Hepatitis c
Hepatitis C Treatment in 2016
Amanda Noska, MD, MPH
Providence VAMC & The Miriam
Hospital & Immunology Center
• I have no financial disclosures.
• I will be referencing trade names in addition
to generic today for ease of understanding and education purposes.
Learning Objectives
Pharmacists• Discuss the epidemiology and immunology of hepatitis C in the
United States.
• Discuss the importance of prior treatment history and liver staging
in determining a HCV treatment regimen.
• Discuss current available hepatitis C treatment options.
• Describe common side effects and drug-drug interactions of
directly-acting antiviral medications for HCV.
• Describe immunizations related to various types of hepatitis.
Review common side effects and therapeutic contraindications
associated with hepatitis C treatment options
Identify patients that may be candidates for hepatitis vaccines
1. What is the most common barrier to patients
accessing hepatitis C treatment currently?
a) Unstable mental health disordersb) Insurance coveragec) Drug-drug interactionsd) A life-expectancy of <1 year
2. Which of the following drugs interacts with Ledipasvir/sofosbuvir (Harvoni) to decrease serum levels of ledipasvir?a) Methadoneb) Levothyroxinec) Levetiracetamd) Omeprazole
3. Based on the ION trials, which of the following patients might be a candidate for 8 weeks of ledipasvir/sofosbuvir (Harvoni)?a) GT 1a, treatment naïve, non-cirrhotic, HCV viral load
b) GT 3, treatment naïve, non-cirrhotic, HCV viral load 3
c) GT 1b, treatment naive, cirrhotic, HCV VL 2 milliond) GT 4, treatment naïve, non-cirrhotic, HCV viral load 6
4. Which of the following is among the most common noted side effect of daclatasvir(Daklinza)? a) Nauseab) Fatiguec) Skin rashd) Diarrhea
5. Which of the following measures are important to preventing morbidity associated with chronic hepatitis C?a) Weekly lab monitoringb) Vaccination against hepatitis B alonec) Vaccination against hepatitis A and Bd) Avoidance of all medications metabolized by the
First described in 1989, Blood screening began in 1990.
Peak prevalence occurs in those born 1945-1965.
Worldwide 350,000-500,000 people die annually from HCV related
Cirrhosis develops in 10-20% of patients with chronic HCV infection
over 20-30 years on average, although rates vary widely (from 2% to
After cirrhosis due to HCV has developed, the
annual risk of
developing HCC is 1-5%.
AASLD. 2015.Westbrook R, J of Hepatotogy. 2014;61:S58-68. Grebely J et al. Hepatology.2014;59:109-120. Alric L. Hepatology. 2014;60(6):Epub.
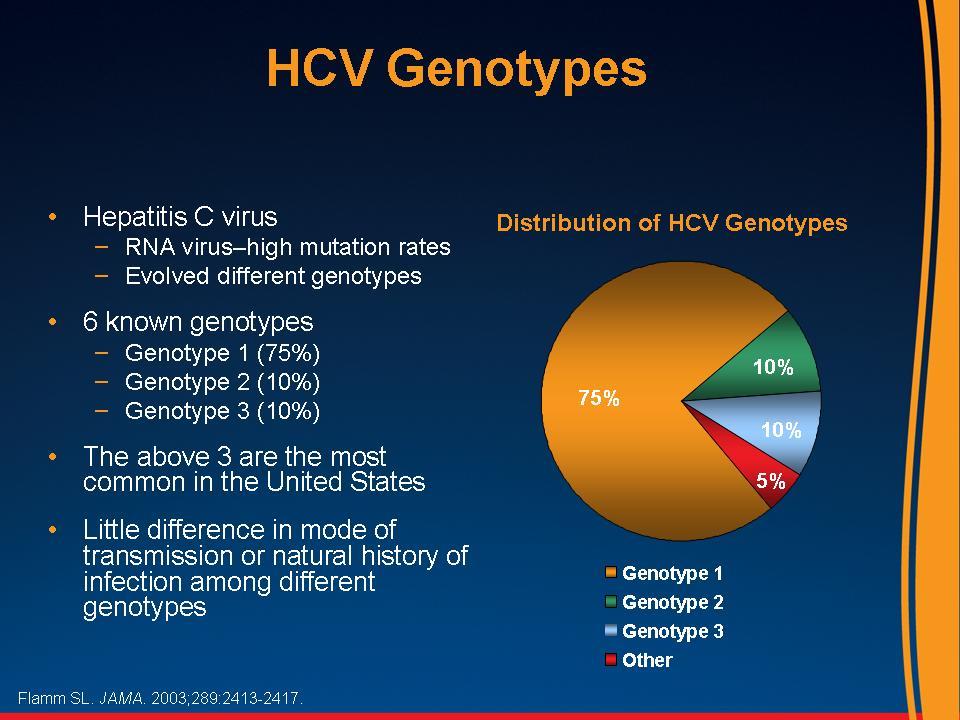
variable geographic distribution
Slide courtesy of Dr. Lynn Taylor
Deaths Due to HCV Infections Now Exceed
Those Due to HIV Infection
Hepatitis C
16,600 deaths
Number of HCV-related deaths may be over 60,000 because of under-reporting on death certificates
Ly KN, Xing J, Klevens RM, Jiles RB, Holmberg SD. Causes of death and characteristics of decedents with viral hepatitis, United States, 2010. Clin Infect Dis. 2014 Jan;58(1):40-9. Mahajan, IDSA 2013
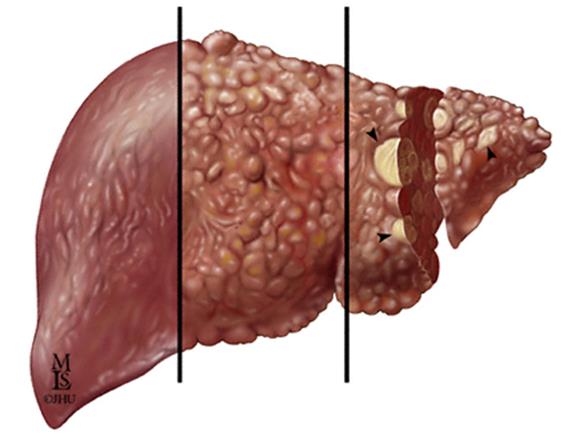
Chronic HCV Infection May Lead to
Chronic Liver Disease and Liver Cancer
Hepatocellular Carcinoma
Chronic HCV
Cancer of the liver
infection can
can develop after
lead to the
years of chronic
development of
fibrous scar
tissue within
the liver
Decompensated cirrhosis:
Over time, fibrosis can
Bleeding gastroesophageal
progress, causing severe
scarring of the liver,
Hepatic encephalopathy
restricted blood flow,
impaired liver function,
and eventually liver failure
*** Slide courtesy of Dr. Camilla Graham***
Chronic liver disease includes fibrosis, cirrhosis, and hepatic decompensation; HCC=hepatocellular carcinoma.
