Microsoft word - project_fond celeghin_2014_def
1. INFORMAZIONI GENERALI
Title of the project:
Glutamate as a therapeutic target and a clinical marker in glioblastoma
Department/Institutions involved in the project:
Unit of Molecular Neuro-Oncology
Unit of Neuroradiology
Fondazione IRCCS Istituto Neurologico C. Besta
Principal Investigator:
Gaetano Finocchiaro
Date of Birth:
30 October 1952
Project duration:
24 months
Budget:
80,000.00
2. PIANO DELLA RICERCA
1. Abstract (ENG)
Background. Glioblastoma(GBM) is the most aggressive brain tumor: treatment with surgery ,
radiotherapy and chemotherapy with temozolomide is associated to 15 months overall survival.
Brain edema and invasive capacity are major determinants of GBM morbidity and lethality. High
levels of glutamate are associated to both features. In particular glutamate may control tumor edema
though interaction with a key molecule in water control, aquaporin 4 (AQP4).We have recently
observed that GLAST, the astrocyte transporter devoted to glutamate homeostasis through
glutamate uptake, is expressed on the surface of GBM cells. We also discovered that, despite
GLAST expression, GBM stem-like cells (GSC) release rather than uptake glutamate.
Immunotherapy experiments with GLAST peptides in the GL261 murine model of GBM
demonstrated prolonged survival without evidence of autoimmunity.
Description of the project. We plan to investigate the therapeutic efficacy of GLAST and/or AQP4
targeting in controlling brain edema and limiting invasion in a preclinical model of GBM and test
the prognostic/predictive value of concentrations of brain glutamate in GBM patients: First, we plan
to target GLAST in GSC using UCPH-101, a novel GLAST inhibitor. In vivo, intratumor delivery
of UCP101 will follow with or without association of peptide immunotherapy plus temozolomide.
Second, we will treat GSC with the AQP4 inhibitor TGN020 and combine this treatment in vivo
with GLAST immunotherapy and temozolomide administration. To follow up in vivo experiments
we will use morphological magnetic resonance imaging (MRI), spectroscopy (MRS) and diffusion
Weighted Imaging (DWI). Third we plan to quantify prospectively glutamate by MRS in GBM
patients, to test its role in predicting patient survival and its potential as an invasion biomarker,
preceding detection of the tumor mass by MRI.
Aims and expected results. One goal of this project is to define whether therapeutic targeting of
GLAST (and AQP4) are able to limit GBM progression by limiting edema formation and invasion.
Another goal is test the clinical utility of glutamate measurement by MRS. The clinical translation
of these results may favor the development of novel treatments and biomarkers improving GBM
prognosis.
Abstract (ITA)
Informazioni preliminari. Il glioblastoma (GBM) è il tumore cerebrale più aggressivo: il
trattamento con chirurgia, radioterapia e chemioterapia con temozolomide si associa ad una
sopravvivenza di 15 mesi. L'edema cerebrale e la capacità di invasione sono due aspetti chiave
della morbidità e letalità del GBM. Alti livelli di glutammato sono associati ad entrambi questi
aspetti. In particolare il glutammato può controllare l'edema peritumorale tramite l'interazione con
una molecola importante nel controllo dell'acqua, l'acquaporina 4 (AQP4). Abbiamo di recente
osservato che GLAST, il trasportatore astrocitario che controlla l'omeostasi del glutammato tramite
il suo uptake, è espresso sulla superficie delle cellule di GBM. Abbiamo anche scoperto che,
nonostante tale espressione, le cellule simil-staminali di GBM (GSC) rilasciano glutammato, invece
di assorbirlo. Esperimenti di immunoterapia con peptidi GLAST nel modello murino di GBM
GL261, hanno dimostrato un prolungamento della sopravvivenza senza evidenze di autoimmunità.
Descrizione del progetto. Ci proponiamo di studiare l'efficacia terapeutica di trattamenti anti
GLAST e/o AQP4 nel controllo dell'edema e della capacità invasiva in un modello pre-clinico di
GBM e di valutare il valore prognostico/predittivo della concentrazione cerebrale di glutammato in
pazienti affetti da GBM. Per prima cosa valuteremo in GSC il nuovo inibitore di GLAST UCPH-
101. Seguirà la valutazione dell'effetto intratumorale di UCPH-101 in vivo, con o senza
l'associazione di immunoterapia con peptidi e temozolomide. Tratteremo poi le GSC con l'inibitore
di AQP4 TGN020e combineremo questo trattamento in vivo con l'immunoterapia anti-GLAST
associata a temozolomide. Per il controllo degli esperimenti in vivo useremo risonanza magnetica
(MRI) morfologica, spettroscopica e la diffusione. Infine ci proponiamo di misurare
prospetticamente con MRI-spettroscopia il glutammato cerebrale in pazienti con GBM, per
verificarne il ruolo nella aspettativa di sopravvivenza e il suo potenziale come marker di invasione,
preliminare al riscontro MRI di una nuova lesione.
Scopo e risultati attesi. Un obiettivo di questo progetto è la verifica della capacità di terapie anti-
GLAST (e anti-AQP4) nel limitare la progressione del GBM, diminuendo l'edema e l'invasione di
tessuto sano. L'altro obiettivo è di verificare l'utilità clinica della misurazione del glutammato
tramite MRI spettroscopia. Il trasferimento alla clinica di questi risultati può favorire lo sviluppo di
nuove terapie e nuovi marker per migliorare la prognosi del GBM.
2.Background and rationale
Among brain tumors, glioblastomas (GBM) are the deadliest. The incurability of GBM is mainly
attributed to their highly invasive ability leading inevitably to recurrence. One of the mechanisms
exploited by GBM to make breach into normal brain is the killing of surrounding normal brain cells
through glutamate release1.
Glutamate, the most abundant free amino acid in the brain and the major excitatory neurotransmitter
of the mammalian central nervous system (CNS), is crucial for several aspects of brain function
including learning, memory, cognition and for CNS development. The Na-dependent transporters
GLAST and GLT-1, also called "excitatory amino acid transporters" (EAAT) are expressed by
astrocytes and play a relevant role in glutamate uptake 1. These transporters are responsible for
maintaining the intracellular levels of glutamate and preventing glutamate-mediated excitotoxicity
in the CNS. Their activity depends on the Na+ electrochemical gradient generated by Na+ K+-
ATPase2,3.
Glutamate metabolism and trafficking are profoundly altered in GBM as reported in numerous cell
and animal models of glioma as well as in patients4–6. Additional glutamate signaling in cell
proliferation and migration implies that glutamate can function as an autocrine and paracrine factor
favoring the spreading ability of glioma cells. Peritumoral glutamate can also contribute to brain
edema7, which amplifies the tumor mass effect, a significant source of patient mortality, and is often
responsible for patient symptoms and signs, including seizures8,9. Very recently glutamate, detected
using MRS, has been described as a negative prognostic marker in pediatric medulloblastomas10.
One reason explaining low glutamate uptake by glioma cells is the low expression of GLT-1, which
is partly repressed by the oncogene AEG-111, as also the mislocalization of GLAST into the
nucleus12. Glioma cells however not only show low-absent uptake of glutamate but are also able to
release it at high levels. So far, the cystine glutamate exchanger xCT has been considered the
unique system through which GBM can release excitotoxic concentration of glutamate5 and high
expression of xCT in GBM was associated to shorter survival13.
As described in preliminary data, we have recently found a novel and relevant role for GLAST in
the invasion of GBM based on glutamate release rather than uptake in the tumor microenvironment
(Pellegatta et al, submitted). The loss of Na+/K+-ATPase in GBM is the key feature underlying
such reversal of GLAST function and the direct correlations of GLAST expression with decreased
survival support the clinical relevance of this observation.
While characterizing expression profiles of GL261 glioma stem cells (GSCs) we previously found
up-regulated expression of GLAST and four other genes of the radial glia, a source of adult neural
stem cells14 15. As surface markers as GLAST would facilitate GSC isolation and characterization
we studied it as a GSC marker. The high expression of GLAST in human GBM and the evidence
that inhibition of its expression impacts on GSC migration, convinced us that GLAST could also be
a target for GBM immunotherapy. Indeed we showed that immunization with GLAST peptides
prolonged survival by promoting specific anti-tumor responses in the murine GL261 glioma16.
Another important player in the generation of brain edema is the water channel protein aquaporin-4
(AQP4)17. AQP4 expression is up-regulated in malignant gliomas18 involved in invasion ability19–
21and expressed in GSCs (Pellegatta et al, unpublished). Up-regulation of AQP-4 in astrocytes after
an injury can be activated by NF-kB22. NF-kB was also shown to up-regulate GLAST23,24. Like
GLAST, AQP4 is functionally and physically linked to Na2KATPase25. Interestingly glutamate
may increases astrocyte water permeability by targeting AQP426. Also supporting the relevance of
glutamate-AQP4 cross-talk is the evidence that in AQP4 knock-out mice intraocular levels of
glutamate increase significantly, and GLAST expression is down-regulated27.These data encourage
investigations on interactions/synergies between GLAST and AQP4 in determining brain edema in
GBM. This could hopefully lead to identify an anti-edema treatment alternative or complementary
to dexamethasone, largely used in the clinical practice to control brain edema in GBM in spite of its
toxicity28.
