Doi:10.1016/j.gaitpost.2006.09.01

GAIPOS-2318; No of Pages 8
Gait & Posture xxx (2006) xxx–xxx
Long-term monitoring of gait in Parkinson's disease
Steven T. Moore ,, Hamish G. MacDougall , Jean-Michel Gracies
Helen S. Cohen William G. Ondo
a Department of Neurology, Mount Sinai School of Medicine, New York, NY 10029, USA
b Bobby R. Alford Department of Otolaryngology—Head and Neck Surgery, Baylor College of Medicine, Houston, TX, USA
c Department of Neurology, Baylor College of Medicine, Houston, TX, USA
Received 4 May 2006; received in revised form 21 July 2006; accepted 8 September 2006
A new system for long-term monitoring of gait in Parkinson's disease (PD) has been developed and validated. The characteristics of every
stride taken over 10-h epochs were acquired using a lightweight ankle-mounted sensor array that transmitted data wirelessly to a small pocketPC at a rate of 100 Hz. Stride was calculated from the vertical linear acceleration and pitch angular velocity of the leg with an accuracy of5 cm. Results from PD patients (5) demonstrate the effectiveness of long-term monitoring of gait in a natural environment. The small, variablestride length characteristic of Parkinsonian gait, and fluctuations of efficacy associated with levodopa therapy, such as delayed onset, wearingoff, and the ‘off/on' effect, could reliably be detected from long-term changes in stride length.
# 2006 Elsevier B.V. All rights reserved.
Keywords: Stride length; Levodopa; Parkinsonian; Locomotion; Accelerometer
medication response usually takes the form of a patientdiary, where the Parkinsonian state is noted as ‘on' (i.e.,
Parkinson's disease (PD) is a common neurodegenerative
effectively medicated), ‘off' or ‘on with dyskinesias'
disorder reflecting a progressive loss of dopaminergic and
However, self-reporting can be unreliable The
other sub-cortical neurons Levodopa, the metabolic
Unified Parkinson's Disease Rating Scale (UPDRS) ,
precursor to dopamine, has commonly been used to manage
although widely utilized in research studies has
the motor symptoms of PD for over 40 years by regenerating
significant limitations. Analysis of gait is limited to
depleted dopamine at the striatum. Although initially
assigning a single value between 0 (normal) and 4 (unable
effective, as the disease advances the duration of each dose
to walk, even with assistance) from brief clinical
shortens (the ‘wearing off' effect), necessitating more frequent
observation. Given the complexity of determining the
levodopa administration. In addition, the development of
optimal levodopa dosing schedule, a more objective means
dyskinesias (involuntary movements) and the ‘off/on'
of assessing gait over longer periods during normal daily
phenomenon (abrupt and unpredictable locomotor responses
life may significantly improve management of locomotor
to individual doses of levodopa) can limit mobility and
dysfunction in PD.
complicate dosing
Wrist or belt mounted accelerometers (activity monitors)
Typically, clinical evaluation involves brief observation
have been used for long-term monitoring of motor
during simple motor tasks, such as getting up out of a chair
fluctuations in PD although ‘on' and ‘off' phases
and walking a short distance. Assessment of long-term
cannot be reliably determined in individual subjects. A more‘brute-force' approach to accelerometry (six tri-axialaccelerometers; mounted on both upper arms, both upper
* Corresponding author at: Mount Sinai School of Medicine, Department
legs, the sternum and one wrist) could distinguish ‘on' and
of Neurology, Box 1135, 1 E 100th Street, New York, NY 10029, USA.
‘off' phases as well as dyskinesias from voluntary
Tel.: +1 212 241 9306; fax: +1 212 831 1610.
E-mail address: (S.T. Moore).
movements . However, the complexity and intrusive
0966-6362/$ – see front matter # 2006 Elsevier B.V. All rights reserved.
Please cite this article in press as: Moore ST, et al., Long-term monitoring of gait in Parkinson's disease, Gait Posture (2006), doi:

GAIPOS-2318; No of Pages 8
S.T. Moore et al. / Gait & Posture xxx (2006) xxx–xxx
nature of multiple body-segment accelerometry limits its use
years [38 (S.D. 7.7)], and height from 153 to 183 cm [167
outside of the research environment.
(S.D. 12.2)]. Seven participants diagnosed with idiopathic
Although gross body acceleration data can provide an
Parkinson's disease (three males and four females) were
objective alternative to periodic self reporting of motor state,
enrolled to verify measurement accuracy (2) and obtain pilot
it does not indicate the functional locomotor capacity of the
data (5) on the efficacy of long-term stride monitoring. Age
individual; i.e., how well the patient is walking. One of the
ranged from 65 to 85 years [72.0 (S.D. 7.4)], age at onset of PD
cardinal features of PD is locomotor dysfunction; shortened
from 40 to 79 years [57.7 (S.D. 13.3)], and height from 160 to
stride length, increased variability of stride
193 cm [172.1 (S.D. 11.8)]. The study was approved by the
shuffling gait, and freezing To characterize patholo-
Institutional Review Boards at the Mount Sinai School of
gical gait in the PD patient it is necessary to accurately
Medicine and Baylor College of Medicine and Affiliated
monitor stride length over extended periods. A number of
Hospitals, and was performed in accordance with the ethical
ambulatory systems have employed gyroscopes to measure
standards of the 1964 Declaration of Helsinki. Participants
the angular velocity of the thigh and/or shank, and integrated
gave informed consent prior to their inclusion in the study.
