Pii: s0926-6410(02)00215-x
Cognitive Brain Research 15 (2002) 47–60
Interactive report
ffects of practice on executive control investigated with fMRI
D.H. Weissman *, M.G. Woldorff , C.J. Hazlett , G.R. Mangun
a
Center for Cognitive Neuroscience and Department of Psychological and Brain Sciences,
Duke University,
Box 90999,
Durham,
NC 27708,
USA
b
Center for Mind Sciences,
University of California,
Davis,
CA 95616,
USA
Various models of executive control predict that practice should modulate the recruitment of executive brain mechanisms. To
investigate this issue, we asked 15 participants to perform a cued global / local attention task while brain activity was recorded withevent-related functional magnetic resonance imaging (fMRI). Practice significantly reduced the recruitment of left inferior parietal regionsthat were engaged when participants oriented attention in response to global and local cue stimuli. In contrast, practice increased therecruitment of midline frontal regions that were engaged by interference between global and local forms during target processing. Thesefindings support models of executive control in which practice increases the tendency for stimuli to automatically evoke task-relevantprocesses and responses.
2002 Elsevier Science B.V. All rights reserved.
Theme: Neural basis of behavior
Topic: Cognition
Keywords: Anterior cingulate; Selective attention; Response interference; Global / local processing; Practice; fMRI
. Introduction
strengthens task-relevant schemas), then correct behavior
Many theories of attention posit an executive control
should be possible with less intervention by the supervis-
system that recruits and oversees task-specific processes in
ory system. For example, practice reduces the time (and,
order to facilitate appropriate behavior [1,10,16,36,46].
by inference, the involvement of executive processes)
This supervisory system is engaged by complex and novel
necessary to switch between different tasks in cued selec-
situations including those that require planning, im-
tive attention paradigms [23,31,45], perhaps by strengthen-
plementation of strategies, switching between stimulus
ing associations between cues and task-appropriate atten-
dimensions and performing multiple tasks at the same
tional processes and responses [31]. Thus, practice appears
time. It is also recruited when new associations (schemas)
to decrease the recruitment of executive processes that are
are formed between task stimuli and task-relevant pro-
engaged during cued attentional orienting.
cesses and responses, especially when new schemas must
On the other hand, when practice strengthens associa-
guide behavior in the face of older, stronger schemas [36].
tions between task-irrelevant stimuli and inappropriate
processes and responses, then correct behavior may require
strengthens new schemas such that stimuli become capable
additional recruitment of executive control mechanisms to
of automatically engaging associated processes and re-
resolve processing conflicts. For example, when particip-
sponses [10,36]. The effect of strengthening schemas on
ants are asked to identify the ink color in which a word is
the recruitment of executive control processes should vary,
printed, they are slower to respond when the irrelevant
however, depending on the specific task situation. When
word names an ink color that is mapped to a competing
practice increases the degree to which task-relevant stimuli
motor response (incongruent trials) than when it names anink color that is mapped to the same response (congruent
trials) (see Ref. [27] for a review). This effect appears to be
E-
mail address: (D.H. Weissman).
due to our extensive experience with reading, which leads
0926-6410 / 02 / $ – see front matter
2002 Elsevier Science B.V. All rights reserved.
D.
H.
Weissman et al. /
Cognitive Brain Research 15 (2002) 47–60
to the word automatically activating task-relevant re-
trials, which reduce interference effects [28]. Practice-
sponses that interfere with color naming [10,11]. Con-
related reductions of interference-related activity in block
sistent with this view, training participants to associate
designs might thus indicate that top-down strategies be-
random polygons with task-relevant color names allows
come easier to implement during blocks with incongruent
those polygons to produce Stroop-like behavioral interfer-
trials. Also, neither of the two block-design studies dis-
ence effects when they serve as distractors in Stroop-like
cussed above specifically manipulated the amount of
tasks [29]. In sum, the behavioral findings discussed above
practice that participants were given at associating distrac-
support the view that practice strengthens new schemas.
tor stimuli with task-relevant responses, which is usually
Further, they demonstrate that strengthening schemas can
necessary for conflict from distractors to increase in
either decrease or increase the recruitment of executive
behavioral studies [29]. Therefore, it is unclear whether
control processes depending on the specific task situation.
practice should have been expected to increase the ten-
Studies of brain–behavior relationships can also provide
dency of incongruent distractors to activate the incorrect
a powerful tool for testing the effects of practice on the
response. Finally, it is possible that participants simply
recruitment of executive control processes. Functional
became less aroused by the more difficult incongruent
neuroimaging, lesion, and single-unit recording studies
trials over the course of the experiment, thereby reducing
have revealed that distinct brain areas contribute differen-
the difference in brain activation between incongruent and
tially to specific executive processes [48]. For example,
either neutral or congruent blocks of trials.
previous studies have indicated that prefrontal regions help
Data from a recent fMRI study are also relevant to our
to maintain and manipulate information held in working
hypothesis that practice should increase interference-re-
memory [40], midline frontal regions help to detect
lated activity. In this study [32], participants were trained
response conflict [27] and superior / inferior parietal regions
to associate polygons with either a pattern or a color patch,
contribute to attentional orienting [42]. Given that such
although associations between polygons and specific but-
relationships exist, it should be possible to determine
ton presses were specifically avoided. Following training,
whether practice modulates the recruitment of distinct
these polygons were used as distractors in a variant of the
brain areas that implement specific executive control
Stroop task. In this task, participants identified a color
patch on each trial that was accompanied by a distractor
In the present study, we used event-related fMRI to
polygon. During training, the distractor polygon had been
investigate whether practice affects the recruitment of
associated with (1) a pattern, (2) a different (i.e. incon-
executive brain mechanisms engaged by (a) cued attention-
gruent) color patch, or (3) had not been presented at all.
al orienting [27,41] and (b) Stroop-like interference be-
Across multiple training and fMRI scanning sessions, the
tween global and local aspects of an object's form [35]. As
presence of polygons associated with incongruent color
discussed earlier, the view that practice strengthens
patches, compared to those associated with patterns,
schemas predicts that practice should reduce neural activity
produced increasing amounts of neural activity, especially
associated with cued attentional orienting while increasing
in the dorsolateral prefrontal cortex.
neural activity associated with Stroop-like interference.
While the finding above is highly consistent with the
Recent advances in event-related fMRI methods have
present hypothesis, the effects of practice on interference
allowed separate estimates of cue and target activity in
were not measured, since congruent trials were not in-
cued attention paradigms [12,19]. To our knowledge,
cluded in the block design (i.e. trials in which the
however, the effects of practice on neural activity associ-
distractor polygon and target color patch were positively
ated with cued attentional orienting have not yet been
associated during training). It could be argued that poly-
investigated. The present study therefore provides the first
gons associated with patterns provided a neutral baseline
test of the hypothesis that practice should reduce the
against which to measure brain activity for polygons
recruitment of executive brain mechanisms that are re-
associated with incongruent color patches. From this
cruited by cued attentional orienting.
perspective, practice increased brain activity related to
The effects of practice on activity associated with
inhibition of incongruent color patches [28]. It is also
Stroop-like interference have been investigated somewhat
possible, however, that practice simply increased all neural
more with fMRI. The results of such studies, however, do
activity associated with task-relevant distractors (i.e. color
not provide clear evidence that practice increases inter-
patches) relative to task-irrelevant distractors (i.e. patterns).
ference-related activity. For instance, using block designs
An effect of task-relevance could be independent of
multiple researchers have reported that practice actually
conflict between two task-relevant representations (i.e.
reduces neural activity that is associated with interference
incongruent versus congruent). Therefore, this experiment
in the Stroop task, contrary to the present hypothesis
also does not provide conclusive evidence that practice
[7,33]. Given the nature of block designs, however,
increases interference-related activity.
participants can predict whether an upcoming trial will
With these issues in mind, we investigated the effects of
contain conflicting distractor information. It has been
practice on neural activity associated with cued attentional
suggested that such predictability often leads to the use of
orienting and interference using a relatively novel event-
top-down strategies during epochs of mostly incongruent
related fMRI approach [53,54]. In the present adaptation of
D.
