An autoimmune disease prevented by anti-retroviral drugs
Beck-Engeser et al. Retrovirology 2011, 8:91http://www.retrovirology.com/content/8/1/91
An autoimmune disease prevented by anti-retroviral drugs
Gabriele B Beck-Engeser1, Dan Eilat2 and Matthias Wabl1*
Background: Both Aicardi-Goutières syndrome, a Mendelian mimic of congenital infection, and the autoimmunedisease systemic lupus erythematosus can result from mutations in the gene encoding the enzyme Trex1. In mice,the absence of Trex1 causes severe myocarditis. The enzyme is thought to degrade endogenous retroelements,thus linking them to autoimmune disease. However, inhibition of reverse transcription by the inhibitor zidovudine(AZT) did not ameliorate the disease, weakening the link to retroelements.
Findings: Here, we show that two other FDA-approved drugs that inhibit reverse transcriptase can ameliorate themyocarditis in Trex1-null mouse.
Conclusions: The result suggests that retroelements contribute to this hereditary form of autoimmunity, and thattreatment with retroelement inhibitors might ameliorate Aicardi-Goutières syndrome in humans.
Keywords: Aicardi-Gouti?è?res syndrome, myocarditis, Trex1, reverse transcriptase inhibitors
disease with anti-retroviral agents. However, treatment
Aicardi-Goutières syndrome (AGS) is a genetically-
of the mice with the reverse transcription inhibitor azi-
determined encephalopathy with remarkable phenotypic
dothymidine (AZT) did not rescue the mice from lethal-
overlap with the sequelae of congenital infection. Sys-
ity [It was argued that the absence of Trex1 may
temic lupus erythematosus (SLE) is an autoimmune dis-
unleash hundreds of diverse reverse transcriptases
ease characterized by the production of autoantibodies
encoded by the mouse genome, some of them being
that target nucleic acids and their associated proteins.
AZT resistant As a single agent, AZT also may
Like AGS [SLE is associated with a perturbation of
leave some retroelements out of its range of activity.
type I interferon metabolism []. Both AGS , and a
Finally, although it leads to premature termination of
cutaneous subtype of SLE called familial chilblain lupus
cDNA synthesis, AZT has only little effect on the synth-
can result from mutations in TREX1. Furthermore,
esis of short reverse transcription intermediates, includ-
mutations in TREX1 represent the single most common
ing those of spliced retroelement products The
cause of monogenic SLE identified to date
interrupted or slowed reverse transcription may create
Trex1 is a ubiquitous DNA 3' exonuclease that can
persistent exposure to cytoplasmic DNA products that
degrade retroelements (retroviruses and retrotranspo-
elicit an antiviral innate immune response coordi-
sons) In Trex1-deficient mice, single-stranded
nated by activation of type I IFNs (the so-called IFN-sti-
DNA derived from retroelement cDNA accumu-
mulatory DNA response [Along this line,
lates in the cytoplasm of cells in the heart and is
raltegravir, a drug that inhibits retroviral integrase and
thought to trigger the sterile inflammatory myocarditis
thus increases the concentration of cDNA in the cell,
On the basis that unrestricted retroelements may
also exacerbates autoimmune disease
cause, or at least contribute to, the disease it was
In Trex1 deficient mice, the inflammation of the heart
reasoned that it ought to be possible to treat or prevent
muscle takes an aggressive course, with mice starting todie after 4 weeks of age (Figure ). We sought to pre-
* Correspondence:
vent the autoimmune disease with anti-retroviral drugs
1Department of Microbiology and Immunology, University of California, San
other than AZT. Keeping in mind that a single drug
Francisco, CA 94143-0414, USAFull list of author information is available at the end of the article
may leave some retroelements out of its range of
2011 Beck-Engeser et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the CreativeCommons Attribution License ), which permits unrestricted use, distribution, andreproduction in any medium, provided the original work is properly cited.