1. Highleyman L. Hepatitis C Support Project. http://www.hcvadvocate.org/hepatitis/factsheets_pdf/Fibrosis.pdf. Accessed August 18, 20112. Bataller R et al. J Clin Invest. 2005;115:209-218; 3. Medline Plus. http://www.nlm.nih.gov/medlineplus/enxy.article/000280.htm. Accessed August 28, 2012; 4. Centers for Disease Control and 12
http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm. Accessed May 8, 2012.
CDC and ACIP Vaccine Schedule: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf
HAV/HCV Co-infection
Acute hepatitis A infection + chronic HCV can be devastating.
In a prospective study by Vento, 432 patients with chronic hepatitis C (183 with cirrhosis) were observed over a 7-year period.
– Of the 17 patients with concurrent HAV infection,
seven (41.4%) developed fulminant hepatitis and 6 patients died (35.3%).
Vento S. J of Viral Hepatitis. 2002; 7(S1):7-8.
Hepatitis A Vaccination
Two doses required
Havrix:1440 ELISA Units (1 ml) IM with a booster dose (1440 units) at 6+ months after the primary immunization
VAQTA : 50 units (1 ml) IM with a booster dose (50 units) given at 6-18 months after primary immunization.
HBV/HCV Co-infection
7-20 million people worldwide are co-infected with HBV/HCV.
Patients with HBV/HCV coinfection have an increased risk for cirrhosis, hepatocellular carcinoma (HCC) and even death.
Potthoff A, Manns MP, Wedemeyer H. Expert Opin on Pharmacotherapy. 2010;11(6):919-28.
Hepatitis B Immunization
– Immunocompetent hosts:
• 1 ml/dose for 3 total doses administered at 0, 1, and 6
– Immunocompromised hosts:
• 20 mcg/ml: administer 2 ml per dose at 0, 1, 2 and 6
Recombivax HB:
– Immunocompromised hosts:
• 40 mcg/ml: administer 1 ml per dose at 0, 1, and 6 months
Twinrix: Hepatitis A and B vaccine
Hepatitis A and recombinant hepatitis B inactivated vaccine
– Hepatitis A virus antigen 720 ELISA units and hepatitis B surface
ag 20 mcg/mL (1 ml)
– Contains aluminum, trace amounts of neomycin, and some
Given as 1 mL intramuscular injections at 0, 1, and 6 months
– Hep A component is ½ that of the Hep A vaccine alone, so it
may be less immunogenic after 1 dose.
– Hep B component is likewise ½ that of the Hep B vaccine alone,
so it may be less immunogenic after a single dose.
– Should not be used as post-exposure prophylaxis.

Developing a HCV Vaccine:
Hepatitis C is highly variable even among strains
HCV mutates quickly
The vaccine likely needs to be specific to only one genotype
Utilization of the T cell response is critical to viral clearance
Hepatitis C Vaccine
• Simian adenovirus vector (ChAd3) + modified vaccinia
Ankara vector (MVA) that encodes NS3, NS4, NS5A and
NS5B proteins of Hepatitis C genotype 1b
• HCV specific T-cells are induced ChAd3, then boosted by
MVA, to generate high levels of CD8+ and CD4+ HCV-
specific T cells that target multiple HCV antigens
• Sustained memory cells and effector T cells are then
• T-cell memory evolves over time to produce anti-HCV
• So far appears to produce a "durable, broad, sustained and
balanced T-cell response to [HCV]… associated with viral
Swadling et al. Science Translational Medicine. 2014; 6(261):261.
FIRST IFN-Free Therapy FDA-approved
Nucleotide Analogue Inhibitor of HCV NS5B polymerase enzyme
Effective for treatment-naives + pts who failed prior IFN treatment, cirrhotics, decompensated cirrhotics
Slide courtesy of Dr. Lynn Taylor
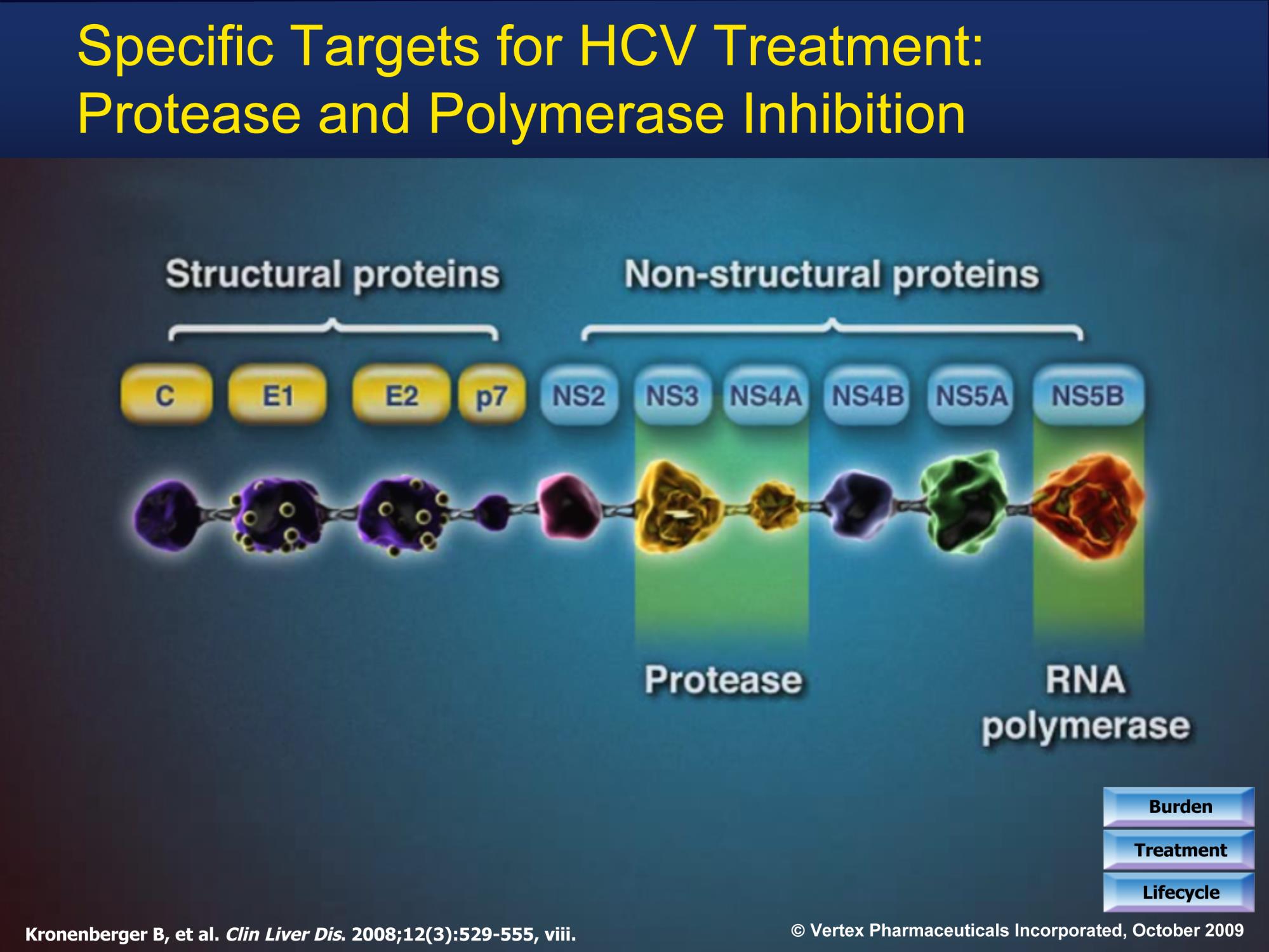
