Plant uptake of pharmaceutical and personal care products from recycled water and biosolids: a review


Contents lists available at
Science of the Total Environment
Plant uptake of pharmaceutical and personal care products from recycled
water and biosolids: a review
Xiaoqin Wu Laurel K. Dodgen, Jeremy L. Conkle, Jay Gan
Department of Environmental Sciences, University of California, Riverside, CA, USA
• We provide an in-depth and up-to-date
overview of the plant uptake of PPCPs.
• We review analytical methods, processes,
mechanisms, and metabolism.
• We identify "priority" PPCPs with a rel-
atively high potential for plant uptake.
• Knowledge gaps and future research
needs are also proposed.
Reuse of treated wastewater for agricultural irrigation is growing in arid and semi-arid regions, while increasing
Received 12 April 2015
amounts of biosolids are being applied to fields to improve agricultural outputs. These historically under-utilized
Received in revised form 25 July 2015
resources contain "emerging contaminants", such as pharmaceutical and personal care products (PPCPs), which
Accepted 26 July 2015
may enter agricultural soils and potentially contaminate food crops. In this review, we summarize recent
Available online xxxx
research and provide a detailed overview of PPCPs in the soil–plant systems, including analytical methods for
Editor: Eddy Y. Zeng
determination of PPCPs in plant tissues, fate of PPCPs in agricultural soils receiving treated wastewater irrigationor biosolids amendment, and plant uptake of PPCPs under laboratory and field conditions. Mechanisms of uptake
and translocation of PPCPs and their metabolisms in plants are also reviewed. Field studies showed that the
concentration levels of PPCPs in crops that were irrigated with treated wastewater or applied with biosolids
Treated wastewater
were very low. Potential human exposure to PPCPs through dietary intake was discussed. Information gaps
and questions for future research have been identified in this review.
2015 Elsevier B.V. All rights reserved.
Plant uptakeHealth risks
⁎ Corresponding author at: Department of Environmental Sciences, University of California, Riverside, 900 University Ave., Riverside, CA 92521, USA.
E-mail address: (X. Wu).
0048-9697/ 2015 Elsevier B.V. All rights reserved.
X. Wu et al. / Science of the Total Environment 536 (2015) 655–666
Analytical methods for determining PPCPs in plant tissues . . . . . . . . . . . . . . . . . . . . .
PPCPs in soil receiving treated wastewater irrigation or biosolids amendment
Fate of PPCPs in soil
Factors affecting the degradation of PPCPs in soil . . . . . . . . . . . . . . . . . . . . .
PPCPs in plants grown under hydroponic conditions . . . . . . . . . . . . . . . . . . . . .
Mechanisms of uptake and translocation of PPCPs in plants
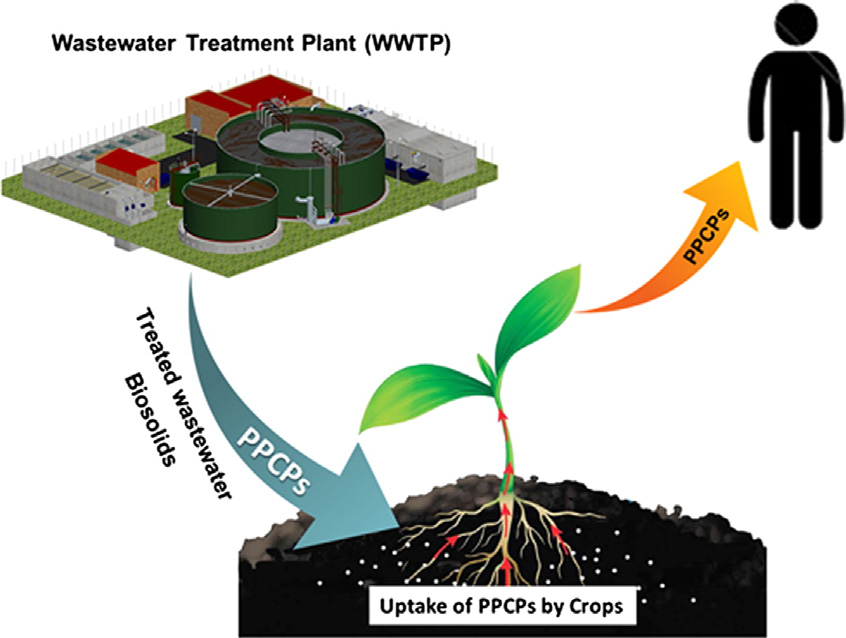
antimicrobial agents triclosan and triclocarban are the most widely de-tected in wastewater and are found to be enriched in biosolids with
Treated wastewater and biosolids, the products of municipal waste-
levels up to 10–40 mg/kg dry weight (
water treatment, have historically been underutilized
). While the effects of environmental PPCPs on terres-
). However, the need to preserve dwindling potable water
trial ecosystems and human health are far from clear, numerous studies
resources and to explore environmentally and economically beneficial
have shown that some PPCPs may exert adverse effects on aquatic ani-
uses of these "waste streams" has led society to reevaluate our disposal
mals such as tadpoles, mussels, and fish at very low levels
of these two resources. As water scarcity is exacerbated by climate
change, population growth, and rapid urbanization, the beneficial
lute concentrations of oxazepam, an anxiolytic drug, was reported to
reuse of treated wastewater for crop irrigation is crucial for relieving
alter behavior of wild fish in the natural aquatic environment
the enormous pressure on water supplies around the globe, especially
in arid and semi-arid regions . In some water-stressed
A major public concern regarding agricultural applications of treated
areas such as California and Israel, treated wastewater reuse is expected
wastewater and biosolids is the introduction of contaminants such as
to increase 2–3 times in the near future (
PPCPs from these "waste streams" to crops via plant uptake. Contamina-
. Along with the population increase and urbanization,
tion of food by these chemicals may pose potential health risks to
the production of treated sewage sludge, or biosolids, has also been on a
humans. Plant uptake of PPCPs has received an increasing amount of
rapid rise, making the issue of disposal and recycling of the biosolids
attention over the last decade, as evidenced from a rapid growth in
important. In the U.S., land application of biosolids currently takes
the number of peer-reviewed publications addressing this issue in
place in all 50 states, with about 50% of all biosolids being recycled to
recent years. To date two review papers have appeared on this subject
land to promote the growth of agricultural crops, fertilize gardens and
matter (Because of the rapid
parks, and reclaim mining sites ). This use is expected
development in this research area, much more information has become
to further increase as traditional disposal options (e.g., landfill) become
available since then.
limited or prohibitively expensive.
In this review, we provide an in-depth and up-to-date overview of
While the use of treated wastewater and biosolids presents multiple
the plant uptake of PPCPs in connection to the use of treated wastewater
economic and environmental benefits, its broad agricultural implemen-
and biosolids in agriculture, including analytical methods for the deter-
tation is faced with a new challenge: the perceived food safety risk from
mination of PPCPs in plant tissues, processes that may affect the fate and
the so-called emerging contaminants, particularly pharmaceutical and
bioavailability of PPCPs in soil, uptake and accumulation of PPCPs from
personal care products (PPCPs) that have been found ubiquitously in
nutrient solutions or soil to plants, mechanisms regulating plant uptake
these resources. PPCPs are a diverse array of chemical substances,
and translocation of different PPCPs, and metabolism of PPCPs in plants.
including over-the-counter and prescription drugs for human and vet-
A primary goal of this review is to identify "priority" PPCPs that have
erinary uses, and products that are used by individuals for personal
shown a relatively high potential for plant uptake. The probable
health or cosmetic purposes. The increasing use of prescription drugs
human exposure by consuming crops grown with treated wastewater
and personal care products, coupled with the incomplete removal of
irrigation or biosolids amendment is also discussed. In addition,
PPCPs by municipal wastewater treatment systems, has led to the
knowledge gaps and future research needs are proposed.
widespread occurrence of these chemicals in treated wastewater andbiosolids (). Among the most frequently occurring
2. Analytical methods for determining PPCPs in plant tissues
drug classes in treated wastewater and biosolids are antibiotics, non-steroidal anti-inflammatories, and anti-convulsants, with concentra-
2.1. Extraction and cleanup
tions between ng/L to low μg/L in treated wastewater () and μg/kg to low
Compared to the analysis of PPCPs in environmental samples such as
mg/kg (dry weight) in biosolids
water, soil, or sediment, detection and quantification of PPCPs in plant
Among personal care products, the
tissues present additional challenges due to the presence of pigments,
Table 1Analytical methods used for determination of PPCPs in plant tissues. ASE: accelerated solvent extraction; d.w.: dry weight; ESI: electrospray ionization; GC: gas chromatography; LC: liquid chromatography; LLE: liquid–liquid extraction; LOD: limit ofdetection; LOQ: limit of quantitation; MS: mass spectrometry; PFE: pressurized fluid extraction; PLE: pressurized liquid extraction; SLE: solid–liquid extraction; SPE: solid-phase extraction; UV: ultraviolet; w.w.: wet weight.
Extracting solvent
Clean-up method Instrumental
Method detection limits
Methanol/HCl (95/5), acetone
Corn, lettuce, potato
Hexane/ethyl acetate (1/1)
Triclosan, triclocarban
T. latifolia, P. cordata, and
4–17 ng/g w.w. (LOD)
Triclosan, triclocarban
Radish, carrot, soybean
2.5–2.8 (LOD), 8.6–9.6 (LOQ) ng/g d.w.