these waveforms to obtain the angular extent of leg swing,which when scaled by subject height yields an estimate of
stride length Stride length estimates were relativelyinaccurate, with an error of 15% . A more recent
The stride monitor consisted of two subsystems. A small
realization utilizing gyroscopes on the shank of both legs
Inertial Measurement Unit (IMU: 28 mm � 38 mm � 54
and a third gyroscope on the right thigh improved stride
mm; MT9, Xsens, Enschede, The Netherlands), with a 9 V
length accuracy to 7 cm, and was capable of logging for up
battery and Bluetooth serial transmitter (BL-819, RS232
to 2.5 h However, cables used to relay data from leg-
Bluetooth Converter, Brainboxes Ltd., Liverpool, United
mounted gyroscopes to a central logging unit create an
Kingdom), was mounted around the shank (just above the
unacceptable trip hazard and interfere with patients' normal
ankle) using an elasticized strap and Velcro. The IMU
daily activity, limiting their use in the community.
transduced 3D linear acceleration and angular velocity of the
In this paper, we describe a novel ambulatory system for
lower limb at a sample rate of 100 Hz. In addition, a Pocket PC
accurate measurement of every stride taken over extended
(iPAQ 2200, Hewlett Packard, Palo Alto, CA), worn in a small
periods (up to 10 h). Clinical features of PD, such as small,
pouch around the waist, acquired the leg movement data
variable stride length and fluctuations in motor performance
wirelessly (via Bluetooth) from the IMU within an effective
with levodopa administration, were well correlated with data
range of 100 m, and stored data files on a secure digital (SD)
obtained from the stride monitor. Long-term stride
flash memory card. The shank-mounted components (IMU,
monitoring may significantly improve pharmacological
battery and Bluetooth transmitter) weighed less than 130 g, or
management of PD symptoms, particularly in the advanced
less than 2% of the mass of the shank and foot , which
stages of the disease where abrupt and unpredictable
should not significantly affect movement of the lower limb.
responses to levodopa complicate dosing.
The PC weighed 146 g, and was slightly larger than a cellphone (119 mm � 77 mm � 16 mm). The stride monitor wasunobtrusive and did not interfere with the participant's normal
2.1. Research participants
2.3. Data processing
Ten healthy participants (five males and five females), with
Vertical linear acceleration and pitch angular velocity
no history of gait abnormalities, provided calibration and
(sagittal plane) of the shank were used to assess gait ().
validation of the stride monitor. Age ranged from 30 to 55
During upright stance there was a DC offset of 9.8 m/s2 in
Fig. 1. Vertical linear acceleration (dashed trace) and pitch angular velocity (solid trace) from the stride monitor during locomotion. The negative portion of theangular velocity trace corresponds to forward rotation of the leg during the swing phase.
Please cite this article in press as: Moore ST, et al., Long-term monitoring of gait in Parkinson's disease, Gait Posture (2006), doi:
GAIPOS-2318; No of Pages 8
S.T. Moore et al. / Gait & Posture xxx (2006) xxx–xxx
the vertical acceleration, and changes in this value were used
evaluated by two different techniques; (1) the pen technique
to distinguish periods where the participant was supine (see
described above, and (2) comparing stride monitor measures
A ‘moving' RMS trace of the vertical acceleration
with those obtained from a video motion analysis system
waveform was calculated using a sliding window of 2 s
tracking horizontal foot movement with an accuracy of
width Locomotor activity was defined as periods where
5 mm (Optitrack, NaturalPoint, Corvallis, OR).
the RMS acceleration was greater than 0.4 m/s2 abovebaseline According to the right-hand rule, negative
2.5. Stride monitoring of PD patients
pitch angular velocity corresponded to the forward rotationof the leg during the swing phase of locomotion ). An
Long-term stride monitoring (left leg) was performed on
initial stride length estimate (SLi) was calculated as follows:
five PD patients at the Baylor College of Medicine
Movement Disorders Clinic, Houston, TX. Stride length
data was collected in two participants over a period of
75 min in the clinic. For the other three participants, thestride monitor was activated at the clinic prior to patient's
where l is the length of the leg from the trochanter (hip joint)
departure and collected from their home after 6 h of data
to the ground, and a is the angular extent of the swing phase
acquisition during normal daily activity. They were also
(determined from integration of the angular velocity trace).
asked to keep a simple diary of activities and PD-related
Determining stride length from leg swing alone is reason-
medication administration at approximately 30-min inter-
ably accurate for small stride lengths (<1 m). However, this
technique underestimates larger strides due to the consider-able forward motion of the body over the stance foot inaddition to the component generated by leg swing. It was
therefore necessary to provide a calibration algorithm basedon the initial stride estimate to correct for longer strides.
