Nickelridge.ca
Multiple daily administrations of low-dose
sublingual immunotherapy in allergic
rhinoconjunctivitis
Vasco Bordignon, MD,* and Samuele E. Burastero, MD†
Background: Sublingual immunotherapy (SLIT) is an efficacious treatment for allergic rhinoconjunctivitis.
Objective: To investigate whether the number of daily administrations of SLIT can affect its efficacy.
Methods: In an open study, 64 patients with allergic seasonal rhinoconjunctivitis to grass or birch pollens were assigned to
the following 2-year daily treatment schedules: "3–3" group, 1 drop 3 times daily for 2 years; "2–3" group, 1 drop twice dailyin year 1 and 1 drop 3 times daily in year 2; "1–3" group, 1 drop once daily in year 1 and 1 drop 3 times daily in year 2; andcontrol group, no treatment. One fifth of the allergen concentration recommended by the manufacturer as maintenance treatmentwas used throughout the study. Patients were monitored for skin reactivity to the allergen used for SLIT using an end pointdilution technique and for drug use.
Results: No treatment-related adverse effects were observed. Skin reactivity to allergen decreased compared with controls in
the first treatment year only in the "3–3" group and in all treated patients in year 2. Drug use decreased in the first treatment yearin the "3–3" and "2–3" groups vs controls. This outcome extended to "1–3" patients in treatment year 2. Antihistamine usedecreased significantly compared with baseline in year 1 in "3–3" and "2–3" patients and in all treated patients in year 2. Nochanges were observed in controls.
Conclusion: The number of daily administrations seems to correlate with the efficacy of SLIT.
Ann Allergy Asthma Immunol. 2006;97:158–163.
that caution should be maintained in attributing a critical role
Sublingual immunotherapy (SLIT) is a safe and efficacious
to SLIT doses to achieve clinical efficacy and that dedicated
treatment for allergic rhinoconjunctivitis.1,2 The efficacy of
studies should instead be focused on the effect of the fre-
SLIT has been reported with a wide range of treatment doses,
quency of immunotherapy administration.
5 to 375 times those used for subcutaneous immunotherapy(SCIT).3,4 It is unclear whether a dose-response relationship
MATERIALS AND METHODS
exists with efficacy, although at least 1 study5 directly sug-gests that this is indeed the case. Furthermore, different
Study Design
weekly schedules are still used (3 times a week, every other
We conducted a 3-year open study of 64 patients (age range,
day, and so on).6 It was previously observed that patients
4 –50 years) with allergic seasonal rhinoconjunctivitis to
treated according to a daily allergen administration schedule,
grass or birch pollens. Patients underwent an accurate anam-
despite a lower cumulative dose, showed a higher reduction
nesis and clinical examination, including rhinologic exami-
in drug intake than patients treated with 3 weekly allergen
nation with the evaluation of anatomical problems (such as
administrations.7 These data suggest that the frequency of
nasal septum deviation), the nature of symptoms (rhinorrhea,
SLIT administration could be crucial to its efficacy and
nasal obstruction, associated ocular problems, cough, or
possibly more so than the absolute amount of the adminis-
asthma), and the seasonality, if any, of each symptom. A
tered dose. To evaluate the relevance of this assumption, we
daily immunotherapy schedule was given, which was started
used markedly low-dose SLIT to evaluate whether this treat-
before the pollen season in the first treatment year (in De-
ment is still efficacious when administered in protocols im-
cember 2000 and February 2001 for patients allergic to birch
plying different numbers of daily dosages. We found a strik-
and grass, respectively) without a build-up phase. Immuno-
ing correlation between the number of daily dosages and both
therapeutic treatment lasted 2 years.
the clinical efficacy and the reduction in skin reactivity to
allergen after only 1 year of SLIT. These results were con-
Patients were enrolled as volunteers after having been in-
solidated in the second treatment year. These data suggest
formed in full detail about the methods and aims of the trial.
Patients or their parents signed an informed consent form.
The procedures followed were in accordance with the ethical
* Bassano del Grappa (VI), Italy.
standards of the responsible institutional committee on hu-
† Department of Biotechnology, San Raffaele Scientific Institute, Milan, Italy.
man experimentation and with the Helsinki Declaration of
Received for publication December 21, 2005.
Accepted for publication in revised form February 27, 2006.
1975, as revised in 1983.
