Doi:10.1016/j.humpath.2006.07.014
Human Pathology (2007) 38, 185 – 189
Temozolomide therapy in a man with an aggressiveprolactin-secreting pituitary neoplasm:morphological findings
Kalman Kovacs MD, PhDa,*, Eva Horvath PhDa, Luis V. Syro MDb, Humberto Uribe MDc,Luis C. Penagos MDd, Leon D. Ortiz MDe, Camilo E. Fadul MDf
aDepartment of Laboratory Medicine, St Michael's Hospital, University of Toronto, Toronto, Ontario, Canada M5B 1W8bDepartment of Neurosurgery, Hospital Pablo Tobon Uribe and Clinica Medellin, Medellin, ColombiacDepartment of Neurosurgery, Clinica SOMA, Medellin, ColombiadDivision of Otolaryngology, Clinica Medellin, Medellin, ColombiaeSection of Neuro-oncology, Instituto de Cancerologia, Clinica Las Americas, Medellin, ColombiafSections of Hematology/Oncology and Neurology, Dartmouth Hitchcock Medical Center, Lebanon, New Hampshire, USA
Received 25 May 2006; revised 24 July 2006; accepted 31 July 2006
Summary Administration of temozolomide to a 46-year-old man with an invasive aggressive prolactin
(PRL)–secreting pituitary neoplasm resulted in improvement of the clinical condition and significant
decrease of blood PRL levels. Histologic, immunohistochemical, and electron microscopic study
Pituitary neoplasms;
demonstrated marked morphological differences in the tumor exposed to temozolomide compared with
the unexposed tumor. Necrosis, hemorrhagic areas, accumulation of connective tissue, focal inflam-
matory infiltration, and neuronal transformation were seen. Immunohistochemical prognostic indicatorsshowed a reduction in growth potential. Based on the clinical, laboratory, and morphological findings,we recommend temozolomide therapy in patients with pituitary tumors not responding adequately toother treatment options.
D 2007 Published by Elsevier Inc.
treatment of patients with growth hormone (GH) and/orthyrotropin (TSH)–secreting pituitary tumors Adminis-
Patients with pituitary tumors can be treated by surgery,
tration of these drugs diminishes blood GH and TSH con-
various forms of radiotherapy, and different drugs. Dopa-
centrations, reduces tumor size, and ameliorates the clinical
mine agonists are effective in many patients with prolactin
conditions. Pegvisomant, a GH receptor blocker, was intro-
(PRL)–producing pituitary tumors They decrease blood
duced for treating patients with acromegaly Temozolo-
PRL levels and cause tumor shrinkage and clinical improve-
mide was recently recommended as a new approach in the
ment. Long-acting somatostatin analogs are used in the
therapy of aggressive pituitary tumors Syro et al administered temozolomide to a 46-year-old man with anaggressive PRL-secreting pituitary neoplasm. The clinicaland laboratory findings were briefly reported in a letter. We
* Corresponding author.
E-mail address:
[email protected] (K. Kovacs).
had the opportunity to investigate and compare the
0046-8177/$ – see front matter D 2007 Published by Elsevier Inc.