H.
Weissman et al. /
Cognitive Brain Research 15 (2002) 47–60
this approach, participants were cued on a trial-by-trial
were controlled by customized software running on a PC.
basis to attend for and identify either the global or the local
Stimuli were projected onto a screen at the back of the
form of an upcoming hierarchical stimulus [35], which
magnet's bore. Participants viewed the stimuli through a
could be either congruent (e.g. a large, global H made up
mirror and responses were recorded with an MR-compat-
of small local Hs) or incongruent (e.g. a large, global S
ible response box.
made up of small local Hs). Since each stimulus dimension(i.e. global and local) was task-relevant on approximatelyone-half of the trials, participants became more practised at
.3.
Paradigm and procedure
identifying and responding to both global and local formsas the experiment progressed. Therefore, we could de-
We used a novel fast-rate event-related fMRI paradigm
termine not only whether practice reduced cue-related
recently developed by Woldorff and co-workers [53,54]
activity, but also whether practice at identifying and
(c.f. Shulman and co-workers [37,38,47]). In this paradigm
responding to target stimuli increased interference-related
(Fig. 1), compound-event trials containing a cue and a
activity from those same stimuli when they served as
target stimulus are randomly interspersed with trials con-
taining only a cue stimulus. On each 3-s trial, participants
We investigated the present hypotheses using a region of
viewed a cue (‘G', ‘L', ‘P', or ‘O'; 1.6831.08 of visual
interest approach that was informed by current knowledge
angle; duration5200 ms), which instructed them to attend
of the functional neuroanatomy of executive control. Much
for and identify either the global (‘G') or local (‘L') aspect
evidence indicates that parietal regions play a crucial role
of an upcoming hierarchical stimulus, or to passively wait
in orienting both spatial and non-spatial attention [20], and
until the next trial (either a ‘P' cue or an ‘O' cue). On
it has been specifically demonstrated that the left inferior
cue-only trials (all passive cue trials and one third of both
parietal lobe is critical for orienting attention in the global /
global task and local task trials), a cue was not followed by
local paradigm [43]. Consequently, we predicted that
a target stimulus. We contrasted neural activity for atten-
practice would decrease neural activity in the left inferior
tion-directing global and local cues with that for passive
parietal cortex that was associated with orienting attention
cues. This contrast isolated neural activity associated with
to global and local stimulus dimensions. Other evidence
executive aspects of cued attentional orienting while
indicates that midline frontal regions (i.e. the anterior
controlling for basic sensory and semantic processing of
cingulate and medial frontal gyri) play a role in detecting
attention-directing cue stimuli.
response conflict between target and distractor stimuli in
On cue-plus-target trials (66% of global-task and local-
selective attention paradigms [2,9,27]. We therefore pre-
task trials), either a congruent (e.g. a large S made of small
dicted that practice would increase interference-related
Ss) or an incongruent (e.g. a large S made of small Hs)
activity in midline frontal regions. Other data from the
hierarchical stimulus appeared for 200 ms, 1500 ms after
present study, which do not include analyses of the
cue onset (Fig. 1). The global and local forms of each
practice effects reported here, are published elsewhere
stimulus subtended 3.3832.18 and 0.6830.48 of visual
angle, respectively. For both the global and the local task,50% of the targets were congruent while the other 50%were incongruent. Participants were instructed to respond
. Materials and methods
to targets with their right hand, using their index finger topress one button if an H appeared at the cued dimension
.1.
Participants
and their middle finger to press a different button if an Sappeared. We contrasted incongruent with congruent
Fifteen participants (nine male, age range 20–36) were
targets to isolate neural activity that was associated with
recruited from the Duke University community in accord-
interference between global and local aspects of target
ance with the rules of the local human subjects committee.
Each participant was told that the study investigated the
In all trials, the fixation dot changed color, from white to
neural bases of selective attention and each gave his or her
red, 1500 ms after cue presentation (i.e. coincident with
written consent to participate. All were right-handed, with
target presentation in cue-plus-target trials). Participants
normal or corrected-to-normal vision, with no history of
were told that if a target did not appear at this point, then
serious neurological traumas or disorders. Prior to the
they should cease attending and simply wait for the next
experiment, informed consent was obtained from each
trial. This manipulation was performed to ensure that
participant. Each participant practised one or two blocks of
demands on pre-target attention-biasing processes would
the experimental task before the MR session. The study
be equated for cue-plus-target and cue-only trials [12].
lasted 2 h and participants were paid $10 per hour for
All seven trial types were included within each run.
They were presented equally often and in a counterbal-anced order such that, within every run, each trial type was
.2.
Apparatus
preceded equally often by every trial type in the design.
Stimulus presentation and the recording of response data
Such counterbalancing allows subtraction of response

D.H. Weissman et al. / Cognitive Brain Research 15 (2002) 47–60
Fig. 1. Examples of the stimuli and timing of stimulus presentation for the seven trial types used (see text for more details).
overlap from adjacent trials when comparing the average
prior to analysis of the functional data. Structural images
time-locked responses to different trial types [6,14,52].
for each participant were also collected using a T1-weight-
Most relevant for the present method of data analysis, the
ed spin echo sequence (TR5500 ms, TE514 ms, flip
presence of cue-only trials allows calculation of indepen-
angle5908, 18 contiguous 7-mm-thick slices—in plane
dent parameter estimates for the response amplitudes to
resolution50.94 mm30.94 mm).
different types of cues (i.e. global, local and passive) andtargets (i.e. global congruent, global incongruent, local
.5. Data analysis
congruent and local incongruent) within a multiple regres-sion framework [37,38].
The software analysis package SPM'99 [18] was used to
correct functional images for temporally asynchronous
.4. fMRI data acquisition
slice acquisition and head motion, to warp the functionalimages to MNI (Montreal Neurological Institute) standard
The blood oxygenation dependent response (BOLD)
space, and to spatially smooth the functional images with a
signal was measured with an echo-planar imaging se-
Gaussian filter (FWHM58 mm in the x, y, and z dimen-
quence (TR51.5 s, TE540 ms, flip angle5908, 18 con-
sions). Next, responses to cue and target stimuli were
tiguous 7-mm-thick slices—in plane resolution53.75
modeled by convolving a vector containing the onset times
mm33.75 mm) during the collection of functional images.
of the different types of cues and targets with a canonical
Each participant completed 10 runs of 5 min duration
hemodynamic response function. This function was com-
each (although one completed only nine). During each run,
posed of the sum of two gamma functions. In total, there
206 brain volumes were collected. The first six functional
were seven regressors: one for each type of cue and target.
images of each run contained no trials and were discarded
Included were regressors for passive cues, global cues,
D.H. Weissman et al. / Cognitive Brain Research 15 (2002) 47–60
local cues, global congruent targets, global incongruent
the t-map for cued attentional orienting was thresholded
targets, local congruent targets, and local incongruent
(t52.625, P,0.01 and 10 contiguous voxels). This con-
targets. Multiple linear regression (SPM'99) was im-
trast revealed voxels that were engaged by interference,
plemented on the data from each participant to determine
major foci of which are indicated in Table 3. The resulting
the parameter estimate for each regressor within each run.
t-map was then decomposed into ROIs using the atlas of
To identify effects of practice on neural activity associ-
Talaraich and Tournoux (1988) [49].