Multiple Validated Drug Targets
MIR 122 Inhibitors
Membraneous web (Preclin)
- MIravirsen
Viral enzyme
Viral enzyme
Viral enzyme
Active site
Replication complex
Active site
Graphic courtesy of Dr John Link,
Slide courtesy of Dr. Camilla Graham.
Ahmed A, Felmlee DJ. Viruses. 2015 Dec 18;7(12):6716-29.
Keeping it Straight
A 56 yo M with diabetes mellitus, peptic ulcer disease due to NSAIDS with prior upper GI bleed, opioid use disorder in remission, tobacco dependence, and chronic hepatitis C genotype 1a, fibrosis stage 2, HCV viral load 2.6 million, presents for evaluation to your office.
Pt's EGD last month shows no active upper GI bleeding and well-healed peptic ulcers. He has never been treated for chronic hepatitis C before, presents today to discuss his treatment options. Pt has no known mental health disorders, is stably housed, and hasn't used injection drugs in over 10 years, stable on methadone.
Provided the patient has no drug-drug interactions…
Which of the following directly-acting antivirals is the best option for treatment?
1. Ledipasvir 90 mg/sofosbuvir 400 mg
(HARVONI) x 8 weeks
2. Sofobuvir 400 mg (SOVALDI) + weight-
based ribavirin x 12 weeks
3. Paritepravir 150 mg/ritonavir 100
mg/ombitasvir 25 mg + dasabavir 250 mg BID (VIEKIRA PAK) twice daily + weight-based ribavirin x 8 weeks
4. Daclatasvir 60 mg (DAKLINZA) +
Sofosbuvir 400 mg (SOVALDI) x 24 weeks
Case #1 continued
This patient's med list includes:omeprazole 40 mg dailylevothyroxine 125 mcg dailylevetiracetam 1000 mg twice dailymethadone 90 mg daily
What changes would you suggest to the patient's provider regarding this patient's medications prior to treatment initiation?
1. Suggest an alternate anti-convulsant
2. Reduce omeprazole to 20 mg daily if
clinically feasible and advise the patient to take the drug at the same time as Ledipasvir/Sofosbuvir(Harvoni)
3. Increase the levothyroxine
4. Reduce the pt's methadone dose
5. No changes needed here
Genotype 1: C-EDGE
382 patients received 12 weeks of elbasvir 50 mg + grazoprevir 100 mg (ZEPATIER) for genotype 1 HCV.
– 50% genotype 1a– 41% genotype 1b
C-EDGE (12 wks Zepatier): SVR12
GT 1a, non-C, naïve
GT 1b, non-C, naïve
Genotype 1: C-WORTHY
74 patients, treatment-naïve, non-cirrhotic, included both HCV mono-infected and HIV/HCV co-infected patients who received 12 weeks of elbasvir 50 mg + grazoprevir 100 mg (ZEPATIER) without ribavirin.
C-WORTHY (12 weeks Zepatier):
GT 1a, non-C, naïve
GT 1b, non-C, naïve
Genotype 1: C-EDGE, Cirrhotic pts
Presence or absence of compensated cirrhosis does not appear to alter the efficacy of the elbasvir 50 mg/grazoprevir 100 mg (ZEPATIER) regimen.
92 (22%) patients in the trial had Metavir F4 disease consistent with cirrhosis.
SVR12 was 97% (90/92) in cirrhotic patients with GT 1 disease.
Baseline NS5A Resistance-associated variants (RAVs)
significantly reduce rates of SVR12 with a 12-week course of
the elbasvir 50 mg/grazoprevir 100 mg (ZEPATIER) regimen in
NS5A RAVs were identified at baseline in 12% (19/154) of GT
1a patients enrolled in the C-EDGE study.
- 58% (11/19) achieved SVR12 compared to - 99% (133/135) SVR12 in patients without RAVs
Recommendation: Patients should be tested for RAVS to NS5A
inhibitors before beginning treatment.
Genotype 1: ION Trials
ION-1: Ledipasvir 90 mg/sofosbuvir 400 mg (HARVONI)
865 treatment-naïve patients, including pts with cirrhosis.