Beck-Engeser et al. Retrovirology 2011, 8:91
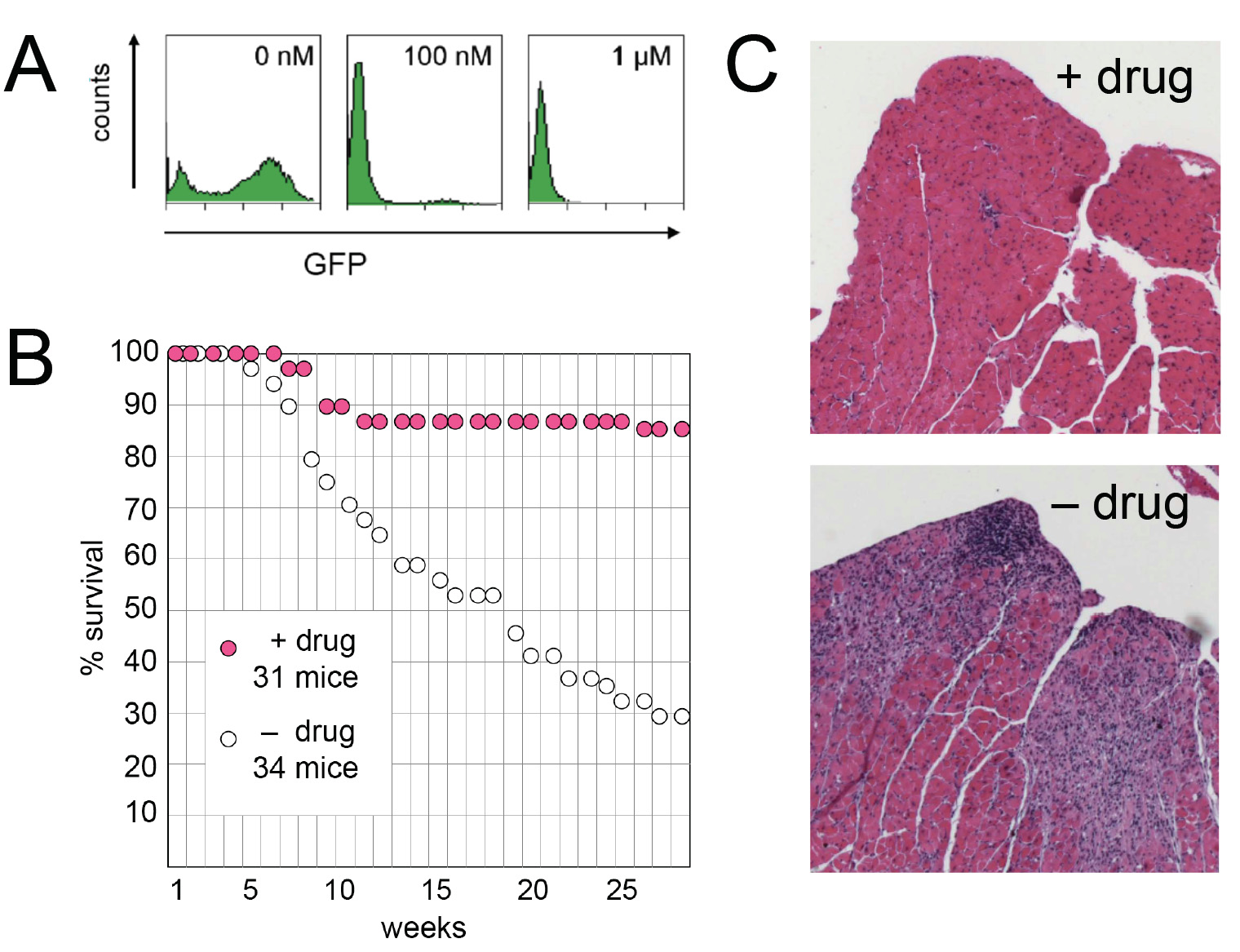
Figure 1 Effect of reverse transcriptase inhibitors on survival of Trex1-deficient mice. A) Inhibition of MLV cDNA synthesis by Truvada/Viramune. Flow cytometry graphs displaying GFP intensity generated by provirus: y-axis, cell number; x-axis, fluorescence intensity on alogarithmic scale. An MLV-based vector encoding GFP was added to NIH/3T3 cell cultures with 0, 100 nM, or 1 μM. B) Survival curves showingthe effect of Truvada/Viramune (+ drug; magenta circles) on Trex1-deficient mice obtained from D. Stetson The drugs were given fromconception via the drinking water as a solution of 3 × 10-4 M nevirapine, 1.6 × 10-4 M emtricitabine and 9.4 × 10-5 M tenofovir. Log rank test forthe drug effect, p = 0.000014. C) Hematoxylin-eosin stained sections of the left heart ventricle of treated (+ drug) and non-treated (- drug) micekilled at 9 and 7 months of age, respectively. Sections from three mice were examined in each category.
