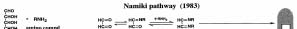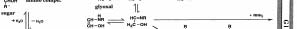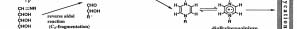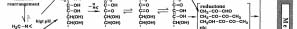Maillard.umin.jp

JMARS News Letter Vol. 1, April 22, 2006
Maillard Reaction Society (IMARS)
Japan Maillard Reaction Society)
The Maillard Reaction and Free Radicals:
Discovery of the Namiki Pathway
Nagoya University
I graduated from university (in Tokyo, 1945) in the
great confusion just after the Second World War and fortunately found work at the Institute of Physical and Chemical Research. The Institute was established in 1917 as the first general research institute in Japan and
achieved much remarkable success for work on, e. g., vitamins and nuclear physics. The institute is now called RIKEN and is one of the world's leading research institutes. However, in those days it had greatly suffered from war damage and most laboratories had been burnt and left without research facilities and budget. At first, I studied on development of new antibiotics, and then started a laboratory
working on radiation biology in 1956 and performed studies on protective substances in food and biological
materials for radiation lethal effect. This work aimed to protect biologically important substances such as DNA
in the living body from damage caused by active
oxygen radicals formed by irradiation using radical scavengers and antioxidants.
Later, I transferred to a laboratory of food chemistry
at Nagoya University (1966) and continued my studies on antioxidants in various health foods such as sesame and tea which offer protection from active oxygen hazards in living systems including aging, carcinogenesis and others. These studies have now
developed into a world-wide trend of the studies on functional foods. Through these studies, I became familiar with the chemistry and biology of free radicals (hereafter referred to as radicals). On the other hand, studies on the Maillard reaction were performed as an important task of food chemistry,
particularly on elucidation of its reaction mechanism at
JMARS News Letter Vol. 1, April 22, 2006
the initial stage. The mechanism of the Maillard
structures of sugars used for the reaction except for the
reaction had been proposed by J.E.Hodge (1953 and
C-3 compounds and varied with the structures of the
1967) and had been introduced in all textbooks and
amino group in the amino compounds. In addition,
assumed to be the only theory (Fig. 1). However, I
analysis of the hyperfine structure revealed that the
believed that there must be some other mechanisms at
radical might be derived from a pyrazine compound.
work in the Maillard reaction other than that of Hodge
Synthesis of the presumed pyrazine compound and its
and challenged to elucidate the mechanism. Another
determination showed complete consistency in ESR
subject that remained to be elucidated was the
spectra. Thus, it was demonstrated that a pyrazinium
differences in the Maillard reaction between processing
cation radical compound formed generally in the initial
food and biological systems. Generally, foods show an
stage of the Maillard reaction. Determination of the
acidic pH range and have a slightly higher pH than
chemical structure of an unknown intermediate radical
neutral in living matter and this difference greatly
in the reaction mixture by analysis of ESR spectrum is
influences the reactions. The major reaction in the
very rare, and the reason why these radical compounds
living body involves a side chain amino group in
are present so stably in the reaction mixture is not yet
protein. This reaction called glycation and it is
somewhat different from the reactions of sugars with
Spectral analysis of the pyrazine skeleton suggested
amino acids in the food system.
that two nitrogen atoms in the pyrazine skeleton may be
The key to the elucidation of the mechanism of the
derived from amino acid, and two carbon atoms (C-2)
Maillard reaction is a radical reaction. ESR (Electron
are presumed to be derived from the sugar. This
Spin Resonance) became popular in 1960s and the
compound is hardly formed according to the
presence of stable radicals in carbonated organic
mechanism of Hodge, so we tried to identify a
substances and roasted coffee beans became known. A
precursor compound of the radical product in the
long-lived radical was also observed in the melanoidin
reaction mixture. After comprehensive analysis of the
of the sugar and amino acid reaction. I hypothesized the
isolated products formed at an early stage of the
formation of a radical product different from the stable
reaction, we confirmed a production of the precursor
radicals in these polymers in the initial stage of the
compound having a nitrogen atom in a C-2 compound
Maillard reaction and tried to verify this in ESR
prior to the formation of the Amadori compound and
proved the formation process of the pyrazinium radical
However, a trace amount of radicals that developed
(Fig. 1). This is quite different from the mechanism of
during the reaction has generally very unstable and
Hodge and a new reaction mechanism that does not
hardly detectable. Therefore, a radical trapping method,
involve the Amadori rearrangement in the Maillard
a rapid flow method and a very low temperature
reaction was clearly found (1975).
determination method was generally used, but these
The characteristic feature of the new found reaction
methods encountered many difficulties in practice.
mechanism was the identification of an unstable
The observed signal merely showed the presence of a
intermediate at the center of the ongoing reaction with
radical if succeeded and no structural information on
the ESR spectrum elucidating the actual condition in
radical product could be obtained given the level of
the new flow of reaction. This procedure was different
radical chemistry in those days.
from the conventional procedures of the time.