doi:10.1016/j.humpath.2006.07.014
K. Kovacs et al.
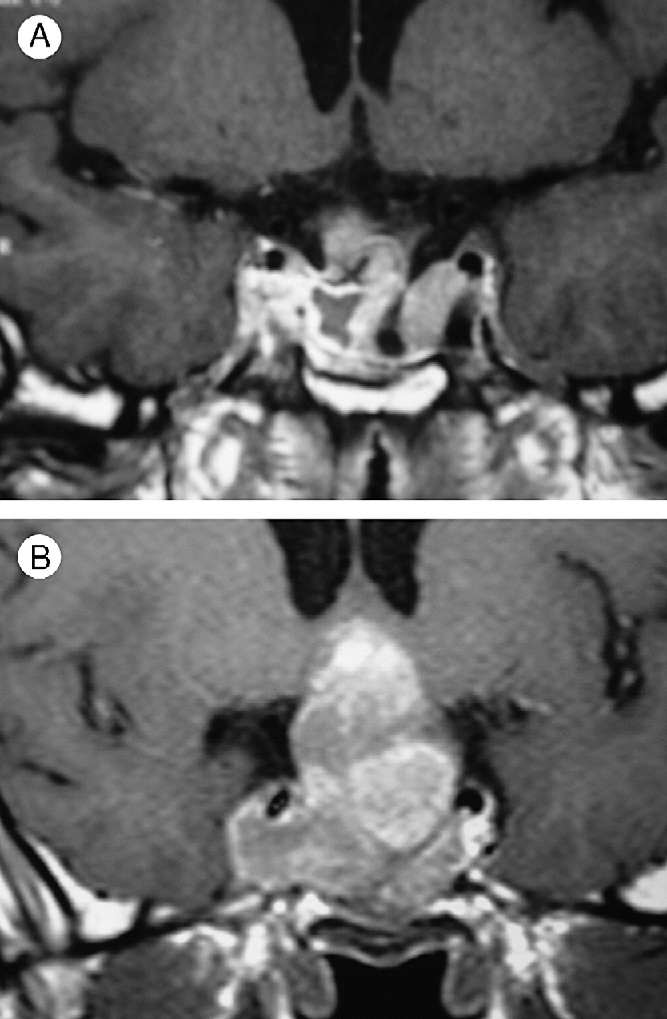
therapy was given. The patient complained of visualdisturbances, which were only temporarily improved aftersurgeries. Magnetic resonance imaging (MRI) disclosed alarge tumor with chiasmal compression and suprasellarextension measuring � � 37 mm, and invasion tothe cavernous sinus (Fig 1A). The fifth surgery was notsuccessful. Only a small portion of the tumor could beresected because of hard consistency. MRI demonstrated alarge neoplasm (50 � 45 � 50 mm), and the postoperativeblood PRL level was 1838 ng/mL.
Temozolomide therapy was started in January 2005 at a
dose of 200 mg/m2/d � 5 days. Treatment was repeatedevery 28 days for 7 months. The clinical conditionimproved, blood PRL levels decreased to 30 ng/mL, andMRI hemorrhage, necrosis, and shrinkage ofthe tumor (Fig. 1B). In October 2005, another surgery wasperformed. The tumor was easily resected because of itsfriable and soft consistency and was submitted for histo-logic, immunohistochemical, and electron microscopicinvestigation. The morphological changes found in thetumor removed after temozolomide treatment were com-pared with those observed in the tumor from the last pre-treatment surgery.
3. Morphological findings
Details of the histologic, immunohistochemical, and
electron microscopic methods were described previouslyThe antibodies used for immunohistochemistry in thisstudy were for PRL (monoclonal; Immunotech, Marseilles,France); GH and corticotropin (polyclonal) as well as
A and B, Demonstrates the coronal magnetic resonance
b-TSH and b-FSH (monoclonal; all 3 from Dakocytoma-
image of the sellar tumor before (A) and after (B) temozolo-
tion, Carpinteria, CA); b-LH (polyclonal; NIDDK-NIH,
mide treatment.
Torrence, CA); and a subunit (monoclonal; Biogenex, San
morphology of the tumor removed by surgery before and
Ramon, CA). For Ki-67, the MIB-1 antibody was used
after temozolomide treatment. Our case is the first to
(Ventana Medical, Tucson, AZ).
provide information on the morphological changes in an
The tumor removed before temozolomide treatment was
aggressive PRL-secreting pituitary neoplasm after temozo-
a cellular well-vascularized neoplasm exhibiting a diffuse
pattern interspersed with narrow strands of connectivetissue. It consisted of chromophobic and slightly acidophilicperiodic acid–Schiff–negative cells. In some areas, marked
2. Clinical and laboratory findings
congestion was evident. Cellular and nuclear pleomorphismwas moderate and mitotic figures were easily identified
The patient was a 46-year-old man with a large, invasive,
(11/1000) nuclei (The streptavidin-biotin-peroxi-
aggressive PRL-secreting pituitary neoplasm. In a period of
dase complex method demonstrated cytoplasmic immuno-
15 years, 5 surgeries were performed, but the tumor could
positivity for PRL in many adenoma cells. Immunostainings
not be completely removed. Radiation therapy and admin-
were negative for GH, corticotropin, TSH, FSH, LH, and
istration of dopamine agonists (bromocriptine and cabergo-
a subunit of the glycoprotein hormones.