Methanol/acetone (1/1)
0.28 (LOD), 1 (LOQ) ng/g
Carbamazepine, diclofenac, fluoxetine, propranolol,
2–11 ng/g d.w. (LOQ)
HCl-KCl buffer solution (pH = 2)
Carbamazepine, ibuprofen
10–20 (LOD), 20–75 (LOQ)
Methanol/HCl, ammonium
Metformin, ciprofloxacin, narasin
15–50 (LOD), 30–100 (LOQ)
acetate/formic acid; Methanol;
Ammonium acetate/formic acid
Acetonitrile with 1% acetic acid
Cucumber, lettuce, bean, radish LLE
Acetonitrile with 1% formic acid
Methanol/HCl (1/1), acetone
Radish, rape, celery, cilantro
0.5–1.5 (LOD), 1.8–4 (LOQ)
chloramphenicol, oxytetracycline, tetracycline,
chlortetracycline, lincomycin, ofloxacin, ciprofloxacin,
Methanol, acetone
Salbutamol, atenolol, lincomycin, cyclophosphamide,
E. sativa L. and Z. mays L.
0.03–0.09 (LOD), 0.06–1.25
carbamazepine, bezafibrate, ofloxacin, ranitidine
(LOQ) ng/g w.w.
Acidified acetonitrile/acetone (1/1)
Tetracycline, sulfamethazine, norfloxacin,
Chinese white cabbage, water
0.8–4.4 ng/g d.w. (LOQ)
spinach, Chinese radish, corn,
Aqueous buffered acetonitrile,
118 PPCPs or PPCP transformation products
Sweet corn, carrot, tomato,
0.12–1403 ng/g d.w. (LOD)
Methyl tertiary butyl ether (MTBE),
Lettuce, spinach, pepper,
0.04–3.0 ng/g d.w. (LOD)
Acetonitrile, methanol with 0.5%
Carbamazepine, ketoprofen, diclofenac, indomethacin,
1.17–6.57 (LOD), 2.73–15.4
(LOQ) ng/g w.w.
sulfamethoxazole, sulfadimethoxine, crotamiton,
gliclazide, losartan, cyclophosphamide,
Acetone/hexane (1/1), ethyl
Ibuprofen, carbamazepine, diclofenac, clofibric acid,
6.6–58.1 (LOD), 7.6–61.7
acetate/hexane (2/1, 1/1)
triclosan, tonalide, nonylphenol, naproxen, hydrocinnamic
(LOQ) ng/g w.w.
MTBE/methanol (90/10), Methanol
Clarithromycin, Azithromycin, roxithromycin,
Bell pepper, bermuda grass,
with 1% acetic acid
cantaloupe, carrot, lettuce,
Methamphetamine, ecstasy, pseudoephedrine
Acetonitrile/water (55/45, 85/15)
Trimethoprim, salbutamol, sulfamethoxazole,
Cabbage, Wisconsin fast plants
1.75–23.04 ng/g w.w. (LOD)
carbamazepine, triclosan, sertraline
Carbamazepine, diphenhydramine, fluoxetine,
Soybean, pepper, collard,
0.10–4.89 ng/g d.w. (LOD)
triclosan, triclocarban
lettuce, radish, tomato
Ketoprofen, naproxen, diclofenac, ibuprofen
Bezafibrate, carbamazepine, diclofenac, gemfibrozil,
Carrot, sweet potato
0.1–1 (LOD), 0.1–5 (LOQ)
ibuprofen, clofibric acid, sulfapyridine, ketoprofen,
Triclosan, triclocarban
Pumpkin, zucchini, and
0.1–1 ng/g d.w. (LOD)
a n/a: not available.
X. Wu et al. / Science of the Total Environment 536 (2015) 655–666
fats, and waxy materials that can result in severe matrix interferences.
presence and concentrations in plant tissues, the mild solvents
Therefore, analytical precision and accuracy is highly dependent on
commonly used do not fully recover antibiotics from plant materials,
effective sample preparation procedures, including compound extrac-
as harsher solvents may not be compatible with ELISA analysis
tion and cleanup, to eliminate interferences while ensuring maximum
(At present, liquid chromatography (LC) in com-
bination with mass spectrometry (MS) is the primary instrument used
The extraction techniques that have been used for extracting PPCPs
to analyze multiple PPCPs in plant tissue extracts ). Methanol
from plant tissues include traditional solid–liquid extraction (SLE), son-
and acetonitrile are usually used as the organic mobile phase,
ication, and accelerated solvent extraction (ASE). ASE is sometimes also
where 0.001-0.1% formic acid or acetic acid is often added to improve
termed "pressurized liquid extraction (PLE)", or "pressurized fluid
LC performance and MS detection sensitivity. Gas chromatography
extraction (PFE)". Sea sand disruption method (SSDM) was also used
(GC)–MS has also been used by several researchers for analyzing acidic
by a few researchers. The published methods used to date for analyzing
PPCPs (e.g., triclosan, ibuprofen, diclofenac, clofibric acid), but
PPCPs in plant tissues are shown in
derivatization using derivatization agents such as trimethylsulfonium
In a basic SLE procedure, PPCPs are extracted from plant tissue sam-
hydroxide (TMSH), bis(trimethylsilyl)trifluoroacetamide (BSTFA),
ples by using polar solvent mixtures via manual or mechanical shaking.
and trimethylchlorosilane (TMCS) is needed before the analysis
In most cases, acids, buffers or salts are added to the extracting solvents
(The MS system is
to enhance the transfer of analytes into the solvent phase. However,
usually equipped with electrospray ionization (ESI) as it pro-
shaking is not always efficient for extracting various types of PPCPs.
vides better sensitivity than atmospheric pressure chemical ionization
Sonication, which uses ultrasonic vibration to ensure high proximity
(APCI) for pharmaceuticals such as cytotoxic drugs cyclophosphamide
between the sample and solvent, is more widely used due to better
and ifosfamide ).
extraction efficiency, low cost, easy operation, and high samplethroughput. ASE uses solvents at high temperatures (80–180 °C) and
3. PPCPs in soil receiving treated wastewater irrigation or
pressures (100–140 bar) to enhance the extraction of chemicals from
biosolids amendment
solid samples. However, the harsh conditions in ASE may also result inmore matrix components than SLE or sonication, which may contribute
When treated wastewater or biosolids is applied to soil, PPCPs may
to increased matrix effects during instrumental analysis. In addition,
be introduced into the soil environment and appear in the μg/kg con-
compounds that are thermally unstable may degrade during ASE
centration range in soil (
extraction (. The method detection limits
. Irrigation of soil with treat-
(MDLs), including limit of detections (LODs) and limit of quantitations
ed wastewater may cause accumulation of PPCPs to much higher levels
(LOQs) are summarized in Generally, sonication and ASE pro-
in soil than in the irrigation water. For example, erythromycin and car-
vide lower MDLs than SLE, suggesting higher extraction efficiencies of
bamazepine were found to accumulate in soil amounting to 305–4060
these two methods.
and 274–1260 times that in the source irrigation water, indicating that
For sample cleanup, liquid–liquid extraction (LLE) with hexane
these compounds were retained and carried over from previous
was used to remove undesirable hydrophobic compounds, such as
irrigation events
chlorophyllic, fatty and waxy materials ).
Biosolids are applied to land less frequently than irrigation water,
However, this cleanup procedure may also remove PPCPs with high or
partly due to the rich nutrients, elevated contents of metals and odors
medium hydrophobicity and should only be used when the target com-
in biosolids that may cause negative impacts on water, soil, and air.
pounds are highly hydrophilic, such as polar antibiotics (
For agricultural land, biosolids are typically applied annually or up to 3
). Due to the wide range of hydrophobicity
times per year ). Therefore, in biosolids-amended soil,
of PPCPs, solid-phase extraction (SPE) has emerged as the method of
more time is available for PPCP degradation to take place between
choice as a clean-up procedure In SPE, hydrophilic–lipophilic-
input events. Due to dilution and degradation, levels of PPCPs in bio-
balanced (HLB) cartridges are frequently employed to clean up plant ma-
solids-amended soils are lower than those in the amendment material.
reported that in soils that had received biosolids appli-
For ionic PPCPs, ion-exchange SPE cartridges have
cations for 33 years, levels of triclocarban, triclosan, and nonylphenol in
also been tested for sample clean-up. proposed
the surface soil layer (0–15 cm) were 1.25, 0.052, 8.83 mg/kg (dry
the use of two sequential cartridges (strong-anion exchange cartridge
weight), respectively, at the cumulative loadings of 2218 tons dry bio-
and polymeric phase cartridge) to clean up cauliflower extract for fluoxe-
solids/ha, while levels in the applied biosolids were 0.23–80, 0.33–61,
tine analysis.
4.85–1380 mg/kg (dry weight), respectively, demonstrating a general
When a broad suite of PPCPs with various physico-chemical proper-
decrease in the soil concentrations as compared to those in the biosolids
ties are analyzed, it is difficult to obtain satisfactory recoveries for all
used for amendment.
target compounds even if a cleanup procedure is applied. Therefore,the use of isotope labeled surrogate for each individual analyte or
3.1. Fate of PPCPs in soil
group of analytes is essential for achieving quantitative measurement.