I was at a loss as to how to elucidate this difficult
In 1982, I presented the results of the new reaction
problem. One day, I proposed to Dr. Tateki Hayashi, a
mechanism at a symposium of the American Chemical
colleague and an expert in ESR, the measurement of an
Society, 3) and received many responses and questions
ESR spectrum of a warmed reaction mixture of a sugar
from the audience. One member of the audience
and an amino acid as is. After a while, Dr. Hayashi
asked whether the mechanism would also occur in vivo,
returned from the ESR laboratory saying that the ESR
and I replied that it was possible. As described above,
imaging had succeeded. Surprisingly, the chart revealed
the mechanism of Hodge proceeds in a lower pH
a spectrum with a fine structure beyond imagination
environment and may face some problems at higher pH.
because the measurement was carried out in an open
However, the new mechanism was advantageous in a
test tube containing the sugar-amino acid mixture
higher pH range. I learned later that the man who asked
heated in a water bath without removal of oxygen or
the question was Dr. V.M.Monnier who later studied the
freezing. I wondered whether the spectrum really
two mechanisms and found that both proceeded equally
showed the radical formed by an amino-carbonyl
at about pH 7.2 and named the new mechanism the
reaction of a sugar-amino acid and tried the reaction
"Namiki pathway." 4) B.Crammerer et al. chemically
with another sugar-amino acid system, obtaining a
studied the mechanism and reported that the Namiki
similarly fine spectrum for the mixture after only about
pathway is predominant at pH 7.0 or over and that
10 minutes of heating with no browning. Thus we
Hodge's mechanism proceeds at about 40% or less.5)
demonstrated the deceptively difficult general
The Namiki pathway is now recognized throughout the
formation of a considerably stable radical compound
world and is often cited in the scientific literature.2)
different from the radical of melanoidins.1,3)
One major difference between the two mechanisms
Another surprising feature was that the determination
is the influence of pH. Glucose is liable to take a ring
of the spectrum of the reaction mixture at ordinary
structure with less of a carbonyl structure at neutral or
temperature showed a hyperfine structure. Radicals in
lower pH and it is difficult to react with amino group.
solid or crystal state sometimes show hyperfine
However, the probability of an open ring form increases
structures, but the observation of the spectrum of the
at pH 7 or over and tends to form a Schiff's base
reaction solution as is was very rare and their hyperfine
together with the formation of a highly reactive C-2
structural patterns were not so different from the
carbonyl-nitrogen compound by the cleavage of the
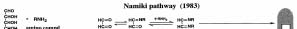
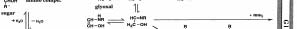
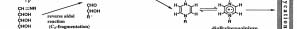

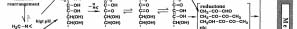
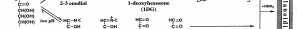

JMARS News Letter Vol. 1, April 22, 2006
Schiff's base by a reverse aldol reaction caused by an
formed biochemically without the Maillard reaction and
anion and leads to browning via the Namiki pathway.
its influence in the living body is the focus of much
In a lower pH range, the Amadori rearrangement occurs
attention. The influence of the Maillard reaction of such
by H+ as in the reaction with amino acid to form
low molecular weight carbonyls in the living body will
3-deoxyglucosone (3DG) as stated by Hodge. This
become more important.
product with an active dicarbonyl compound also takes
In any case, the effects of the Maillard reaction vary
on a cyclic structure and exists with comparative
depending on the conditions. In foods the reaction
stability in foods.