line) were ineffective. Blood PRL levels were markedly
The morphological features of the tumor removed after
elevated (1885 ng/mL). After surgeries, blood PRL levels
temozolomide treatment markedly differed from those
temporarily decreased but started to rise again, and the
reported before temozolomide administration. By histology,
tumor increased in size. Blood follicle stimulating hormone
the chromophobic slightly acidophilic periodic acid–Schiff–
(FSH), luteinizing hormone (LH), and cortisol levels were
negative tumor cells were separated by edema and
low. Blood GH, TSH, and thyroxine (T4) concentrations
hemorrhage. In several areas, accumulation of connective
were within the normal range. Hormone replacement
tissue was noted. The tumor cells were irregular, showing

Temozolomide therapy in a man with an aggressive prolactin-secreting pituitary neoplasm
A and B, A cellular neoplasm with frequent mitoses is seen before temozolomide treatment (A). A markedly different morphology
is apparent with large neuronlike cells after temozolomide administration (hematoxylin-eosin, original magnification �40). C and D, A veryhigh Ki-67 nuclear labeling is depicted in the tumor before temozolomide administration (C). The number of labeled nuclei is markedlyreduced in the posttreatment specimen (D). Immunostaining for Ki-67 using the MIB-1 antibody (original magnification �40).
moderate cellular and nuclear pleomorphism. Mitotic
temozolomide-treated tumor specimen. In the pretreated
figures were also encountered (2/1000 nuclei). In few areas,
tumor, the Ki-67 nuclear labeling index using the MIB-1
mild to moderate mononuclear cell infiltration was noted.
antibody was 40% to 60% (topoisomerase-2-a,
Several tumor cells were very large and resembled nerve
90%; P-27, 25%; P-53, 15%; vascular endothelial growth
cells (. By immunohistochemistry, many tumor cells
factor, 100%. In the tumor cells after temozolomide therapy,
were immunopositive for PRL. Few tumor cells appeared to
the Ki-67 index markedly decreased to 5% (
express GH as well. Immunostainings were negative for
topoisomerase-2-a, 80%; P-27, 70%; P53, 4%; and vascular
corticotropin, TSH, FSH, LH, and a subunit of the glyco-
endothelial growth factor, 95%.
protein hormones. Several mononuclear inflammatory cells
By electron microscopy (the tumor (before
were immunopositive for CD3, a T-cell marker, and for
temozolomide treatment) was highly atypical and undiffer-
CD68, a macrophage marker. Few inflammatory cells were
entiated. The characteristic features of PRL-producing cells
immunopositive for CD20, a B-cell marker. Immunostain-
were not apparent. The cells were spherical or irregular,
ing for CD34, an endothelial cell marker, showed that the
closely apposed without well-formed intercellular junctions.
tumor was well vascularized. Several capillaries were
The nuclei were ovoid, irregular, and occasionally bizarre,
abnormal in shape and size. E-cadherin expression in the
containing 1 or more variably developed nucleoli and small
cytoplasm of tumor cells was slightly decreased. Expression
to moderate quantities of stippled or clumped heterochro-
of neurofilament antigen, S-100 protein, and glial fibrillary
matin. The prominence of rough-surfaced endoplasmic
acidic protein was apparent in several cells.
reticulum (RER) was variable, and well-organized arrays
Comparison of immunohistochemical prognostic indica-
of RER were only seen in occasional tumor cells. In a few
tors showed marked differences between the pretreated and
tumor cells, annulate lamellae were noted; this special RER

K. Kovacs et al.
formation occurs in atypical cells. The Golgi complexeswere rarely prominent. The secretory granules were small,extremely scant, and spherical, and it did not exceed 200 nmin most of the tumor cells. Granule exocytoses were onlyrarely detectable. Numerous tumor cells displayed variabledegrees of oncocytic change. The ultrastructural features ofthe mitochondria were wnormal range.
Electron microscopy (Fig. 3B) documented a surprising-
ly heterogeneous tumor tissue (after temozolomide treat-ment), the components of which were (1) small cellsdisplaying poorly developed membranous organelles and afew minute (50-150 nm) secretory granules. These cellsresembled null cells, (2) middle-sized cells having propor-tionally larger nucleus and a prominent nucleolus. The well-developed cytoplasm contained appreciable quantities ofRER and a sizeable Golgi complex. However, the 50- to150-nm secretory granules were very sparse, and these cellsdid not possess markers of PRL cell differentiation either;(3) admixed with the tumor cells described hereinabove,there was a neural component consisting of neuropil(masses of what appeared to be neuronal processesincluding varying amounts of cytoplasmic constituents) aswell as large neuronlike cells. The latter had very large,often pleomorphic, nucleus with markedly large nucleolus.