These techniques depend on the similar physical and chemical proper-
The fate of PPCPs in soil affects their concentrations available for
ties of the analyte and surrogate to account for analyte loss during sam-
plant uptake. After introduction into soil, PPCPs undergo sorption/de-
ple preparation, and signal suppression, enhancement, or interference
sorption and transformations. Sorption and transformations of PPCPs
caused by the sample matrix during instrumental analysis. Using HLB
in soil can result in the formation of non-exchangeable or bound resi-
for clean up and stable isotope labeled references as surrogates, the
due, which has much reduced bioavailability. Of the limited informa-
corrected recoveries were found to be 56.3-129.6% for a wide range of
tion, formation of bound residues varied greatly among PPCPs. For
PPCPs in several vegetable tissues ).
example, acetaminophen was rapidly converted to bound residue insoils, with the bound residue fraction reaching 73.4–93.3% of the initial-
2.2. Instrumental analysis
ly spiked amount In comparison, only a small fraction(b4.2%) of carbamazepine was found as bound residue under similar
Some of the early studies utilized enzyme-linked immunosorbent
assay (ELISA) to evaluate plant uptake of antibiotics (e.g., tylosin, chlor-
The freely dissolved and exchangeable (or reversibly sorbed)
tetracycline, sulfamethazine) ().
fractions of PPCPs in soil may be available for migration, microbial utili-
Although ELISA analysis offers a rapid, low-cost assessment of antibiotic
zation and plant uptake . The migration of PPCPs in
X. Wu et al. / Science of the Total Environment 536 (2015) 655–666
soil may lead to the leaching of these chemicals to groundwater or
biodegradation in soil. The microbial populations and activities also
contributed to the degradation of PPCPs in different soils (
Some PPCPs have been frequently detected in
leachate or runoff from lands irrigated with treated wastewater, includ-
When biosolids are used for soil amendment in agriculture, degrada-
ing sulfamethoxazole, carbamazepine, meprobamate, trimethoprim,
tion of PPCPs may be affected by the biosolids. It was reported that
irrigation with treated wastewater had no discernable effect on the
), implying their high mobilities in soil. However, mobility
biodegradation of PPCPs in soil ), whereas
is dependent on various conditions as evidenced by decreasing trans-
biosolids amendment generally inhibited the degradation of PPCPs
port of PPCPs in the presence of soil organic matter (SOM) due to en-
therefore prolonging
hanced sorption ) and either an
their persistence in soil. The reason for this inhibition of degradation
increase or decrease in movement due to dissolved organic matter
may be that biosolids amendment usually increases the organic matter
(DOM) depending on chemical and environmental properties (
content in soil, leading to increased sorption of PPCPs to soil (and hence
decreased bioavailability) ). In a previous study, adsorp-
reported that the application of biosolids to arable land in-
tion of carbamazepine to soil was found to increase significantly in bio-
creased the content of SOM and therefore increased the retardation of
solids-amended soils (Also, biosolids may serve
pharmaceuticals, whereas treated wastewater irrigation increased the
as a more readily available nutrient or carbon source over PPCPs for mi-
mobilities of weakly acidic pharmaceuticals, including naproxen, gemfi-
croorganisms, which may contribute to the decreased degradation of
brozil, and diclofenac, in biosolids-amended soils. The authors indicated
PPCPs in soil ).
that the enhanced mobilities of weakly acidic pharmaceuticals weremainly due to the increased soil solution pH (the pH of the treated
3.3 Knowledge gaps
wastewater was 8.37), and not complexation of the chemicals with dis-solved organic carbon (DOC) in the treated wastewater.
Agricultural application of treated wastewater and biosolids can lead
PPCPs in soil may be degraded or transformed as a result of biotic or
to the accumulation of PPCPs in arable soil and may have influence on
abiotic reactions. Biodegradation has been reported to play a major role
the fate and transport process of PPCPs in soil. An examination of
in the removal of PPCPs from environments
existing literature suggests at least two weaknesses in our current
). For example, after 45-day incubation, the fraction
knowledge on the fate of PPCPs in soil. First, the majority of published
of degraded clofibric acid and diclofenac in nonsterile and sterilized
studies used non-labeled compounds and consequently, there are few
agricultural soils was 88-100% (nonsterile) and 33-43% (sterilized), re-
mechanistic insights into the entire pathways of PPCP degradation/
spectively, indicating a significant role of microorganisms in degrading
dissipation in soil. Knowledge of mineralization and bound residue
these PPCPs in the soils Besides biodegradation, abiotic
formation of PPCPs will not only provide information on the long-
process such as photodegradation and hydroly-
term or terminal fate of PPCPs, but also on the availability of parent
sis () may also contribute to the transformations of
and/or metabolites for plant uptake. However, the fraction of minerali-
PPCPs in the environment. During decomposition, a part of the com-
zation and bound residue of PPCPs in soil cannot be quantified without
pound in soil may undergo mineralization (i.e., converted to CO2),
the use of 14C. The second significant gap is in the understanding of the
which is viewed as complete detoxification. So far little information is
factors (e.g., physicochemical properties of the chemical and soil charac-
available on mineralization of PPCPs in soil due to the limited availabil-
teristics) that may affect the fate and movement of PPCPs in soil,
ity of 14C-labeling compounds. 14C-naproxen and 14C-diclofenac were
especially under conditions that represent treated wastewater irriga-
found to be mainly (up to 50-80%) mineralized to 14CO2 in different
tion or biosolids application. At present the mechanisms and effects of
soils (), while only a small part of
ionization on the behavior of PPCPs in soil is inconclusive and further
14C-carbamazepine (b1.2%) and 14C-acetaminophen (17%) were miner-
investigations are needed to better correlate persistence and mobility
alized (Some metabolites of pharmaceuticals in
of PPCPs with their basic properties.
soils were identified by using a combination of techniques, including
14C labeling, LC fractionation, and LC–MS/MS structural elucidation
4. Uptake of PPCPs by plants
). It was reported thatacetaminophen was rapidly converted to various metabolites in soils
4.1. PPCPs in plants grown under hydroponic conditions
), while slow and limited transformation was observedfor carbamazepine ().
A number of studies have been performed under hydroponic condi-
tions to evaluate the bioaccumulation potentials of PPCPs in plants and
3.2. Factors affecting the degradation of PPCPs in soil
explore uptake mechanisms under simplified conditions (
(e.g., hydrophobicity and dissociation) of PPCPs may significantly affect
). The extent of PPCP uptake by
their degradation pathways and their interactions with SOM
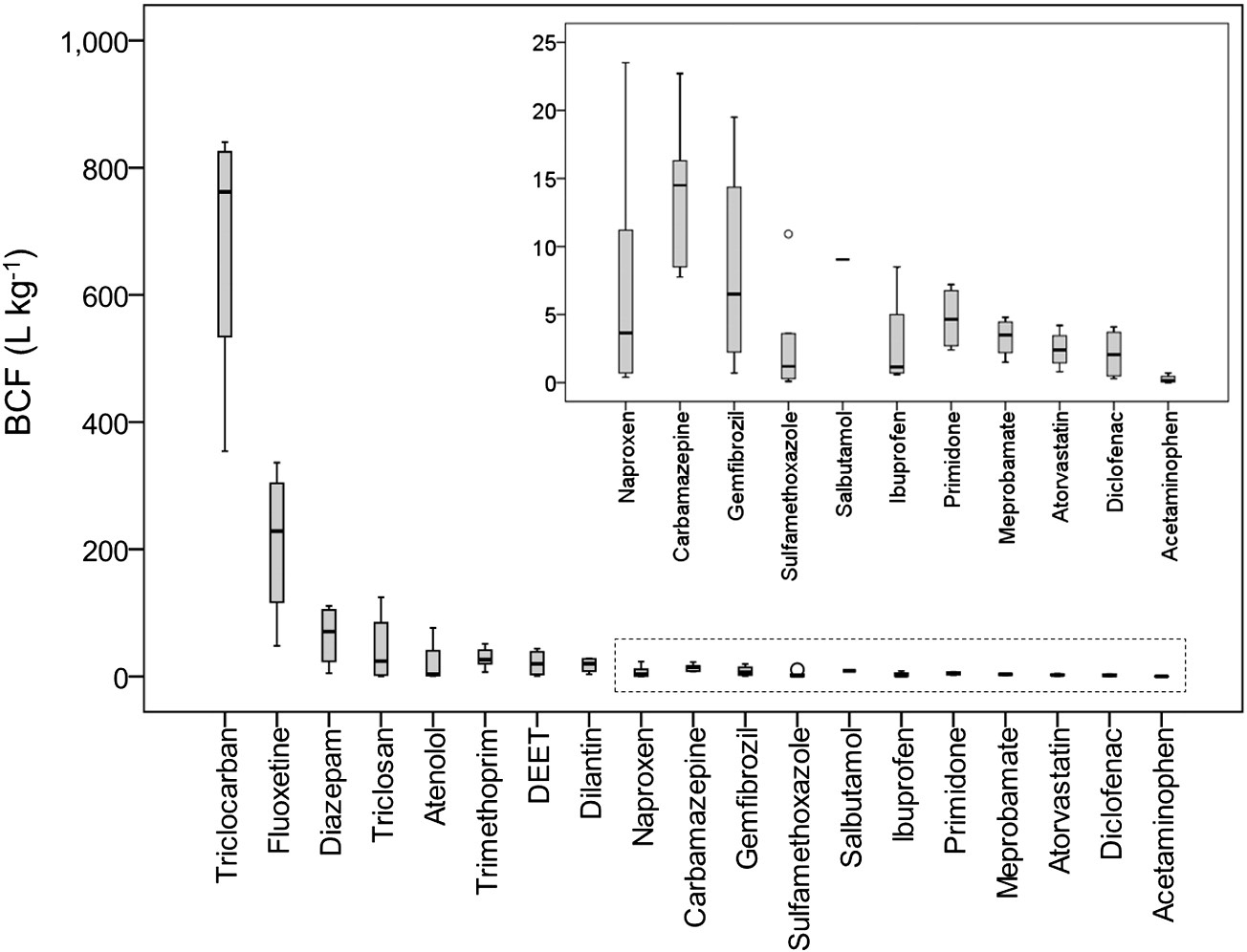
plants is usually evaluated using bioconcentration factor (BCF), which
). For example, antibiotics containing labile carbonyl moieties such
is the ratio of the analyte concentration detected in the plant tissue to
as lactams, esters, carbamates, and amides are likely to undergo hydro-
the spiked concentration in the growth medium. The BCF values obtain-
lysis (). observed that neu-
ed from these studies, which were carried out with different exposure
tral PPCPs were more recalcitrant and persistent in soil irrigated with
concentrations and periods, are summarized and shown in
treated wastewater, while weakly acidic pharmaceuticals, namely
Throughout these studies, it is evident that the BCF values of PPCPs
diclofenac, ibuprofen, bezafibrate, gemfibrozil and naproxen, exhibited
in roots varied widely. Some PPCPs such as triclocarban, fluoxetine,
more rapid degradation, probably because these acidic pharmaceuticals
diazepam, and triclosan may be highly concentrated in roots, with BCF
contained carboxylic groups that are more susceptible to microbial
values up to 111-840 L/kg, while some other PPCPs such as meprobam-
ate, atorvastatin, diclofenac, and acetaminophen were less concentrated
On the other hand, soil properties, such as organic carbon content,
in roots, with BCF values generally less than 5 L/kg (. PPCPs
also play an important role in the degradation of PPCPs.
exhibiting high BCF values in leaves include carbamazepine, fluoxetine,
and reported that high organic carbon content
dilantin, and diazepam (), implying that these chemicals have a
in soil reduced bioavailability of chemicals and hence inhibited their
comparatively high potential for translocation within plants.