– SVR12 was 97-99% overall in all groups
– There was no significant difference in SVR12 based on:
• Use of RBV• HCV genotype 1 subtype • Length of treatment (12 vs. 24 week regimens)
– 16% of subjects had cirrhosis
• SVR12 was 97% in cirrhotic patients
Genotype 1: ION Trials
ION-3: Ledipasvir 90 mg/sofosbuvir 400 mg (Harvoni)
647 treatment-naïve patients, non-cirrhotic only
– There were lower relapse rates in patients
receiving 8 weeks of ledipasvir/sofosbuvir(Harvoni) who had baseline HCV RNA levels below 6 million IU/mL (2%; 2 of 123)
Genotype 1: PEARL-IV, SAPPHIRE-1,
SAPPHIRE-I: Paritaprevir 150 mg/ ritonavir 100 mg/ ombitasvir
25 mg + Dasabavir 250 mg BID x 12 wks (VIEKIRA PAK) +
weight-based ribavirin
322 treatment-naïve, non-cirrhotic patients with genotype 1a
– SVR12 was 95% with 12 weeks of Viekira pak and ribavirin– Virologic failure was higher in GT1a (7 of the 8 failures were GT
PEARL-IV:305 treatment-naïve, non-cirrhotic patients with genotype 1a
– This trial provided the rationale for recommendation to use
ribavirin with all GT1a disease if using Viekira pak
Genotype 1: PEARL-IV, SAPPHIRE-1,
PEARL-IV:305 treatment-naïve, non-cirrhotic patients with genotype 1a
– This trial provided the rationale for recommendation to
use ribavirin with all GT1a disease if using Viekira pak
PEARL-IV: Viekira pak for GT1a
+/- RBV: SVR12
GT1a, naïve, non-C, no RBV
GT1a, naïve, non-C, +RBV
Genotype 1: PEARL-IV, SAPPHIRE-1,
TURQUOISE-II: Paritaprevir 150 mg/ ritonavir 100 mg/ ombitasvir 25 mg + Dasabavir 250
mg BID x 12 wks (Viekira pak) + weight-based ribavirin261 treatment-naïve and -experienced patients with genotype 1a and cirrhosis.
TURQUOISE-II (Viekira pak + RBV
12 vs. 24wks): SVR12
GT1, 12 wks, all pts
GT1, 24 wks, all pts
Due to at least 2 cases of CTP class A compensated cirrhotic patients dying or requiring
liver transplant after receipt of Viekira pak or Technivie, this regimen is now
contraindicated in patients with Child Turcotte Pugh (CTP) class B or C hepatic
impairment (decompensated liver disease).
Genotype 1: OPTIMIST-1
OPTIMIST-1: 310 treatment-naïve and -experienced patients without cirrhosisSimeprevir 150 mg (OLYSIO) and sofosbuvir 400 mg (SOVALDI) in chronically infected patients with HCV genotype 1
OPTIMIST 1: SIM + SOF for GT1,
OPTIMIST-1: SIM + SOF for GT1,
8 vs. 12 weeks: SVR12
Treatment-naive vs. -exp'd: SVR12
SIM + SOF x 8 wks
SIM + SOF x 12 wks
SIM + SOF, naïve, 12 wks
SIM + SOF, exp'd, 12 wks
Genotype 1: OPTIMIST -2
Simeprevir 150 mg (OLYSIO) and sofosbuvir 400 mg (SOVALDI) in chronically infected patients with HCV genotype 1
OPTIMIST-2:103 treatment-naïve and -experienced patients with cirrhosis
OPTIMIST-2: SIM + SOF x 12 weeks,
Cirrhotic patients, Treatment-naive
and experienced: SVR12
SIM + SOF overall
SIM + SOF, cirrhotic,
SIM + SOF, cirrhotic,
Genotype 1: ALLY-1
ALLY-1: Daclatasvir 60 mg daily (DACLINZA) + sofosbuvir
400 mg daily (SOVALDI) + weight-based RBV in 60 patients
with advanced cirrhosis
ALLY-1: DAC + SOF + RBV x 12 weeks
for cirrhotic pts- SVR12
Therefore 24 weeks of treatment is recommended for GT1a
with cirrhosis, although the SVR12 remains unclear in this
A 65 yo M with history of anemia of chronic disease, GERD, asthma, CAD and
chronic hepatitis C genotype 2, fibrosis stage 3, HCV viral load 4 million,
presents for evaluation. Pt is interested in treatment. Pt's anemia has been
thoroughly evaluated and appears to be anemia of chronic disease. His last
hemoglobin was 9.5. He denies having ever had any bleeding, melena,
BRBPR, hematemesis, epistaxis or hemoptysis.
Meds: albuterol inhaler as neededomeprazole 20 mg dailymetoprolol tartrate 100 mg dailylisinopril 10 mg daily aspirin 325 mg dailysimvastatin 10 mg daily
Which of the following is a contraindication to the use of ribavirin in this patient?
1. Drug-drug interaction
2. Hemoglobin baseline
3. Coronary artery
4. Patient's HCV
Genotype 2: FISSION, VALENCE,
Sofosbuvir 400 mg daily (SOVALDI) and weight-based ribavirin
FISSION: 499 treatment-naïve pts with GT 2 or 3, randomized to daily PEG-
IFN/RBV x 24 wks vs. Sofosbuvir 400 mg daily (SOVALDI) + RBV x 12 weeks.
FISSION: SOF + RBV vs. PEG-
IFN + RBV for GT 2- SVR12
PEG-IFN + RBV
POSITRON: 278 interferon-ineligible or unwilling, treatment-naïve and
treatment-experienced GT2 and GT3 pts randomized to 12 weeks Sofosbuvir
(SOVALDI) + RBV vs. placebo x 12 weeks.
- SVR12 was 93% (101/109) among GT2s
Genotype 2: FISSION, VALENCE,
VALENCE: 419 treatment-naïve and treatment-experienced
patients with HCV genotype 2 or 3. GT 2 patients received 12
weeks of sofosbuvir 400 mg daily (SOVALDI) + RBV versus
• SVR12 for GT2 was 97% (31/32) for SOF + RBV x12
The overall SVR12 was 94% in a pooled analysis of all 3 trials
with SOF/RBV x 12 weeks (for GT 2)
• Patients with cirrhosis tended to do worse in all 3
• Thus therapy was extended to 16 weeks in pts with
cirrhosis (despite limited data)
A 45 yo M w/ a seizure disorder, hypothyroidism, and treatment-experienced hepatitis C genotype 3 without cirrhosis (null response to PEG-IFN + RBV after 12 weeks), presents for treatment. His provider decides to treat him with 12 weeks of daclatasvir(DAKLINZA) + sofosbuvir (SOVALDI).
Which of the following drug-drug interactions are you most concerned about?