The ample cytoplasm harbored peripheral parallel stacks ofRER, heavily studded with ribosomes—the ultrastructuralequivalent of Nissl substance. The Golgi apparatus seemedto be prominent, but the secretory granules were verysmall (50-150 nm) and scant. These cells, resembling theperikarya of secretory neurons, contained also aggregates oflow-density microfilaments.
Temozolomide is an imidazotetrazine derivative, an
alkylating compound that depletes MGMT (0-6-methylgua-nine-DNA methyltransferase), and a DNA repair enzyme,which methylates DNA and exerts an antineoplastic effectagainst various experimental tumors It absorbs rapidlyafter oral administration and crosses readily the blood-brainbarrier. Because of easy penetration to the central nervoussystem, patients with various gliomas and cerebral metas-tases of malignant melanoma were treated with the drug,and the results showed reduction of the tumor mass, clinicalimprovement, and prolonged survival A group ofpatients with pheochromocytoma, pancreatic endocrineneoplasms, and carcinoid tumors showed objective bio-chemical and radiologic improvement followed by oraltreatment with temozolomide and thalidomide, suggesting
A and B, Electron micrograph of the pretreatment tumor
that the combination of these 2 drugs appeared to be an
demonstrates small undifferentiated tumor cells possessing few
option in the treatment of neuroendocrine tumors
minute secretory granules, but no markers of PRL cell differen-
Fadul et al administered temozolomide to 2 patients with
tiation (A). Part B depicts 2 intermediate cells surrounded byaggregates of neuropil. The large cells possess considerable
pituitary carcinoma. The first patient had a PRL-secreting
quantities of RER and sparse small secretory granules (original
pituitary carcinoma with bone metastases. The second
patient had a large clinically nonfunctioning metastasizing
Temozolomide therapy in a man with an aggressive prolactin-secreting pituitary neoplasm
pituitary carcinoma. In both patients, temozolomide medi-
for their generous support; Mrs Maureen Molson for the
cation impressive improvement. The patient of Zhu
secretarial work; Ms Corinne Holubovich for the literature
et al [5] was a 61-year-old man with a PRL-secreting
search; and Mr Fabio Rotondo for participation in the
pituitary carcinoma. Temozolomide administration to this
patient decreased blood PRL levels, caused clinical provement, and significant tumor shrinkage. Zhu et al [5]recommended temozolomide treatment as a novel approach
in patients with pituitary carcinoma.
In our case, after temozolomide administration, clinical
[1] Iva´n G, Szigetti-Csu´cs N, Olah M, Nagy GM, Go´th MI. Treatment of
improvemwas obvious and blood PRL ls markedly
pituitary tumors—dopamine agonists. Endocrine 2005;28:101 - 10.
eased [6] similar to the cases of Fadul et al [4] and Zhu et al
[2] Tichomirowa MA, Daly AF, Beckers A. Treatment of pituitary
[5]. However, our publication is the first that reports
tumors—somatostatin. Endocrine 2005;28:93 - 9.
the morphological findings and compares the changes in
[3] Paisley AN, Drake WM. Treatment of pituitary tumors—pegvisomant.
the tumor before and after temozolomide therapy. The
Endocrine 2005;28:111 - 4.
[4] Fadul CE, Kominsky AL, Meyer LP, et al. Pituitary carcinomas
presence of hemorrhages, necrosis, fibrosis, and shrinkage
respond to temozolomide. Neuro-oncol 2004;6:374.
in the tumor confirmed the antitumoral effect of temozolo-
[5] Zhu Y, Shahinian H, Hakimian B, Bonert V, Lim S, Heaney A.
mide. The study of prognostic indicators also showed that
Temodar: novel treatment for pituitary carcinoma. Abstr US Endocr
temozolomide significantly decreased tumor cell proliferation.
Soc 2004;138:43 - 5.
The most intriguing finding was the presence of apparent
[6] Syro LV, Uribe H, Penages LC, et al, Antitumor effects of
temozolomide in a man with a large, invasive prolactin producing
neuronal transformation in the tumor removed after treat-
pituitary neoplasm. Clin Endocrinol [in press].
ment with temozolomide. No neural elements were identi-
[7] Horvath E, Vidal S, Syro LV, Kovacs K, Smyth HS, Uribe H. Severe
fied in the pretreated specimen. Neuronal transformation
lymphocytic adenohypophysitis with selective disappearance of
that was unexpected in our case was described in several
prolactin cells: a histologic, ultrastructural and immunoelectron
tumor types, including pituitary adenomas, mainly GH-
microscopic study. Acta Neuropathol 2001;101:631 - 7.