ANNALS OF ALLERGY, ASTHMA & IMMUNOLOGY
The study inclusion criteria were as follows: allergic sea-
the diagnostic extract prepared in sterile solution immediately
sonal rhinoconjunctivitis (without asthma) for at least 2 years;
before the assay. Saline solutions and histamine hydrochlo-
sensitization limited to either grass or birch pollens, as eval-
ride (ALK-Abello ) were used as negative and positive con-
uated by means of skin prick testing and serum specific IgE
trols, respectively. All the skin tests were performed by the
measurement; living in northern Italy; and only partial control
same individual (V.B.).
of allergic symptoms, never complete and satisfactory to the
To avoid any interference due to exposure to the relevant
patient, with the use of antihistamines (either cetirizine or
environmental allergen, the skin end point test was performed
loratadine). The study exclusion criteria were as follows:
outside the pollination period of the sensitizing species (ie, in
asthma or nasal polyps or obstructive nasal septum deviation;
February and December for patients allergic to grass and
a clinical history of significant symptomatic perennial aller-
trees, respectively). The test was performed at the beginning
gic rhinitis or asthma caused by an allergen to which the
of the first (observational) year to evaluate baseline reactivity
patient was regularly exposed; a clinical history of significant
and was repeated in the same months after the symptomatic
recurrent acute sinusitis (defined as 2 episodes per year for
seasons of each treatment year. Positivity was expressed as
the past 2 years, all of which required antibiotic drug treat-
the highest dilution yielding a wheal diameter greater than 3
ment) or chronic sinusitis or chronic obstructive lung disease;
mm. Using this end point dilution skin test, all patients
and, at randomization, current symptoms of, or treatment for,
included in the study scored positive at the 1:64 dilution (or
upper respiratory tract infection, acute sinusitis, acute otitis
above) at baseline.
media, or other relevant infectious processes.
Patients who met the study criteria and who were willing to
participate in the study were consecutively assigned to 1 of 3
In each treatment group, mixed birch or grass allergen ex-
treatment schedules: "3–3" group (n ⫽ 18), 1 drop 3 times
tracts (ALK Abello ) were used at one fifth of the concentra-
daily for 2 years; "2–3" group (n ⫽ 18), 1 drop twice daily in
tion recommended by the manufacturer as maintenance ther-
year 1 and 1 drop 3 times daily in year 2; and "1–3" group
apy (ie, 200 standard treatment units/mL). For each
(n ⫽ 18), 1 drop once daily in year 1 and 1 drop 3 times daily
administration, a single drop of the extract at this concentra-
in year 2. Patients who met the inclusion criteria but who did
tion was used 1, 2, or 3 times a day, according to the
not want to perform immunotherapy served as untreated
experimental group. In the conventional immunotherapy pro-
controls (n ⫽ 10). Patients were not informed that different
tocol, this dose would have to be scaled up to 5 drops and
numbers of drops were prescribed to different patients to
followed by a further step to a dosage 5 times higher to reach
prevent the possibility that "the more the better" hypothesis
maintenance. The timing of immunotherapy administration
could affect the individual perception of the need for antihis-
was as follows: (1) 8 AM for the once daily schedule (the first
tamines. Patient characteristics are summarized in Table 1.
year of treatment in the "1–3" group), (2) 8 AM and 8 PM forthe twice-daily schedule (the first year of treatment in the
Skin Testing and Allergen Extracts Used for Diagnosis
"2–3" group), and (3) 8 AM, 2 PM, and 10 PM for the 3 times
The skin response to the sensitizing allergen was quantified
daily schedule (the first year of treatment in the "3–3" group
using skin prick tests with mixed grasses (
Poa pratensis,
and the second year treatment in all the treated groups).
Phleum pratense, Dactylis glomerata, Festuca pratensis, and
Lolium perenne) and mixed Betulaceae (
Betula alba, Alnus
Drug Use Score
glutinosa, and
Corylus avellana) commercial extracts (ALK
As stated, at enrollment, all patients had a history of at least
Abello , Milan, Italy) by means of an end point dilution
2 years of allergic symptoms, which required exclusively the
technique. Each allergen extract was prepared in a saline
administration of oral antihistamines. Drug use was quanti-
solution containing glycerol, 50% vol/vol, and phenol, 0.4%
fied as the number of days during which the antihistamine
wt/vol, and was preliminarily used undiluted to evaluate the
(cetirizine or loratadine) was taken in each symptomatic
monosensitization of the patient. Then, the previously de-
season. In the geographic area where patients were living, the
scribed end point technique was used.7 Briefly, this method
average duration of the allergic season is approximately 90
implied 12 consecutive half dilutions in a saline solution of
days for birch and grass pollens.