Finally the identification of glutamate as a predictive biomarker of survival in pediatric
medulloblastoma by MRS encourages its investigation in GBM and may offer a future tool for
disease monitoring if therapeutic targeting of GLAST becomes achievable10.
3.Preliminary results
The results reported in this section are part of a manuscript recently submitted entitled "GLAST
expression and loss of Na+/K+-ATPase enhance the invasive ability of GBM through reversal of
glutamate uptake", Pellegatta et al.
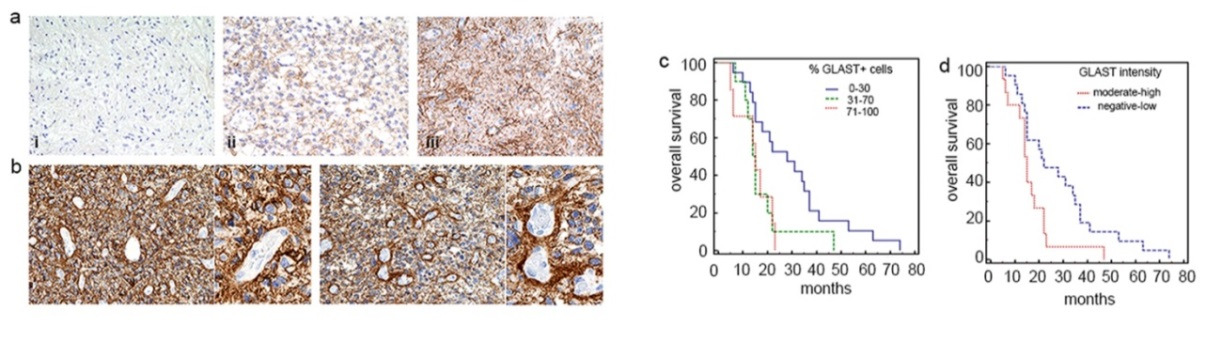
Figure 1. GLAST is expressed in glioblastoma specimens and correlates with patient survival.
(a) Representative examples of GLAST expression levels and reactivity in i. grade I; ii. grade II; iii. grade III glioma specimens (20X magnification) and (b) glioblastomas (20X and 40X magnification). (c, d) Kaplan Meier survival curves showing a significant correlation between GLAST expression and poor prognosis (% GLASTpos cells: P = 0.01 71-100 vs. 0-30; P = 0.04 % 31-70 vs 0-30; GLAST intensity P = 0.02 moderate high vs negative-low).
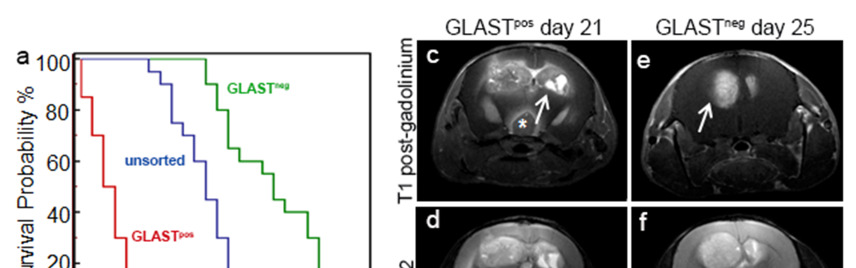
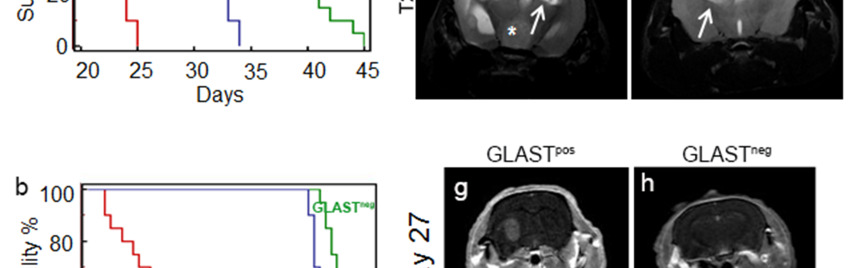
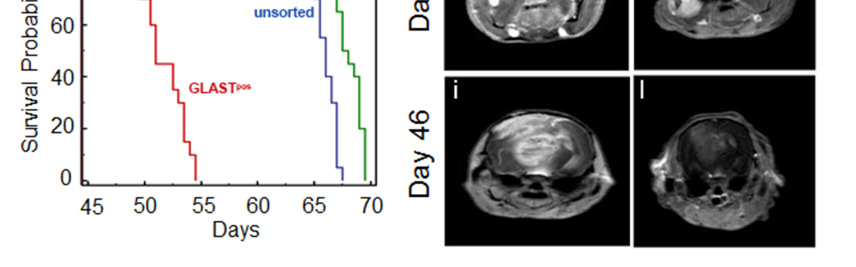
Figure 2. GLAST-enriched cells are more aggressive in vivo that depleted cells.
(a, b) Kaplan Meier survival curves showing that murine and human GLASTpos cells are more aggressive than GLASTneg and unsorted cells. (c-f) T1-weighted images (T1-wi) with contrast medium injection (c, e) and T2-weighted images (T2-wi) (d, f): murine GLASTpos gliomas on day 21(c, d) showed extensive lesions infiltrating the contralateral hemispheres (arrow) and disseminating away from the tumor mass (asterisk); GLASTneg gliomas on day 25 (e, f) are less infiltrative and developed preferentially in the homolateral hemisphere (arrow). (g-l) T1-wi with contrast medium injection in xenograft model: GLASTpos and GLASTneg on day 27 (g, h), GLASTpos and GLASTneg on day 46 (i, l) after tumor cell
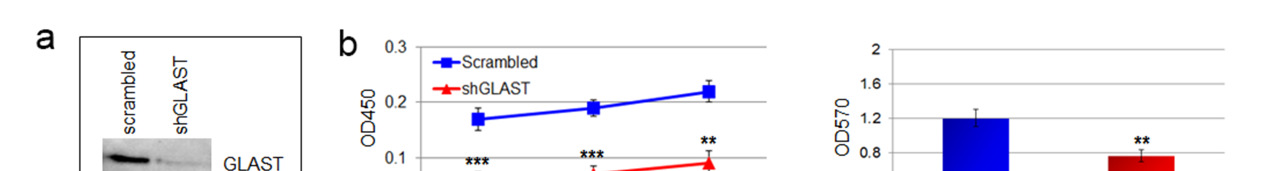
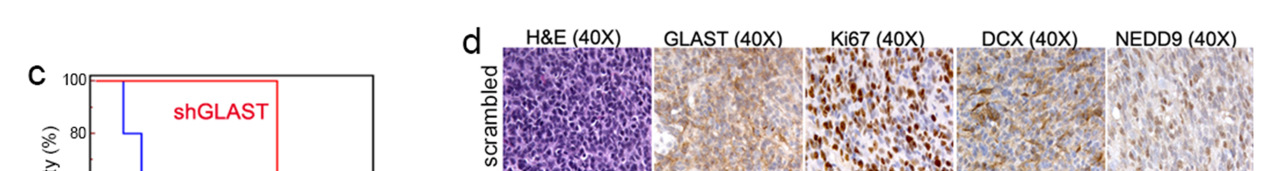
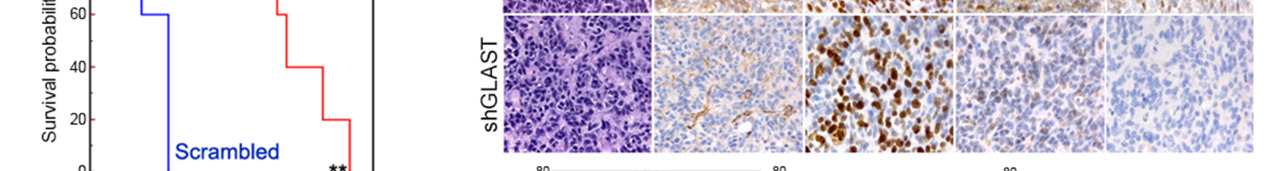
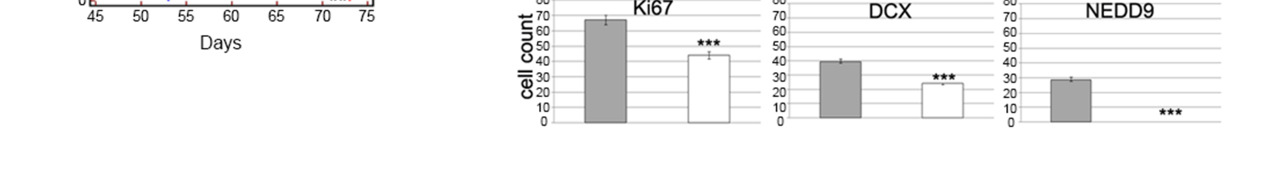
Figure 3. GLAST is involved in invasive ability and release of glutamate.