X. Wu et al. / Science of the Total Environment 536 (2015) 655–666
Fig. 1. BCFroot of PPCPs in plants grown under hydroponic conditions ). The BCF value was calculated as the ratio of the analyte concentration in plant roots to the spiked concentration in the growth medium. The open circle representsthe mild outlier.
Hydroponic experiments can be used to quickly screen and identify
hydroponic conditions (), it was not found in soybean
"priority" PPCPs exhibiting relatively high potential for plant uptake.
plants grown in soils irrigated with water containing up to 10 μg/L of
For PPCPs that may preferentially accumulate in roots, it may be argued
fluoxetine (), indicating the low bioavailability of fluox-
that higher residues may be found in tuber vegetables such as carrot and
etine in soil, probably due to sorption to soil particles.
radish. On the other hand, PPCPs with a high translocation potentialmay result in higher levels in leaves or fruits.
4.2. PPCPs in plants grown in soil
Due to the complex processes of PPCPs in soil, plant uptake of PPCPs
from nutrient solutions and from soil can be very different. Therefore it
An increasing number of studies have considered the plant uptake of
should be cautious to make predictions of plant uptake of PPCPs in real
PPCPs from soils spiked with PPCP standards (
environment based on hydroponic experiments. For example, although
), irrigated with PPCP-contaminated water
fluoxetine was found to highly accumulate in plants grown under
or treated wastewater
Fig. 2. BCFleaf/stem of PPCPs in plants grown under hydroponic conditions ). The BCF value was calculated as the ratio of the analyte concentration in plant leaves/stems to the spiked concentration in the growth medium. The open circlerepresents the mild outlier and the star represents the extreme outlier.
X. Wu et al. / Science of the Total Environment 536 (2015) 655–666
or amended with manure
transformation products by tomato, carrot, potato and sweet corn from
), sewage sludge
field soils treated with municipal biosolids. The results suggested that
the potential for micropollutants to enter edible parts of food crops
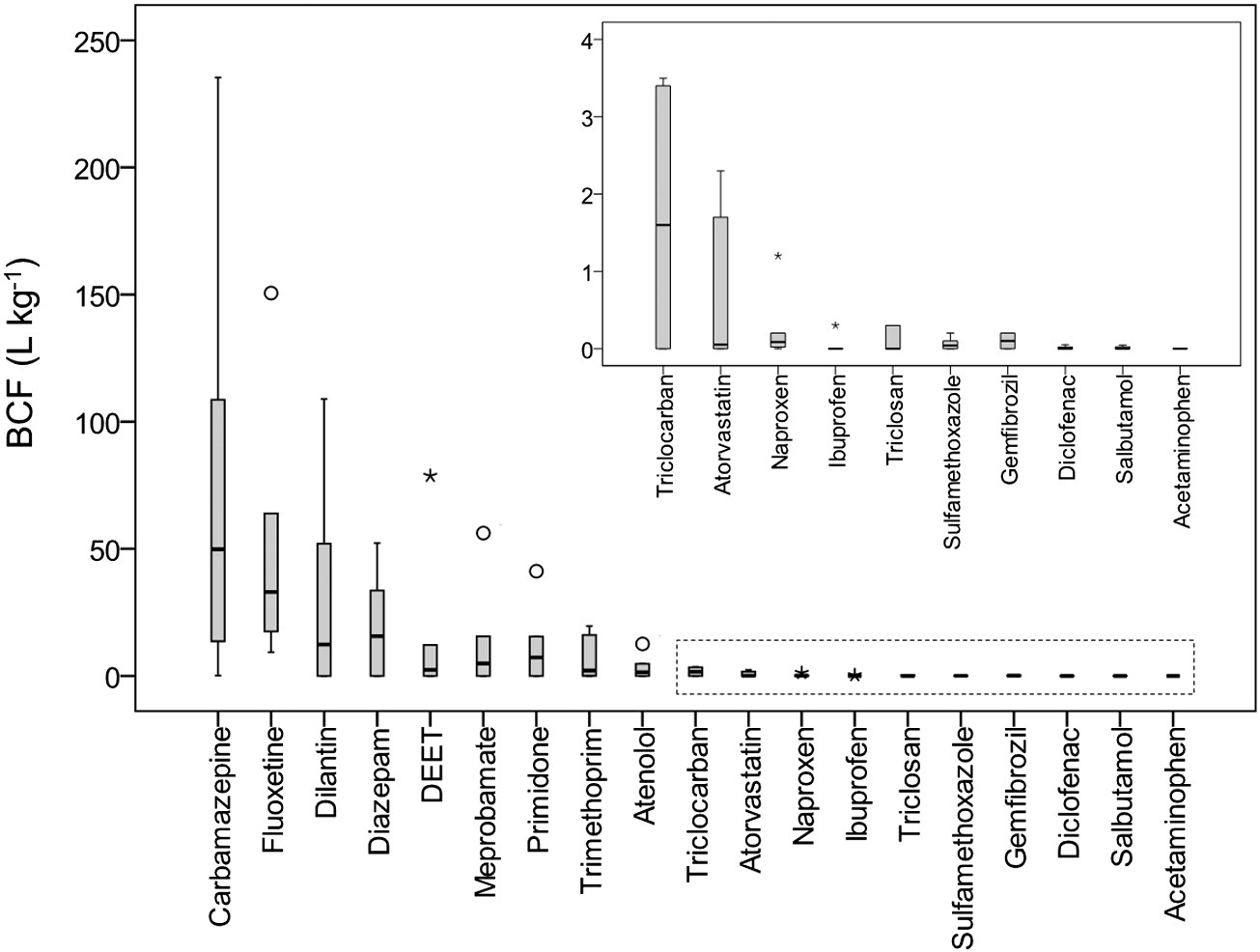
). The BCFs between plant tissues and soil are pre-
was generally low under normal farming conditions. The detected phar-
sented in . Similar to hydroponic studies, triclocarban had
maceuticals included atenolol, cocaine, ciprofloxacin, metformin,
the highest BCF in roots, and carbamazepine had the highest BCF in
minocycline, norfloxacin, DEET, naproxen, glyburide, sulfamerazine,
leaves/stems. Compared to BCFs obtained from hydroponic studies
penicillin G, triamterene, and trimethoprim, with concentrations ranging
), BCFs from soil studies were much lower, indicating that
from 0.02 to 14 ng/g (dry weight). Although triclocarban and triclosan
interactions between PPCPs and soil as well as degradation of PPCPs in
were the predominant PPCPs existing in biosolids, they were not found
soil significantly decreased the bioavailability of PPCPs in soil.