ated with cued attentional orienting, a voxelwise analysiswas used to create regions of interest (ROIs). The vox-
.6. Region of interest (ROI) analyses
elwise analysis contrasted the mean parameter estimate forglobal and local attention-directing cues with the parameter
We first performed an ROI analysis to determine
estimate for passive sensory / semantic control cues (termed
whether practice significantly reduced neural activity in the
the cue difference) in each voxel separately. This contrast
left inferior parietal lobe that was associated with cued
revealed voxels that were involved in cued attentional
attentional orienting. In this analysis, the average cue
orienting [53,54]. The t-map was thresholded at a level
difference across all voxels in the left parietal ROI was
(t52.625, P,0.01 and 10 contiguous voxels) that revealed
calculated separately for runs 1–3, runs 4–6, and runs 6–9
several clusters of activation, major foci of which are
in each participant. To determine whether practice affected
revealed in Table 1. These clusters were then divided into
neural activity that was associated with cued attentional
different regions of interest (ROIs) using the atlas of
orienting, we performed paired t-tests to contrast the
Talaraich and Tournoux (1988).
average cue difference for runs 1–3 versus runs 4–6 and
To identify effects of practice on neural activity associ-
runs 4–6 versus runs 7–9. Since we hypothesized that
ated with interference, a second voxelwise analysis was
activity associated with cued attentional orienting would
performed. This analysis contrasted the mean parameter
decrease with practice, each t-test was one-tailed. Since
estimate for incongruent targets with that for congruent
two t-tests were performed for each ROI, however, only
targets (termed the target difference) in each voxel separ-
P-values less than 0.05 / 250.025 were considered signifi-
ately. The target difference was calculated by averaging
cant (t.2.15). For completeness, we also calculated sepa-
across global and local targets to increase statistical power.
rate t-values for the average cue differences in runs 1–3,
The resulting t-map was thresholded in the same way that
runs 4–6, and runs 7–9 (Table 2). Exploratory analyses inother ROIs activated by cued attentional orienting were
performed in the same way. We make no strong conclu-
Major foci activated by cued attentional orienting
sions, however, regarding the significance of effects in
these other ROIs (Table 2) since they were not predicted apriori.
We also performed two ROI analyses to determine
whether practice significantly increased neural activity
associated with interference in midline frontal regions (i.e.
the anterior cingulate and medial frontal gyri). In these
analyses, the average target differences across all voxels in
(1) the medial frontal gyrus and (2) the anterior cingulate
cortex were calculated separately for runs 1–3, runs 4–6,
and runs 6–9 in each participant. To determine whether
practice affected neural activity associated with interfer-
ence in each of these two ROIs, we performed paired
t-tests to contrast the average target differences for runs
1–3 with runs 4–6 and runs 4–6 with runs 7–9. As for our
analyses of cue activity, each of these two t-tests was
one-tailed and therefore only P-values less than 0.05 / 25
0.025 were considered significant (t.2.15). For complete-
ness, we also calculated separate t-values for the average
target differences in runs 1–3, runs 4–6, and runs 7–9(Table 4). Exploratory analyses in other ROIs were
BA, Brodmann area; x, y, z, coordinates of peak activation from Talaraichand Tournoux's (1988) atlas [49]. T, peak voxel T-score within a region;
performed in exactly the same way. We make no strong
P, probability that a voxel T-score occurred by chance. L, left; R, right;
conclusions, though, regarding the significance of effects
SFG, superior frontal gyrus; MFG, middle frontal gyrus; MeFG, medial
in these other ROIs (Table 4) since they were not predicted
frontal gyrus; ACC, anterior cingulate cortex; PrCG, precentral gyrus;
PostCG, postcentral gyrus; SPL, superior parietal lobule; IPL, inferior
To further illustrate the findings from the ROI analyses
parietal lobule; Prec, precuneus; SMG, supramarginal gyrus; AG, angulargyrus; MOG, middle occipital gyrus; Cun, cuneus; LG, lingual gyrus.
above, we performed selective averaging to determine the
D.H. Weissman et al. / Cognitive Brain Research 15 (2002) 47–60
Table 2ROIs for which practice significantly reduced activity related to cued attentional orienting
T-1, 2, 3 5 T-score indicating reliability of activation for runs 1–3, runs 4–6, and runs 7–9. T-1 vs. 25T-score testing whether activity was greater for runs1–3 versus 4–6 (t .2.15, P ,0.025). T-2 vs. 35T-score testing whether activity was greater for runs 4–6 than runs 7–9 (t .2.15, P ,0.025). ROI, regionof interest; N 5number of voxels; BA, Brodmann area; x, y, z, geographic center of mass coordinates from Talaraich and Tournoux's (1988) atlas [49]. L,left; R, right; SFG, superior frontal gyrus; MFG, middle frontal gyrus; MeFG, medial frontal gyrus; ACC, anterior cingulate cortex; PrCG, precentral gyrus;SPL, superior parietal lobule; IPL, inferior parietal lobule; AG, angular gyrus; MOG, middle occipital gyrus; Cun, cuneus; LG, lingual gyrus.
average time-locked response to each of the seven trial
measures ANOVA using three factors: block (runs 1–3,
types across all voxels within each ROI. We then plotted
runs 4–6, runs 7–9), level (global, local) and distractor
the difference in peak amplitude (in units of percent
type (congruent, incongruent). As expected [21,22,25],
change) between (a) attention-directing and passive cues
RTs were significantly faster for congruent (520 ms) than
(Fig. 2) and (b) incongruent and congruent targets (Fig. 3)
for incongruent (579 ms) trials, F(1,11)535.718, P ,
for ROIs with a P-value less than 0.025.
0.001. Consistent with prior findings [35], RTs weresignificantly faster for global (533 ms) than for local (567
.7. Conversion from MNI to Talaraich coordinates
ms) trials, F(1,11)58.585, P ,0.02. No other main effectsor interactions reached significance (P .0.50 in all cases).
Following statistical analyses in MNI space, we con-
An analogous ANOVA with percent correct as the
verted sites of activation to Talaraich coordinates to allow
dependent measure revealed that overall performance was
better comparison with activations from prior studies.
quite high (90.7%). As in the RT analysis, there was a
Conversion from MNI to Talaraich coordinates was im-
significant main effect of distractor type, F(1,11)57.003,
plemented with a non-linear combination of two linear
P ,0.025, because responses were less accurate for incon-
transformations that has been used in other published
gruent (87.1%) than for congruent (94.2%) cue-plus-target
trials. All other possible main effects and interactions
Coordinates above the anterior commis-
failed to achieve statistical significance (P .0.16 in all
sure (AC) were transformed as follows: X9 5 0.99X; Y9 5
0.9688Y 1 0.0460Z; Z9 5 2 0.0485Y 1 0.9189Z. Coordi-nates below the AC were transformed with these equa-
.2. Imaging
X9 5 0.99X;
Y9 5 0.9688Y 1 0.0420Z;
2 0.0485Y 1 0.8390Z. Talaraich coordinates in the tables
We contrasted neural activity for attention-directing (i.e.
indicate the location(s) of peak activity within each region.