(a) GLAST silencing efficacy in GCS line as evaluated by western blot. (b) cell proliferation evaluated by a colorimetric method using the WST reagent performed on shGLAST compared with scrambled cells (** P < 0.005; *** P < 0.0005) and quantification of migrating cells using matrigel-coated transwells (P < 0.005 shGLAST vs. scrambled cells). (c) Kaplan Meier survival curves showing that mice injected with shGLAST GSCs survive less than scrambled GSCs. (d) Representative histological analysis for GLAST, ki67, DCX and NEDD9 performed on serial sections of gliomas from mice injected with scambled or shGLAST GSCs. Immunohistochemistry quantification of Ki67, DCX, NEDD9 positive cells in shGLAST vs. scrambled gliomas (*** P = 0.0001).
Figure 4. GSCs show compromised glutamate uptake.
(a, b) Evaluation of uptake and release of glutamate using a colorimetric assay performed on 15 GSC cell lines indicated as GBM-NS (uptake: 0.2±0.09, release: 23.0±4.0 ng/microl respectively), GL261-NS (uptake: 0.3±0.07 release: 15.0±2.3 ng/microl respectively) normal (uptake: 39.8±3.0, release 0.3±0.03 ng/microl respectively) and hypoxic astrocytes (uptake: 0.09±0.01, release 33.4±2.0 ng/microl respectively) (**** P < 0.0001; *** P < 0.001; ** P < 0.01 vs astrocytes). (c) Western Blot analysis showing GLAST expression in astrocytes and GSCs under normoxic and hypoxic conditions. (d) Detection of glutamate release performed on shGLAST vs scrambled GSCs (P < 0.01).
Figure 5. Glutamate concentration is higher in GLASTpos than GLASTneg gliomas.
(a, b; e, f) Variation over time of brain metabolites concentration measured at the injection site of GL261 cells and on the contralateral hemisphere in GLASTpos (a, b) and Glastneg gliomas (e, f). Data were acquired one week before the MRI signs of the tumor (time point -1), at the tumor onset on MRI (time point 0) and one and two weeks after the tumor onset (time point +1 and +2). Glutamate concentration increased before tumor appearance from 8.0 mM (time point -2) to11.4 mM (time point -1) in the tumor injection site (a) and from 8.5 mM to 11.0 mM in the contralateral hemispheres (b). The value at the first time point (time point -2 on the graphs) is the concentration of metabolites before tumor cell injection. (c, d; g, h). 1H-MRS spectra from the single voxel pointed out on T2-wi on the injection site (c, g) and contralateral hemispheres (d, h) in a GLASTpos and GLASTneg gliomas.
Figure 6. GSCs and glioblastoma specimens fail to express Na+/K+-ATPase.
(a) Expression of ATP1A1-4 and ATP1B1-4 subunits evaluated by real Time PCR
performed on 29 GB specimens. (b) Western blot analyses of α1 and α2 expression
performed on GL261- GSCs, human GSC lines and (c) on astrocytes under normoxic and
hypoxic condition.
4. Experimental plans and expected outcomes
We plan to pursue this research by three distinct, yet fully integrated, work packages (WPs).
WP1. Pharmacological targeting of GLAST in GBM (Months 1-24)
In vitro experiments will be performed using a commercially available specific inhibitor of GLAST,
UCPH-101 (Tocris), previously investigated in normal cells29.
GSCs will be treated with different concentrations of UCPH-101 in the micromolar range, at
different time points and glutamate release will be measured in the cell culture medium as
previously described (see preliminary data). Based on preliminary data obtained on GSCs where the
expression of GLAST was silenced, we expect to find a significant reduction of glutamate
concentration supporting the direct involvement of GLAST in glutamate release caused by a
functional reversal. If this will be confirmed a pilot study in vivo will be performed by direct
intratumoral injection of UCPH-101 using stereotaxic coordinates, as previously performed by our
group with DC immunotherapy30 or intracranial guide screws31. Systemic delivery will be
considered after in vitro experiments testing the bioavailability of the drug after admixture with
liposomes. Difficulties in crossing the BBB may be decreased in GL261 GBM, where BBB is
disrupted32. Another series of in vivo experiments will include treatment with GLAST peptides with
temozolomide and local or systemic UCPH-101, using protocols established in our laboratory16,33.
WP2. Evaluation of GLAST and AQP4 involvement in edema formation (Months 12-24)
Based on data suggesting the relevance of glutamate/AQP4cross talk in generating brain edema we
want to correlate the role of GLAST with AQP-4. To address the potential of GLAST and AQP4 as
therapeutic targets we will perform experiments with the AQP4 inhibitor TGN-020 in C57BL6
mice bearing intracranial GL261 gliomas34.We will try to treat mice before and at the time the
edema appearance (day 9 and 15 after tumor implantation, respectively). Animals will receive a
single intraperitoneal injection of TGN-020 (200mg/kg, dissolved in 0.1 ml normal saline), and the
effects will be monitored by MRI. In addition we will perform combined experiments with the
GLAST peptides, temozolomide and TGN-020.
We also plan to inject three "scrambled" and three shGLAST GSCs and to investigate the presence
of edema in correlation with GLAST expression (5 nude mice/cell line/treatment. Tot: 30).
Histological analysis at different time points will be performed to evaluate the expression and co-
expression of GLAST and AQP-4. To confirm the co-localization of GLAST and AQP4, and
consequently their potential synergy, we will perform co-immunoprecipitation experiments on
GSCs ("scrambled" vs shGLAST) and on gliomas explanted by xenograft models.
By MRI, the tumor mass volume and edema will be evaluated by manual segmentation on T2wi and
T1wi pre and T1wi post-contrast agent enhancement. All MRI studies will include Diffusion
Weighted Imaging (DWI) sequences; the derived diffusion parameters will be performed by
comparing shGLAST and "scrambled" gliomas in order to detect microstructural differences in the
peritumoral edema.
WP 3. Prognostic value of glutamate levels in GBM (Months 1-24)
We already studied by MRI 5 healthy volunteers in order test a long (144 ms) and a short (35 ms)
TE PRESS sequence for glutamate quantification. The sequences were repeated twice without
moving the acquisition volume. The spectra were analyzed with LCModel software79 for
metabolites absolute quantification. Starting from this preliminary set up, we will select at least 30
patients before surgery, affected by glioblastoma as preliminarily diagnosed by MRI and confirmed
by histology. The number of patients is a reasonable estimate based on the number of MRI actually
performed in 2 years at the Unit of Neuroradiology. Patients will be re-tested by MRS 6 months
after surgery. Each patient will undergo an 1H-MRS protocol by 3 T clinical MR scanner including
3 single voxel (4,5 ml) PRESS sequence localized in tumor mass, in tumor peripheral area and in
the contralateral hemisphere. We plan to detect the concentration of glutamate by 3T MRS before
and six months after surgery and to follow the radiological and clinical evolution of these patients in
order to realize whether glutamate concentrations can predict the survival patients with GBM and
the tumor evolution.
Brain peritumoral edema will be evaluated by morphological MRI on T2wi. Glutamate values will
be correlated with progression free survival (PFS), as determined by MRI with RANO criteria.
The expected outcome is the definition of glutamate as an early biomarker in GBM patients. Based
on preliminary observations in mice, we also plan verify preliminary investigation on the possibility
to predict tumor infiltration before evidence of a novel contrast-enhancing lesion at recurrence.
References
Danbolt, N. C. Glutamate uptake. Prog. Neurobiol. 65, 1–105 (2001).
Szatkowski, M., Barbour, B. & Attwell, D. Non-vesicular release of glutamate from glial
cells by reversed electrogenic glutamate uptake. Nature 348, 443–6 (1990).
Attwell, D., Barbour, B. & Szatkowski, M. Nonvesicular release of neurotransmitter. Neuron
11, 401–7 (1993).
Takano, T. et al. Glutamate release promotes growth of malignant gliomas. Nat. Med. 7,
1010–5 (2001).
Lyons, S. A., Chung, W. J., Weaver, A. K., Ogunrinu, T. & Sontheimer, H. Autocrine
glutamate signaling promotes glioma cell invasion. Cancer Res. 67, 9463–71 (2007).
Ye, Z. C. & Sontheimer, H. Glioma cells release excitotoxic concentrations of glutamate.
Cancer Res. 59, 4383–91 (1999).
Savaskan, N. E. et al. Small interfering RNA-mediated xCT silencing in gliomas inhibits
neurodegeneration and alleviates brain edema. Nat. Med. 14, 629–32 (2008).
Buckingham, S. C. et al. Glutamate release by primary brain tumors induces epileptic
activity. Nat. Med. 17, 1269–74 (2011).
Yuen, T. I. et al. Glutamate is associated with a higher risk of seizures in patients with
gliomas. Neurology 79, 883–9 (2012).
Wilson, M. et al. Non-invasive detection of glutamate predicts survival in pediatric
medulloblastoma. Clin. Cancer Res. 20, 4532–4539 (2014).