Changes in stride length following levodopa adminis-
tration in participants with Parkinson's disease were
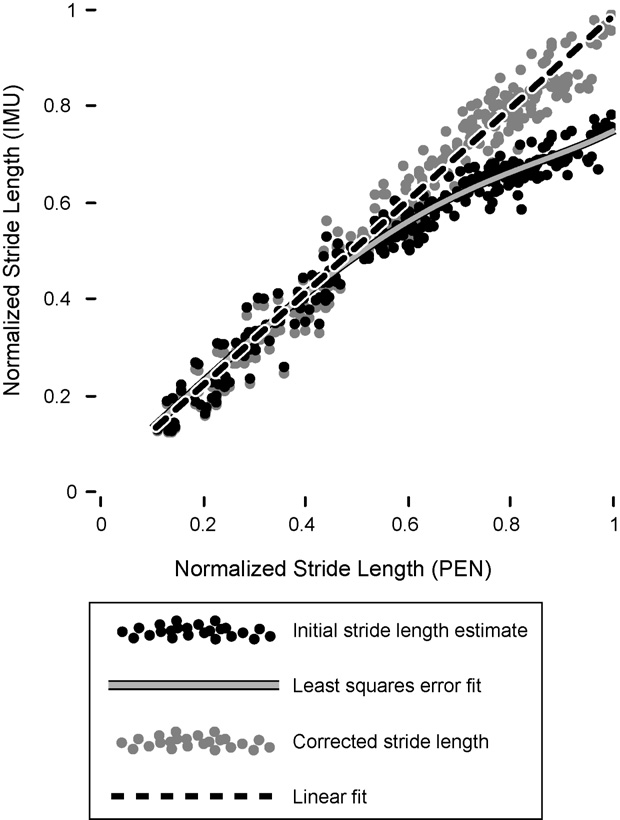
Ten healthy controls travelled 27.9 m (S.D. 1.8) over 27
assessed by fitting an exponential function to binned mean
strides (S.D. 3.6) while traversing the 30-m corridor. Plotting
stride data (each bin comprising 60 sequential strides) using
height-normalized true-versus-estimated stride lengths from
the Levenberg–Marquardt algorithm The time
the 10 controls revealed a non-linear but consistent
constant of the exponential rise or decay of stride length was
relationship, such that it was possible to generalize a
estimated from the best fit (see
calibration algorithm applicable to all participants ).
To correct for underestimation of large (>1 m) strides due to
forward motion of the body over the stance foot, a least-squares fit (Labview Advanced Analysis Package, National
The stride monitor was primarily calibrated using a direct
Instruments, Austin, TX) was applied to the height-
measure of stride length obtained from 10 healthy
normalized initial stride length estimates (SLni) (,
participants walking along a 30-m hallway. Healthy controls
solid black circles) of the form:
were utilized as it was necessary to acquire angular velocity
data over a wide range of stride lengths (�0.2–1.5 m) to
SLnc ¼ a0 þ a1 sin ðSL2 Þ þ a
23 cos ðSLniÞ þ
determine the calibration algorithm; varying stride length on
demand is beyond the capabilities of most PD patients,
particularly in the ‘off' state. An aluminum tube was taped tothe heel of the left shoe and a whiteboard marker inserted
where SLnc is the height-normalized corrected stride length
such that the tip left a single dot on the floor during each foot
solid grey circles), and the coefficients ai were
placement. Simultaneous estimates of stride length were
(�43.3, 21.9, 14.9, �1.4, 2.3). The resultant corrected stride
obtained from the stride monitor, also attached to the left leg.
length measures exhibited a highly linear relationship to
Actual stride length was determined from measurement of
true stride length (r = 0.98) (dashed black line). The
the distance between successive dots on the floor.
mean error was 2.8% (CI 1.1) of participant height (max-
Participants were instructed to walk at a natural pace but
imum error 9%), or 5 cm for the average participant height
to vary gait according to verbal commands to produce a
of 167 cm. The error per stride was also estimated by
range of stride lengths, including small shuffling steps
comparing the total distance traveled down the hallway
typical of Parkinson's disease. The pen technique was
(cumulative stride length of the true and corrected values)
chosen as it allowed calibration of the stride monitor over a
and dividing by the number of strides taken for each
wide range, was relatively accurate (�5 mm error), and
participant. Mean error was similar to that calculated from
facilitated calibration outside of the laboratory. Accuracy of
the height-normalized data at 4.8 cm (CI 1.1), with a max-
the device to monitor pathological (Parkinsonian) gait was
imum error of 8 cm.