activity, we decided to use a combination of drugs that
We first determined that the combination of Truvada
inhibit reverse transcriptase. Because nucleoside reverse
and Viramune is effective against MLV. Using flow cyto-
transcription inhibitors also inhibit human LINE-1 retro-
metry, we titrated the drug concentration for its ability
transposition , we assumed that a Truvada/Viramune
to inhibit expression of green fluorescence protein
combination (both FDA-approved drugs) would inhibit
encoded by MLV provirus upon infection; the EC50 was
both classes of retroelements–retroviruses and retrotran-
well below 100 nM (Figure When fed to Trex1-
sposons. Truvada is a fixed-dose combination tablet con-
deficient mice at a dose comparable to that given to
taining emtricitabine and tenofovir disoproxil fumarate
patients with HIV, the drugs substantially reduced mor-
Emtricitabine is a synthetic nucleoside analog of
tality (Figure On sections of the heart from 9-
cytidine. Tenofovir disoproxil fumarate is converted in
month old treated mice, there were some mild patchy
vivo to tenofovir, an acyclic nucleoside phosphonate
inflammatory infiltrates with little myocyte injury; but
(nucleotide) analog of adenosine 5'-monophosphate. Vir-
the difference to the marked inflammatory infiltrates
amune (nevirapine) blocks the reproduction of retro-
with myocyte necrosis and dropout in 7-month old
virus earlier in its cycle than Truvada. It binds directly to
non-treated mice (at 9 months all untreated mice were
reverse transcriptase and blocks the RNA-dependent and
dead) was striking (Figure
DNA-dependent DNA polymerase activities by disrupt-
Almost half of the human genome consists of retroele-
ing the enzyme's catalytic site. Viramune does not com-
ments, many of them active. There are several ways that
pete with template or nucleoside triphosphates, or inhibit
retroelements might trigger an autoimmune response,
the cellular DNA polymerases tested so far
including (i) sensing of retroelement RNA and cDNA,
Beck-Engeser et al. Retrovirology 2011, 8:91
(ii) generation of mimetopes through error-prone
Lindahl T, Barnes DE, Yang YG, Robins
reverse transcription of mRNA encoding retroelement
Biochem Soc Trans 2009, 37:535-538.
proteins, and (iii) insertional mutagenesis. We showed
Stetson DB, Ko JS, Heidmann T, Medzhitov
here that a hereditary autoimmune inflammation in the
Cell 2008, 134:587-598.
mouse that is likely caused by accumulation of retroele-
Beck-Engeser GB, Eilat D, Harrer T, Jack HM, Wabl Proc
ment cDNA can be treated with reverse transcriptase
Natl Acad Sci USA 2009, 106:20865-20870.
inhibitors. Other autoimmune diseases might be amen-
Yan N, Regalado-Magdos AD, Stiggelbout B, Lee-Kirsch MA, Lieberman J:
able to different interventions of retroelement activities.
Nat Immunol 2010,11:1005-1013.
Yang YG, Lindahl T, Barnes
AZT: zidovudine; AGS: Aicardi-Goutières syndrome; IFN: interferon; MLV:
2007, 131:873-886.
murine leukemia virus; SLE: systemic lupus erythematosus.
Morita M, Stamp G, Robins P, Dulic A, Rosewell I, Hrivnak G, Daly G,Lindahl T, Barnes DE:
We thank Dan Stetson for the Trex1-deficient mice; Jean Olson for the
2004, 24:6719-6727.
micrographs; Cliff Wang and Jay Lalezari for suggestions; and Mary
Quan Y, Liang C, Inouye P, Wainberg MA:
McKenney for editing the manuscript. Supported by grants from the NIH
(R01AI041570) and the Lupus Research Institute to MW; and from the United
Nucleic Acids Res 1998,
States - Israel Binational Foundation (BSF) to MW and DE.
Houzet L, Morichaud Z, Mougel M:
1Department of Microbiology and Immunology, University of California, San
Retrovirology 2007, 4:30.