in the plant tissue samples (This was different
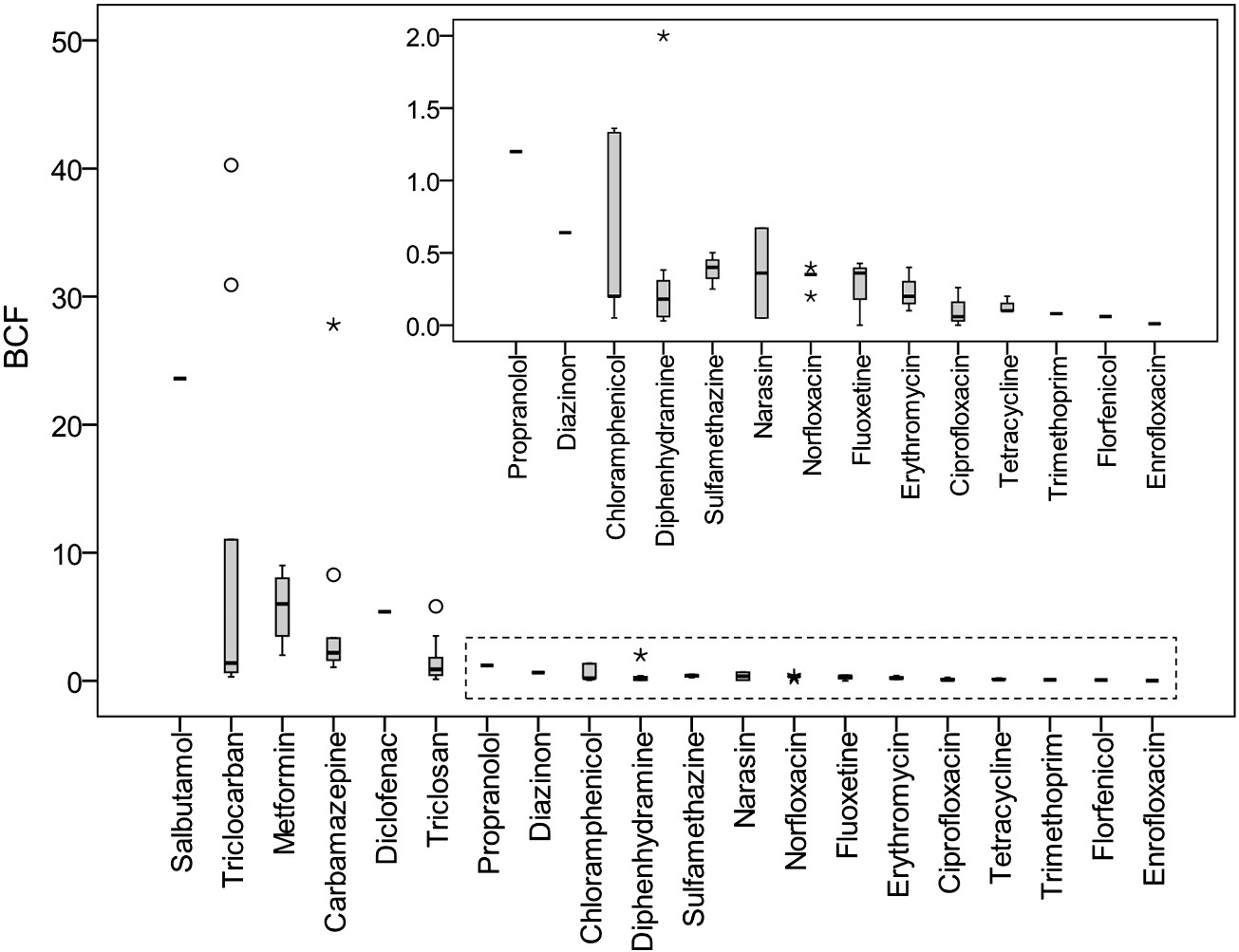
When treated wastewater or spiked water was used for irrigation,
with the results reported by who examined con-
carbamazepine appeared to be commonly detected in various plant
centrations of triclosan and triclocarban in edible portions of green pep-
per, carrot, cucumber, tomato, radish, and lettuce plants grown in a field
suggesting that carbamazepine has high bioavailability in soil and is
with biosolids application. Triclosan was detected in cucumber and rad-
relatively easy to transfer from soil to plants. On the other hand, when
ish up to 5.2 ng/g (dry weight), and triclocarban was detected in carrot,
biosolids were used for soil amendment, triclocarban and triclosan are
green pepper, tomato, and cucumber up to 5.7 ng/g (dry weight).
usually of concern because of their abundance in biosolids (accounting
reported the occurrence of six PPCPs,
for up to 65% of the total PPCPs in biosolids) (
i.e., hydrocinnamic acid, salicylic acid, caffeine, ibuprofen, methyl
). Triclosan and triclocarban were found to be taken up by plant
dihydrojasmonate, and galaxolide, in apple tree leaves and alfalfa irri-
roots and subsequently translocated to stems, leaves, and even fruits
gated with reclaimed wastewater, with concentrations of 0.016–
16.9 ng/g (wet weight). In another field study,
detected a stimulant, N,N-dimethylphenethylamine (DMPEA),
PPCPs have also been detected in plants grown in soils after application
in four crops that were irrigated with effluent from a local wastewater
of biosolids as a fertilizer, such as carbamazepine
treatment plant (WWTP), at 48–180 ng/g (dry weight). Very recently,
two field studies on uptake of PPCPs by crops irrigated with treated
), and diphenhydramine (),
wastewater were reported by and
while sulfamethoxazole and trimethoprim were reported to have limit-
, respectively. found that in two root crops
ed accumulation in plants grown in biosolids-amended soils (
(carrots and sweet potatoes), the nonionic pharmaceuticals (carbamaz-
epine, caffeine, and lamotrigine) were detected at significantly higher
Most studies on plant uptake of PPCPs from soil were carried out in
concentrations than ionic pharmaceuticals (metoprolol, bezafibrate,
laboratory or greenhouse settings. So far the information on accumula-
clofibric acid, diclofenac, gemfibrozil, ibuprofen, ketoprofen, naproxen,
tion of PPCPs in crops receiving biosolids application or treated wastewa-
sulfamethoxazole, and sildenafil). detected 8 PPCPs
ter irrigation under realistic field conditions is limited.
in the edible tissues of 8 vegetables, with a detection frequency of
examined over 20 PPCPs in the grain of wheat grown in the
64%, and the total PPCP concentrations were in the range of 0.01–
field for about 1 year following a high single application of municipal
3.87 ng/g (dry weight). These PPCPs included caffeine, meprobamate,
biosolids, but no PPCPs was detected. studied the
primidone, DEET, carbamazepine, dilantin, naproxen, and triclosan.
uptake of organic micropollutants including 118 PPCPs and PPCP
Basic PPCPs were found at comparable levels with neutral PPCPs,
Fig. 3. BCFroot of PPCPs in plants grown in soil (). The BCF value was calculated as the ratio of the analyte concentration in plant roots to its concentration in soil. The BCFs of triclosan reported by (874-1822) were abnormally higher than those reported in other references and were not included in this figure. The open circle represents the mild outlier and the star representsthe extreme outlier.
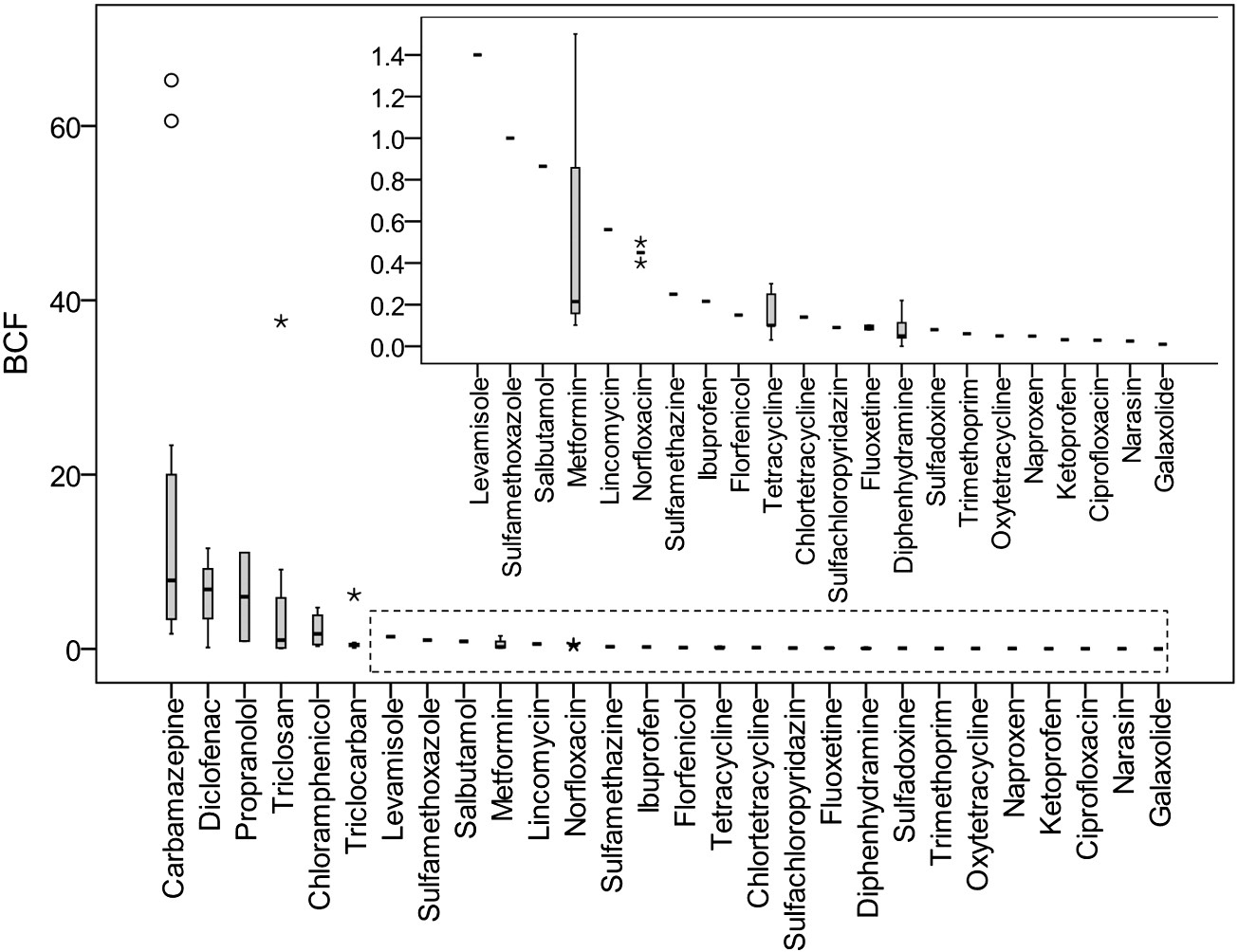
X. Wu et al. / Science of the Total Environment 536 (2015) 655–666
Fig. 4. BCFleaf/stem of PPCPs in plants grown in soil (The BCF value was calculated as the ratio of the analyte concentration in plant leaves/stems to itsconcentration in soil. The open circle represents the mild outlier and the star represents the extreme outlier.
while acidic PPCPs existed at significantly lower levels ().
influence the uptake and translocation of an organic compound in plants
In some places, untreated wastewater was also used for agricultural irri-
gation. investigated five antibiotics in five edible crops
difference in plant accumulation among various PPCPs is believed to be
that were irrigated with either domestic wastewater (largely untreated)
driven by the difference in physico-chemical properties of PPCPs, particu-
or fishpond water. Norfloxacin was consistently found at the highest con-
larly a compound's dissociation and hydrophobicity.
centrations (4.6–23.6 μg/kg) in crop tissues, followed by chloramphenicol(2.6–22.4 μg/kg) and tetracycline (4.0–10.1 μg/kg), while sulfamethazineand erythromycin were not detected in most of the vegetable crops.