global and local) cues to that for passive cues in order tofunctionally define left inferior parietal regions that wereengaged by cued attentional orienting as well as other
. Results
regions of interest (ROIs) in which we performed explorat-ory analyses (Table 1; Figs. 2 and 3). To investigate
.1. Behavior
practice effects with sufficient statistical power, we com-bined data across groups of three runs. Specifically, we
Data from three of the 15 participants were lost due to a
determined whether neural activity associated with cued
problem with the response box. Mean reaction times (RTs)
attentional orienting varied across runs 1–3, runs 4–6, and
for the remaining participants were analyzed with repeated
runs 7–9. In line with predictions, there was a significant

15 (2002) 47–60
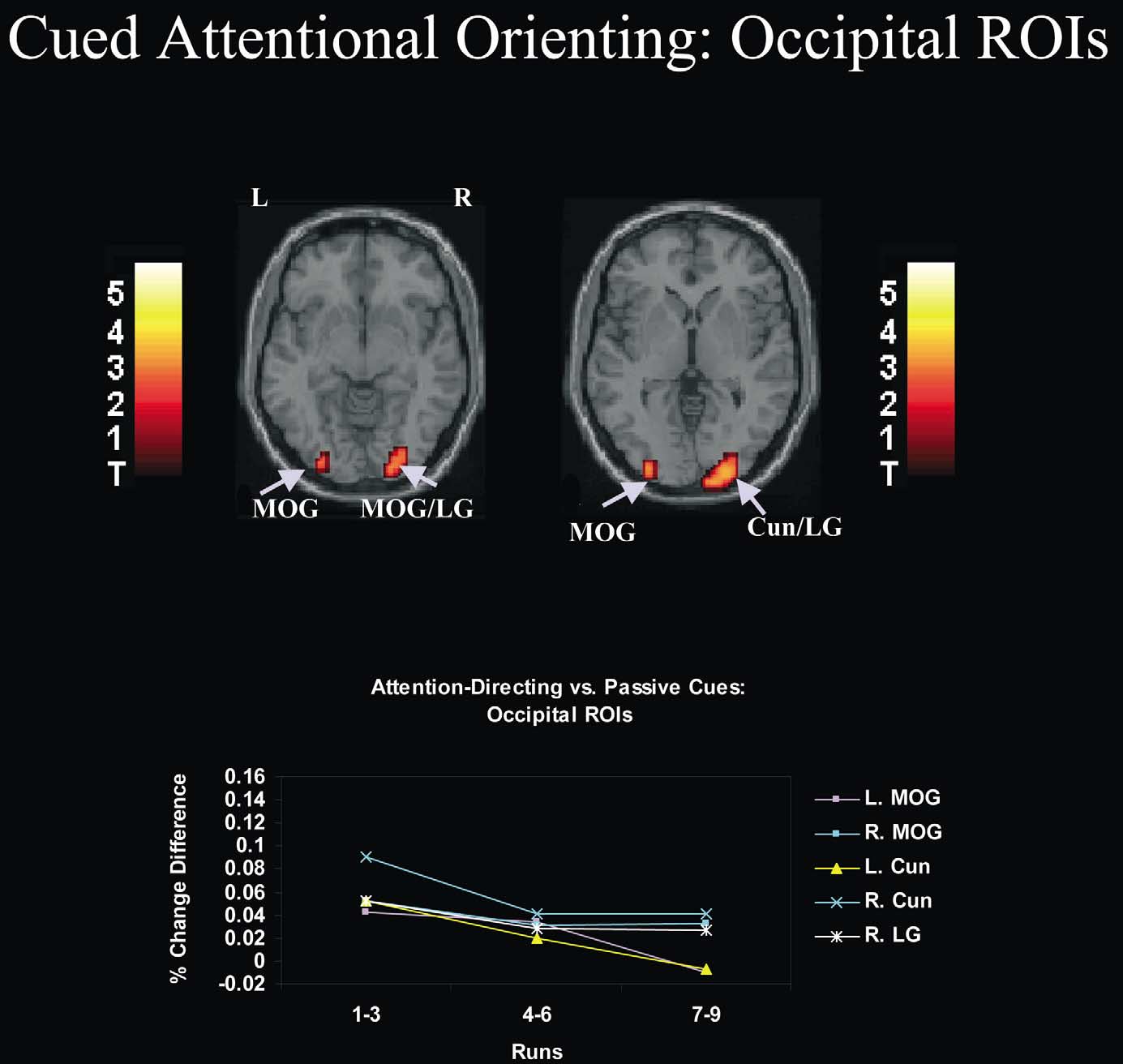
Fig. 2. Effects of practice on neural activity associated with cued attentional orienting in frontal and parietal regions of interest (ROIs). As shown in the figure, cued attentional orienting activatedregions of frontal and parietal cortex (see also Table 1). ROI analyses revealed that practice significantly reduced the size of the hemodynamic response evoked by cued attentional orienting in severalROIs including the left inferior parietal lobe. This reduction is illustrated in separate insets for frontal and parietal ROIs. In each graph, the difference in peak percent signal change betweenattention-directing and passive cues is depicted as a function of practice (runs 1–3, runs 4–6, runs 7–9) for individual brain ROIs (see Table 2 for a list of abbreviations for these ROIs). Thesedifferences in peak percent signal change were derived from the time-locked average responses to global and local cue-only and passive cue-only trials. In most of these ROIs, there was a large andsignificant decrease in neural activity associated with cued attentional orienting after runs 1–3 that was sustained throughout the remainder of the experiment (see Table 2 for relevant statisticsperformed on parameter estimates). All activations are overlaid on the canonical MNI normalized anatomical template provided by SPM'99. Anatomical slices range from Z 535 mm (top left) to
Z 563 mm (top right). L, left; R, right.
D.H. Weissman et al. / Cognitive Brain Research 15 (2002) 47–60
Fig. 3. Effects of practice on neural activity associated with cued attentional orienting in occipital regions of interest (ROIs). As shown in the figure, cuedattentional orienting activated regions of occipital cortex (see also Table 1). ROI analyses revealed that practice significantly reduced the size of thehemodynamic response evoked by cued attentional orienting in several occipital ROIs. In the inset graph, the difference in peak percent signal changebetween attention-directing and passive cues is depicted as a function of practice (runs 1–3, runs 4–6, runs 7–9) for individual brain ROIs (see Table 2 fora list of abbreviations for these ROIs). These differences in peak percent signal change were derived from the time-locked average responses to global andlocal cue-only and passive cue-only trials. In most of these ROIs, there was a large and significant decrease in neural activity associated with cuedattentional orienting after runs 1–3 that was sustained throughout the remainder of the experiment (see Table 2 for relevant statistics performed onparameter estimates). All activations are overlaid on the canonical MNI normalized anatomical template provided by SPM'99. Anatomical slices rangefrom 27 mm (top left) to 0 mm (top right) in intervals of 7 mm. L, left; R, right.
decrease (t .2.15, P ,0.025) of neural activity associated
well as several occipital ROIs (Table 2; Fig. 3). Thus, the
with cued attentional orienting between runs 1–3 and runs
pattern of effects observed in all ROIs indicates an early
4–6 for the left inferior parietal cortex with no further
reduction in the recruitment of executive mechanisms
significant changes between runs 4–6 and runs 7–9 (Table
following runs 1–3 that was sustained throughout the
2; Fig. 2). Exploratory analyses revealed similar effects in
remainder of the experiment. We make no strong conclu-
several other frontal and parietal ROIs (Table 2; Fig. 2) as
sions regarding statistical significance in ROIs identified
D.H. Weissman et al. / Cognitive Brain Research 15 (2002) 47–60
by the exploratory analyses, however, since they were not
because they were not predicted at the outset of the study.
predicted at the outset of the study. Finally, no ROIs
Finally, no ROIs showed decreases of interference-related
showed increases of neural activity associated with cued
We next contrasted neural activity for incongruent
targets with that to congruent targets (averaged across the
. Discussion
global and local tasks to increase statistical power) in orderto functionally define medial frontal and anterior cingulate
We investigated a key prediction of various models of
regions that were activated by interference (Table 3; Figs.