Lee, S.-G. et al. Oncogene AEG-1 promotes glioma-induced neurodegeneration by
increasing glutamate excitotoxicity. Cancer Res. 71, 6514–23 (2011).
Ye, Z. C., Rothstein, J. D. & Sontheimer, H. Compromised glutamate transport in human
glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and
enhanced activity of cystine-glutamate exchange. J. Neurosci. 19, 10767–77 (1999).
Takeuchi, S. et al. Increased xCT expression correlates with tumor invasion and outcome in
patients with glioblastomas. Neurosurgery 72, 33–41; discussion 41 (2013).
Merkle, F. T., Tramontin, A. D., García-Verdugo, J. M. & Alvarez-Buylla, A. Radial glia
give rise to adult neural stem cells in the subventricular zone. Proc. Natl. Acad. Sci. U. S. A.
101, 17528–32 (2004).
Pellegatta, S. et al. Neurospheres enriched in cancer stem-like cells are highly effective in
eliciting a dendritic cell-mediated immune response against malignant gliomas. Cancer Res.
66, 10247–52 (2006).
Cantini, G., Pisati, F., Pessina, S., Finocchiaro, G. & Pellegatta, S. Immunotherapy against
the radial glia marker GLAST effectively triggers specific antitumor effectors without
autoimmunity. Oncoimmunology 1, 884–893 (2012).
Fukuda, A. M. & Badaut, J. Aquaporin 4: a player in cerebral edema and neuroinflammation.
J. Neuroinflammation 9, 279 (2012).
Saadoun, S., Papadopoulos, M. C., Davies, D. C., Krishna, S. & Bell, B. A. Aquaporin-4
expression is increased in oedematous human brain tumours. J. Neurol. Neurosurg.
Psychiatry 72, 262–5 (2002).
Ding, T., Gu, F., Fu, L. & Ma, Y. Aquaporin-4 in glioma invasion and an analysis of
molecular mechanisms. J. Clin. Neurosci. 17, 1359–61 (2010).
Yang, L. et al. Aquaporin-4 upregulated expression in glioma tissue is a reaction to glioma-
associated edema induced by vascular endothelial growth factor. Oncol. Rep. 28, 1633–8
(2012).
McCoy, E. S., Haas, B. R. & Sontheimer, H. Water permeability through aquaporin-4 is
regulated by protein kinase C and becomes rate-limiting for glioma invasion. Neuroscience
168, 971–81 (2010).
Rao, K. V. R., Reddy, P. V. B., Curtis, K. M. & Norenberg, M. D. Aquaporin-4 expression in
cultured astrocytes after fluid percussion injury. J. Neurotrauma 28, 371–81 (2011).
Lin, C.-H., You, J.-R., Wei, K.-C. & Gean, P.-W. Stimulating ERK/PI3K/NFκB signaling
pathways upon activation of mGluR2/3 restores OGD-induced impairment in glutamate
clearance in astrocytes. Eur. J. Neurosci. 39, 83–96 (2014).
Tai, Y.-H. et al. Amitriptyline induces nuclear transcription factor-kappaB-dependent
glutamate transporter upregulation in chronic morphine-infused rats. Neuroscience 153, 823–
31 (2008).
Illarionova, N. B. et al. Functional and molecular interactions between aquaporins and Na,K-
ATPase. Neuroscience 168, 915–25 (2010).
Gunnarson, E. et al. Identification of a molecular target for glutamate regulation of astrocyte
water permeability. Glia 56, 587–96 (2008).
Li, X.-M. et al. Abnormal glutamate metabolism in the retina of aquaporin 4 (AQP4) knockout mice upon light damage. Neurol. Sci. (2013). doi:10.1007/s10072-013-1610-7
McGirt, M. J. et al. Persistent outpatient hyperglycemia is independently associated with
decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery 63,
286–91; discussion 291 (2008).
Abrahamsen, B. et al. Allosteric modulation of an excitatory amino acid transporter: the
subtype-selective inhibitor UCPH-101 exerts sustained inhibition of EAAT1 through an
intramonomeric site in the trimerization domain. J. Neurosci. 33, 1068–87 (2013).
Pellegatta, S. et al. Intra-tumoral dendritic cells increase efficacy of peripheral vaccination by
modulation of glioma microenvironment. Neuro. Oncol. 12, 377–88 (2010).
Lal, S. et al. An implantable guide-screw system for brain tumor studies in small animals. J.
Neurosurg. 92, 326–33 (2000).
Leten, C., Struys, T., Dresselaers, T. & Himmelreich, U. In vivo and ex vivo assessment of
the blood brain barrier integrity in different glioblastoma animal models. J. Neurooncol. 119,
297–306 (2014).
Favaro, R. et al. Sox2 is required to maintain cancer stem cells in a mouse model of high-
grade oligodendroglioma. Cancer Res. 74, 1833–44 (2014).
Igarashi, H., Huber, V. J., Tsujita, M. & Nakada, T. Pretreatment with a novel aquaporin 4
inhibitor, TGN-020, significantly reduces ischemic cerebral edema. Neurol. Sci. 32, 113–6
(2011).
5. Research team: personnel involved in the project
Role in the
Effort (%)
Role in the project
As PI of the project, he will participate to
data analysis and to the discussion of
progress reports, abstracts and papers deriving from this project.
She will be in charge of patient selection.
She will collaborate to data interpretation
and correlation with the clinical outcome and survival of patients.
She will collaborate to Spectroscopy and
neuroradiologist 0.1
Maria Grazia Radiology
Magnetic Resonance Imaging interpretation.
She will be in charge of the animal
treatments and in vitro GSC silencing.
She will coordinate TBD who will mainly work at the project.
6. Methodology
Prelinical studies. Animal models will be injected with 50,000 GSCs in 2 microl of PBS1X. The
coordinates, with respect to the bregma, will be 0.7 mm post, 3 mm left lateral, 3.5 mm deep, and
within the nucleus caudatum. Animal experimentation will be performed in accordance to the
Italian Principle of Laboratory Animal Care (D. Lgs. 26/2014) and European Communities Council
Directives (86/609/EEC and 2010/63/UE).
Experimental magnetic resonance imaging and spectroscopy. MRI/MRS studies will be
performed on a 7 Tesla BioSpec 70/30 USR scanner equipped with a 12 cm inner diameter actively
shielded gradient system reaching a maximum amplitude of 400 mT/m. (Bruker BioSpin, Ettlingen,
Germany). Mice will be anaesthetized with 1.5–2% isoflurane (60:40 N2O:O2 (vol:vol), flow rate
0.8 L/min). To detect the depth of anaesthesia and the animal health condition during the MRI
study, the respiratory rate and temperature will be monitored by a pneumatic and rectal sensor
respectively. For anatomical references, T2-wi will be acquired in three orthogonal planes: axial,
sagittal, and coronal. MRI investigation will be performed with the following protocol: high
resolution axial T2-weighted images (T2-wi) acquired using a rapid acquisition with relaxation
enhancement (RARE) sequence (TR/TE=3000/13.5 ms, matrix size=256×256, RARE factor=8,
slice thickness=0.8 mm, FOV=2.2×2.2 cm2, in plane resolution=86 µm, number of averages
(NA)=8, acquisition time (AT)=9 min 30 s); 1H spectra carried out by a PRESS sequence (Point
RESolved Spectroscopy, TR/TE = 5000/13 ms, adjustment of the first and second order shims
conducted beforehand by Mapshim macro of Paravision 5.1 software) from a single voxel ( 10µL)
located inside a tumor (or, before detecting solid tumor, at the injection site) and symmetrically in
the contralateral hemisphere; contrast agent induced signal enhancement, interpreted as being due to
a BBB lesion, was highlighted by 2D T1-weighted (T1-w) sequence (TR/TE=390/11 ms, matrix
sixe=256×256, slice thickness=1 mm, FOV=3×3 cm2, in plane resolution=117 µm, NA=4, AT=6
min 36 s); acquired before and after intraperitoneal administration of contrast medium (Gadolinium
DTPA).
Clinical magnetic resonance imaging and spectroscopy. MRI protocol performed on a 3 T
clinical scanner (Achieva TX, Philips Medical System BV, Best, NL) and equipped with a 32
channels head coil. The scanning protocol will include morphological imaging and three single-
voxel 1H-MRS sequence. A Sagittal T1-w Inversion Recovery (FOV = 240x240, voxel = 0.9 x1.1
mm2, slice thickness = 4 mm, TR/TI = 2000/800 ms, TE = 20 ms), an axial T2-w spin echo (FOV =
230x200 mm2, voxel = 0.6 x 0.7 mm2, slice thickness = 4 mm, TR = 4000 ms, TE = 95 ms) and a
coronal FLAIR (Fluid Attenuated Inversion Recovery) (FOV = 230x200 mm2, voxel = 0.9 x 1.0
mm2, slice thickness = 4 mm, TR = 11000 ms, TI = 2800 ms, TE = 125 ms) will be used for lesion
localization and voxel positioning; the three single-voxel PRESS sequences (voxel volume = 2x2x2
cm3, TR = 2000 ms, TE = 30 ms, NSA = 128, bandwidth = 2048 Hz, complex points = 2000) will
be placed one inside the lesion one in peripheral area and the last one in controlateral brain area.