Table 1. Characteristics of 64 Patients With Allergic Seasonal Rhinoconjunctivitis to Grass or Birch Pollens*
Allergic patients, No.
Sex, M/F, No.
Age, mean (range), y
* See the "Patient Enrollment" subsection for definitions of the treatment groups.
VOLUME 97, AUGUST, 2006
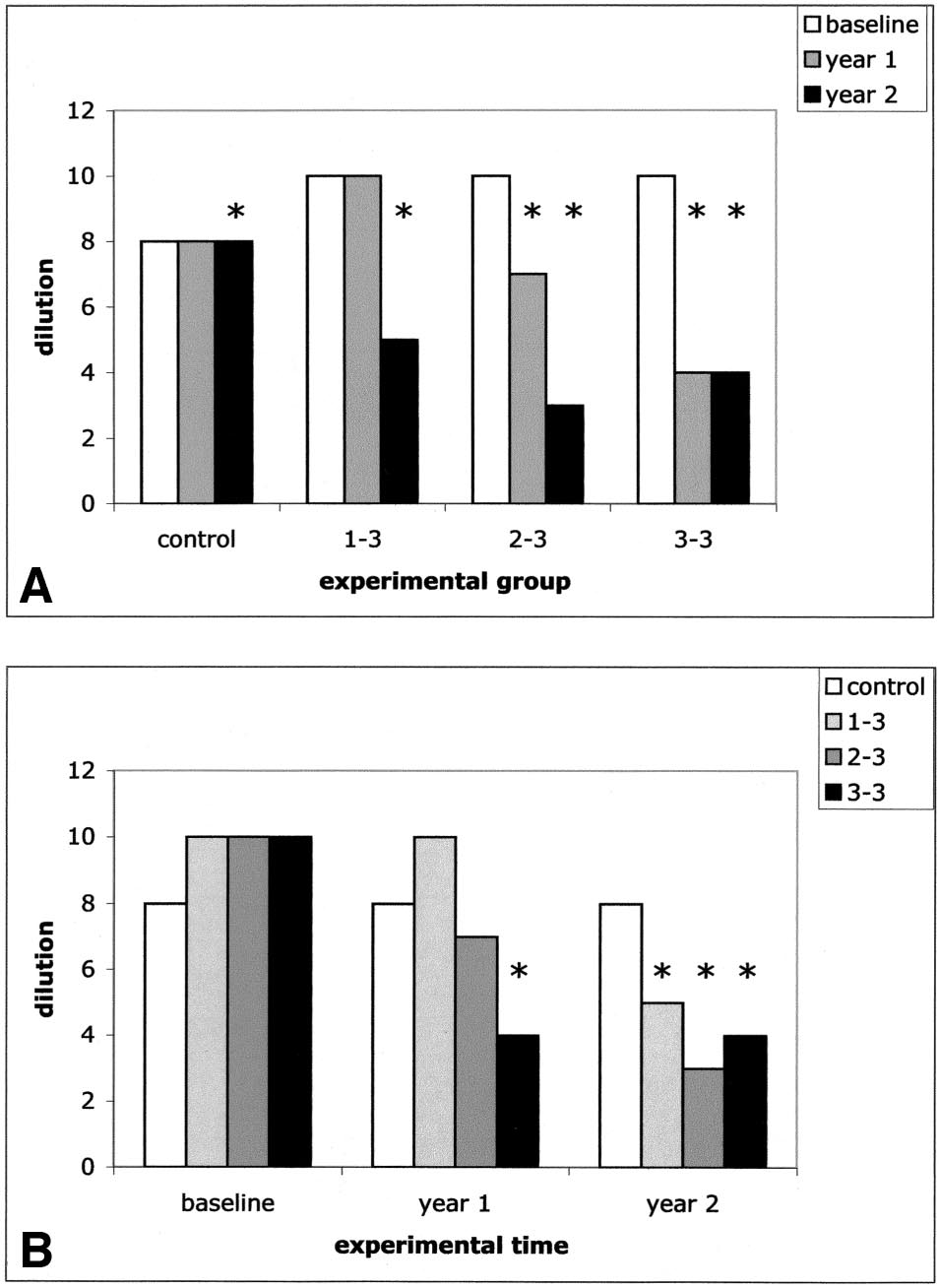
Skin Test Reactivity
Statistical analysis was performed by means of nonparametric
In the intragroup analysis, skin reactivity significantly de-
tests (the Wilcoxon test for intragroup comparison and the
creased compared with baseline in only "3–3" and "2–3"
Mann-Whitney test for intergroup comparison) because none
patients after the first treatment year (P ⬍ .001 and P ⫽ .002,
of the examined data could be considered for normal distri-
respectively) and in all treated patients after the second treat-
bution either directly or following common mathematical
ment year (P ⬍ .001) (Fig 1A and Table 2). In contrast, in the
transformations. The statistical analysis was performed using
control group, skin reactivity did not change in the first year
a software program (GraphPad; GraphPad Software, San
and increased compared with baseline in the second year of
Diego, CA). P ⱕ .05 was considered statistically significant.