4 and 5). As for the analysis of cue-related practice effects,
strengthens associations between task stimuli and task-
we divided the experiment into three parts (runs 1–3, runs
relevant processes and responses. To do so, we determined
4–6, and runs 7–9). In line with predictions (and opposite
whether practice affects the recruitment of brain regions
to the findings for cued attentional orienting), practice
that are engaged by (a) cued attentional orienting and (b)
significantly (t .2.15, P ,0.025) increased interference-
interference between target and distractor stimuli. As
related activity between runs 1–3 and runs 4–6 in bilateral
predicted, practice reduced neural activity associated with
regions of the medial frontal gyrus (Table 4; Fig. 4), with
cued attentional orienting in left inferior parietal regions
no further changes between runs 4–6 and runs 7–9.
and increased interference-related activity in medial frontal
Exploratory analyses revealed similar effects in several
regions. Both of these findings support models in which
other frontal ROIs (Table 4; Fig. 4) and in some subcorti-
practice strengthens schemas as we describe below.
cal ROIs (Table 4; Fig. 5). We make no strong conclusionregarding statistical significance in these regions, though,
.1. Effects of practice on cue-related activity
Attention-directing cues engage processes that interpret
Major foci activated by interference
the meaning of cues [47,53,54], orient attention [41], andactivate task-relevant stimulus–response mappings [44]. If
practice strengthens associations between cues and these
processes, then cues should become able to evoke these
processes more automatically, with less intervention by the
supervisory system. Practice should therefore reduce the
recruitment of executive brain mechanisms that are en-
gaged by cued attentional orienting (i.e. attention-directing
cues versus passive sensory / semantic control cues).
Consistent with this prediction, practice reduced neural
activity that was associated with cued attentional orienting
in left inferior parietal regions that have been specifically
linked to the control of attention toward global and local
aspects of hierarchical stimuli [43]. In addition, this effect
occurred relatively quickly. Neural activity associated with
cued attentional orienting was greatest in runs 1–3,
significantly lower in runs 4–6, and remained low in runs
7–9. Exploratory analyses suggested that the same pattern
of effects was present in several other brain areas, includ-
ing regions of frontal, parietal and occipital cortex. Given
that we controlled for basic sensory and semantic process-
ing of attention-directing cues with passive cues, our
findings provide support for the view that practice
strengthens cue schemas, thereby allowing cues to activate
appropriate processes with less intervention from executive
BA, Brodmann area; x, y, z, coordinates of peak activation from Talaraich
brain mechanisms.
and Tournoux's (1988) atlas [49]. T, peak voxel T-score within a region;
Definitive interpretations of the decreased cue-related
P, probability that a voxel T-score occurred by chance. L, left; R, right;SFG, superior frontal gyrus; MFG, middle frontal gyrus; MeFG, medial
activity in frontal, parietal and occipital cortices must
frontal gyrus; ACC, anterior cingulate cortex; PrCG, precentral gyrus;
await further investigation, but the existent literature
IFG, inferior frontal gyrus; SPL, superior parietal lobule; IPL, inferior
suggests several possibilities. Practice may engender more
parietal lobule; Prec, precuneus; FFG, fusiform gyrus; ITG, inferior
efficient focusing of attention on task-relevant information,
temporal gyrus; PhG, parahippocampal gyrus; MOG, middle occipital
thereby leading to decreased activity within inferior pariet-
gyrus; Cereb, cerebellum; Thal, thalamus; Pulv, pulvinar nucleus of thethalamus.
al regions that orient attention [12,13,19,26,42,51]. It may
15 (2002) 47–60
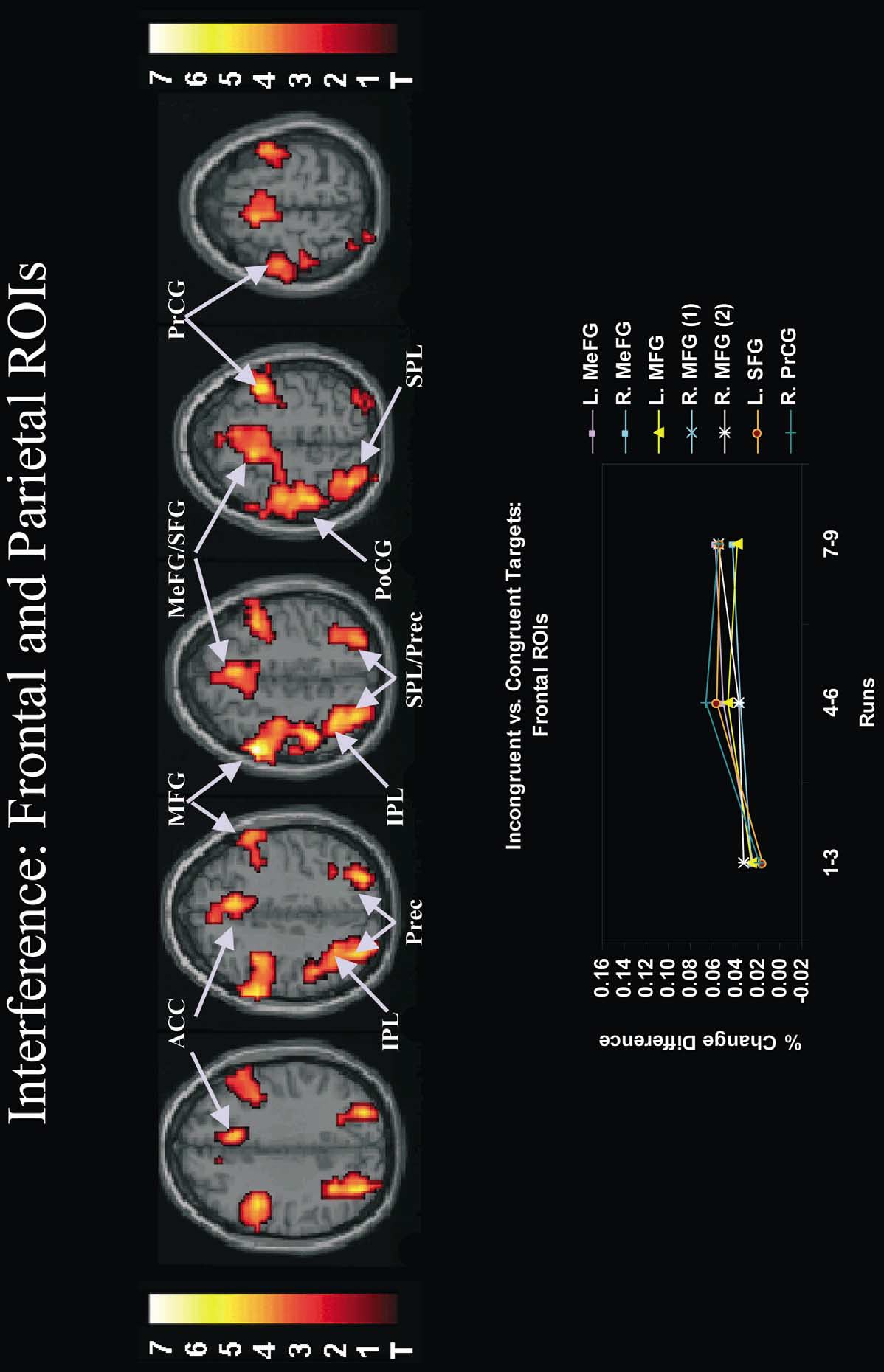
Fig. 4. Effects of practice on neural activity associated with interference in frontal and parietal regions of interest (ROIs). Interference activated regions of frontal and parietal cortex (see also Table 3).
ROI analyses revealed that practice significantly increased the size of the hemodynamic response evoked by interference in several frontal ROIs including the medial frontal gyrus. This increase isillustrated in the inset graph. In this inset, the difference in peak percent signal change between incongruent and congruent targets is depicted as a function of practice (runs 1–3, runs 4–6, runs 7–9)for individual brain ROIs (see Table 4 for a list of abbreviations for these ROIs). These differences in peak percent signal change were derived from the time-locked average responses tocue-plus-incongruent and cue-plus-congruent trials, averaged across the global and local tasks. In most ROIs, there was a large and significant increase in neural activity associated with interferenceafter runs 1–3 that was sustained throughout the remainder of the experiment (see Table 4 for relevant statistics performed on parameter estimates). All activations are overlaid on the canonical MNInormalized anatomical template provided by SPM'99. Anatomical slices range from Z 535 mm (top left) to Z 563 mm (top right). L, left; R, right.