Please cite this article in press as: Moore ST, et al., Long-term monitoring of gait in Parkinson's disease, Gait Posture (2006), doi:
GAIPOS-2318; No of Pages 8
S.T. Moore et al. / Gait & Posture xxx (2006) xxx–xxx
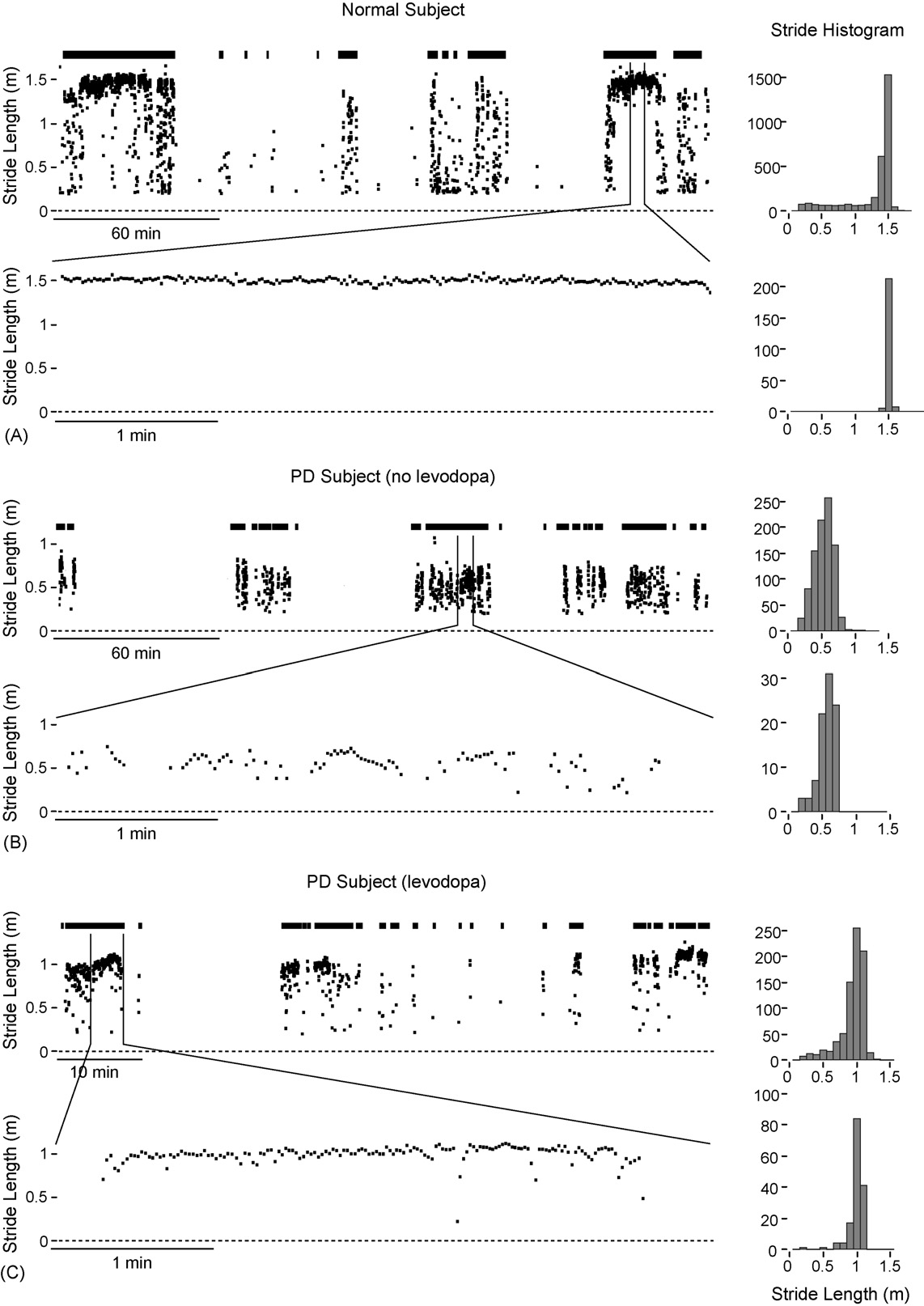
participant (37-year-old male) covered a total of 3.9 km with3071 strides, including two 1.5-km walks in an urbanenvironment (Manhattan) at the start and end of the epochupper trace). In the intervening period theparticipant walked periodically while working in alaboratory. Stride length was stable at 1.5 m, as indicatedby the stride histogram, consistent with the typical value foradult males This is clearly seen in the 4 min of stridedata while walking home (A, lower trace). In contrast,4 h of data from a PD patient (85-year-old female, age atonset 79 years) during normal daily activities outside of theclinic demonstrates the cardinal features of Parkinsoniangait; namely a small (�0.5 m), highly variable stride lengthB, upper trace and histogram), covering a distance of492 m with 923 strides. Note that this particular patient wasnot prescribed levodopa at the time of testing. Stride datafrom a well-managed PD patient (65-year-old female, age atonset 51 years) in the ‘on' phase approximately 2 h afterlevodopa administration (levodopa 150 mg, pramipexole1.5 mg) demonstrates the effectiveness of dopamine-replacement therapy (Over a 75-min period inthe clinic stride length was relatively stable at �1 m asobserved in a 4-min interval lower trace) as theparticipant walked along a corridor (although still less thanthe mean value of 1.3 m for adult females ).