The PRESS sequences will be optimized to evaluate variation in absolute concentration of
glutamate. A volumetric 3D T1-w Turbo Field Echo (FOV = 240x240x240 mm3, voxel = 1x1x1
mm3, TR = 9.9 ms, TE = 4.6 ms) for neurosurgery navigator system will be acquired before and
after administration of gadolinium contrast agent (Magnevist 0.1 mol/kg).
Statistical analysis. Kaplan–Meier survival analysis will be performed considering the PFS of
patients and the glutamate levels, dividing the patient cohort into two groups: cases with glutamate
concentrations above and below a cutoff value. The optimal cutoff value will be found by
performing a χ2 significance test over a range of values10.
3. BUDGET
Direct Costs
Consumables and
€ 22.000,00 Mice purchase and
transportation; animal maintenance. Reagents for cell biology, molecular biology. Antibodies.
Personnel costs
€ 50.000,00 Two "to be defined" working
on: in vivo experiments and in vitro studies (TBD1); the in-vivo clinical MRS analysis and acquisition (TBD2) none
National or international
Meeting-workshop
€ 700,00 € 800,00 € 1.500,00 meeting and fees
Paper submission and reprint
Publications/Others
€ 1.500,00 € 1.500,00
€ 36.700,00
€ 43.300,00 € 80.000,00
Co-Fund: AIRC - IG2013 N.14323: "GLAST, a neural stem cell marker, as a novel target for
glioblastoma immunotherapy" (2013-2015 - €185.000,00- PI: Pellegatta). (see attachment)
Pending: Cariplo 2014 - Young Researchers: "Investigations on molecular biology of GLAST-
mediated release of glutamate in glioblastoma" (Request: 237,248.00 euro-PI Pellegatta)
Timeline
WP1. 0-3 months: in vitro experiments with GLAST inhibitor UCPH-101; 6-12 months: in vivo
experiments; 12-24 months: in vivo combination of different treatments.
WP2. 12-15 months: in vivo treatment with AQP4 inhibitor TGN-020; 15-24 months:
investigations on edema formation in xenograft models; in vitro studies in order to characterize
GLAST and AQP4 synergy.
WP3. 0-6 months: patient identifications; 6-12 months: MRI/MRS studies; 12-24 months: follow-
up and survival correlation with glutamate detection.
4. Education, research and professional experience of the PI
Gaetano Finocchiaro, MD
GF is active in the main field of basic and translational brain tumor research, aiming at the
development of new approaches in prevention, diagnosis and therapy of brain tumors. As PI of this
project, he will provide its expertise in glioma biology and will be involved for supervision of all
the project with a translational emphasis.
PROFESSIONAL EXPERIENCE
Present-2009 Director, Unit of Molecular Neuro-Oncology, Istituto Neurologico Besta
Present-2008 Director, Department of Neuro-Oncology, Istituto Neurologico Besta
Present-2008 Scientist at IFOM IEO Campus
Present-2006 Scientist in the PhD Program of Translational Medicine (DIMET: www.dimet.org)
2008-1998 Neurologist, Director, Unit of Experimental Neuro-Oncology and Gene Therapy, Istituto
Neurologico Besta
1987-1997 Neurologist, Unit of Biochemistry and Genetics, Istituto Neurologico Besta
EDUCATION
1988 Specialized in Biochemistry and Clinical Chemistry, University of Milan
1984-1987 Research fellow, Department of Human Genetics, Yale Medical School, New Haven,
CT, USA
1984 Specialized in Neurology, University of Milan
1979 Degree in Medicine, University of Milan
AWARDS
Member of the international committee evaluating research activities of the CRP-Santé in
Luxembourg in 2008. Coordinator for the group proposing guidelines for the treatment of gliomas
for regione Lombardia in 2010. Member of the delegation of Italian scientists received by the Italian
President Giorgio Napolitano (International Day of Cancer Research in 2011).
Member of the international committee evaluating research activities of the ICM-Dalpetriere (Paris)
in 2013.
Other CV Informations: GF obtained a number of grants from Italian and International Agencies
for his research work: MDA, AIRC, European Community, TeleThon, Italian Minister of Health,
Italian Minister of Reaserch and University. He acted as the European Editor of Neuro-Oncology; is
now in the Editorial Board of Journal of Neuro-Oncology and of Neuro-Oncology.
Reviewer of the most relevant journals in his fields of research and reviewer of National and
European grants. GF acted as a PI in 11 clinical studies, 9 with international sponsors.
Papers published in the last five years (max 30):
1: Finocchiaro G, Pellegatta S. Perspectives for immunotherapy in glioblastoma treatment. Curr
Opin Oncol. 2014 Sep 10
2: Labussière M, Boisselier B, Mokhtari K, Di Stefano AL, Rahimian A, Rossetto M, Ciccarino P,
Saulnier O, Paterra R, Marie Y, Finocchiaro G, Sanson M. Combined analysis of TERT, EGFR,
and IDH status defines distinct prognostic glioblastoma classes. Neurology. 2014 Aug 22
3: Bertolino N, Marchionni C, Ghielmetti F, Burns B, Finocchiaro G, Anghileri E, Bruzzone MG,
Minati L. Accuracy of 2-hydroxyglutarate quantification by short-echo proton-MRS at 3Â T: A
phantom study. Phys Med. 2014 Sep;30(6):702-7
4: Aquino D, Di Stefano AL, Scotti A, Cuppini L, Anghileri E, Finocchiaro G,Bruzzone MG, Eoli
M. Parametric response maps of perfusion MRI may identify recurrent glioblastomas responsive to
bevacizumab and irinotecan. PLoS One. 2014 Mar 27;9(3):e90535
5: Favaro R, Appolloni I, Pellegatta S, Sanga AB, Pagella P, Gambini E, Pisati F, Ottolenghi S, Foti
M, Finocchiaro G, Malatesta P, Nicolis SK. Sox2 is required to maintain cancer stem cells in a
mouse model of high-grade oligodendroglioma.Cancer Res. 2014 Mar 15;74(6):1833-44
6: Nava F, Tramacere I, Fittipaldo A, Bruzzone MG, Dimeco F, Fariselli L, Finocchiaro G, Pollo B,
Salmaggi A, Silvani A, Farinotti M, Filippini G. Survival effect of first- and second-line treatments
for patients with primary glioblastoma: a cohort study from a prospective registry, 1997-2010.
Neuro Oncol. 2014 May;16(5):719-27
7: Patanè M, Porrati P, Bottega E, Morosini S, Cantini G, Girgenti V, Rizzo A, Eoli M, Pollo B,
Sciacca FL, Pellegatta S, Finocchiaro G. Frequency of NFKBIA deletions is low in glioblastomas
and skewed in glioblastoma neurospheres. Mol Cancer. 2013 Dec 11;12:160
8: Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng
S,Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G,
Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W,
Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL,
Eschbacher J, Finocchiaro G, et al. TCGA Research Network. The somatic genomic landscape of
glioblastoma. Cell. 2013 Oct 10;155(2):462-.
9: Cuppini L, Calleri A, Bruzzone MG, Prodi E, Anghileri E, Pellegatta S, Mancuso P, Porrati P, Di
Stefano AL, Ceroni M, Bertolini F, Finocchiaro G, Eoli M. Prognostic value of CD109+ circulating
endothelial cells in recurrent glioblastomas treated with bevacizumab and irinotecan. PLoS One.
2013 Sep 12;8(9):e74345. doi: 10.1371/journal.pone.0074345.
10: Schneider L, Pellegatta S, Favaro R, Pisati F, Roncaglia P, Testa G, Nicolis SK, Finocchiaro G,
d'Adda di Fagagna F. DNA Damage in Mammalian Neural Stem Cells Leads to Astrocytic
Differentiation Mediated by BMP2 Signaling through JAK-STAT. Stem Cell Reports. 2013 Jul
25;1(2):123-38
11: Frattini V, Trifonov V, Chan JM, Castano A, Lia M, Abate F, Keir ST, Ji AX,Zoppoli P, Niola
F, Danussi C, Dolgalev I, Porrati P, Pellegatta S, Heguy A, Gupta G, Pisapia DJ, Canoll P, Bruce
JN, McLendon RE, Yan H, Aldape K, Finocchiaro G, et al. The integrated landscape of driver
genomic alterations in glioblastoma. Nat Genet. 2013 Oct;45(10):1141-9
12: Frattini V, Pisati F, Speranza MC, Poliani PL, Frigà G, Cantini G, Kapetis D, Cominelli M,
Rossi A, Finocchiaro G, Pellegatta S. FOXP3, a novel glioblastoma oncosuppressor, affects
proliferation and migration. Oncotarget. 2012 Sep 22;3(10):1146-57
13: Pellegatta S, Eoli M, Frigerio S, Antozzi C, Bruzzone MG, Cantini G, Nava S, Anghileri E,
Cuppini L, Cuccarini V, Ciusani E, Dossena M, Pollo B, Mantegazza R, Parati EA, Finocchiaro G.