D.H. Weissman et al. / Cognitive Brain Research 15 (2002) 47–60
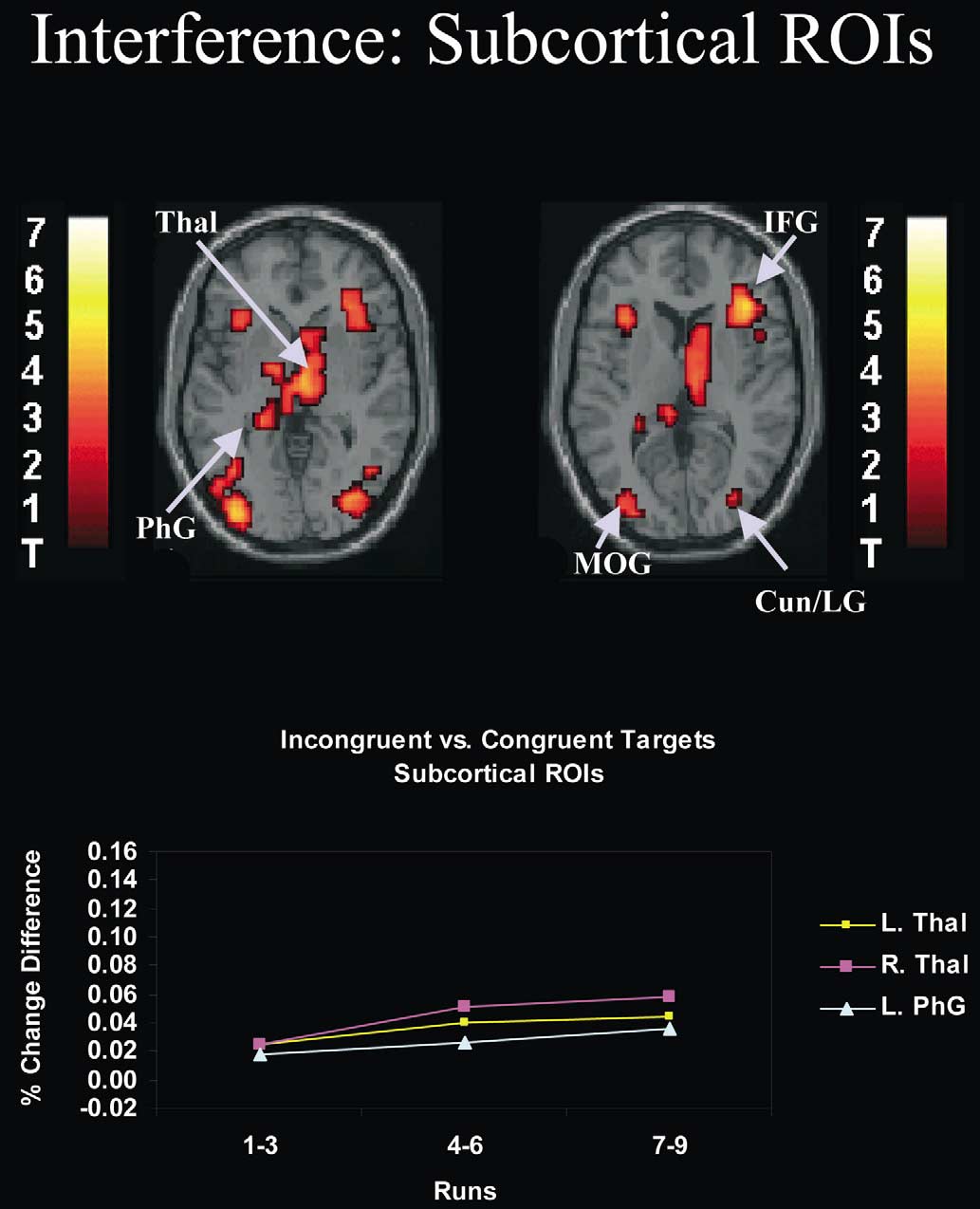
Fig. 5. Effects of practice on neural activity associated with interference in subcortical regions of interest (ROIs). As shown in the figure, interferenceactivated subcortical regions including the thalamus and parahippocampal gyrus (see also Table 3). ROI analyses revealed that practice significantlyincreased the size of the hemodynamic response evoked by interference in several ROIs. This increase is illustrated in the inset graph. In this inset, thedifference in peak percent signal change between incongruent and congruent targets is depicted as a function of practice (runs 1–3, runs 4–6, runs 7–9) forindividual brain ROIs (see Table 4 for a list of abbreviations for these ROIs). These differences in peak percent signal change were derived from thetime-locked average responses to cue-plus-incongruent and cue-plus-congruent trials, averaged across the global and local tasks. In most ROIs, there was alarge and significant increase in neural activity associated with interference after runs 1–3 that was sustained throughout the remainder of the experiment(see Table 4 for relevant statistics performed on parameter estimates). All activations are overlaid on the canonical MNI normalized anatomical templateprovided by SPM'99. Anatomical slices range from 0 mm (top left) to 7 mm (top right) in intervals of 7 mm. L, left; R, right.
also increase the efficiency of encoding processes in
[36], such that brain regions that interpret the meaning of
occipital regions [5], where attention can facilitate the
linguistic (cue) stimuli, such as the angular gyrus [4],
encoding of task-relevant stimuli [19,30], such that less
become less recruited by executive mechanisms. Task
attention is necessary during cue processing to prepare for
repetition may also lead to more efficient maintenance of
upcoming task stimuli. In addition, repeated performance
task-relevant representations [46], thereby reducing activi-
of a task may strengthen associations between task cues
ty in brain regions that keep currently relevant representa-
and the processes they activate at non-supervisory levels
tions active in working memory, such as the middle frontal
D.H. Weissman et al. / Cognitive Brain Research 15 (2002) 47–60
Table 4ROIs for which practice significantly increased activity associated with interference
T-1, 2, 3 5 T-score indicating reliability of activation for runs 1–3, runs 4–6, and runs 7–9. T-1 vs. 25T-score testing whether activity was smaller for runs1–3 than runs 4–6 (t .2.15, P ,0.025). T-2 vs. 35T-score testing whether activity was smaller for runs 4–6 than runs 7–9 (t .2.15, P ,0.025). ROI,region of interest; N, number of voxels; BA, Brodmann area; x, y, z, geographic center of mass coordinates from Talaraich and Tournoux's (1988) atlas[49]. L, left; R, right; MeFG, medial frontal gyrus; MFG, middle frontal gyrus; PrCG, precentral gyrus; SFG, superior frontal gyrus; PhG, parahippocampalgyrus; Thal, thalamus.
gyrus [40]. Finally, practice may strengthen associations
cesses that keep track of current task goals [3,15]. In the
between target stimuli and their appropriate responses.
present study, practice at associating distractor stimuli with
Such effects may reduce neural activity in midline frontal
motor responses that conflicted with those required by
regions that is related to motor preparation following cue
targets on incongruent trials may have increased the
presentation [39]. These interpretations are highly con-
demands on such working memory processes. Practice
sistent with current views on the functional neuroanatomy
effects in thalamic regions, which included the left pulvi-
of cued attentional orienting.
nar, may indicate that strengthening associations betweendistractors and specific motor responses leads to increased
.2. Effects of practice on interference-related activity
focusing of attention on target features [24] during incon-gruent trials. Therefore, the present findings support the
Target stimuli engage processes that select, identify and
view that practice at associating distractor stimuli with
respond to task-relevant stimulus features. If practice
specific responses increases the recruitment of executive
strengthens associations between targets and such pro-
brain systems that detect / resolve interference on incon-
cesses, then targets should come to evoke identification
gruent trials.
and response processes relatively automatically, with less
One may wonder why, if our interpretation of the
intervention by the supervisory system. In the present
imaging data is correct, practice failed to increase be-
experiment, we used a cued attention paradigm in which
havioral measures of interference. One possibility is that
participants became increasingly practised at selecting,
practice increased the ability of distractors to evoke
identifying and responding to every possible target
conflict, but that the increased conflict was resolved before
stimulus (e.g. global H, global S, local H, local S). We
it affected behavioral performance. From this perspective,
therefore predicted that practice would increase the recruit-
at least some of the brain regions that showed increased
ment of brain regions that implement conflict detection
interference-related activity with practice likely make a
and / or resolution processes when those same stimuli
functional contribution to detecting / resolving interference
served as incongruent distractors.
rather than causing it.