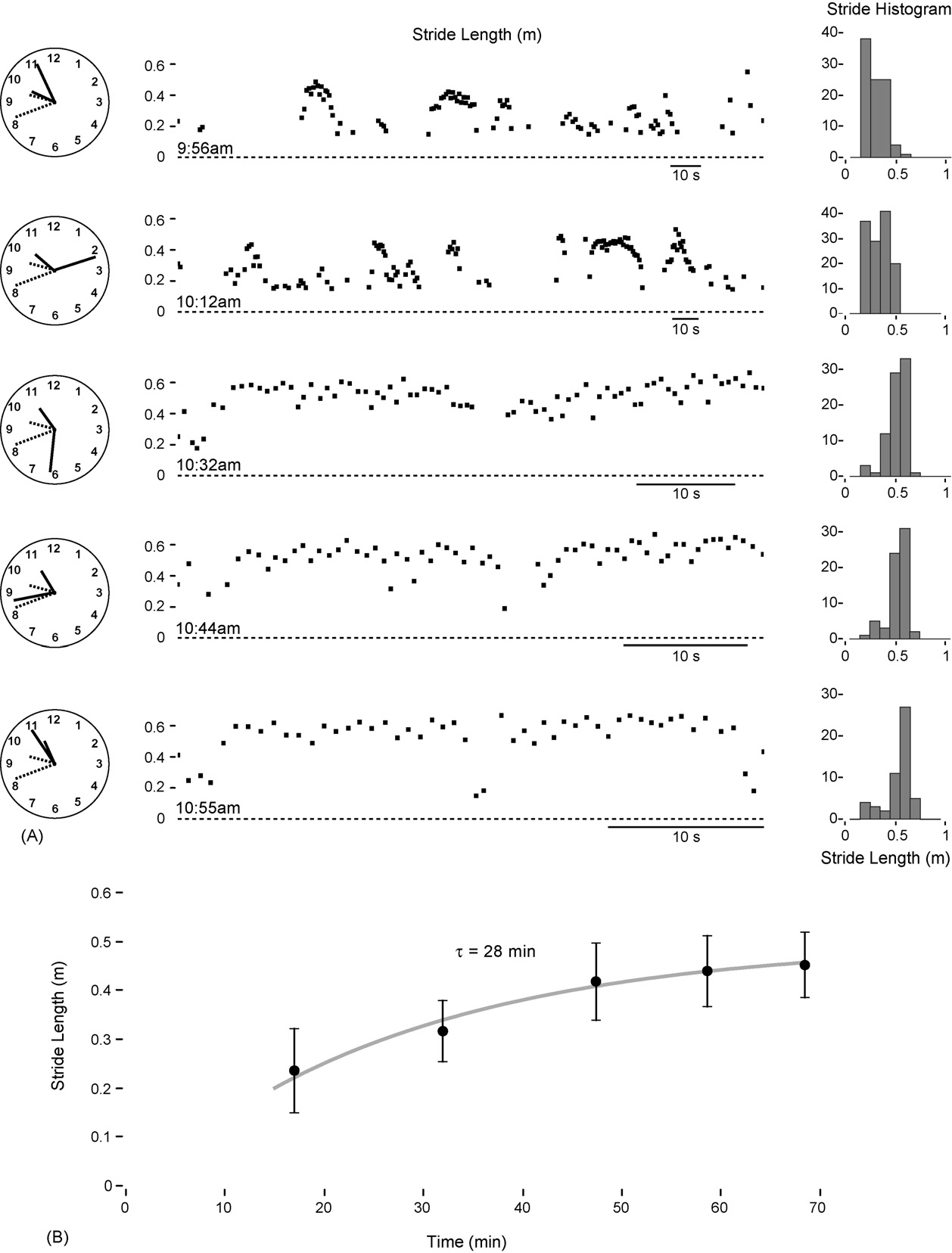
A standard dose of levodopa typically becomes effective
Fig. 2. True (pen) versus estimated (stride monitor) stride length from 10healthy controls, normalized by height (filled black circles). A general least-
20–40 min after drug ingestion , although onset can be
square error fit (grey trace) to these data was used to derive a calibration
considerably delayed and inconsistent in patients with
algorithm that produced a highly linear relationship with actual stride values
advanced PD. The effect of levodopa on stride length was
(black dashed line).
monitored in the clinic (during intermittent 30-m walks alonga corridor) in an advanced PD patient (66-year-old male, age
Stride data obtained from two PD participants in the ‘off'
at onset 40 years). Over a period of 75 min post-adminis-
state (no dopaminergic medication in the previous 12 h)
tration (levodopa 100 mg, pramipexole 0.5 mg) stride length
demonstrated similar measurement accuracy. A participant
increased (and variability decreased) from 24 cm (S.D. 9) to
with a relatively mild form of PD (69-year-old female, age at
45 cm (S.D. 6) A). Freezing occurred up to 30 min post-
onset 59 years, height 173 cm) walked a distance of 4.5 m
medication, but had ceased by 50 min. The time constant of
(five strides) and simultaneous pen and stride monitor
levodopa onset (28 min) was estimated from an exponential fit
measures of stride length (left leg) were obtained. Average
to the mean stride data B).