[8] Newlands ES, Stevens MFG, Wedge SR, Wheelhouse RT, Brock C.
producing tumors The causative mechanisms for
Temozolomide: a review of its discovery, chemical properties, pre-
this transformation are unknown. In pituitary cells, exposure
clinical development and clinical trials. Cancer Treat Rev 1997;23:
to nerve growth factor resulted in neuronal transformation
It is not clear whether the process represents
[9] Agarwala SS, Kirkwood JM. Temozolomide, a novel alkylating agent
differentiation, which slows tumor growth. Temozolomide
with activity in the central nervous system, may improve the treatmentof advanced metastatic melanoma. Oncologist 2000;5:144 - 51.
may act similarly to nerve growth factor and cause neuronal
[10] Stupp R, Gander M, Leyvraz S, Newlands E. Current and future
metaplasia, that is, transformation of pituitary tumor cells to
developments in the use of temozolomide for the treatment of brain
nerve cells.
tumours. Lancet Oncol 2001;2:552 - 60.
Our work is based on the study of 1 single case only.
[11] Hegi ME, Diserens AC, Godard S, et al. Clinical trial substantiates the
Thus, no definitive conclusions can be drawn. Despite
predictive value of 0-6-methylguanine-DNA methyltransferase pro-moter methylation in glioblastoma patients treated with temozolo-
this limitation, the encouraging results support the view
mide. Clin Cancer Res 2004;10:1871 - 4.
that if other options fail, temozolomide should be adminis-
[12] Kulke MH, Stuart K, Enzinger PC, et al. Phase II study of
tered to patients with large, rapidly growing, invasive,
temozolomide and thalidomide in patients with metastatic neuroen-
and aggressive PRL-secreting pituitary tumors. Our prelim-
docrine tumors. Clin Oncol 2006;24:401 - 6.
inary findings did not provide an answer whether other
[13] Horvath E, Kovacs K, Scheithauer BW, Lloyd RV, Smyth HS.
Pituitary adenoma with neuronal choristoma (PANCH): composite
pituitary tumor types would respond to temozolomide
lesion or lineage infidelity? Ultrastruct Pathol 1994;18:565 - 74.
therapy as well.
[14] Thodou E, Kontogeorgos G, Horvath E, Kovacs K. Prolactin-
producing pituitary adenoma with incomplete neuronal transforma-tion: an intermediate adenoma-neuronal tumor. Acta Neuropathol
2004;108:115 - 20.
[15] Missale C, Boroni F, Sigala S, et al. Nerve growth factor in the
anterior pituitary: localization in mammotroph cells and cosecretion
We thank the Jarislowsky Foundation, the Lloyd Carr-
with prolactin by a dopamine-regulated mechanism. Proc Natl Acad
Harris Foundation, Dr Mannicio Velez, and Dr Susalind Eps
Sci U S A 1996;93:4240 - 5.
Source: http://mail.idclasamericas.co/Documentos/Investigacion/Publicaciones/Neuro%20Oncologia/Kovacs_et_al._Temozolomide_treatment_in_a_man_with_an_aggressive_prolactin_pituitary_neoplasm.[1].pdf
PROYECTO PARA LA CONSERVACION Y USO SOSTENIBLE DEL SISTEMA ARRECIFAL MESOAMERICANO Belice – Guatemala – Honduras - México SAM / MBRS Manual de Métodos para la Elaboración de Programas de Uso Público en Áreas Protegidas de la Región del Sistema Arrecifal Mesoamericano.
Table 1. By type of infection, microorganisms to be suspected in relation to the presence or not of risk factors for multidrug resistance and suggested empirical treatments [VAP: ventilador-associated pneumonia; MDR: multidrug resistance; ESBL: extended-spectrum β-lactamase; ESCPM group (Enterobacter cloacae, Enterobacter aerogenes, Serratia marcescens, Citrobacter freundii, Providencia rettgeri and Morganella morganii); MRSA: methicillin-resistant S. aureus; HACEK (Haemophilus spp., Aggregatibacter -formerly Actinobacillus- actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella spp)] INFECTION TYPE