1. Carbamazepine2. Pantoprazole3. Levothyroxine4. Levetiracetam5. Omeprazole
Genotype 3: ALLY-3 Trial
ALLY-3: 101 treatment-naïve patients with and without cirrhosis, daclatasvir 60 mg daily (Daklinza) + sofosbuvir 400 mg daily (Sovaldi) x 12 weeks (no ribavirin)
This data suggests that cirrhotic patients might benefit from extension of therapy to 24 weeks.
ALLY-3: DAC + SOF x 12 weeks for
Genotype 3
GT3, non-C, naïve GT3, Cirrhotic, naïve
Genotype 3: ALLY-3 Trial
Daclatasvir + Sofosbuvir + RBV x 12 vs. 16 weeks in those with cirrhosis:
– SVR12 rates were 88% (15/17) for those in the 12
– SVR12 of 89% (16/18) in the 16 week arm
Genotype 3: BOSON Trial
592 patients total, both treatment-naïve and treatment-experienced (IFN-eligible ONLY)
196 received sofosbuvir 400 mg daily (SOVALDI) and RBV for 16 weeks 199 received sofosbuvir 400 mg daily (SOVALDI) and RBV for 24 weeks197 received sofosbuvir 400 mg daily (SOVALDI) + PEG-IFN/RBV for 12 weeks
BOSON: SOF + RBV for Genotype 3
BOSON Trial: SOF + RBV compared
(Cirrhotic vs. non-Cirrhotic)
to SOF + RBV + PEG-IRN x 12 weeks
SOF + RBV x
SOF + RBV x
SOF + RBV x
SOF + RBV x
16 wks, non-C 24 wks, non-C
SOF + PEG-IFN+ RBV x 12 wks
SOF + RBV x 12 wks
Genotype 3: VALENCE Trial
250 treatment-naïve (42%) and -experienced (58%) subjects with genotype 3 (cirrhotic (n=45) and non-cirrhotic (n=100)) received sofosbuvir 400 mg daily (SOVALDI) plus weight-based RBV x 24 weeks.
VALENCE Trial: SOF + RBV x 24 wks
for Tx-naive and -exp'd, Cirrhotic and
non-C Genotype 3
Cirrhosis had no significant impact on overall SVR12.
Genotype 3: C-SWIFT Trial
40 patients with GT 3, treatment-naïve, with and without cirrhosis, randomized to 8 versus 12 weeks of triple therapy with elbasvir 50 mg/grazoprevir 100 mg (ZEPATIER) + sofosbuvir 400 mg daily (SOVALDI).
C-SWIFT: Zepatier + Sovaldi (8 vs. 12 wks)
for Genotype 3
12 weeks, Cirrhotic
Genotype 4: SYNERGY Trial
21 patients with GT4, both treatment-naïve and - experienced, both cirrhotic and non-cirrhotic, randomized to 12 weeks of ledipasvir 90 mg/sofosbuvir 400 mg (HARVONI)
• 60% were treatment-naïve • 43% had advanced fibrosis (F3 or F4)
Overall SVR12 was 100% for all 20 patients.
Genotype 4: PEARL-1 Trial
PEARL-I:86 treatment-naïve GT4 patients, non-cirrhotic received 12 weeks of the daily fixed-dose combination of paritaprevir 150 mg/ritonavir 100 mg/ombitasvir 25 mg (TECHNIVIE) +/- RBV
PEARL-1: 12 weeks Technivie +/- RBV
for Genotype 4 (12 wks, Tx-naïve,
Genotype 4: AGATE-I and –II Trials
AGATE-1:
120 treatment-naïve and -experienced patients with GT4 + cirrhosis
- 12 versus 16 weeks of paritaprevir/ritonavir/ombitasvir (TECHNIVIE) + RBV- SVR12 was 96% in the 12 week Technivie + RBV - SVR12 was 100% in the 16 week Technivie + RBV arm
AGATE-II:
100 treatment-naïve and -experienced non-cirrhotic GT4 patients received 12
weeks of paritaprevir/ritonavir/ombitasvir (TECHNIVIE )+ RBV
- Overall SVR12 was 94% for 12 weeks of TECHNIVIE + RBV
AGATE-II:
60 treatment-naïve and -experienced GT4 patients with cirrhosis
12 versus 24 weeks of paritaprevir/ritonavir/ombitasvir (TECHNIVIE) + RBV
- SVR12 was 97% for 12 weeks of PrO + RBV in cirrhotic pts
Genotype 4: C-EDGE Trial
66 treatment-naïve GT4 patients, with and without
cirrhosis, received elbasvir 50 mg/grazoprevir 100 mg
(ZEPATIER) x 12 weeks
• 6 were cirrhotic (9.1%)• 28 were co-infected with HIV (42.4%)• 10 also received RBV• 56 did not receive RBV
- Overall SVR12 was 97% (64/66) regardless of status of
cirrhosis or coinfection
- 1 treatment failure - Baseline RAVs did not impact SVR12 rates
Genotype 4: NEUTRINO Trial
28 treatment-naïve patients with GT4 with and without cirrhosis received 12 weeks of sofosbuvir 400 mg daily (SOVALDI) + PEG-IFN 2a + RBV
- SVR12 was 96% (27/28)- The one treatment failure was in a cirrhotic pt
Important to carefully consider the patient's baseline comorbidities.
- If you have to stop RBV, you have to stop treatment.
- Pts with prior CVA, CAD, COPD, etc… may be risky candidates due to anemia
and low oxygen carrying capacity that can result
Avoid ribavirin in pts w/ anemia or thalassemia
- Anyone with hemoglobin <11.0 should not receive RBV- Particularly problematic in women (Pregnancy category X); 2 forms of
contraception needed
Ribavirin needs to be dosed according to renal function.
CrCl >50, no dose adjustment CrCl 30-50: Alternate 200 mg and 400 mg every other dayCrCl <30: 200 mg once dailyESRD: 200 mg once daily
Ribavirin's half-life is very long.
Capsule, single dose; 44 hours in HCV pts Tablet: 120-170 hours
www.uptodate.com, Ribavirin Drug Information, 2015.
Barriers to Treatment
• Insurance coverage is the major barrier to
treatment access.
• Drug-drug interactions (esp. antacids with
• Provider misconceptions• Competing interests at patient level
Multidisciplinary team approach
Involvement of a multidisciplinary team (including specialty-trained HCV pharmacists, RNs, social workers and counselors) improves HCV-related outcomes and overall SVR rates.
Nurse practitioners and MDs/DOs can work in common to provide high-quality care to pts living with hepatitis C, and outcomes are equivalent.