stride length was 90.1 cm (pen) and 89.2 cm (stride
The levodopa cycle, characterized by changes in stride
monitor). Mean difference was 3.3 cm (maximum error
length, was also assessed from long-term monitoring in the
8 cm). A second participant (68-year-old male, age at onset
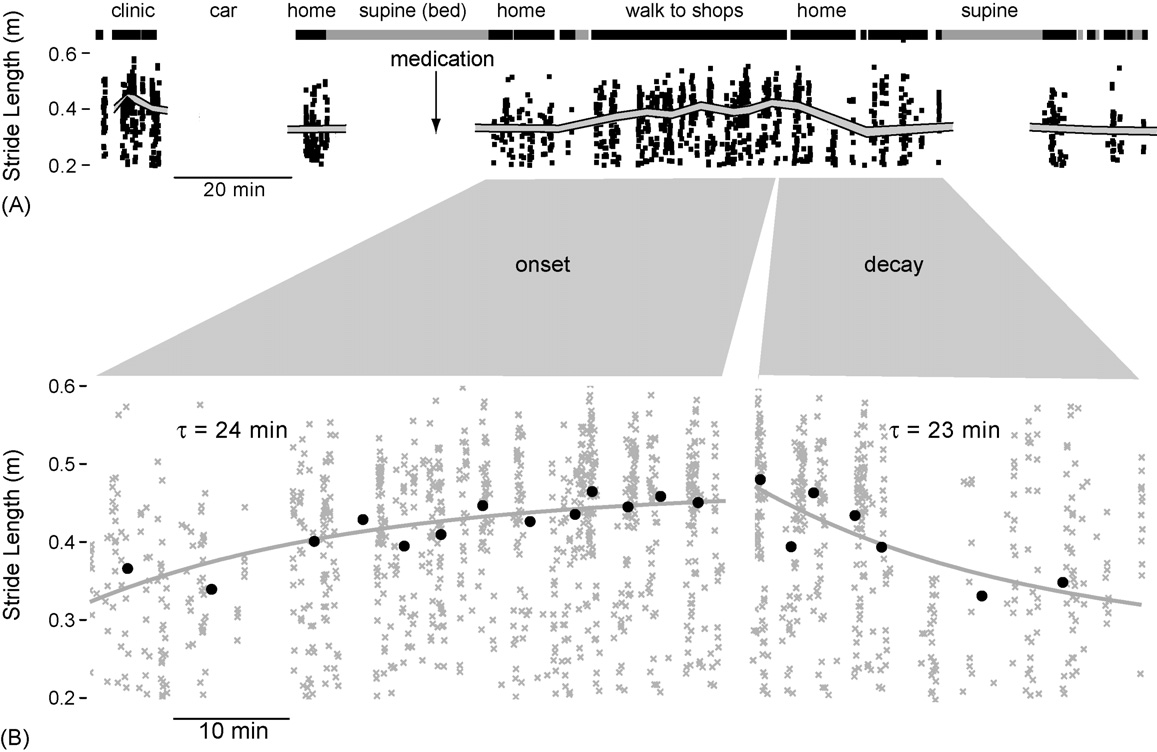
community. A participant with advanced PD (79-year-old
52 years, height 168 cm) with severe locomotor impairment
female, age at onset 69 years) wore the stride monitor for 6 h
traversed a distance of 89 cm utilizing small shuffling steps
following a morning clinic visit. Stride length was
(seven strides). Stride length (left leg) was measured using
decreasing at the clinic as the patient came off a morning
the stride monitor, and from a post hoc video motion analysis
dose of levodopa (levodopa 100 mg, ropinirole 2 mg). The
of the horizontal displacement of the left foot. Average stride
patient went to bed shortly after being driven home
length was 12.7 cm (video analysis) and 10.4 cm (stride
Approximately 10 min prior to getting out of
monitor); mean difference was 2.5 cm (maximum 4.7 cm).
bed the participant took a second dose of levodopa (levodopa
Thus, at two extremes of locomotor impairment in the PD
100 mg, ropinirole 2 mg) then walked to a local shopping
‘off' state, the accuracy of the stride monitor was within that
mall. Stride length increased steadily over 60 min following
established in the 10 healthy controls.
levodopa administration, and then declined as the participantwalked home A). The time constants of the onset and
3.2. Monitoring of gait in Parkinson's disease
decay of levodopa B) were estimated at 24 and23 min, respectively, using an exponential fit to the mean
illustrates the differences between healthy and
binned stride data (each bin comprising 60 sequential
Parkinsonian gait over extended periods. Over 4 h a healthy
Please cite this article in press as: Moore ST, et al., Long-term monitoring of gait in Parkinson's disease, Gait Posture (2006), doi:
GAIPOS-2318; No of Pages 8
S.T. Moore et al. / Gait & Posture xxx (2006) xxx–xxx
Fig. 3. (A) Four hours of stride data from a healthy participant. (B) Four hours of stride data from an unmedicated (i.e., no levodopa) PD patient during naturaldaily activity outside of the clinic. (C) Stride data (75 min of intermittent walking around the clinic) from a well-managed PD patient in the ‘on' phaseapproximately 2 h post levodopa administration.