4.3.1. Root uptake of PPCPs
At present, factors influencing plant uptake of PPCPs from soil are not
The hydrophobicity of a compound is usually used for interpretation
well understood. In a recent study, reported that
of uptake of organic compounds into plant roots. For neutral PPCPs, a pos-
crops grown in soils with low SOM and clay contents were at greater
itive linear relationship between the root uptake and chemical hydropho-
risk for uptake and accumulation of PPCPs. They also found that the up-
bicity was observed (), suggesting that hydrophobicity was
take of acidic pharmaceuticals by cucumber was inhibited, probably be-
a primary factor affecting uptake of neutral PPCPs by roots. However, this
cause of the interactions between the acidic pharmaceuticals and DOM
model cannot be applied to ionic PPCPs ). For ionizable
present in treated wastewater. Therefore, the influence of factors such
PPCPs, additional mechanisms such as electrical attraction or repulsion,
as SOM, DOM, soil pH, and PPCP sources should be further evaluated in
and ion trap may affect accumulation in roots. Usually ions cross
biomembranes (e.g., plasma membrane, tonoplast) at a slower rate than
Additionally, little information exists on the plant uptake of PPCP me-
a neutral molecule and therefore molecular dissociation
tabolites, which may be present at levels similar to or even greater than
may lead to reduced accumulation by roots, as shown in a recent study
the parent compounds in WWTP effluents
that ionic pharmaceuticals exhibited lower uptake compared to neutral
or in soils from biotic/abiotic transformations. Considering
that some PPCP metabolites have similar biological activity to the parent
Acidic PPCPs could partly dissociate and form at least two species: the
compound ), the uptake behaviors of these metabo-
undissociated acid and its corresponding anion. Anions are generally
lites and their ecotoxicological and human health risks merit further
poorly taken up by plants ), due to the fact that plants cells
have a negative electrical potential at the cell membrane ), and this leads to repulsion to the negatively charged
4.3. Mechanisms of uptake and translocation of PPCPs in plants
anion. A mechanism that may lead to accumulation of acidic compoundsin plant cell is called ion trap. When the pH of external solution is below
The driving mechanism for uptake and transport of PPCPs within the
the pH of cells, the undissociated acid outside the cell may diffuse rapidly
plant is transpiration and the properties of PPCPs
into the cell. However, once inside the cell, dissociation of the weak acid
play a vital role during this process. PPCPs represent a broad variety of
may occur due to the higher pH. Since an ion is much less able to perme-
chemicals with a wide range of physico-chemical properties, from ex-
ate membranes than its neutral molecule, the acid is thus trapped inside
treme hydrophilic (e.g., atenolol at log Kow 0.16 and sulfamethoxazole at
the cell . As an example, an overall low root accumulation
log Kow 0.89) to highly hydrophobic chemicals (e.g., atorvastatin at log
of acidic PPCPs, including naproxen, diclofenac, atorvastatin, gemfibrozil,
Kow 6.36 and triclocarban at log Kow 4.90). Additionally, most PPCPs are
and ibuprofen, was observed in a hydroponic study ).
ionizable compounds, either acids or bases, which may undergo dissocia-
However, an opposite phenomenon was observed by
tion in soil or water depending on the specific pH. Studies on pesticides
who found that these acidic PPCPs were accumulated significant-
suggest that properties such as hydrophobicity and dissociation greatly
ly more than basic or neutral PPCPs in plant roots.
X. Wu et al. / Science of the Total Environment 536 (2015) 655–666
For basic PPCPs, which may dissociate and form neutral and cationic
) were among the first to study the metabolism
fractions, there are three possible processes that may lead to plant uptake:
of pharmaceuticals in plant tissues. They proposed that mechanisms for
(1) electrical attraction of the cation due to the negative charge on the
the detoxification of xenobiotics in plants were closely related to those
plasmalemma. This can be used to explain the moderate root uptake of
in the mammalian system. Briefly, detoxification may be divided into
basic polar PPCPs such as trimethoprim, atenolol, caffeine, primidone,
three phases: phase I is an activation reaction; in phase II the compound
and meprobamate; (2) accumulation into the vacuole by ion trap; and
conjugates with small biomolecules (e.g., glutathione, glucose, amino
(3) partitioning on to the root solids, substantial only for the most lipo-
acids) that would increase the hydrophilicity and mobility of the parent
philic compounds ().
compound. The conjugated molecules may then undergo phase III,during which storage in the plant vacuole, cleavage/degradation, and
4.3.2. Translocation of PPCPs within plants
formation of bound residues in cell walls or transport within plants
Translocation of PPCPs within plants is also related to a compound's
may take place. Huber and coworkers exposed barley and a hairy root
dissociation potential and hydrophobicity. A generally negative correla-
cell culture of horseradish to diclofenac and acetaminophen, and identi-
tion was observed between translocation of PPCPs from roots to leaves
fied two metabolites of diclofenac in plants 4-OH-
and the pH-adjusted octanol–water partition coefficient (log Dow), indi-
diclofenac (phase I metabolite) and 4-OH-diclofenac glucopyranoside
cating that hydrophobic compounds tended to remain in the roots with
(phase II metabolite); and three phase II metabolites of acetaminophen
limited in-plant redistribution, while hydrophilic compounds were sus-
acetaminophen glucoside, acetaminophen gluta-
ceptible to movement to leaves in the direction of the transpiration
thione, and the corresponding cysteine conjugate. In Indian mustard,
stream ). reported that pharmaceu-
the same authors observed that concentrations of acetaminophen in
ticals with an intermediate polarity (log Dow between 0.5 and 3) could be
plant tissues significantly decreased after a 24-h exposure, which was
easily transported to plant shoots. studied the effect
concurrent to an increase in the glutathione S-transferase (GST) activity
of transpiration on plant accumulation and translocation of acidic, basic
in leaves and the appearance of the acetaminophen–glutathione
and neutral PPCPs. They found that for basic and neutral PPCPs, a signifi-
cant positive correlation was observed between translocation factor
Two metabolites of carbamazepine, 10,11-epoxide carbamazepine
values and the transpired masses, while no such relationship was ob-
and 10,11-dihydroxy carbamazepine, were identified in plant leaves
served for acidic PPCPs, suggesting that translocation of basic and neutral
and fruits with comparable levels to carbamazepine
PPCPs from root to leaves was influenced by transpiration. For acidic
In their field study,
PPCPs, other mechanisms rather than transpiration, such as ion trap (as
detected these two carbamazepine metabolites in carrots and sweet
discussed above), may lead to their accumulation in plants. They also
potatoes irrigated with treated wastewater. These metabolites were
found that basic PPCPs had significantly greater translocation factors
found mainly in the leaves, where the concentration of 10,11-epoxide
than neutral or acidic PPCPs, indicating that basic PPCPs were more likely
carbamazepine was significantly higher than the parent compound.
to translocate from root to leaf tissues compared to neutral or acidic
studied the biotransfomation of two benzimid-
azole anthelmintics (used for killing parasitic worms), albendazole and
Recently, examined the ability of two models:
flubendazole, in reed in vitro, and identified 10 albendazole and 5
dynamic plant uptake (DPU) model and biosolids-amended soil level IV
flubendazole metabolites that were mostly phase II metabolites.
(BASL4) model to predict the concentration of eight PPCPs in the tissue
investigated the metabolism of triclocarban, tri-
of plants grown in biosolids-amended soil. For PPCPs including triclosan,
closan, and methyl triclosan in carrot cell cultures as well as in intact car-
triclocarban, miconazole, carbamazepine, and diphenhydramine, their
rot plants. Although triclocarban and methyl triclosan remained
concentrations in various plant tissue predicted by DPU were in the
unaltered in the cell cultures, fast metabolism of triclosan was observed
same or within one order of magnitude of residues observed in the liter-
and all metabolites were phase II metabolites (i.e., conjugates). Moreover,
ature. For ionizable PPCPs, such as cimetidine, fluoxetine, and gemfibrozil,
the total amount of triclosan conjugates in intact carrot plants was
more empirical data are needed to make a definitive conclusion on the
assessed to exceed the amount of triclosan itself by a factor of 5.
ability of DPU to model their translocation in plants. Metabolism of
studied the plant uptake of two PPCPs (diclofenac and
PPCPs in plant tissues were not considered in both models, which may
naproxen) and two endocrine disrupting chemicals (bisphenol A and
contribute to their overprediction for some PPCPs.
nonylphenol) using 14C-labeled compounds. They found that nearly all14C-residues in plant tissues were non-extractable, indicating that these
4.4. Knowledge gaps
chemicals predominantly existed as conjugated residues after beingtaken up by plants, and only a small fraction was extractable.
Studies to date show that various PPCPs can be taken up by plants
from nutrient solution or soil. The accumulation and translocation of
5.1. Knowledge gaps
PPCPs in plants are greatly influenced by the physico-chemical propertiesof the chemical. The following are two apparent limitations in our current
A limited few studies to date show that PPCPs can undergo extensive
understanding. 1) Although relevant research is starting to appear, at
transformations after being taken up by plants. A disregard of PPCP conju-
present our knowledge on plant accumulation of PPCPs is inadequate, es-
gates and metabolites in plant tissues may severely underestimate the ex-
pecially under realistic field conditions (i.e., without fortification). This
tent of PPCP uptake into plants and, eventually, the potential human
lack of knowledge impedes a proper assessment of the probable risks to
exposure to these chemicals via dietary intake. The use of approaches
consumers from the use of treated wastewater and biosolids in agricul-
such as 14C-labeling is essential for mechanistically understanding accu-
ture. And 2) The behavior of ionic PPCPs during plant uptake and translo-
mulation, translocation, and fate of PPCPs in plants. However, at present
cation, and the underlying mechanism are poorly understood. Since many
only a few 14C labeled PPCPs are available commercially, which prevents
PPCPs are ionizable compounds, more research is needed to focus on ion-
more comprehensive experimentation. Similarly, there is a general lack of
izable and persistent compounds.
authentic standards for the metabolites of PPCPs, inhibiting the ability toidentify primary metabolites and their contributions to residues in plants.
