Pharmacotherapy impacts functional connectivity among affective circuits during response inhibition in pediatric mania


Contents lists available at
Behavioural Brain Research
Pharmacotherapy impacts functional connectivity among affective circuits
during response inhibition in pediatric mania
Mani N. Pavuluri , James A. Ellis , Ezra Wegbreit , Alessandra M. Passarotti , Michael C. Stevens
a Pediatric Brain Research and Intervention Center, Institute for Juvenile Research, Berger-Colbeth Clinic, University of Illinois at Chicago, IL, USA
b Olin Neuropsychiatry Research Center, The Institute of Living/Hartford Hospital, Yale University School of Medicine, CT, USA
Objective: The aim of the current study was to determine the influence of implicated affective circuitry
Received 17 August 2011
disturbance in pediatric bipolar disorder (PBD) on behavioral inhibition. The differential influence of
Received in revised form
an antipsychotic and an anti-epileptic medication on the functional connectivity across affective and
23 September 2011
cognitive neural operations in PBD was examined.
Accepted 3 October 2011
Methods: This was a six-week double blind randomized fMRI trial of risperidone plus placebo vs. dival-
Available online 8 October 2011
proex plus placebo for patients with mania (n = 22; 13.6 ± 2.5 years). Healthy controls (HC; n = 14,
14.5 ± 2.8 years) were also scanned for normative comparison. Participants performed a response inhibi-
tion fMRI task where a motor response, already ‘on the way' to execution, had to be voluntarily inhibited
Functional connectivity
on trials where a stop signal was presented. Independent component analysis was used to map functional
connectivity across the whole brain.
Response inhibition
Results: While there were no behavioral differences between the groups at pre- or post-drug trial, there
was significant improvement on manic symptoms in the patient groups. All participants engaged an
evaluative affective circuit (EAC: bilateral inferior frontal gyrus, middle frontal gyrus, anterior cingulate
cortex (ACC), middle temporal gyrus, insulae, caudate and putamen) and a reactive affective circuit (RAC:
bilateral occipital cortex, amygdala, medial frontal gyrus and insula) during task performance. Within the
EAC, post-treatment and relative to HC, greater engagement was seen in left insula in risperidone group
and left subgenual ACC in divalproex group. Within the RAC, greater baseline amygdala connectivity in
patients did not alter with treatment.
Conclusion: EAC and RAC are two key circuits that moderate emotional influence on response inhibition
in PBD. Risperidone and divalproex differentially engage the EAC. Limited change in amygdala activity
with treatment in all patients indicates a likely trait deficit in PBD.
2011 Elsevier B.V. All rights reserved.
circuit consisting of the amygdala, posterior cingulate/precuneus,
and fusiform/parahippocampal gyrus further dys-
Overcoming the important challenges in understanding how
function at multiple circuitry-wide levels. Similarly, decreased
affective abnormalities contribute to disinhibition and impulsivity
functional connectivity during mania between DLPFC and tem-
in pediatric bipolar disorder (PBD) will help discover how interven-
poral circuitry has even been found while participants were in a
tions can alter these neural operations. To date, numerous studies of
resting state Further, pharmacological fMRI studies of pedi-
pediatric mania have focused on affective processing with or with-
atric mania with risperidone, divalproex and lamotrigine have
out cognitive challenge which have typically shown under activity
shown increased activity during cognitive control under emo-
in ventrolateral prefrontal cortex (VLPFC), medial PFC (MPFC), dor-
tional challenge in VLPFC, DLPFC, MPFC, subgenual ACC, temporal
solateral PFC (DLPFC) overactivity in anterior cingulate
lobe, and striatum but the amygdala remained overac-
cortex (ACC), amygdala, and striatum addition, reduced
tive relative to healthy controls (HC) affective circuitry
connectivity in PBD was found in an emotional face response
level disturbance in PBD is likely to influence cognitive function,
given our previous findings illustrating a strong interlink between
affective and cognitive systems Therefore, in the cur-
rent study, we sought to further examine how affective neural
Corresponding author at: Pediatric Brain Research and Intervention Center, 1747
systems commonly involved in PBD influence impulsivity and dis-
West Roosevelt Road, Department of Psychiatry, Chicago, IL 60608, USA.
Tel.: +1 312 413 0064, fax: +1 312 413 0063.
inhibition using cognitive paradigms without any affective stimuli
E-mail address: (M.N. Pavuluri).