Please cite this article in press as: Moore ST, et al., Long-term monitoring of gait in Parkinson's disease, Gait Posture (2006), doi:
GAIPOS-2318; No of Pages 8
S.T. Moore et al. / Gait & Posture xxx (2006) xxx–xxx
Fig. 4. The transition from ‘off' to ‘on' following levodopa administration was assessed in the clinic in a participant with advanced PD. (A) Stride data fromperiodic walking along a corridor of length 15 m (up and back) following levodopa administration at 9:41 a.m. (B) The time constant (t) of the onset of levodopawas estimated at 28 min using an exponential fit to the mean stride data.
Please cite this article in press as: Moore ST, et al., Long-term monitoring of gait in Parkinson's disease, Gait Posture (2006), doi:
GAIPOS-2318; No of Pages 8
S.T. Moore et al. / Gait & Posture xxx (2006) xxx–xxx
Fig. 5. The effect of levodopa administration during natural daily activities outside of the clinic. (A) Three hours of activity (mean and 90%CI of stride length,plus individual values); thick black lines above the stride data indicate locomotion; thick grey lines show periods where the participant was supine. (B) Anexponential fit to binned mean stride length was used to estimate the time constant of onset (24 min) and decay (23 min) of levodopa.
setting eliminates this confound and exhibits greatersensitivity to the dynamic effects of dopamine replacement
The results of this study demonstrate the feasibility of
therapy on stride length.
accurate stride length measurement using a single shank-
Locomotor impairment is one of the cardinal features of
mounted stride monitor, and the applicability of this
PD but certainly not the only one. Many other PD symptoms,
technique to long-term monitoring of gait in Parkinson's
such as rigidity, difficulty swallowing, stooped posture,
disease. Improved accuracy (mean error 5 cm), relative to
olfactory dysfunction, and upper-body tremor and dyski-
previous techniques utilizing both single (15% error)
nesias, cannot be detected with the stride monitor; however,
and multiple (7 cm error) gyroscopes , was obtained
no objective measures of these indicators are routinely used
using a combined accelerometer/gyroscope sensor array and
in the clinic. Tremor can readily be measured with an
a calibration algorithm to account for the forward motion of
accelerometer but provides limited sensitivity to motor
the body over the stance foot. The stride monitor is small and
complications in PD patients . The complexity of
unobtrusive, and did not interfere with natural daily
identifying ‘off' and ‘on' states and upper-body dyskinesias
activities during extended monitoring of gait outside of
(requiring six triaxial accelerometers ) effectively
curtails its use outside of the research environment. Despite
Stride data obtained from PD patients demonstrated
these recent attempts at objectivity, the essentially subjective
many facets of Parkinsonian gait, such as small stride length
UPDRS remains the current standard of PD assessment.
and larger stride-to-stride variability. Fluctuations of
Clinicians typically see a ‘snapshot' of the patient's
efficacy associated with levodopa therapy, such as delayed
motor state and management of PD often involves a trial and
onset, wearing off, and the ‘off/on' effect, could also be
error approach, relying heavily on the patient's subjective
detected from long-term changes in stride length. The time
feedback to optimize the levodopa dosage regime. Objective
constants of onset and decay of levodopa were estimated
long-term data obtained from stride monitoring may provide
from stride length data acquired both at the clinic and in the
a faster and more valid end-point.
real world. In contrast, a previous laboratory study periodically assessed gait on a fixed 7-m walkway over thelevodopa cycle and found no consistent changes in stride
length, likely due to the contrived nature of the laboratorywalking task that can temporarily enhance performance in
This work was supported by NASA grant NNJ04HF51G
PD patients . Long-term gait assessment in a community
(Steven Moore).
Please cite this article in press as: Moore ST, et al., Long-term monitoring of gait in Parkinson's disease, Gait Posture (2006), doi:
GAIPOS-2318; No of Pages 8
S.T. Moore et al. / Gait & Posture xxx (2006) xxx–xxx
[15] Hausdorff JM, Schaafsma JD, Balash Y, Bartels AL, Gurevich T,
Giladi N. Impaired regulation of stride variability in Parkinson's
[1] Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in
disease subjects with freezing of gait. Exp Brain Res 2003;149:
the development of Parkinson's disease-related pathology. Cell Tissue
[16] Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL.
[2] Ondo W. Investigational pharmacological treatments for Parkinson's
Gait variability and basal ganglia disorders: stride-to-stride variations
In: Pahwa R, Lyons K, Koller W, editors. Handbook of
of gait cycle timing in Parkinson's disease and Huntington's disease.
Parkinson's disease. New York: Marcel Dekker; 2003.
Mov Disord 1998;13:428–37.
[3] Brown RG, MacCarthy B, Jahanshahi M, Marsden CD. Accuracy of
[17] Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of
self-reported disability in patients with parkinsonism. Arch Neurol
gait in Parkinson's disease: a review of two interconnected, episodic
phenomena. Mov Disord 2004;19:871–84.
[4] Golbe LI, Pae J. Validity of a mailed epidemiological questionnaire
[18] Miyazaki S. Long-term unrestrained measurement of stride length and
and physical self-assessment in Parkinson's disease. Mov Disord
walking velocity utilizing a piezoelectric gyroscope. IEEE Trans
Biomed Eng 1997;44:753–9.