5. Metabolism of PPCPs in plants
6. Potential human exposure
After being taken up, organic compounds, such as pesticides and
PAHs, have been found to metabolize in plant tissues (
Several researchers tried to assess the human health risks of con-
suming PPCP-contaminated crops based on their own experimental
X. Wu et al. / Science of the Total Environment 536 (2015) 655–666
data carried out in greenhouses or under field conditions. Due to varia-
xenobiotics have significantly reduced toxicity and are not considered
tions in experimental conditions, such as growing medium, exposure
to be bioavailable to animal metabolism, conjugated compounds may
concentration, exposure time, plant species, among others, the estimat-
be cleaved during animal metabolism and potentially exert biological
ed human exposure values for human varied widely.
activity. The health risks of conjugated and transformed PPCPs in plants
Based on hydroponic experiments, and
should be more thoroughly evaluated. Finally, data derived from field
estimated an individual's annual exposure to PPCPs for av-
trials are urgently needed to afford more representative estimations of
erage daily consumption of leafy vegetables, and the estimated annual
potential human exposure to PPCPs introduced via treated wastewater
exposure values ranged from 0.04 to 350 μg for an average, 70-kg
reuse and biosolids applications.
individual residing in the United States. These estimates of annualexposure were much smaller than that expected in a single medical
7. Conclusions and future research needs
dose (typically in the 20–200 mg range).
For plants grown in soil, calculated human expo-
Studies to date have provided clear evidence to suggest that PPCPs can
sure to carbamazepine, diclofenac, fluoxetine, propranolol, and triclosan
transfer from soil to plants when treated wastewater or biosolids are used
based on a laboratory study. They estimated that humans could con-
in agriculture. For PPCPs that have relatively high bioaccumulation factors
sume 0.01–0.21% of an acceptable daily intake (ADI) for each compound
in roots, e.g., triclocarban, triclosan, metformin, and carbamazepine, high
in root vegetables and 0.09–3.81% in leaf vegetables. A major exception
residues may be found in tuber vegetables such as carrot and radish. On
was the high accumulation of triclosan, which was predicted to reach
the other hand, PPCPs with high translocation potential and accumulation
83.8% of ADI in leaf tissues, nearing the acceptable limit.
tendency in leaves/stems, e.g., carbamazepine, dilantin, diclofenac, pro-
So far data from studies under typical agricultural condition are rath-
pranolol, triclosan, and chloramphenicol, may result in relatively high
er limited. carried out a field study to investigate
levels in leafy vegetables such as lettuce, spinach and cabbage, and may
the uptake of triclosan and triclocarban by crops including radish, car-
further transfer to fruits.
rot, green bell pepper, tomato, cucumber, and lettuce, from biosolids-
Although the human health and ecological risks of plants contami-
amended soil. For both compounds, exposure from plant tissue con-
nated with low levels of PPCPs are still far from clear, based on the
sumption was estimated to account for b0.13–0.39% and b0.73–1.5%
adverse effects of PPCPs observed on non-target organisms such as
of the ADI for an adult and toddler, respectively. Another field study
aquatic organisms, potential risks still exist through dietary intake of
by showed that carbamazepine and caffeine
PPCP-contaminated crops by human or animals, and therefore uptake
would require an adult to consume hundreds of kilograms of treated
of PPCPs by plants should be explored more thoroughly. The following
wastewater-irrigated sweet potatoes or carrots daily to reach the
are a summary of research needs that warrant study in the near and in-
threshold of toxicological concern (TTC) level. However, the TTC level
termediate future:
of lamotrigine and 10,11- epoxycarbamazepine would be easilysurpassed for an adult or child, indicating a demand for specific toxicity
1. Field-based data on accumulation of PPCPs in plants, including vege-
analysis of these chemicals. estimated dietary exposure
tables and fruit trees: First-hand and comprehensive data for a range
values for 7 PPCPs through consumption of treated wastewater
of vegetables and fruit trees and under typical field and agronomic
(fortified with 250 ng/L PPCPs)-irrigated vegetables grown under realis-
conditions are imperative for a more accurate assessment of human
tic field conditions. The estimated total annual PPCPs exposure value
exposure via dietary intakes. With data obtained under field condi-
was 3.69 μg per capita. This amount was more than 3 orders of magni-
tions representative of geographic regions where reuse of treated
tude smaller than that in a single medical dose for one compound (typ-
wastewater and biosolids is happening, such as California, it will
ically in the 10–200 mg range).
then become possible to analyze the potential risks to human from
In a review paper, predicted that human
dietary intake of PPCPs via such beneficial reuses. This knowledge
consumption of vegetable crops irrigated with water containing PPCPs
is crucial for enhancing adoption of such beneficial reuses while
would cause an exposure of 500 ng/day of each compound, a level
well below the therapeutic dose for individual pharmaceuticals. Prosser
2. Given that PPCPs encompass numerous compounds from diverse
et al. ) reviewed litera-
chemical classes, it is valuable to develop a priority list of PPCPs
tures and assessed the concentrations of PPCPs in edible tissue of plants
exhibiting the greatest plant uptake potential under realistic field
grown in soil amended with biosolids or manure or irrigated with
conditions so that research and evaluation may become more fo-
wastewater. They concluded that the concentrations of the majority of
cused. This prioritization maximizes investment in research and
PPCPs in the edible plant tissue represent a de minimis risk to human
may help produce information to guide future research efforts.
3. In-depth understanding of the behavior and fate of PPCPs in plants:
Based on research findings reported to date, human exposure to
PPCPs include a huge number of chemicals with vastly different
PPCPs was likely to be small through daily consumption of crops
physical and chemical properties (e.g., Kow, pKa). Their physico-
grown in biosolids-amended or treated wastewater irrigated soil. How-
chemical properties greatly influence their potential for uptake,
ever, it may be still premature to draw a concrete conclusion, as there
accumulation, translocation, and transformation in plants. Under-
lacks a comprehensive assessment of the human health risks associated
standing the effect of physico-chemical properties of PPCPs in these
with exposure to PPCPs through edible crops ).
processes is valuable for obtaining unifying concepts and conclusionsfor addressing plant accumulation of PPCPs. Additionally, knowledge
6.1. Knowledge gaps
on the metabolism pathway of PPCPs in plants may be used to im-prove the risk assessment of dietary intake of PPCP-contaminated
These limited few studies only predicted the probable exposure to
individual PPCPs. In reality, however, numerous PPCPs may be present
4. Influences of soil and plant factors on plant accumulation of PPCPs:
in the reclaimed water or biosolids, and it is likely that the edible pro-
For the same PPCPs, soil properties such as organic matter content,
duce may be contaminated with trace levels of multiple PPCPs. The si-
and plant species, may greatly affect the actual uptake and distribu-
multaneous exposure to multiple PPCPs needs to be better predicted.
tion. It is important to elucidate the interactions of these factors
In addition, in these studies, only the extractable parent compound
with plant uptake of PPCPs in the context of treated wastewater
was measured. It is likely that a large portion of accumulated PPCPs
irrigation or biosolids amendment. In particular, plants should be
may be in the form of transformation products, conjugated compounds,
categorized into root-edible, leaf-edible and fruit-bearing plants, as
and bound residue that escaped the analysis. While bound residues of
different PPCPs may selectively accumulate in these plant parts.
X. Wu et al. / Science of the Total Environment 536 (2015) 655–666
The knowledge of interactions of soil properties and plant types may
Dodgen, L.K., Li, J., Parker, D., Gan, J.J., 2013.
be further used to develop guidelines and mitigation options for
Dodgen, L.K., Li, J., Wu, X., Lu, Z., Gan, J.J., 2014.
minimizing the potential risk associated with the reuse of treated
wastewater and biosolids.
Dodgen, L.K., Ueda, A., Wu, X., Parker, D.R., Gan, J., 2015.
5. Potential effects of PPCPs on terrestrial systems: Irrigation with
Dolliver, H., Kumar, K., Gupta, S., 2007.
treated wastewater or amendment with biosolids is an important
route for exposing managed and natural terrestrial ecosystems to
Dordio, A.V., Belo, M., Martins Teixeira, D., Palace Carvalho, A.J., Dias, C.M.B., Pico, Y.,
PPCPs. However, it is unknown whether PPCPs from irrigation or
Pinto, A.P., 2011.
amendment would adversely affect terrestrial species such as insects
at environmentally relevant levels. The general lack of information
Eggen, T., Asp, T.N., Grave, K., Hormazabal, V., 2011.
on the effects of treated wastewater/biosolids-borne PPCPs on
Gibson, R., Duran-Alvarez, J.C., Leon Estrada, K., Chavez, A., Jimenez, Cisneros B., 2010.
terrestrial ecology is of major concern to not only agroecosystems
where irrigation or amendment may serve as a major conduit for
contamination, but also the natural surrounding ecosystems.
Goldstein, M., Shenker, M., Chefetz, B., 2014.
Gottschall, N., Topp, E., Metcalfe, C., Edwards, M., Payne, M., Kleywegt, S., Russell, P.,
Lapen, D.R., 2012.
Aryal, N., Reinhold, D.M., 2011.
Grossberger, A., Hadar, Y., Borch, T., Chefetz, B., 2014.