follow-up (P ⫽ .03). In the intergroup analysis, values of skinreactivity to each sensitizing allergen were homogeneous in
the 4 experimental groups at baseline (Fig 1B). After 1 year
Adverse Effects
of treatment, skin reactivity decreased compared with con-
No SLIT-related adverse effects were observed in any of the
trols in the "3–3" group (P ⬍ .001) but not in the "2–3" and
treated groups. Although no build-up phase was performed at
"1–3" groups. After 2 years of treatment, skin reactivity
the beginning of the study, neither local (oral) adverse effects
decreased compared with controls in all the treated groups
nor systemic symptoms (rhinoconjunctivitis, asthma, urti-
(P ⬍ .001) (Fig 1B and Table 2).
caria, or pruritus) were observed. No dropouts were observed.
Comparing skin reactivity in the "1–3" group vs the "2–3"
group, the "1–3" group vs the "3–3" group, and the "2–3"group vs the "3–3" group at baseline, no differences wereobtained, indicating a homogeneous level of allergen-specificsensitization at the recruitment of patients. At year 1, "2–3"and "3–3" patients reached a lower level of sensitizationcompared with "1–3" patients (P ⬍ .001). Furthermore, atyear 1, "3–3" patients lowered their sensitization comparedwith "2–3" patients (P ⬍ .001). At year 2, "2–3" and "3–3"patients reached a lower level of sensitization compared with"1–3" patients (P ⬍ .001), whereas no difference occurredbetween "2–3" and "3–3" patients.
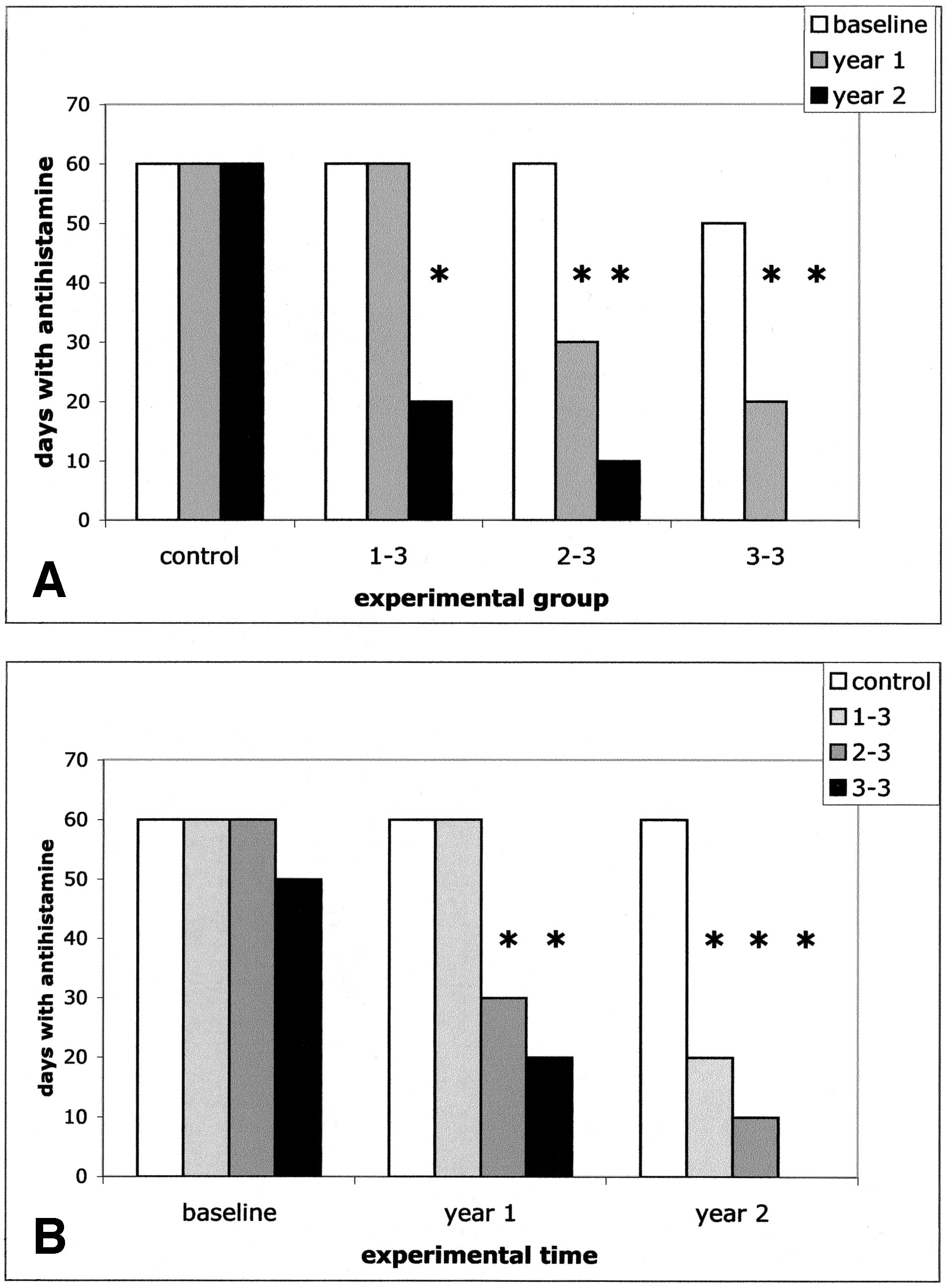
Drug UseIn the intragroup analysis, antihistamine use decreased sig-nificantly compared with baseline in the first treatment yearonly in the "3–3" and "2–3" groups but not in "1–3" patients(P ⬍ .001) and in all treated patients in the second treatmentyear (P ⬍ .001). No changes were observed in controls (Fig2A and Table 2). In the intergroup analysis, at baseline,treated patients were similar to controls regarding antihista-mine use (Fig 2B and Table 2). In contrast, in the first
Table 2. Skin Reactivity and Drug Use in the Experimental Groups*
Value, median (IQR)
Skin reactivity (reverse of dilution)
Figure 1. Median values of skin reactivity at the 3 considered experimen-
Drug use (days with antihistamine therapy)
tal times. The median values of the reverse of dilution yielding wheals with
a diameter greater than 3 mm are indicated for representative purposes. A,
The intragroup analysis shows the modification across time of skin reactivity
in each experimental group. B, The intergroup analysis shows the compar-
ison at each experimental time of skin reactivity in the 3 experimentalgroups. Asterisks indicate the significant differences of the considered values
Abbreviation: IQR, interquartile range.