Consistent with this prediction, practice significantly
increased interference-related activity in the medial frontalgyrus (just dorsal to the anterior cingulate cortex), which
. Summary
has previously been linked to the detection of responseconflict [34]. This effect occurred relatively quickly in that
The present findings make several important contribu-
interference-related activity was quite low in runs 1–3,
tions to our understanding of executive brain mechanisms.
significantly higher for runs 4–6, and remained high
Firstly, to our knowledge, they constitute the first direct
(possibly at ceiling) for runs 7–9. Exploratory analyses
evidence from functional neuroimaging that practice de-
revealed similar effects in a number of other regions
creases the recruitment of executive brain mechanisms
including the middle frontal gyrus and the thalamus. The
engaged by cued attentional orienting. Secondly, our
middle frontal gyrus is engaged when the presence of
results are the first to show that practice increases the
conflicting distractor stimuli increases demands on pro-
recruitment of executive brain mechanisms that are en-
D.H. Weissman et al. / Cognitive Brain Research 15 (2002) 47–60
[9] C.S. Carter, A.M. Macdonald, M. Botvinick, L.L. Ross, V.A.
gaged by interference when the task-relevance of distractor
Stenger, D. Noll, J.D. Cohen, Parsing executive processes: strategic
information is controlled. These findings illustrate that
vs. evaluative functions of the anterior cingulate cortex, Proc. Natl.
practice can decrease the recruitment of executive brain
Acad. Sci. USA 97 (2000) 1944–1948.
mechanisms for one component of a task (e.g. cued
[10] J.D. Cohen, K. Dunbar, J.L. McClelland, On the control of
attentional orienting) while increasing it for another (e.g.
automatic processes: a parallel distributed processing account of theStroop effect, Psychol. Rev. 97 (1990) 332–361.
detecting / resolving interference). This finding is more
[11] J.D. Cohen, D. Servan-Schreiber, J.L. McClelland, A parallel
distributed processing approach to automaticity, Am. J. Psychol. 105
strengthens task schemas than with alternative explanations
(1992) 239–269.
related to arousal. For example, it is unclear how a uniform
[12] M. Corbetta, J.M. Kincade, J.M. Ollinger, M.P. McAvoy, G.L.
Shulman, Voluntary orienting is dissociated from target detection in
decrease in arousal over the course of the experiment
human posterior parietal cortex, Nat. Neurosci. 3 (2000) 292–297.
would predict both an increase of interference-related
[13] J.T. Coull, C.D. Frith, C. Buchel, A.C. Nobre, Orienting attention in
activity and a decrease of activity associated with cued
time: behavioural and neuroanatomical distinction between exogen-
attentional orienting. The present results therefore demon-
ous and endogenous shifts, Neuropsychologia 38 (2000) 808–819.
[14] A.M. Dale, R.L. Buckner, Selective averaging of rapidly presented
strate how functional neuroimaging studies of practice
individual trials using fMRI, Hum. Brain Mapp. 5 (1997) 329–340.
effects can be extremely useful for testing theoretical
[15] J.W. de Fockert, G. Rees, C.D. Frith, N. Lavie, The role of working
models of executive control.
memory in visual selective attention, Science 291 (2001) 1803–1806.
[16] R. Desimone, Visual attention mediated by biased competition in
extrastriate visual cortex, Phil. Trans. R. Soc. Lond. Ser. B: Biol.
Sci. 353 (1998) 1245–1255.
[17] J. Duncan, R.J. Seitz, J. Kolodny, D. Bor, H. Herzog, A. Ahmed,
This research was supported by a postdoctoral National
F.N. Newell, H. Emslie, A neural basis for general intelligence,Science 289 (2000) 457–460, see comments.
Research Service Award to D.W. (1 F32 NS41867-01) and
[18] K.J. Friston, A.P. Holmes, K.J. Worsley, J.P. Poline, C.D. Frith,
by NIMH grants to M.G.W. (MH60415) and G.R.M.
R.S.J. Frackowiak, Statistical parametric maps in functional imag-
(MH55714 and MH02019). We wish to thank Barry
ing: a general linear approach, Hum. Brain Mapp. 2 (1995) 189–
Giesbrecht, Kevin Wilson, Sean Fannon, Wayne Khoe,
[19] J.B. Hopfinger, M.H. Buonocore, G.R. Mangun, The neural mecha-
Laura Busse and Heleen Slagter for useful discussions. We
nisms of top-down attentional control, Nat. Neurosci. 3 (2000)
also wish to thank Linsay Warner for her careful proof-
reading of the manuscript.
[20] N. Kanwisher, E. Wojciulik, Visual attention: insights from brain
imaging, Nat. Rev. Neurosci. 1 (2000) 91–100.
[21] R. Kimchi, Selective attention to global and local levels in the
comparison of hierarchical patterns, Percept. Psychophys. 43 (1988)
eferences
[22] R. Kimchi, Primacy of wholistic processing and global / local
[1] A.D. Baddeley, Working Memory, Clarendon Press, Oxford, 1986.
paradigm: a critical review, Psychol. Bull. 112 (1992) 24–38.
[2] M.T. Banich, M.P. Milham, R. Atchley, N.J. Cohen, A. Webb, T.
[23] A.F. Kramer, S. Hahn, D. Gopher, Task coordination and aging:
Wszalek, A.F. Kramer, Z.P. Liang, A. Wright, J. Shenker, R. Magin,
explorations of executive control processes in the task switching
FMRI studies of Stroop tasks reveal unique roles of anterior and
paradigm, Acta Psychol. 102 (1999) 339–378.
posterior brain systems in attentional selection, J. Cogn. Neurosci.
[24] D.L. LaBerge, Attention, Psychol. Sci. 1 (1990) 156–162.
12 (2000) 988–1000.
[25] M.R. Lamb, L.C. Robertson, R.T. Knight, Attention and interference
[3] M.T. Banich, M.P. Milham, R.A. Atchley, N.J. Cohen, A. Webb, T.
in the processing of global and local information: effects of
Wszalek, A.F. Kramer, Z. Liang, V. Barad, D. Gullett, C. Shah, C.
unilateral temporal–parietal junction lesions, Neuropsychologia 27
Brown, Prefrontal regions play a predominant role in imposing an
(1989) 471–483.
attentional ‘set' evidence from fMRI, Cogn. Brain Res. 10 (2000)
[26] T.H. Le, J.V. Pardo, X. Hu, 4 T-fMRI study of nonspatial shifting of
selective attention: cerebellar and parietal contributions, J. Neuro-
[4] J. Binder, C.J. Price, Functional neuroimaging of language, in: R.
physiol. 79 (1998) 1535–1548.