Chopra A, Klein PL, Drinnan T, Lee SS. Liver Int. 2013 Feb;33 Suppl 1:30-4.
Manos MM, Ho CK, Murphy RC, Shvachko VA. Patient. 2013;6(1):23-34.
Backus L, Belperio PS, Shahoumian TA, Mole LA. J Clin Gastroenterol. 2015 Apr;49(4):329-35.
Role of Pharmacists in Improving
• Careful evaluation for drug-drug interactions• Adherence checks: Evaluation of refill records• Counselling regarding possible side effects of
• Continuity of care for hospitalized patients• Evaluation for proper dose and frequency of
• Expertise in pharmacology; best therapies and
dose adjustments for folks with renal disease, etc.
H2 Blockers and Proton Pump Inhibitors with
H2 blockers
H2 blocker may be administered at the
same time with LED/SOF OR 12 hours apart
Famotidine 40mg BID
from LED/SOF at a dose that does not
Ranitidine 150mg BID
exceed doses comparable to famotidine
Tagamet 800mg BID
Proton Pump Inhibitors
PPI doses comparable to omeprazole 20mg or lower can be administered at the same
Omeprazole 20mg daily
time with LED/SOF under fasting conditions.
Prevacid 30mg daily
Aciphex 20mg daily
Protonix 40mg daily
Nexium 20 to 40mg daily (try
to stay with lower dose if
possible)
*** Slide courtesy of Dr. Camilla Graham***
Ritonavir: Drug-Drug Interactions
• Phenobarbital, phenytoin, oxcarbazepine• Amiodarone• Quetiapine• Efavirenz• Atorvastatin, simvastatin• Many, many others • Check for drug-drug interactions, then double-
Common Side Effects
Sofosbuvir (NS5B Polymerase inhibitor):
Neutropenia (<1%-17%)
Insomnia (15-25%)
Irritability (10-13%)
Flu-like symptoms (6-16%)
Pruritus (11-27%)
Skin rash (8-18%)
Increased Lipase (<2%)
Increased CPK (1-2%)
Increased serum bilirubin (3%)
Severe depression (<1%)
Suicidal ideation (<1%)
Common Side Effects
Ledipasvir (NS5A Inhibitor):
Daclatasvir (NS5A inhibitor):
Fatigue (14%)
Increased CPK (1%)
Weakness (18-31%)
Irritability (8%)
Increased lipase (2%)
Skin rash (including
blistering skin disease,
Depression (<5%)
angioedema) (<1%)
Nausea (6-9%)Diarrhea (3-7%)Increased lipase (<9%)Elevated bilirubin (<3%)
Common Side Effects
Paritepravir/ritonavir/ombitasvir + Dasabuvir:
Fatigue (34%)
Headache (44%)
Insomnia (5-26%)
Skin reaction/rash (7-24%)
Pruritus (7-18%)
Anemia (11-29%)
Increased serum bilirubin (15-54%)
Weakness (4-14%)
Muscle spasm (21%)
Cough (11-32%)
Irritability (10%)
Icterus (10%)
Dyspnea (<1%)
Hepatic failure/liver decompensation (in pt's with underlying cirrhosis- FDA issued a
safety alert on 10/22/15)
Hypersensitivity reaction (<1%)
1. What is the most common barrier to patients
accessing hepatitis C treatment currently?
a) Unstable mental health disordersb) Insurance coveragec) Drug-drug interactionsd) A life-expectancy of <1 year
1. What is the most common barrier to patients
accessing hepatitis C treatment currently?
a) Unstable mental health disordersb) Insurance coveragec) Drug-drug interactionsd) A life-expectancy of <1 year
2. Which of the following drugs interacts with Ledipasvir/sofosbuvir to decrease serum levels of ledipasvir?a) Methadoneb) Levothyroxinec) Levetiracetamd) Omeprazole
2. Which of the following drugs interacts with Ledipasvir/sofosbuvir to decrease serum levels of ledipasvir?a) Methadoneb) Levothyroxinec) Levetiracetamd) Omeprazole
3. Based on the ION trials, which of the following patients might be a candidate for 8 weeks of ledipasvir/sofosbuvir?a) GT 1a, treatment naïve, non-cirrhotic, HCV viral load
b) GT 3, treatment naïve, non-cirrhotic, HCV viral load 3
c) GT 1b, treatment naive, cirrhotic, HCV VL 2 milliond) GT 4, treatment naïve, non-cirrhotic, HCV viral load 6
3. Based on the ION trials, which of the following patients might be a candidate for 8 weeks of ledipasvir/sofosbuvir?a) GT 1a, treatment naïve, non-cirrhotic, HCV viral load
b) GT 3, treatment naïve, non-cirrhotic, HCV viral load 3
c) GT 1b, treatment naive, cirrhotic, HCV VL 2 milliond) GT 4, treatment naïve, non-cirrhotic, HCV viral load 6
4. Which of the following is among the most common noted side effect of daclatasvir? a) Nauseab) Fatiguec) Skin rashd) Diarrhea
4. Which of the following is among the most common noted side effect of daclatasvir? a) Nauseab) Fatiguec) Skin rashd) Diarrhea
5. Which of the following measures are important to preventing morbidity associated with chronic hepatitis C?a) Weekly lab monitoringb) Vaccination against hepatitis B alonec) Vaccination against hepatitis A and Bd) Avoidance of all medications metabolized by the
5. Which of the following measures are important to preventing morbidity associated with chronic hepatitis C?a) Weekly lab monitoringb) Vaccination against hepatitis B alonec) Vaccination against hepatitis A and Bd) Avoidance of all medications metabolized by the
World Health Organization. 2009. Retrieved online at: http://hepcbc.ca/wp-content/uploads/2012/08/GlobalDist_HCV_genotypes.jpg, Retrieved 6/15/15.
AASLD. Recommendations for testing, managing and treating hepatitis C. Retrieved online at: http://www.hcvguidelines.org/full-report/when-and-whom-initiate-hcv-therapy, Retrieved 6/15/15.
CDC. Hepatitis C. Retrieved online at: http://www.cdc.gov/hepatitis/hcv/hcvfaq.htm#section1, Retrieved 7/30/15.
Afdhal et al. The new paradigm of hepatitis C therapy: integration of oral therapies into best practices. J Viral Hepat. 2013 Nov; 20(11): 745–760.