The natural killer cell response and tumor debulking are associated with prolonged survival in
recurrent glioblastoma patients receiving dendritic cells loaded with autologous tumor lysates.
Oncoimmunology. 2013 Mar 1;2(3):e23401
14: Ferroli P, Schiariti M, Finocchiaro G, Salmaggi A, Castiglione M, Acerbi F,
Tringali G, Farinotti M, Broggi M, Roberto C, Maccagnano E, Broggi G. Operability of
glioblastomas: "sins of action" versus "sins of non-action". Neurol Sci. 2013 Dec;34(12):2107-16
15: Nava S, Dossena M, Pogliani S, Pellegatta S, Antozzi C, Baggi F, Gellera C, Pollo B, Parati
EA, Finocchiaro G, Frigerio S. An optimized method for manufacturing a clinical scale dendritic
cell-based vaccine for the treatment of glioblastoma. PLoS One. 2012;7(12):e52301
16: De Rosa A, Pellegatta S, Rossi M, Tunici P, Magnoni L, Speranza MC, Malusa F, Miragliotta
V, Mori E, Finocchiaro G, Bakker A. A radial glia gene marker, fatty acid binding protein 7
(FABP7), is involved in proliferation and invasion of glioblastoma cells. PLoS One.
2012;7(12):e52113
17: Cantini G, Pisati F, Pessina S, Finocchiaro G, Pellegatta S. Immunotherapy against the radial
glia marker GLAST effectively triggers specific antitumor effectors without autoimmunity.
Oncoimmunology. 2012 Sep 1;1(6):884-893
18: Speranza MC, Frattini V, Pisati F, Kapetis D, Porrati P, Eoli M, Pellegatta S, Finocchiaro G.
NEDD9, a novel target of miR-145, increases the invasiveness of glioblastoma. Oncotarget. 2012
Jul;3(7):723-34
19: Singh D, Chan JM, Zoppoli P, Niola F, Sullivan R, Castano A, Liu EM, Reichel J, Porrati P,
Pellegatta S, Qiu K, Gao Z, Ceccarelli M, Riccardi R, Brat DJ, Guha A, Aldape K, Golfinos JG,
Zagzag D, Mikkelsen T, Finocchiaro G, Lasorella A, Rabadan R, Iavarone A. Transforming fusions
of FGFR and TACC genes in human glioblastoma. Science. 2012 Sep 7;337(6099):1231-5
20: De Bacco F, Casanova E, Medico E, Pellegatta S, Orzan F, Albano R, Luraghi P, Reato G,
D'Ambrosio A, Porrati P, Patanè M, Maderna E, Pollo B, Comoglio PM, Finocchiaro G,
Boccaccio C. The MET oncogene is a functional marker of a glioblastoma stem cell subtype.
Cancer Res. 2012 Sep 1;72(17):4537-50
21: Bruzzone MG, D'Incerti L, Farina LL, Cuccarini V, Finocchiaro G. CT and MRI of brain
tumors. Q J Nucl Med Mol Imaging. 2012 Apr;56(2):112-37
22: Anghileri E, Eoli M, Paterra R, Ferroli P, Pollo B, Cuccarini V, Maderna E, Tringali G, Saini
M, Salsano E, Finocchiaro G. FABP4 is a candidate marker of cerebellar liponeurocytomas. J
Neurooncol. 2012 Jul;108(3):513-9
23: Eoli M, Bianchessi D, Di Stefano AL, Prodi E, Anghileri E, Finocchiaro G. Central nervous
system lymphoma occurring in a patient with neurofibromatosis type 1 (von Recklinghausen
disease). Neurol Sci. 2012 Dec;33(6):1429-33
24: Pellegatta S, Cuppini L, Finocchiaro G. Brain cancer immunoediting: novel examples provided
by immunotherapy of malignant gliomas. Expert Rev Anticancer Ther. 2011 Nov;11(11):1759-74
25: Finocchiaro G, Pellegatta S. Immunotherapy for glioma: getting closer to the clinical arena?
Curr Opin Neurol. 2011 Dec;24(6):641-7
26: Menghi F, Orzan FN, Eoli M, Farinotti M, Maderna E, Pisati F, Bianchessi D, Valletta L,
Lodrini S, Galli G, Anghileri E, Pellegatta S, Pollo B, Finocchiaro G. DNA microarray analysis
identifies CKS2 and LEPR as potential markers of meningioma recurrence. Oncologist.
2011;16(10):1440-50.
27: Cantini G, Pisati F, Mastropietro A, Frattini V, Iwakura Y, Finocchiaro G, Pellegatta S. A
critical role for regulatory T cells in driving cytokine profiles of Th17 cells and their modulation of
glioma microenvironment. Cancer Immunol Immunother. 2011 Dec;60(12):1739-50.
28: Orzan F, Pellegatta S, Poliani PL, Pisati F, Caldera V, Menghi F, Kapetis D,Marras C, Schiffer
D, Finocchiaro G. Enhancer of Zeste 2 (EZH2) is up-regulated in malignant gliomas and in glioma
stem-like cells. Neuropathol Appl Neurobiol. 2011 Jun;37(4):381-94
29: Ferroli P, Acerbi F, Finocchiaro G. From standard treatment to personalized medicine: role of
IDH1 mutations in low-grade glioma evolution and treatment. World Neurosurg. 2010
Apr;73(4):234-6
30: Pellegatta S, Poliani PL, Stucchi E, Corno D, Colombo CA, Orzan F, Ravanini M, Finocchiaro
G. Intra-tumoral dendritic cells increase efficacy of peripheral vaccination by modulation of glioma
microenvironment. Neuro Oncol. 2010 Apr;12(4):377-88
Other collaborator publications: papers published in the last five years
Marica Eoli, MD
1: Acerbi F, Broggi M, Eoli M, Anghileri E, Cavallo C, Boffano C, Cordella R, Cuppini L, Pollo B,
Schiariti M, Visintini S, Orsi C, La Corte E, Broggi G, Ferroli P. Is fluorescein-guided technique
able to help in resection of high-grade gliomas? Neurosurg Focus. 2014 Feb;36(2):E5
2: Acerbi F, Cavallo C, Broggi M, Cordella R, Anghileri E, Eoli M, Schiariti M, Broggi G, Ferroli
P. Fluorescein-guided surgery for malignant gliomas: a review. Neurosurg Rev. 2014 Oct;37
3: Pensato V, Castellotti B, Gellera C, Pareyson D, Ciano C, Nanetti L, Salsano E, Piscosquito G,
Sarto E, Eoli M, Moroni I, Soliveri P, Lamperti E, Chiapparini L, Di Bella D, Taroni F, Mariotti C.
Overlapping phenotypes in complex spastic paraplegias SPG11, SPG15, SPG35 and SPG48. Brain.
2014 Jul;137(Pt 7):1907-20
4: Aquino D, Di Stefano AL, Scotti A, Cuppini L, Anghileri E, Finocchiaro G, Bruzzone MG, Eoli
M. Parametric response maps of perfusion MRI may identify recurrent glioblastomas responsive to
bevacizumab and irinotecan. PLoS One. 2014 Mar 27;9(3):e90535
5: Patanè M, Porrati P, Bottega E, Morosini S, Cantini G, Girgenti V, Rizzo A,Eoli M, Pollo B,
Sciacca FL, Pellegatta S, Finocchiaro G. Frequency of NFKBIA deletions is low in glioblastomas
and skewed in glioblastoma neurospheres. Mol Cancer. 2013 Dec 11;12:160
6: Cuppini L, Calleri A, Bruzzone MG, Prodi E, Anghileri E, Pellegatta S, Mancuso
P, Porrati P, Di Stefano AL, Ceroni M, Bertolini F, Finocchiaro G, Eoli M. Prognostic value of
CD109+ circulating endothelial cells in recurrent glioblastomas treated with bevacizumab and
irinotecan. PLoS One. 2013 Sep 12;8(9):e74345
7: Pellegatta S, Eoli M, Frigerio S, Antozzi C, Bruzzone MG, Cantini G, Nava S, Anghileri E,
Cuppini L, Cuccarini V, Ciusani E, Dossena M, Pollo B, Mantegazza R,Parati EA, Finocchiaro G.
The natural killer cell response and tumor debulking
are associated with prolonged survival in recurrent glioblastoma patients receiving dendritic cells
loaded with autologous tumor lysates. Oncoimmunology. 2013 Mar 1;2(3):e23401
8: Acerbi F, Broggi M, Eoli M, Anghileri E, Cuppini L, Pollo B, Schiariti M, Visintini S, Orsi C,
Franzini A, Broggi G, Ferroli P. Fluorescein-guided surgery for grade IV gliomas with a dedicated
filter on the surgical microscope: preliminary results in 12 cases. Acta Neurochir (Wien). 2013
Jul;155(7):1277-86.