0166-4328/$ – see front matter
2011 Elsevier B.V. All rights reserved.
M.N. Pavuluri et al. / Behavioural Brain Research 226 (2012) 493–503
There have been several fMRI studies of motor inhibition in
this study was to use functional connectivity to map PBD patho-
pediatric mania, including one treatment study. Leibenluft et al.
physiology within the hypothesized EAC and RAC. We predicted
that failed inhibition during a Stop signal task in PBD
that PBD patients would show less functional connectivity within
involved decreased activation in right VLPFC and bilateral stria-
each of these key networks during response inhibition.
tum when compared to healthy controls. In addition, a recent study
A second goal was to link the hypothesized functional connec-
employing a blocked Go/No-Go task similar to the Stop signal task
tivity deficits to clinical improvement in response to two different
used in the current study found increased activation in the DLPFC
classes of medications known to stabilize affect in PBD. One of the
in participants with PBD relative to controls However, the
medications, risperidone, an antipsychotic that acts by serotonin
Go/No-Go task employed in the Singh et al. study involved with-
dopamine antagonism, known to reduce manic symptoms
holding a prepotent response, rather than interrupting a response
in PBD, improves VLPFC and MPFC activity pre-
that was already on the way to completion, so the results of that
dicted that risperidone would improve EAC functional connectivity
study might not be directly comparable with the present study.
and that the treatment-induced reduction in manic symptoms will
Recent studies by Passarotti et al. that emotion-
correlate with the change in EAC connectivity. The other medi-
ally linked brain regions such as the VLPFC and pregenual ACC are
cation, divalproex sodium (divalproex), is an anti-epileptic that
implicated both in the cognitive control of emotional processing
modulates intracellular pathways also serves as a tradi-
and in behavior inhibition in pediatric mania relative to atten-
tional mood stabilizer known to reduce both manic and depressive
tion deficit hyperactivity disorder (ADHD). Therefore, a particular
symptoms that a similar anti-epileptic, lamotrigine,
challenge in PBD studies is to unravel the relationship between
led to greater subgenual and MPFC activity in pediatric mania dur-
emotional systems and inhibitory control systems in manic states.
ing response inhibition predicted that divalproex would
In addition, understanding the effects of medication treatments on
also improve functional connectivity in EAC and that reduction
the neural networks involved in response inhibition in PBD would
in both the manic and depressive symptoms will correlate with
help us understand the intervention effects on the neural func-
the change in EAC. With regards to the RAC, previous functional
tion. For example, lamotrigine monotherapy has been shown to
imaging results are equivocal, with persistent increase in amygdala
enhance underactive MPFC and temporal lobe regions during a
activity, relative to HC, with treatment of mania decreased
response inhibition task, suggesting that mood stabilizers which
activity with reduction in depression within patients find-
improve function in affective regions can also influence the brain
ings will inform if functional connectivity in this circuit will be
networks involved in response inhibition how dys-
altered by either of the medications. Essentially, given that this
function in the neural networks central to affective disturbance in
is the first study comparing divalproex and risperidone effect on
PBD motor inhibition will more conclusively link the
behavior inhibition in pediatric mania, we began with the premise
affective disturbance that is the hallmark of PBD to its behavioral
that both medications would have an equal impact on EAC and RAC.
Informed by the aforementioned fMRI studies of PBD, we have
2. Methods
proposed theoretical models of functional networks
regions of higher cortical evaluation of emotional and behavioral
control in the VLPFC, evaluation in the MPFC,
This was an NIH-funded (1 K23 RR018638-01) six-week outpatient double blind
executive function of emotion modulation in the DLPFC
randomized controlled trial (DBRCT) of risperidone plus placebo (that resembled a
divalproex capsule) vs. divalproex plus placebo (that resembled a risperidone tablet)
emotional and cognitive control and mod-
for manic and mixed episodes of bipolar disorder. This study was approved by the
ulation is accompanied by greater activity in the ACC in PBD
University of Illinois at Chicago's Institutional Review Board (IRB).
in both compensatory error correction and the
complex interface of affective and cognitive processing
collection of brain regions can be considered an evaluative affective
Inclusion criteria were a DSM-IV diagnosis of mixed or manic bipolar disorder;
circuit (EAC) that is likely to contribute to behavioral disinhibition
12–18 years old; and medication free or currently clinically unstable on medication,
in PBD by interfering with oversight of behavioral control. Another
justifying termination of the ineffective regimen (with consent, all subjects were
proposed complementary posterior circuit was an occipito-limbic
washed out and free of any medication for a week prior to baseline scanning, and
associative circuit the occipital cortex and amygdala,
four weeks in case of fluoxetine or aripiprazole). Prior exposure to SGAs and anti-
epileptic medications was acceptable. Exclusion criteria included: active substance
which is activated in response to incidental emotional process-
abuse; serious medical problems; autism and non-affective psychotic disorders. Par-
ing This reactive affective circuit (RAC) contributes to
ticipants who had a diagnosis of ADHD preceding the onset of PBD were excluded to
impulsive automatic response tendencies, which are moderated by
reduce the confound of comorbid attentional disorders, relevant specifically in prob-
evaluative MPFC region.
ing response inhibition. Using these criteria, we recruited 44 subjects into the study.