compared with baseline values of the same experimental group (A) or
* See the "Patient Enrollment" subsection for definitions of the treat-
compared with time-matched values of controls (B).
ment groups.
ANNALS OF ALLERGY, ASTHMA & IMMUNOLOGY
DISCUSSION
Sublingual immunotherapy is a safe and effective alternative
to injection immunotherapy for respiratory allergic diseas-
es.8–16 A rigorous meta-analysis1 of evidence-based studies
has recently established this conclusion. Herein, we confirm
the efficacy of SLIT, which induced the reduction of 2 end
points (skin reactivity to allergen and antihistamine use) in
monosensitized patients with seasonal allergic rhinoconjunc-
tivitis.
We found a direct correlation between the improvement of
end points and the number of daily administrations. To makeit unlikely that the slightly different allergen doses related tothe different number of daily dispensations could explain theresults, we administered an amount of allergen approximatelyone fiftieth of that recommended by the manufacturer(namely, 2%, 4%, and 6% of the daily recommended dose inthe first treatment year of "1–3," "2–3," and "3–3" patients,respectively). As a whole, the administered dose corre-sponded to 0.56, 0.37, and 0.18 times the cumulative monthlySCIT dose in the "3–3," "2–3," and "1–3" groups, respec-tively. The SLIT/SCIT ratio of dosages is an imperfect way toexpress the allergen dose, which should be accepted withsome caution until the content in recombinant allergen isprovided for all available extracts. However, these figures arefar below the lower value of the wide dose range (5–375times), which seemed efficacious in a meta-analysis3 of evi-denced-based SLIT studies with schedules implying singledaily administrations. Indeed, in a consensus document from
Figure 2. Median values of antihistamine use at the 3 considered exper-
the Allergic Rhinitis and Its Impact on Asthma group, effi-
imental times. The number of days with antihistamine use is indicated. A,
cacy was attributed to even higher SLIT doses, in the 50 to
The intragroup analysis shows the modification across time of drug use in
100 SLIT/SCIT ratio, or above.17 In contrast to these argu-
each experimental group. B, The intergroup analysis shows the comparisonat each experimental time of drug use in the 3 experimental groups. Asterisks
ments, with the extremely low doses we used, the impact of
indicate the significant differences of the considered values compared with
SLIT on skin reactivity to the relevant allergen and on the
baseline values of the same experimental group (A) or compared with
drug use scores was critically dependent on the number of
time-matched values of controls (B).