9: Speranza MC, Frattini V, Pisati F, Kapetis D, Porrati P, Eoli M, Pellegatta S,Finocchiaro G.
NEDD9, a novel target of miR-145, increases the invasiveness of glioblastoma. Oncotarget. 2012
Jul;3(7):723-34
10: Eoli M, Bianchessi D, Di Stefano AL, Prodi E, Anghileri E, Finocchiaro G. Central nervous
system lymphoma occurring in a patient with neurofibromatosis type 1 (von Recklinghausen
disease). Neurol Sci. 2012 Dec;33(6):1429-33
11: Menghi F, Orzan FN, Eoli M, Farinotti M, Maderna E, Pisati F, Bianchessi D, Valletta L,
Lodrini S, Galli G, Anghileri E, Pellegatta S, Pollo B, Finocchiaro G. DNA microarray analysis
identifies CKS2 and LEPR as potential markers of meningioma recurrence. Oncologist.
2011;16(10):1440-50
12: Bruzzone MG, Eoli M, Cuccarini V, Grisoli M, Valletta L, Finocchiaro G.Genetic signature of
adult gliomas and correlation with MRI features. Expert Rev Mol Diagn. 2009 Oct;9(7):709-20.
doi: 10.1586/erm.09.44
13: Yin D, Ogawa S, Kawamata N, Tunici P, Finocchiaro G, Eoli M, Ruckert C, Huynh T, Liu G,
Kato M, Sanada M, Jauch A, Dugas M, Black KL, Koeffler HP. High-resolution genomic copy
number profiling of glioblastoma multiforme by single nucleotide polymorphism DNA microarray.
Mol Cancer Res. 2009
14: Silvani A, Gaviani P, Lamperti EA, Eoli M, Falcone C, Dimeco F, Milanesi IM, Erbetta A,
Boiardi A, Fariselli L, Salmaggi A. Cisplatinum and BCNU chemotherapy in primary glioblastoma
patients. J Neurooncol. 2009 Aug;94(1):57-62.
15: Silvani A, Gaviani P, Fiumani A, Scaioli V, Lamperti E, Eoli M, Botturi A,Salmaggi A.
Systemic sagopilone (ZK-EPO) treatment of patients with recurrent malignant gliomas. J
Neurooncol. 2009 Oct;95(1):61-4
Maria Grazia Bruzzone, MD.
1: A Superfluorinated Molecular Probe for Highly Sensitive in Vivo(19)F-MRI.Tirotta I,
Mastropietro A, Cordiglieri C, Gazzera L, Baggi F, Baselli G, Grazia Bruzzone M, Zucca I, Cavallo
G, Terraneo G, Baldelli Bombelli F, Metrangolo P, Resnati G.J Am Chem Soc. 2014 Jun
18;136(24):8524-7
2: Preoperative mapping of the sensorimotor cortex: comparative assessment of task-based and
resting-state FMRI. Rosazza C, Aquino D, D'Incerti L, Cordella R, Andronache A, Zacà D,
Bruzzone MG, Tringali G, Minati L. PLoS One. 2014 Jun 10;9(6):e98860
3: Frontal cortex BOLD signal changes in premanifest Huntington disease: A possible fMRI
biomarker. Ferraro S, Nanetti L, Piacentini S, Mandelli ML, Bertolino N, Ghielmetti F, Epifani F,
Nigri A, Taroni F, Bruzzone MG, Donato SD, Savoiardo M, Mariotti C, Grisoli M. Neurology.
2014 Jun 4
4: Cerebrovascular reactivity by quantitative magnetic resonance angiography with a Co₂
challenge. Validation as a new imaging biomarker. Caputi L, Ghielmetti F, Faragò G, Longaretti F,
Lamperti M, Anzola GP, Carriero MR, Charbel FT, Bruzzone MG, Parati E, Ciceri E. Eur J Radiol.
2014 Jun;83(6):1005-10
5: Accuracy of 2-hydroxyglutarate quantification by short-echo proton-MRS at 3 T: A phantom
study. Bertolino N, Marchionni C, Ghielmetti F, Burns B, Finocchiaro G, Anghileri E, Bruzzone
MG, Minati L. Phys Med. 2014 Mar 27.
6: Parametric response maps of perfusion MRI may identify recurrent glioblastomas responsive to
bevacizumab and irinotecan. Aquino D, Di Stefano AL, Scotti A, Cuppini L, Anghileri E,
Finocchiaro G, Bruzzone MG, Eoli M. PLoS One. 2014 Mar 27;9(3)
7: The Italian Alzheimer's Disease Neuroimaging Initiative (I-ADNI): validation of structural MR
imaging. Cavedo E, Redolfi A, Angeloni F, Babiloni C, Lizio R, Chiapparini L, Bruzzone MG,
Aquino D, Sabatini U, Alesiani M, Cherubini A, Salvatore E, Soricelli A, Vernieri F, Scrascia F,
Sinforiani E, Chiarati P, Bastianello S, Montella P, Corbo D, Tedeschi G, Marino S, Baglieri A, De
Salvo S, Carducci F, Quattrocchi CC, Cobelli M, Frisoni GB. J Alzheimers Dis. 2014;40(4):941-52.
8: Survival effect of first- and second-line treatments for patients with primary glioblastoma: a
cohort study from a prospective registry, 1997-2010. Nava F, Tramacere I, Fittipaldo A, Bruzzone
MG, Dimeco F, Fariselli L, Finocchiaro G, Pollo B, Salmaggi A, Silvani A, Farinotti M, Filippini
G. Neuro Oncol. 2014 May;16(5):719-27
9: Substantia nigra in Parkinson's disease: a multimodal MRI comparison between early and
advanced stages of the disease. Aquino D, Contarino V, Albanese A, Minati L, Farina L, Grisoli M,
Elia A, Bruzzone MG, Chiapparini L. Neurol Sci. 2014 May;35(5):753-8. doi: 10.1007/s10072-
013-1595-2. Epub 2013 Dec 11.
10: Preoperative language lateralization in temporal lobe epilepsy (TLE) predicts peri-ictal, pre-
and post-operative language performance: An fMRI study. Rosazza C, Ghielmetti F, Minati L,
Vitali P, Giovagnoli AR, Deleo F, Didato G, Parente A, Marras C, Bruzzone MG, D'Incerti L,
Spreafico R, Villani F. Neuroimage Clin. 2013 Jul 11;3:73-83
11: Prognostic value of CD109+ circulating endothelial cells in recurrent glioblastomas treated with
bevacizumab and irinotecan. Cuppini L, Calleri A, Bruzzone MG, Prodi E, Anghileri E, Pellegatta
S, Mancuso P, Porrati P, Di Stefano AL, Ceroni M, Bertolini F, Finocchiaro G, Eoli M. PLoS One.
2013 Sep 12;8(9):e74345
12: The natural killer cell response and tumor debulking are associated with prolonged survival in
recurrent glioblastoma patients receiving dendritic cells loaded with autologous tumor lysates.
Pellegatta S, Eoli M, Frigerio S, Antozzi C, Bruzzone MG, Cantini G, Nava S, Anghileri E, Cuppini
L, Cuccarini V, Ciusani E, Dossena M, Pollo B, Mantegazza R, Parati EA, Finocchiaro G.
Oncoimmunology. 2013 Mar 1;2(3):e23401.
13: Connectivity of the amygdala, piriform, and orbitofrontal cortex during olfactory stimulation: a
functional MRI study. Nigri A, Ferraro S, D'Incerti L, Critchley HD, Bruzzone MG, Minati L.
Neuroreport. 2013 Mar 6;24(4):171-5
14: In medication-overuse headache, fMRI shows long-lasting dysfunction in midbrain areas.
Ferraro S, Grazzi L, Muffatti R, Nava S, Ghielmetti F, Bertolino N, Mandelli ML, Visintin E,
Bruzzone MG, Nigri A, Epifani F, Bussone G, Chiapparini L. Headache. 2012 Nov-
Dec;52(10):1520-34. doi: 10.1111/j.1526-4610.2012.02276.x. Epub 2012 Oct 23.
15. CT and MRI of brain tumors. Bruzzone MG, D'Incerti L, Farina LL, Cuccarini V, Finocchiaro
G. Q J Nucl Med Mol Imaging. 2012 Apr;56(2):112-37
16: Thoughts turned into high-level commands: Proof-of-concept study of a vision-guided robot
arm driven by functional MRI (fMRI) signals. Minati L, Nigri A, Rosazza C, Bruzzone MG. Med
Eng Phys. 2012 Jun;34(5):650-8.
17: Functional connectivity during resting-state functional MR imaging: study of the
correspondence between independent component analysis and region-of-interest-based methods.
Rosazza C, Minati L, Ghielmetti F, Mandelli ML, Bruzzone MG. AJNR Am J Neuroradiol. 2012
Jan;33(1):180-7.