Cabeza, A. Kingstone (Eds.), Handbook of Functional Neuroimag-
[27] A.W. MacDonald, J.D. Cohen, V.A. Stenger, C.S. Carter, Dissociat-
ing of Cognition, Bradford, Cambridge, 2001, pp. 187–251.
ing the role of the dorsolateral prefrontal and anterior cingulate
[5] R.L. Buckner, J. Goodman, M. Burock, M. Rotte, W. Koutstaal, D.
cortex in cognitive control, Science 288 (2000) 1835–1838.
Schacter, B. Rosen, Functional–anatomic correlates of object prim-
[28] C.M. MacLeod, Half a century of research on the Stroop effect: an
ing in humans revealed by rapid presentation event-related fMRI,
integrative review, Psychol. Bull. 109 (1991) 163–203.
Neuron 20 (1998) 285–296.
[29] C.M. MacLeod, K. Dunbar, Training and Stroop-like interference:
[6] M.A. Burock, R.L. Buckner, M.G. Woldorff, B.R. Rosen, A.M.
evidence for a continuum of automaticity, J. Exp. Psychol. Learn.
Dale, Randomized event-related experimental designs allow for
Mem. Cogn. 14 (1988) 126–135.
extremely rapid presentation rates using functional MRI, Neurore-
[30] A. Martin, Semantic memory and the brain: structure and processes,
port 9 (1998) 3735–3739.
Curr. Opin. Neurobiol. 11 (2001) 194–201.
[7] G. Bush, P. Whalen, B.R. Rosen, M.A. Jenike, S.C. McInerney, S.L.
[31] N. Meiran, Reconfiguration of processing mode prior to task
Rauch, The counting Stroop: an interference task specialized for
performance, J. Exp. Psychol.: Learn. Mem. Cogn. 22 (1996)
functional neuroimaging—validation study with functional MRI,
Hum. Brain Mapp. 6 (1998) 270–282.
[32] M.P. Milham, An fMRI Analysis of Dorsolateral Prefrontal Cortex's
[8] A.J. Calder, A.D. Lawrence, A.W. Young, Neuropsychology of fear
Involvement in Attentional Control, Unpublished Doctoral Disserta-
and loathing, Nat. Rev. Neurosci. 2 (2001) 352–363.
D.H. Weissman et al. / Cognitive Brain Research 15 (2002) 47–60
[33] M.P. Milham, M.T. Banich, E. Claus, N.J. Cohen, Practice-related
[45] E. Ruthruff, J.C. Johnston, M. Van Selst, Why practice reduces
effects demonstrate complementary roles of anterior cingulate and
dual-task interference, J. Exp. Psychol. Hum. Percept. Perform. 27
prefrontal cortices in attentional control, in preparation.
(2001) 3–21.
[34] M.P. Milham, M.T. Banich, A. Webb, V. Barad, The relative
[46] R. Shiffrin, W. Schneider, Controlled and automatic human in-
involvement of anterior cingulate and prefrontal cortex in attentional
formation processing: II. Perceptual learning, automatic attending,
control depends on nature of conflict, Cogn. Brain Res. 12 (2001)
and a general theory, Psychol. Rev. 84 (1977) 127–189.
[47] G.L. Shulman, J.M. Ollinger, E. Akbudak, T.E. Conturo, A.Z.
[35] D. Navon, Forest before trees: the precedence of global features in
Snyder, S.E. Petersen, M. Corbetta, Areas involved in encoding and
visual perception, Cogn. Psychol. 9 (1977) 353–383.
applying directional expectations to moving objects, J. Neurosci. 19
[36] D.A. Norman, T. Shallice, Attention to action: willed and automatic
(1999) 9480–9496.
control of behavior, in: G.E.S.D.S.R.J. Davidson (Ed.), Conscious-
[48] D.T. Stuss, T. Shallice, M.P. Alexander, T.W. Picton, A multidiscip-
ness and Self-regulation, Plenum Press, New York, 1986, pp. 1–18.
linary approach to anterior attentional functions, Ann. NY Acad. Sci.
[37] J.M. Ollinger, M. Corbetta, G.L. Shulman, Separating processes
769 (1995) 191–211.
within a trial in event-related functional MRI, Neuroimage 13
[49] J. Talaraich, P. Tournoux, Co-planar Stereotactic Atlas of the
(2001) 218–229.
Human Brain, 2nd ed., Thieme Stuttgart, 1998.
[38] J.M. Ollinger, G.L. Shulman, M. Corbetta, Separating processes
[50] D.H. Weissman, M.G. Woldorff, G.R. Mangun, A role for top-down
within a trial in event-related functional MRI, Neuroimage 13
attentional orienting during interference between global and local
(2001) 210–217.
aspects of hierarchical stimuli, NeuroImage (2002) in press.
[39] L. Petit, S.M. Courtney, L.G. Ungerleider, J.V. Haxby, Sustained
[51] E. Wojciulik, N. Kanwisher, The generality of parietal involvement
activity in the medial wall during working memory delays, J.
in visual attention, Neuron 23 (1999) 747–764.
Neurosci. 18 (1998) 9429–9437.
[52] M.G. Woldorff, Distortion of ERP averages due to overlap from
[40] M. Petrides, Functional organization of the human frontal cortex for
temporally adjacent ERPs: analysis and correction, Psychophysiolo-
mnemonic processing, Ann. NY Acad. Sci. 769 (1995) 85–96.
gy 30 (1993) 98–119.
[41] M.I. Posner, Orienting of attention, Q. J. Exp. Psychol. 32 (1980)
[53] M.G. Woldorff, H.M. Fichteholtz, C.J. Hazlett, T. Tran, D.H.
Weissman, A.W. Song, G.R. Mangun, Functional parcellation of
[42] M.I. Posner, J.A. Walker, F.J. Friedrich, R.D. Rafal, Effects of
attentional control regions in the brain, submitted.
parietal injury on covert orienting of attention, J. Neurosci. 4 (1984)
[54] M.G. Woldorff, H.M. Fichtenholtz, T. Tran, D.H. Weissman, A.W.
Song, G.R. Mangun, Separation of cue- and target-related process-
[43] L.C. Robertson, M.R. Lamb, R.T. Knight, Effects of lesions of
ing in a fast-rate visual spatial attention cueing paradigm, Neuroim-
temporal–parietal junction on perceptual and attentional processing
age 13 (2001) S372.
in humans, J. Neurosci. 8 (1988) 3757–3769.
[44] R.D. Rogers, S. Monsell, Costs of a predictable switch between
simple cognitive tasks, J. Exp. Psychol. Gen. 124 (1995) 207–231.
Source: http://woldorfflab.org/pdfs/BR_PractEff_2002.pdf
Dystonia: A Guide to Good Practice for Health and Social Care Professionals The Dystonia Society 2nd Floor, 89 Albert Embankment London SE1 7TP Office: 0845 458 6211Email enquiries: [email protected]: www.dystonia.org.ukHelpline: 0845 458 6322Email helpline: [email protected] Registered Charity No. 1062595 and SC042127 Company limited by guarantee No. 3309777
Glucuronidated Quercetin Lowers Blood Pressure inSpontaneously Hypertensive Rats via Deconjugation Pilar Galindo1, Isabel Rodriguez-Go´mez2, Susana Gonza´lez-Manzano3, Montserrat Duen˜as3, Rosario Jime´nez1, Carmen Mene´ndez4,5, Fe´lix Vargas2, Juan Tamargo4, Celestino Santos-Buelga3, Francisco Pe´rez-Vizcaı´no4,5, Juan Duarte1* 1 Department of Pharmacology, School of Pharmacy, University of Granada, Granada, Spain, 2 Department of Physiology, School of Medicine, University of Granada,