daily assumptions. Indeed, despite these remarkably lowdoses, a formally more appropriated experimental designwould have been to compare the usual once-daily dose with
treatment year, "3–3" and "2–3" patients reached lower drug
the same amount divided into 2 or 3 doses rather than simul-
use compared with controls (P ⬍ .001 and P ⬍ .02, respec-
taneously changing frequency and dose. However, the results
tively). The reduction in drug use compared with controls
suggest that in determining SLIT efficacy, allergen persis-
extended to "1–3" patients in the second treatment year (P ⬍
tence represents a far more relevant factor than its concen-
.001) (Fig 2B and Table 2).
Comparing the use of drugs in the "1–3" group vs the
Although this is an open study, the fact that such an
"2–3" group, the "1–3" group vs the "3–3" group, and the
objective end point as skin reactivity was modulated in par-
"2–3" group vs the "3–3" group at baseline, no differences
allel to drug use suggests that indeed the immune response
were observed, indicating a homogeneous level of clinical
mutated in response to the number of daily drops taken by
severity at the recruitment of patients. At year 1, "2–3" and
patients, bringing about the observed clinical consequences.
"3–3" patients decreased drug use compared with "1–3"
Furthermore, although symptom scores were not collected
patients (P ⬍ .001). Furthermore, at year 1, "3–3" patients
with daily diaries, a clear-cut improvement in "3–3" patients
had lower drug use compared with "2–3" patients (P ⬍ .002).
was observed after a single year of treatment compared with
At year 2, "2–3" and "3–3" patients had lower drug use
the previous season in terms of overall self-evaluation of
compared with "1–3" patients (P ⬍ .003 and P ⬍ .001,
symptoms and with respect to answers to questions about
respectively). Furthermore, at year 2, "3–3" patients used
quality of life. Furthermore, this improvement extended to the
fewer drugs than "3–3" patients (P ⬍ .04).
other groups when the "3–3" dosage was applied to them.
VOLUME 97, AUGUST, 2006
Recently, the dose-response dependence of the efficacy
would also lower the cost of treatment and avoid the induc-
and severity of adverse effects has been considered in 2
tion phase. This issue deserves to be investigated more ex-
dedicated studies,5,18 although within a narrow dose range,
tensively in double-blind, placebo-controlled studies of ho-
and in 1 meta-analysis.19 Furthermore, the possibility that
mogenous cohorts (ie, either adults or children with
precoseasonal treatment may be as efficacious as a perennial
sensitization to a single allergen). This approach should par-
treatment has been reported.6,20 However, the relevance of the
allel current efforts with high-dose regimens.5,18
number of daily administrations in obtaining efficacious clin-ical results has never been addressed directly. It was previ-
ously observed that patients treated according to a daily
1. Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy
allergen administration schedule, despite a lower cumulative
for allergic rhinitis: systematic review and meta-analysis. Al-lergy. 2005;60:4 –12.
dose, showed a significantly greater decrease in skin reactiv-
2. Novembre E, Galli E, Landi F, et al. Coseasonal sublingual
ity, a higher rate of no use of any drug, and a more than 50%
immunotherapy reduces the development of asthma in children
reduction in yearly drug intake compared with patients
with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004;
treated with 3 weekly allergen administrations.7 These data
114:851– 857.
were obtained as side results in a study focused on the
3. Canonica GW, Passalacqua G. Noninjection routes for immu-
modulation of skin reactivity to the allergen. Nevertheless, it
notherapy. J Allergy Clin Immunol. 2003;111:437– 449.