proceeds usually under serious conditions such as
One more important feature of the Namiki pathway
heating or long-term storage at lower pH causing
is the presence of a radical in the reaction product
discoloration, development of roast flavors or burned
which reacts with oxygen and probably gives an active
smells and the formation of pyrolyzate carcinogens,
oxygen species. I have observed a very weak
while the reaction under mild conditions in living body
chemiluminescence in the early stage of the Maillard
induces the accumulation of slight changes in living
reaction and assumed it to be caused by the reaction of
material causing serious damage in physiological
the radical product and oxygen. G.T.Wondrak also
functions related to aging and carcinogenesis. The
proved the generation of chemiluminescence based on
reaction mechanism of Hodge may be dominant in the
the Namiki pathway and further proved by ESR the
former system and that of the Namiki pathway in living
formation of a pyrazinium radical bridge between
-amino groups of lysine in a protein followed by the
Extensive investigations over a long period of time
formation of active oxygens by the reaction of the
on the radical reactions and a will to meet scientific
pyrazinium radical with oxygen.6) It is possible that the
challenges led to the discovery of a previously
opposite reaction of the pyrazinium radical with the
unknown mechanism other than the conventional
active oxygens may occur, so further studies on the
mechanism of the Maillard reaction, and the
influence of oxygen in the Maillard reaction should be
development of a new scientific field. Studies in
science are often like climbing a mountain for the first
T. Hofmann studied the Maillard reaction in detail
time, and no breakthrough can be accomplished without
at pH 7.0 and proved once again our mechanism
careful preparation and a spirit of challenge. Successful
involving the formation of a pyrazinium radical
studies involving close cooperation in chemistry,
intermediate from a sugar through glyoxal. Furthermore,
biology and medicine are eagerly awaited.
he named the intermolecular pyrazinium radical bridge
of lysine molecules the "CROSSPY radical" and
References
elucidated its high reactivity with SH and OH
Namiki, M, A New Development of the Maillard
compound in food and living matter.7) These facts show
Reaction and Glycation. SEIKAGAKU (The
various possible influences of the Maillard reaction
Journal of Biochemistry), 75 (1) 37-42, (2003)
product and functional substances in the living body
Rizzi, G.P., Free Radicals in the Maillard Reaction.
and future studies are awaited.
Food Reviews Intern., 19, 375-395 (2003)
One more influence of the Namiki pathway is the
Namiki, M., Hayashi, T., The Maillard Reaction in
re-presentation of the importance of a low molecular
Food and Nutrition, ACS Symp. Ser., 215, 21-46
weight carbonyl compound. As described above,
glucose takes on a ring structure with hardly any
Glomb, M.A. and Monnier, V. M., J. Biol. Chem.
reactivity of a carbonyl group. It is thus the most stable
270, 10017-10026 (1995)
sugar in the Maillard reaction. In contrast, low
Crammerer, B., and Kroch, L.W., Food Chem., 57,
molecular weight dicarbonyl compounds such as glycol
aldehyde, glyceraldehyde, glyoxal and methyl glyoxal
Wondrak, G.T., Pier, T. and Tressel, R., J. Biolumi.
have experimentally been proved to have several
Chemilumi. 10, 277-284 (1995)
hundred or thousand times reactivity compared with
Hofmann, T., Bore, W., and Stettmaier, K., J.
glucose. Compounds such as methyl glyoxal may be
Agric. Food Chem. 47, 391-396 (1999)
Fig. 1: Main pathway of the
Maillard reaction.
JMARS News Letter Vol. 1, April 22, 2006
COST-IMARS Joint Workshop
Napoli, May 24-27, 2006
II. 9th International Maillard Symposium
Munich, September 1-5, 2007
JMARS News Letter Vol. 1, April 22, 2006
Source: http://www.maillard.umin.jp/1st%20JMARS%20newsletter.pdf
Issue date: January 2007 Review date: March 2010 Naltrexone for the management of opioid dependence NICE technology appraisal guidance 115 NICE technology appraisal guidance 115 Naltrexone for the management of opioid dependence Ordering information You can download the following documents from www.nice.org.uk/TA115
Food intake and social habits in male patients and its relationship to intracytoplasmic sperm injection outcomesDaniela Paes de Almeida Ferreira Braga, D.V.M., M.Sc.,a,b Gabriela Halpern, M.Sc.,a Rita de C� assia S. Figueira, M.Sc.,a Amanda S. Setti, B.Sc.,b Assumpto Iaconelli Jr., M.D.,a and Edson Borges Jr., M.D., Ph.D.a,b a Fertility-Assisted Fertilization Centre and b Sapientiae Institute-Educational and Research Centre in Assisted Reproduction, S ao Paulo,Brazil