18: Neuroradiological diagnosis of Chiari malformations. Chiapparini L, Saletti V, Solero CL,
Bruzzone MG, Valentini LG. Neurol Sci. 2011 Dec;32 Suppl 3:S283-6
19: Pain processing in medication overuse headache: a functional magnetic resonance imaging
(fMRI) study. Ferraro S, Grazzi L, Mandelli ML, Aquino D, Di Fiore D, Usai S, Bruzzone MG, Di
Salle F, Bussone G, Chiapparini L. Pain Med. 2012 Feb;13(2):255-62
20: Hemophagocytic lymphohistiocytosis with neurological presentation: MRI findings and a
nearly miss diagnosis. Chiapparini L, Uziel G, Vallinoto C, Bruzzone MG, Rovelli A, Tricomi G,
Bizzi A, Nardocci N, Rizzari C, Savoiardo M. Neurol Sci. 2011 Jun;32(3):473-7
21: Abnormal ERD/ERS but unaffected BOLD response in patients with Unverricht-Lundborg
disease during index extension: a simultaneous EEG-fMRI study. Visani E, Minati L, Canafoglia L,
Gilioli I, Granvillano A, Varotto G, Aquino D, Fazio P, Bruzzone MG, Franceschetti S, Panzica
F.Brain Topogr. 2011 Mar;24(1):65-77
22: Quantitation of normal metabolite concentrations in six brain regions by in-vivoH-MR
spectroscopy. Minati L, Aquino D, Bruzzone MG, Erbetta A. J Med Phys. 2010 Jul;35(3):154-63
23: Chronic migraine with medication overuse pre-post withdrawal of symptomatic medication:
clinical results and FMRI correlations. Grazzi L, Chiapparini L, Ferraro S, Usai S, Andrasik F,
Mandelli ML, Bruzzone MG, Bussone G. Headache. 2010 Jun;50(6):998-1004
24: Effect of diffusion-sensitizing gradient timings on the exponential, biexponential and
diffusional kurtosis model parameters: in-vivo measurements in the rat thalamus. Minati L, Zucca I,
Carcassola G, Occhipinti M, Spreafico R, Bruzzone MG. MAGMA. 2010 Apr;23(2):115-21
25: Simultaneous EEG-fMRI in patients with Unverricht-Lundborg disease: event-related
desynchronization/synchronization and hemodynamic response analysis. Visani E, Minati L,
Canafoglia L, Gilioli I, Salvatoni L, Varotto G, Fazio P, Aquino D, Bruzzone MG, Franceschetti S,
Panzica F. Comput Intell Neurosci. 2010:164278. doi: 10.1155/2010/164278. Epub 2010 Jan 6.
26: Decreased diffusivity in the caudate nucleus of presymptomatic huntington disease gene
carriers: which explanation? Mandelli ML, Savoiardo M, Minati L, Mariotti C, Aquino D, Erbetta
A, Genitrini S, Di Donato S, Bruzzone MG, Grisoli M. AJNR Am J Neuroradiol. 2010
Apr;31(4):706-10
27: Genetic signature of adult gliomas and correlation with MRI features. Bruzzone MG, Eoli M,
Cuccarini V, Grisoli M, Valletta L, Finocchiaro G. Expert Rev Mol Diagn. 2009 Oct;9(7):709-20.
28: Functional-MRI evaluation of pain processing in chronic migraine with medication overuse.
Chiapparini L, Grazzi L, Ferraro S, Mandelli ML, Usai S, Andrasik F, Bruzzone MG, Bussone G.
Neurol Sci. 2009 May;30 Suppl 1:S71-4
29: Diffusion tensor imaging shows different topographic involvement of the thalamus in
progressive supranuclear palsy and corticobasal degeneration. Erbetta A, Mandelli ML, Savoiardo
M, Grisoli M, Bizzi A, Soliveri P, Chiapparini L, Prioni S, Bruzzone MG, Girotti F. AJNR Am J
Neuroradiol. 2009 Sep;30(8):1482-7.
30: Age-related iron deposition in the basal ganglia: quantitative analysis in healthy subjects.
Aquino D, Bizzi A, Grisoli M, Garavaglia B, Bruzzone MG, Nardocci N, Savoiardo M, Chiapparini
L. Radiology. 2009 Jul;252(1):165-72
Serena Pellegatta, PhD (10 most relevant papers)
1: Finocchiaro G, Pellegatta S. Perspectives for immunotherapy in glioblastoma treatment. Curr
Opin Oncol. 2014 Sep 10
2: Favaro R, Appolloni I, Pellegatta S, Sanga AB, Pagella P, Gambini E, Pisati F, Ottolenghi S, Foti
M, Finocchiaro G, Malatesta P, Nicolis SK. Sox2 is required to maintain cancer stem cells in a
mouse model of high-grade oligodendroglioma. Cancer Res. 2014 Mar 15;74(6):1833-44
3: Pellegatta S, Eoli M, Frigerio S, Antozzi C, Bruzzone MG, Cantini G, Nava S, Anghileri E,
Cuppini L, Cuccarini V, Ciusani E, Dossena M, Pollo B, Mantegazza R, Parati EA, Finocchiaro G.
The natural killer cell response and tumor debulking are associated with prolonged survival in
recurrent glioblastoma patients receiving dendritic cells loaded with autologous tumor lysates.
Oncoimmunology. 2013 Mar 1;2(3):e23401
4: Frattini V, Pisati F, Speranza MC, Poliani PL, Frigé G, Cantini G, Kapetis D, Cominelli M,
Rossi A, Finocchiaro G, Pellegatta S. FOXP3, a novel glioblastoma oncosuppressor, affects
proliferation and migration. Oncotarget. 2012 Sep 22;3(10):1146-57
5: Cantini G, Pisati F, Pessina S, Finocchiaro G, Pellegatta S. Immunotherapy against the radial glia
marker GLAST effectively triggers specific antitumor effectors without autoimmunity.
Oncoimmunology. 2012 Sep 1;1(6):884-893
6: Pellegatta S, Cuppini L, Finocchiaro G. Brain cancer immunoediting: novel examples provided
by immunotherapy of malignant gliomas. Expert Rev Anticancer Ther. 2011 Nov;11(11):1759-74.
7: Finocchiaro G, Pellegatta S. Immunotherapy for glioma: getting closer to the clinical arena? Curr
Opin Neurol. 2011 Dec;24(6):641-7
8: Cantini G, Pisati F, Mastropietro A, Frattini V, Iwakura Y, Finocchiaro G, Pellegatta S. A critical
role for regulatory T cells in driving cytokine profiles of Th17 cells and their modulation of glioma
microenvironment. Cancer Immunol Immunother. 2011 Dec;60(12):1739-50
9: Pellegatta S, Poliani PL, Stucchi E, Corno D, Colombo CA, Orzan F, Ravanini M, Finocchiaro
G. Intra-tumoral dendritic cells increase efficacy of peripheral vaccination by modulation of glioma
microenvironment. Neuro Oncol. 2010 Apr;12(4):377-88
10: Pellegatta S, Poliani PL, Corno D, Menghi F, Ghielmetti F, Suarez-Merino B, Caldera V, Nava
S, Ravanini M, Facchetti F, Bruzzone MG, Finocchiaro G. Neurospheres enriched in cancer stem-
like cells are highly effective in eliciting a dendritic cell-mediated immune response against
malignant gliomas. Cancer Res. 2006 Nov 1;66(21):10247-52.
Attachment: Cofund
Source: http://fondazioneceleghin.it/wp-content/uploads/2015/12/project_fondazione_celeghin2014_finocchiaro.pdf
N° 10 Abril 2013 Publicación de contenido científico editada por GT Laboratorio S.R.L. Necochea 3274 Rosario Método enzimático UV para la determinación cuantitativa de lactato en suero, plasma o líquido LACTATO Liquid Plus cefalorraquídeo. El nuevo método enzimático UV GT Lab para la determinación de lactato permite la cuantificación rápida y precisa, empleando un reactivo de tra-bajo único de sencilla preparación. Dicha preparación se hace por disolución de un polvo en el buffer provis-to listo para usar. El reactivo de trabajo es estable 24 horas a temperatura ambiente o 15 días refrigerado. Se resume seguidamente el documento "Lactato: utilidad clínica y reco-mendaciones para su medición", de la Sociedad Española de Química Clí-nica preparado por P. Guevara Ramírez, R. Díaz García, A. Galán Ortega, E. Guillén Campuzano, S. Malumbres, J.L. Marín Soria, M. Muñoz Pérez, X. Navarro Segarra, P. Oliver Sáez, E. Oujo, N. del Río Barcenilla y A. Buño Soto en 2010.
Activity of the new quinolone WCK 771 against pneumococciP. C. Appelbaum1, G. A. Pankuch1, B. Bozdogan1, G. Lin1, M. R. Jacobs2, M. V. Patel3, S. V. Gupte3,M. A. Jafri3, N. J. De Souza3 and H. F. Khorakiwala3 1Department of Pathology, Hershey Medical Center, Hershey, PA, 2Department of Pathology, CaseWestern Reserve University, Cleveland, OH, USA and 3Wockhardt Research Centre, Aurangabad,India









