south and east. Cameroon is often described as "Africa in Miniature" because of its ethnic, linguistic, and geographic diversity. • The Bank of Central African States (BEAC) sets some aspects of monetary policy for Cameroon and other CEMAC members. CEMAC's currency, the Central African CFA Franc (CFA), is managed by BEAC and guaranteed at a rate of 655.957 CFA to the euro by the French Treasury. Cameroon is also a member of a much larger economic zone called Economic Community of Central African States (ECCAS or CEEAC), which includes the Democratic Republic of Congo and Angola, and which represents a market of 120 million people. • Cameroon's major exports are oil, timber, and cash crops such as cocoa, coffee, rubber, cotton, and bananas. Cameroon imports mainly semi-processed products, industrial inputs, machinery, food products, pharmaceuticals, automobile, machinery, and light crude oil from neighboring countries. The European Union is Cameroon's main trading partner. Nigeria and France are Cameroon's major suppliers of imported goods and services. • A committee commissioned by the Government of the Republic of Cameroon (GRC) in May 2007 found the United States to be the largest single foreign investor in Cameroon, in large part due to the substantial American equity in the Chad-Cameroon pipeline and the power sector. Trade between the two countries has been on a steady increase since 2009, almost doubling by 2012, as Cameroonians continue to discover and appreciate the high standards and quality of U.S. goods. • Yaounde is the nation's political capital, but Douala, the largest city, serves as the country's commercial center. Almost all transport in and out of Cameroon, Chad, and
Acne: Past, Present, and Emma Taylor, M.D Assistant Clinical Professor Dermatology and Dermatopathology, UCLA Cofounder, President, and CEO of Acne: A Historical Perspective Ancient Egyptian Aku-t -boils, blains, sores, pustules, inflamed swelling Fuchs 1840 coined-Acne Vulgaris, Acne Mentagra and Acne Rosacea Bloch 1931 proved relationship