Francisco, CA 94143-0414, USA. 2Department of Medicine, Hadassah
Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML,
University Hospital and The Hebrew University Faculty of Medicine,
Hebbeler AM, Greene
Jerusalem 91120, Israel.
Authors' contributions
Stetson DB, Medzhitov R:
GBE, DE, and MW planned the study; GBE carried out the experiments; and
Immunity 2006, 24:93-103.
MW wrote the manuscript. All authors read and approved the final
Jones RB, Garrison KE, Wong JC, Duan EH, Nixon DF, Ostrowski MA:
PLoS ONE 2008, 3:e1547.
Competing interests
The authors declare that they have no competing interests.
Expert Opin Pharmacother 2006,7:793-802.
Received: 12 September 2011 Accepted: 8 November 2011
Merluzzi VJ, Hargrave KD, Labadia M, Grozinger K, Skoog M, Wu JC, Shih CK,
Published: 8 November 2011
Eckner K, Hattox S, Adams J, Rosenthal AS, Faanes R, Eckner RJ, Koup RA,Sullivan JL:
Science 1990, 250:1411-1413.
Aicardi J, Goutieres F:
Neuropediatrics 2000, 31:113.
Lebon P, Badoual J, Ponsot G, Goutieres F, Hemeury-Cukier F, Aicardi J:
Cite this article as: Beck-Engeser et al.: An autoimmune diseaseprevented by anti-retroviral drugs. Retrovirology 2011 8:91.
J Neurol Sci 1988, 84:201-208.
Pascual V, Banchereau J, Palucka Curr Opin Rheumatol 2003, 15:548-556.
Crow YJ, Hayward BE, Parmar R, Robins P, Leitch A, Ali M, Black DN, vanBokhoven H, Brunner HG, Hamel BC, Corry PC, Cowan FM, Frints SG,Klepper J, Livingston JH, Lynch SA, Massey RF, Meritet JF, Michaud JL,Ponsot G, Voit T, Lebon P, Bonthron DT, Jackson AP, Barnes DE, Lindahl T:Nat Genet 2006,38:917-920.
Rice G, Newman WG, Dean J, Patrick T, Parmar R, Flintoff K, Robins P,Harvey S, Hollis T, O'Hara A, Herrick AL, Bowden AP, Perrino FW, Lindahl T,Barnes DE, Crow YJ:
Submit your next manuscript to BioMed Central
and take full advantage of:
Genet 2007, 80:811-815.
Lee-Kirsch MA, Chowdhury D, Harvey S, Gong M, Senenko L, Engel K,
• Convenient online submission
Pfeiffer C, Hollis T, Gahr M, Perrino FW, Lieberman J, Hubner
• Thorough peer review
J Mol Med 2007, 85:531-537.
• No space constraints or color figure charges
Lee-Kirsch MA, Gong M, Chowdhury D, Senenko L, Engel K, Lee YA, de
• Immediate publication on acceptance
Silva U, Bailey SL, Witte T, Vyse TJ, Kere J, Pfeiffer C, Harvey S, Wong A,Koskenmies S, Hummel O, Rohde K, Schmidt RE, Dominiczak AF, Gahr M,
• Inclusion in PubMed, CAS, Scopus and Google Scholar
Hollis T, Perrino FW, Lieberman J, Hübner
• Research which is freely available for redistribution
Nat Genet 2007, 39:1065-1067.
Submit your manuscript at www.biomedcentral.com/submit
Source: http://www.wabllab.com/wp-content/uploads/2012/08/An-autoimmune-disease-prevented-by-anti-retroviral-drugs.pdf
Fermentation Process Kinetics* Elmer L. Gaden, Jr. Department of Chemical Engineering, Columbia University,New York 27, N.Y. Abstract: Information on fermentation process kinetics is especially true for kinetics. Although the study of fermen- potentially valuable for the improvement of batch pro- tation rates is relatively new, it promises much for the fuller
PROJECT REPORT "ANALYTICAL METHOD DEVELOPMENT AND VALIDATION OF SOME ANTI-CANCER DOSES FORMS' UGC REFERENCE No. 47-114/07 SUBMITTED TO UNIVERSITY GRANT COMMISSION Dr. JWALANT J. VORA M. G. SCIENCE INSTITUTE, AHMEDABAD-380009 PRINCIPAL INVESTIGATOR,