was indicating that the number of daily administrations of a
4. Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis
sublingual vaccine could be more critical in obtaining a
and its impact on asthma. J Allergy Clin Immunol. 2001;108:
favorable modulation of immunity to allergens than the
amount of the administered allergen itself. The biological
5. Marcucci F, Sensi L, Di Cara G, et al. Dose dependence of
immunological response to sublingual immunotherapy. Allergy.
mechanisms that underlie SLIT efficacy are still largely un-
known and remain a matter of speculation.21 Thus, it should
6. Di Rienzo V, Puccinelli P, Frati F, et al. Grass pollen specific
not be surprising that dose-response rules cannot be applied
sublingual/swallow immunotherapy in children: open-
tout-court to the allergic response involving such an immune-
controlled comparison among different treatment protocols. Al-
privileged site as the oral mucosa. Indeed, our results can
lergol Immunopathol (Madr). 1999;27:145–151.
easily be integrated with the established knowledge on oral
7. Bordignon V, Parmiani S. Variation of the skin end-point in
allergen absorption in SLIT. In fact, a study from Bagnasco
patients treated with sublingual specific immunotherapy. J In-
et al22 demonstrated that when performing SLIT, only a
vestig Allergol Clin Immunol. 2003;13:170 –176.
minimal amount of allergen is absorbed orally, but this has to
8. Tari MG, Mancino M, Monti G. Efficacy of sublingual immu-
be considered critical to the efficacy of SLIT. In contrast, the
notherapy in patients with rhinitis and asthma due to house dustmite: a double-blind study. Allergol Immunopathol (Madr).
gastrointestinal absorption, which is prevalent in SLIT itself
and represents the exclusive modality of absorption in oral
9. Troise C, Voltolini S, Canessa A, et al. Sublingual immuno-
immunotherapy, is not associated with any clinical improve-
therapy in Parietaria pollen-induced rhinitis: a double-blind
ment.23–28 Along this line, the allergen persistence in the oral
study. J Investig Allergol Clin Immunol. 1995;5:25–30.
mucosa may be a far more relevant factor for gaining efficacy
10. Sabbah A, Hassoun S, Le Sellin J, et al. A double-blind,
than allergen concentration. It would be interesting to exper-
placebo-controlled trial by the sublingual route of immunother-
imentally compare with radiolabeled allergens the amount
apy with a standardized grass pollen extract. Allergy. 1994;49:
that is actually absorbed through the oral mucosa when using
a dose as low as one fiftieth of the recommended one.
11. Clavel R, Bousquet J, Andre C. Clinical efficacy of sublingual-
We suggest that multiple daily doses may provide a much
swallow immunotherapy: a double-blind, placebo-controlledtrial of a standardized five-grass-pollen extract in rhinitis. Al-
greater impact on the overall allergen absorption through the
lergy. 1998;53:493– 498.
oral mucosa than the concentration of the allergen or its
12. Feliziani V, Lattuada G, Parmiani S, et al. Safety and efficacy
cumulative amount. In principle, it cannot be excluded that
of sublingual rush immunotherapy with grass allergen extracts:
extremely high concentrations of allergen used in some SLIT
a double blind study. Allergol Immunopathol (Madr). 1995;23:
trials may also imply longer persistence of the extract in the
mouth. This increased persistence could be responsible for
13. Quirino T, Iemoli E, Siciliani E, et al. Sublingual versus injec-
relatively greater efficacy5 rather than the dose itself, but at a
tive immunotherapy in grass pollen allergic patients: a double
given point this would be achieved at the cost of many and
blind (double dummy) study. Clin Exp Allergy. 1996;26:
annoying oral and gastrointestinal adverse effects.3,29
In conclusion, these data indicate that SLIT is safe and
14. Passalacqua G, Albano M, Fregonese L, et al. Randomised
controlled trial of local allergoid immunotherapy on allergic
efficacious in patients with allergic rhinoconjunctivitis also at
inflammation in mite-induced rhinoconjunctivitis. Lancet.
a low-dose regimen. The number of doses seems to be far
1998;351:629 – 632.
more crucial for SLIT efficacy than the absolute amount of
15. Vourdas D, Syrigou E, Potamianou P, et al. Double-blind,
administered allergen. Two daily doses are necessary to
placebo-controlled evaluation of sublingual immunotherapy
achieve clinical improvement, which is more consistent with
with standardized olive pollen extract in pediatric patients with
3 doses. Adoption of the concentration used in this study
allergic rhinoconjunctivitis and mild asthma due to olive pollen
ANNALS OF ALLERGY, ASTHMA & IMMUNOLOGY
sensitization. Allergy. 1998;53:662– 672.
grass pollen to hay fever patients: an efficacy study in oral
16. Bousquet J, Scheinmann P, Guinnepain MT, et al. Sublingual-
swallow immunotherapy (SLIT) in patients with asthma due to
25. Taudorf E, Laursen LC, Lanner A, et al. Oral immunotherapy in
house-dust mites: a double-blind, placebo-controlled study. Al-
birch pollen hay fever. J Allergy Clin Immunol. 1987;80:
lergy. 1999;54:249 –260.