[5] Goetz CG, Stebbins GT, Blasucci LM, Grobman MS. Efficacy of a
[19] Tong K, Granat MH. A practical gait analysis system using gyro-
patient-training videotape on motor fluctuations for on-off diaries in
scopes. Med Eng Phys 1999;21:87–94.
Parkinson's disease. Mov Disord 1997;12:1039–41.
[20] Aminian K, Najafi B, Bula C, Leyvraz PF, Robert P. Spatio-temporal
[6] Fahn S, Elton RL. Members of the UPDRS Development Committee.
parameters of gait measured by an ambulatory system using miniature
In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Unified
gyroscopes. J Biomech 2002;35:689–99.
Parkinson's Disease Rating Scale. Recent Developments in Parkin-
[21] Salarian A, Russmann H, Vingerhoets FJ, Dehollain C, Blanc Y,
son's disease. Florham Park: Macmillan Healthcare Information;
Burkhard PR, et al. Gait assessment in Parkinson's disease: toward
1987 . p. 153–63. 293–304.
an ambulatory system for long-term monitoring. IEEE Trans Biomed
[7] Mitchell SL, Harper DW, Lau A, Bhalla R. Patterns of outcome
measurement in Parkinson's disease clinical trials. Neuroepidemiol-
[22] US Navy. Anthropometry and mass distribution for human analogues
New Orleans: Naval Biodynamics Laboratory; 1988.
[8] van Hilten JJ, Middelkoop HA, Kerkhof GA, Roos RA. A new
[23] MacDougall HG, Moore ST. Marching to the beat of the same
approach in the assessment of motor activity in Parkinson's disease.
drummer: the spontaneous tempo of human locomotion. J Appl
J Neurol Neurosurg Psychiatry 1991;54:976–9.
[9] Saito N, Yamamoto T, Sugiura Y, Shimizu S, Shimizu M. Lifecorder: a
[24] Levenberg K. A Method for the solution of certain problems in least
new device for the long-term monitoring of motor activities for
squares. Quart Appl Math 1944;2:164–8.
Parkinson's disease. Intern Med 2004;43:685–92.
[25] Marquardt D. An algorithm for least-squares estimation of nonlinear
[10] Hoff JI, van den Plas AA, Wagemans EA, van Hilten JJ. Accelero-
parameters. SIAM J Appl Math 1963;11:431–41.
metric assessment of levodopa-induced dyskinesias in Parkinson's
[26] Murray MP, Drought AB, Kory RC. Walking patterns of normal men. J
disease. Mov Disord 2001;16:58–61.
Bone Joint Surg 1964;46A:335–60.
[11] Hoff JI, van der Meer V, van Hilten JJ. Accuracy of objective ambulatory
[27] Murray MP, Kory RC, Sepic SB. Walking patterns of normal women.
accelerometry in detecting motor complications in patients with Par-
Arch Phys Med Rehabil 1970;51:637–50.
kinson's disease. Clin Neuropharmacol 2004;27:53–7.
[28] Chana P, Kuntsmann C, Reyes-Parada M, Saez-Briones P. Delayed
[12] Keijsers NL, Horstink MW, Gielen SC. Ambulatory motor assessment
early morning turn ‘‘ON'' in response to a single dose of levodopa in
in Parkinson's disease. Mov Disord 2006;21:34–44.
advanced Parkinson's disease: pharmacokinetics should be consid-
[13] Keijsers NL, Horstink MW, Gielen SC. Automatic assessment of
ered. J Neurol Neurosurg Psychiatry 2004;75:1782–3.
levodopa-induced dyskinesias in daily life by neural networks. Mov
[29] MacKay-Lyons M. Variability in spatiotemporal gait characteristics
over the course of the L-dopa cycle in people with advanced Parkin-
[14] Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff
son's disease. Phys Ther 1998;78:1083–94.
JM. Gait dynamics in Parkinson's disease: relationship to Parkinsonian
[30] Yekutiel MP. Patients fall records as an aid in designing and assessing
features, falls and response to levodopa. J Neurol Sci 2003;212:47–53.
therapy in Parkinsonism. Disabil Rehabil 1993;15:189–93.
Please cite this article in press as: Moore ST, et al., Long-term monitoring of gait in Parkinson's disease, Gait Posture (2006), doi:
Source: http://www.xsens.biz/images/stories/PDF/PD_gait_monitor.pdf
Information Critical Care Management of the Adult Patient In Ireland with Ebola Virus Disease 2014 / 2015 Report of : Critical Care Advisory Group on Ebola Virus Disease Intensive Care Society of Ireland Interim Guidelines Update 4th January 2015 (to be updated with evolving international guidelines)
A survey of tobacco dependence treatment guidelines and systems in 45 countries Martin Raw 1, 2 and Catherine Slevin 2 1 Freelance consultant; Special Lecturer; Manage2 Division of Epidemiology and Public Health, University of Nottingham, England Sao Paulo and Nottingham Friday 7 December 2007 Contents1 Key messages.32 Introduction.5 3 Methods.74 Results of guidelines survey.95 Results of treatment survey.186 Summary of results.307 Discussion and conclusions.358 Recommendations.379 Acknowledgements.3810 References.3911 Appendices.40