McNamara B, Losikoff P, Huguenin L, Macalino G, Rich J, Gregory SH. Increasing hepatitis C prevalence and associated risk behaviors among Incarcerated young adults. J Urban Health. 2013; 91(2): 376-82.
Stockman LJ, Guilfoye SM, Benoit AL, Vergeront JM, Davis JP. Rapid hepatitis C testing among persons at increased risk for infection– Wisconsin, 2012-2013. MMWR Morb Mortal Wkly Rep. 2014 Apr 11; 63(14): 309-11.
CDC. Use of enhanced surveillance for hepatitis C virus infection to detect a cluster among young injection-drug users--new York, November 2004-April 2007. MMWR Morb Mortal Wkly Rep. 2008 May 16;57(19):517-21.
CDC. Hepatitis C virus infection among adolescents and young adults:Massachusetts, 2002-2009. MMWR MorbMortal Wkly Rep. 2011 May 6;60(17):537-41.
Barua S, Greewald R, Grebely J, Dore GJ, Swan T, Taylor LE. Retrictions for Medicaid reimbursement of Sofosbuvirfor the treatment of hepatitis C in the United States. Annals of Int Med. 2015;163(3):215-223.
Ly KN, Xing J, Klevens RM, Jiles RB, Holmberg SD. Causes of death and characteristics of decedents with viral hepatitis, United States, 2010. Clin Infect Dis. 2014 Jan;58(1):40-9
Kramer B. et al. Meeting vaccination quality measures for hepatitis A and B virus in patients with
chronic hepatitis C infection. Hepatology. 2011 Jan;53(1):42-52. doi: 10.1002/hep.24024. Epub 2010 Dec 13.
PDA. Zepatier press release. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm483828.htm, 1/29/16.
Strickland GT, El-Kamary SS, Klenerman P, Nicosia A. Hepatitis C vaccine: supply and demand. Lancet Inf Dis. 8(6):379-86.
Scripps Research Institute scientists achieve most detailed picture ever of key part of hepatitis C virus. Scripps Research Institute. 12/6/2013.
Kong L, Ward A, Wilson I, Law M, Giang E. Hepatitis C virus E2 envelope glycoprotein core structure. 2013. Science. 342(6162):1090-94.
Chen JY, Li F. Development of hepatitis C virus vaccine using hepatitis B core antigen as immuno-carrier. 2006. World J Gastro. 12(48):7774-78.
Halliday J, Klenerman P, Barnes E. Vaccination for hepatitis C vvirus: closing in on an elusive target. Expert Rev Vaccines. 10(5):659-72.
Potthoff A, Manns MP, Wedemeyer H. Treatment of HBV/HCV Coinfection. Expert Opin on Pharmacotherapy. 2010;11(6):919-28.
Swadling L, Capone S, Antrobus RD, et al. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Science Translational Medicine. 2014;6(261):261.
Vento S. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. J of Viral Hepatitis. 2002; 7(S1):7-8.
Gyarmathy VA, Neaigus A, Ujhelyi. Vulnerability to drug-related infections and co-infections among injecting drug users in Budapest, Hungary
The European Journal of Public Health. 2009, 19 (3) 260-265.
Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevir-Elbasvir Combination Therapy for Treatment-Naive Cirrhotic and Noncirrhotic Patients With Chronic Hepatitis C Virus
Genotype 1, 4, or 6 Infection: A Randomized Trial. Ann Intern Med. 2015 Jul 7;163(1):1-13. doi: 10.7326/M15-0785.
Sulkowski, MS., et al. 2015b. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial. Lancet 385(9973):1087-97.
Kwo P, Gane E, Peng CY, et al. Efficacy and safety of grazoprevir/elbasvir +/- RBV for 12 weeks in patients with HCV G1 or G4 infection who previously failed peginterferon/RBV: C-edge treatment-experienced trial [Abstract PO886] 50th Annual Meeting of the European Association for the Study of the Liver (EASL). April 22-26, 2015b; Vienna, Austria.
Jacobson IM, Asante-Appiah E, Wong P et al. Prevalence and Impact of Baseline NSA Resistance Associated Variants (RAVs) on the Efficacy of Elbasvir/Grazoprevir (EBR/GZR) Against GT1a Infection [Abstract LB-22]. 66th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD). November 13-17, 2015b; San Francisco, CA.
Thompson A, Zeuzem S, Rockstroh J, Kwo P, Roth D, Lawitz E, Sulkowski M, Forns X, Wahl J, Nguyen B, Barr E, Howe A, Miller M, Hwang P, Robertson M. 2015. The Combination of Grazoprevir and Elbasvir + RBV is highly effective for the treatment of GT1a-Infected patients. American Association for the Study of Liver Diseases, The Liver Meeting 2015, San Francisco, Abstract 703.
Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014a;370(20):1889-1898.
Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879-1888.
Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1594-1603.
Ferenci P, Bernstein D, Lalezari J, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014;370:1973-1982.
Kwo P, Gitlin N, Nahass R et al. Simeprevir Plus Sofosbuvir (12 and 8 Weeks) in HCV Genotype 1-Infected Patients Without Cirrhosis: OPTIMIST-1, a Phase 3, Randomized Study. Hepatology. 2016 Jan 22. doi: 10.1002/hep.28467. [Epub ahead of print]
Lawitz E, Matusow G, DeJesus E et al. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: A Phase 3 study (OPTIMIST-2). Hepatology. 2015 Dec 24. doi: 10.1002/hep.28422. [Epub ahead of print]
Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015.
Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014a,16;370(3):211-21
Welzel TM, Herzer K, Ferenci P et al. Daclatasvir plus sofosbuvir with or without ribavirin for the treatment of HCV in patients with severe liver disease: interim results of a multicenter compassionate use program. [Abstract P0072.] 50th Annual Meeting of the European Association for the Study of the Liver (EASL). April 22-26, 2015;S619; Vienna, Austria.
de Ledinghen V, Fontaine H, Dorival C et al. Safety and efficacy of sofosbuvir-containing regimens in the French obervational cohort ANRS C022 hepather. [Abstract P0795.] 50th Annual Meeting of the European Association for the Study of the Liver (EASL). April 22-26, 2015;S631; Vienna, Austria.
Poordad F, Schiff ER, Vierling JM, et al. Daclatasvir With Sofosbuvir and Ribavirin for HCV Infection With Advanced Cirrhosis or Post-Liver Transplant Recurrence. Hepatology 2016; DOI: 10.1002/hep.28446. [Epub ahead of print]
Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015.
Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014a,16;370(3):211-21.
Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013a;368(20):1878-1887.
US FDA. FDA Antiviral Drugs Advisory Committee Meeting October 25, 2013a: Background Package for NDA 204671 Sofosbuvir (GS-7977). http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/ucm371875.htm. Accessed onNovember 15, 2013a.
Dieterich D, Bacon B, Flamm SL et al. Evaluation of sofosbuvir and simeprevir-based regimens in the TRIO network: academic and community treatment of a real-world, heterogeneous population. [Abstract 46.] 65th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD). November 7-11, 2014a;220A; Boston, MA.
Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med.
2013c;368(20):1867-1877.
Foster GR, Pianko S, Brown A, et al. Efficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in patients with hepatitis C virus genotype 3 infection and treatment-experienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology 2015;149(6):1462-70.
Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015b;61(4):1127-1135.
Hezode C, Asselah T, Reddy KR, Hassanein T, Berenguer M, Fleischer-Stepniewska K, Marcellin P, et al. Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL-I): a randomised, open-label trial. Lancet 2015.
Leroy V, Angus P, Bronowicki JP, et al. Daclatasvir, Sofosbuvir, and Ribavirin for Hepatitis C Virus Genotype 3 and Advanced Liver Disease: A Randomized Phase III Study (ALLY-3+). Hepatol. 2016; DOI:10.1002/hep.28473. [Epub ahead of print].
Foster GR, Pianko S, Brown A, et al. Efficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in patients with hepatitis C virus genotype 3 infection and treatment-experienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology 2015;149(6):1462-70.
Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, Illeperuma A, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. The New England journal of medicine 2014b;370:1993-2001.
Ahmed A, Felmlee DJ. Mechanisms of hepatitis C viral resistance to directly acting antivirals. Viruses. 2015 Dec 18;7(12):6716-29.
Poordad F, Schiff ER, Vierling JM, et al. Daclatasvir With Sofosbuvir and Ribavirin for HCV Infection With Advanced Cirrhosis or Post-Liver Transplant Recurrence. Hepatology 2016; DOI: 10.1002/hep.28446. [Epub ahead of print].
Wong KA, Worth A, Martin R, et al. Characterization of Hepatitis C virus resistance from a multiple-dose clinical trial of the novel NS5A inhibitor GS-5885. Antimicrob Agents Chemother. 2013;57(12):6333-6340.
Kohler JJ, Nettles JH, Amblard F, et al. Approaches to hepatitis C treatment and cure using NS5A inhibitors. Infect Drug Resist.
2014;7:41-56.
Kohli A, Kapoor R, Sims Z et al. Ledipasvir and sofosbuvir for hepatitis C genotype 4: a proof-of-concept, single-centre, open-label phase 2a cohort study. Lancet Infect Dis. 2015;15(9):1049-54.
Abergel A, Loustaud-Ratti V, Metivier S et al. Ledipasvir/sofosbuvir for the treatment of patients with chronic genotype 4 or 5 HCV infection. 50th Annual Meeting of the European Association for the Study of the Liver (EASL). April 22-26, 2015; Vienna, Austria.
Esmat G, Doss W, Qqish RB, et al. Efficacy and Safety of Co-Formulated Ombitasvir/Paritaprevir/Ritonavir with Ribavirin in Adults with Chronic HCV Genotype 4 Infection in Egypt (AGATE-II) [Abstract 708]. 66th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD). November 13-17, 2015; San Francisco, CA.
Zeuzem S, Ghalib R, Reddy KR, Pockros PJ, Ari ZB, Zhao Y, Brown DD, Wan S, DiNubile MJ, Nguyen BY, Robertson MN, Wahl J, Barr E, Butterton JR. Ann Intern Med. 2015f Jul 7;163(1):1-13. doi: 10.7326/M15-0785. PMID: 25909356] Grazoprevir-ElbasvirCombination Therapy for Treatment-Naive Cirrhotic and Noncirrhotic Patients With Chronic Hepatitis C Virus Genotype 1, 4, or 6 Infection: A Randomized Trial.
Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med.
2013a;368(20):1878-1887.
Chopra A, Klein PL, Drinnan T, Lee SS. How to optimize HCV therapy in genotype 1 patients: management of side-effects. Liver
Int. 2013 Feb;33 Suppl 1:30-4. doi: 10.1111/liv.12080.
Manos MM, Ho CK, Murphy RC, Shvachko VA. Physical, social, and psychological consequences of treatment for hepatitis C : a
community-based evaluation of patient-reported outcomes. Patient. 2013;6(1):23-34. doi: 10.1007/s40271-013-0005-4.
Backus L, Belperio PS, Shahoumian TA, Mole LA. Impact of provider type on hepatitis C outcomes with boceprevir-based and
telaprevir-based regimens. J Clin Gastroenterol. 2015 Apr;49(4):329-35. doi: 10.1097/MCG.0000000000000124.
Source: http://www.ripharmacists.org/resources/Documents/2016%20Spring%20Seminar/Noska.pdf
Type II Diabetes Targets:• HbA1c ≤7% (53 mmol/mol)** HbA1c can be used for screening, diagnosis and ongoing • Lipids: TC<4, TG<2, HDL>1, LDL<1.8 mmol/L Early detection and glycaemic control can prevent serious monitoring of diabetes. (See FLOWCHART) Interpretation of HbA1c: • BMI <25kg/m2 • All Aboriginal people over 15 years of age.
Recent Trends in Class Action and Aggregate Litigation in the Life Sciences Industry www.morganlewis.com © 2013 Morgan, Lewis & Bockius LLP For the last several years, the life sciences industry has been fertile ground for class action and aggregate litigation. Developments in this area have driven several trends, including state consumer fraud claims, securities class actions, antitrust class actions, and aggregate litigation brought by private healthcare insurers and state attorneys general. These recent trends have been driven, in part, by legislative and doctrinal developments. For example, in 2005—based on legislative findings of abuse in class action practice in state courts—Congress enacted the Class Action Fairness Act (CAFA), permitting defendants to remove to federal court putative class actions that previously may have been subject to less stringent standards in state court. In Standard Fire Insurance Co. v. Knowles,1 the U.S. Supreme Court held that a plaintiff's stipulation that he would not accept more than $5 million in damages could not be used to avoid CAFA's amount in controversy requirement. In other words, a class representative may not agree to seek less money to try to keep a case in state court.