17. Bousquet J, Van Cauwenberge P. Allergic rhinitis and its impact
26. Oppenheimer J, Areson JG, Nelson HS. Safety and efficacy of
on asthma. J Allergy Clin Immunol. 2001;108:S1–S315.
oral immunotherapy with standardized cat extract. J Allergy
18. Andre C, Perrin-Fayolle M, Grosclaude M, et al. A double-blind
Clin Immunol. 1994;93:61– 67.
placebo-controlled evaluation of sublingual immunotherapy
27. Van Deusen MA, Angelini BL, Cordoro KM, et al. Efficacy and
with a standardized ragweed extract in patients with seasonal
safety of oral immunotherapy with short ragweed extract. Ann
rhinitis: evidence for a dose-response relationship. Int Arch
Allergy Asthma Immunol. 1997;78:573–580.
Allergy Immunol. 2003;131:111–118.
28. Litwin A, Flanagan M, Entis G, et al. Oral immunotherapy with
19. Gidaro GB, Marcucci F, Sensi L, et al. The safety of sublingual-
short ragweed extract in a novel encapsulated preparation: a
swallow immunotherapy: an analysis of published studies. Clin
double-blind study. J Allergy Clin Immunol. 1997;100:30 –38.
Exp Allergy. 2005;35:565–571.
29. Burastero S. Regarding Gidaro GB, Marcucci F, Sensi L, In-
20. Gozalo F, Martin S, Rico P, et al. Clinical efficacy and tolerance
corvaia C, Frati F, Ciprandi G. The safety of sublingual-
of two year Lolium perenne sublingual immunotherapy. Aller-
swallow immunotherapy: an analysis of published studies. Clin
gol Immunopathol (Madr). 1997;25:219 –227.
Exp Allergy 2005;35:565–571. Clin Exp Allergy. 2005;35:
21. Akdis CA, Blaser K. Mechanisms of allergen-specific immu-
1407–1408; author reply 1409.
22. Bagnasco M, Mariani G, Passalacqua G, et al. Absorption and
distribution kinetics of the major Parietaria judaica allergen(Par j 1) administered by noninjectable routes in healthy human
Requests for reprints should be addressed to:
beings. J Allergy Clin Immunol. 1997;100:122–129.
Samuele E. Burastero, MD
23. Cooper PJ, Darbyshire J, Nunn AJ, et al. A controlled trial of
San Raffaele Scientific Institute
oral hyposensitization in pollen asthma and rhinitis in children.
via Olgettina 58
Clin Allergy. 1984;14:541–550.
20132 Milano, Italy
24. Taudorf E, Laursen LC, Djurup R, et al. Oral administration of
VOLUME 97, AUGUST, 2006
Source: http://nickelridge.ca/wp-content/uploads/2013/03/Bordignon-study.pdf
Design of the HIV Prevention Trials Network (HPTN) Protocol 054: A cluster randomized crossover trial to evaluate combined access to Nevirapine in developing countriesJim HughesUniversity of Washington, [email protected] Robert L. GoldenbergUniversity of Alabama, [email protected] Catherine M. WilfertElizabeth Glaser Pediatric AIDS Foundation/Duke University, [email protected]
STRUCTURE SEARCHING DERWENT WORLD PATENTS INDEX® (DWPISM) USING STN EXPRESS®: Part I INTELLECTUAL PROPERTY SOLUTIONS BRIAN LARNER JANUARY 2013 – THE PROBLEM; WHY IS IT DIFFICULT TO SEARCH CHEMICAL STRUCTURES IN PATENTS? – SOLUTION; DWPI STRUCTURAL INDEXING – DWPI PATENT COVERAGE – COMPOUND COVERAGE – WHICH COMPOUNDS ARE – DETAILED LOOK AT BCE FRAGMENTATION CODING – DETAILED LOOK AT DCR – STRUCTURAL MANUAL CODES