Arye, G., Dror, I., Berkowitz, B., 2011.
Avisar, D., Lester, Y., Ronen, D., 2009.
Haham, H., Oren, A., Chefetz, B., 2012.
Bahlmann, A., Brack, W., Schneider, R.J., Krauss, M., 2014.
Hazelton, P.D., Cope, W.G., Mosher, S., Pandolfo, T.J., Belden, J.B., Barnhart, M.C., Bringolf,
Bartha, B., Huber, C., Harpaintner, R., Schroeder, P., 2010.
Herklotz, P.A., Gurung, P., Heuvel, B.V., Kinney, C.A., 2010.
Holling, C.S., Bailey, J.L., Heuvel, B.V., Kinney, C.A., 2012.
Bennett, G.D., Amore, B.M., Finnell, R.H., Wlodarczyk, B., Kalhorn, T.F., Skiles, G.L., Nelson,
S.D., Slattery, J.T., 1996.
Hu, X., Zhou, Q., Luo, Y., 2010.
Bondarenko, S., Gan, J., Ernst, F., Green, R., Baird, J., McCullough, M., 2012.
Huber, C., Bartha, B., Harpaintner, R., Schroeder, P., 2009.
Borgman, O., Chefetz, B., 2013.
Huber, C., Bartha, B., Schroeder, P., 2012.
Boxall, A.B.A., Johnson, P., Smith, E.J., Sinclair, C.J., Stutt, E., Levy, L.S., 2006.
Inoue, J., Chamberlain, K., Bromilow, R.H., 1998.
Briggs, G.G., Bromilow, R.H., Evans, A.A., 1982.
Jones-Lepp, T.L., Sanchez, C.A., Moy, T., Kazemi, R., 2010.
Briggs, G.G., Rigitano, R.L.O., Bromilow, R.H., 1987.
Kawabata, K., Sugihara, K., Sanoh, S., Kitamura, S., Ohta, S., 2013.
Brodin, T., Fick, J., Jonsson, M., Klaminder, J., 2013.
Calderón-Preciado, D., Jiménez-Cartagena, C., Peñuela, G., Bayona, J.M., 2009.
Kim, S.D., Cho, J., Kim, I.S., Vanderford, B.J., Snyder, S.A., 2007.
Kinney, C.A., Furlong, E.T., Zaugg, S.D., Burkhardt, M.R., Werner, S.L., Cahill, J.D., Jorgensen,
Calderón-Preciado, D., Jiménez-Cartagena, C., Matamoros, V., Bayona, J.M., 2011.
Kinney, C.A., Furlong, E.T., Werner, S.L., Cahill, J.D., 2006b.
Calderón-Preciado, D., Renault, Q., Matamoros, V., Cañameras, N., Bayona, J.M., 2012.
Kolb, M., Harms, H., 2000.
Carter, L.J., Harris, E., Williams, M., Ryan, J.J., Kookana, R.S., Boxall, A.B.A., 2014.
Kumar, K., Gupta, S.C., Baidoo, S.K., Chander, Y., Rosen, C.J., 2005.
Carvalho, P.N., Basto, M.C.P., Almeida, C.M.R., Brix, H., 2014. A review of plant–pharmaceu-
tical interactions: from uptake and effects in crop plants to phytoremediation in
Li, J., Dodgen, L., Ye, Q., Gan, J., 2013.
constructed wetlands. Environ. Sci. Pollut. Res. http://dx.doi.or
Li, J., Ye, Q., Gan, J., 2014.
Chefetz, B., Mualem, T., Ben-Ari, J., 2008.
Lin, K., Gan, J., 2011.
Clarke, B.O., Smith, S.R., 2011.
Llewellyn, N., Lloyd, P., Jurgens, M.D., Johnson, A.C., 2011.
Conners, D.E., Rogers, E.D., Armbrust, K.L., Kwon, J.-W., Black, M.C., 2009.
Macherius, A., Eggen, T., Lorenz, W., Moeder, M., Ondruschka, J., Reemtsma, T., 2012.
Cortés, J.M., Larsson, E., Jönsson, J.A., 2013.
Malchi, T., Maor, Y., Tadmor, G., Shenker, M., Chefetz, B., 2014.
Das, B., Lee, L., Rao, P., Hultgren, R., 2004.
Malchi, T., Maor, Y., Chefetz, B., 2015.
X. Wu et al. / Science of the Total Environment 536 (2015) 655–666
Marsoni, M., De Mattia, F., Labra, M., Bruno, A., Bracale, M., Vannini, C., 2014.
Tanoue, R., Sato, Y., Motoyama, M., Nakagawa, S., Shinohara, R., Nomiyama, K., 2012.
Matamoros, V., Calderón-Preciado, D., Dominguez, C., Bayona, J.M., 2012.
Ternes, T.A., Joss, A., Siegrist, H., 2004.
Tolls, J., 2001.
McClellan, K., Halden, R.U., 2010.
Topp, E., Hendel, J.G., Lapen, D.R., Chapman, R., 2008.
Miao, X.S., Yang, J.J., Metcalfe, C.D., 2005.
Trapp, S., 2000.
Migliore, L., Cozzolino, S., Fiori, M., 2003.
Trapp, S., 2009.
Mitchell, S.M., Ullman, J.L., Teel, A.L., Watts, R.J., 2014.
Monteiro, S.C., Boxall, A.B.A., 2009.
Navon, R., Hernandez-Ruiz, S., Chorover, J., Chefetz, B., 2011.
Available atU.S. EPA, 2012.
Pan, M., Wong, C.K., Chu, L.M., 2014.
Van Eerd, L.L., Hoagland, R.E., Zablotowicz, R.M., Hall, J.C., 2003.
Vanderford, B., Snyder, S., 2006.
Pannu, M.W., Toor, G.S., O'Connor, G.A., Wilson, P.C., 2012.
Podlipna, R., Skalova, L., Seidlova, H., Szotakova, B., Kubicek, V., Stuchlikova, L., Jirasko, R.,
Walters, E., McClellan, K., Halden, R.U., 2010.
Vanek, T., Vokral, I., 2013.
Water Authority, State of Israel, 2012.
Prosser, R.S., Sibley, P.K., 2015.
Prosser, R.S., Lissemore, L., Topp, E., Sibley, P.K., 2014a.
Williams, C.F., Williams, C.F., Adamsen, F.J., 2006.
Winker, M., Clemens, J., Reich, M., Gulyas, H., Otterpohl, R., 2010.
Prosser, R.S., Trapp, S., Sibley, P.K., 2014b.
Wu, C., Spongberg, A.L., Witter, J.D., Fang, M., Czajkowski, K.P., 2010.
Redshaw, C.H., Wootton, V.G., Rowland, S.J., 2008.
Wu, C., Spongberg, A.L., Witter, J.D., 2011.
Ruiz, S.H., Wickramasekara, S., Abrell, L., Gao, X., Chefetz, B., Chorover, J., 2013.
Wu, X., Conkle, J.L., Gan, J., 2012a.
Sabourin, L., Duenk, P., Bonte-Gelok, S., Payne, M., Lapen, D.R., Topp, E., 2012.
Wu, C., Spongberg, A.L., Witter, J.D., Sridhar, B.B.M., 2012b.
SCCWRP (Southern California Coastal Water Research Project), 2012.
Wu, X., Ernst, F., Conkle, J.L., Gan, J., 2013.
Schopfer, P., Brennicke, A., 1999.
Wu, X., Conkle, J.L., Ernst, F., Gan, J., 2014.
Schultz, M.M., Painter, M.M., Bartell, S.E., Logue, A., Furlong, E.T., Werner, S.L., Schoenfuss,
Xia, K., Hundal, L.S., Kumar, K., Armbrust, K., Cox, A.E., Granato, T.C., 2010.
Semple, K.T., Doick, K.J., Jones, K.C., Burauel, P., Craven, A., Harms, H., 2004.
Xu, J., Wu, L., Chang, A.C., 2009.
Shenker, M., Harush, D., Ben-Ari, J., Chefetz, B., 2011.
Ying, G.G., Yu, X.Y., Kookana, R.S., 2007.
Snyder, S.A., Leising, J., Westerhoff, P., Yoon, Y., Mash, H., Vanderford, B., 2004.
Yu, Y., Liu, Y., Wu, L., 2013.
Sui, Q., Huang, J., Deng, S., Chen, W., Yu, G., 2011.
Zarate Jr., F.M., Schulwitz, S.E., Stevens, K.J., Venables, B.J., 2012.
Source: http://conklelab.tamucc.edu/Resources/Wu-2015a.pdf
Mattie Lou Koster, 1912-2001 QUESTIONS AND ANSWERS BEBRF Founder MEDICAL ADVISORY BOARD Q What are the early symptoms of benign essential blepharospasm? Botox ® (onabotulinumtoxinA) Mark Hallett, M.D., Chair . . . . . Bethesda, MD Xeomin ® (incobotulinumtoxinA) Brian D. Berman, M.D., M.S. . . . . .Denver, CO A • Dry eyes
Esta es una publicación del Instituto de Cardiología de Corrientes "Juana F. Cabral " y la FUNDACION CARDIOLOGICA CORRENTINA PUBLICACION Vuelve la Clínica Intensiva para Bajar de Peso GRATUITA Año 7 Nº54 Recientemente se ha llevado a cabo en la ciudad de Buenos Aires el JUNIO 2008 Congreso Mundial de Cardiología, que reunió a 15000 especialistas de todo el mundo y del que muchos miembros del Instituto de Cardio-logía hemos tenido el honor de participar.Sin lugar a duda, una de las afirmaciones mas contundentes de este congreso ha sido justamente el título de esta nota: "La obesidad es el factor de riesgo más importante para el corazón".