The regions within EAC and RAC inter-communicate and are
After excluding subjects whose data were unusable due to motion artifacts (HC:
n = 2; risperidone group: n = 3; divalproex group: n = 3), the final sample included
important for task success. We implemented a novel approach to
in the analyses consisted of 14 HC, and 22 patients randomized to either risperi-
study functional connectivity among specific brain regions within
done (n = 11) or divalproex (n = 11). No subjects dropped out of the study. Sample
the EAC and RAC networks that influence activity in distal regions
characteristics are summarized in
within each network during task performance Independent
2.3. Assessment and efficacy measures
component analysis (ICA), used in the current study, is a model-free
technique that robustly identifies distinct spatiotemporal profiles
Each child and their parent or legal guardian were interviewed using the
of distributed brain function that closely correspond to known
Washington University in St. Louis Kiddie Schedule for Affective Disorders and
anatomical neural networks. This approach was used as a means to
Schizophrenia (WASH-U-KSADS) supplemented by the episode characterization of
test hypotheses regarding regional functional connectivity during
bipolar disorder from the KSADS – Present and Lifetime version
interviews were completed by doctoral-level clinicians with established inter-rater
response inhibition. Although previous studies have documented
reliability. The primary clinical efficacy measure was the Young Mania Rating Scale
impairments in specific brain regions underlying response inhi-
(YMRS) Child Depression Rating Scale-Revised was also administered
bition in PBD and other studies have documented
networks involved in response inhibition in normal adolescents,
2.4. Study dosing of risperidone and divalproex
date, there have been no direct tests of whether brain regions
in the EAC or RAC show disrupted functional connectivity in PBD
The mean (standard deviation, SD) risperidone dose at endpoint was
during demands for behavioral control. Therefore, the first goal of
1.43 (±0.35) mg/day in non-responders and 1.33 (±0.43) mg/day in responders
M.N. Pavuluri et al. / Behavioural Brain Research 226 (2012) 493–503
Demographic variables and clinical characteristics.
Age in years (Age range: 10–19)
Estimated range: 70–139)
88.54, p < 0.001
16.73, p < 0.001
17.04, p < 0.001
DVPX = Divalproex; RISP = Risperidone; HC = Healthy control; YMRS = Young Mania Rating Scale; CDRS-R = Child Depression Rating Scale-Revised; SES = Socioeconomic status.
a Estimated with Wechsler Abbreviated Scale of Intelligence (WASI; Matrix Reasoning and Vocabulary Subtests).
(defined as improvement ≥50% on the YMRS scores). The mean (SD) divalproex
displacement from the median head position was greater than 1.5 mm, or if head
dose at endpoint was 863.64 (±210.54) mg/day in non-responders and 855.14
rotation from the median head position was greater than 0.5◦. There were no sig-
(±245.23) mg/day in responders. The mean serum valproic level at end point was
nificant group differences in the number of volumes retained after discarding those
98 g/mL, and 95% of patients achieved a therapeutic serum valproic level of
with motion artifact. Individual volumes were excluded from analyses if, relative to
>75 g/mL by the 5th day. No titration of medications was allowed after day 7.
median head position, head displacement was greater than 1.5 mm or head rotation
One subject in the divalproex group received lorazepam as a rescue medication at
was greater than 0.5◦. T-tests revealed no significant group differences in the num-
a dose of 2 mg for severe agitation during the first week of the trial. No other rescue
ber of volumes retained after discarding those with motion artifact. After motion
medications or stimulants were used during the trial
correction and de-trending using FIASCO, the functional images were preprocessed
with SPM5 Slice timing correc-
2.5. fMRI session: response inhibition task
tion was applied to the data to remove signal variation due to slice acquisition
temporal onset differences. The first functional image volume of each participant
was used to determine parameters for spatial normalization into Montreal Neu-
The fMRI behavioral paradigm was a block design task in which a motor
rological Institute (MNI) standardized space employed in SPM5 using non-linear
response, already ‘on the way' from planning to execution, had to be voluntarily
transformation. The normalization parameters determined for the first functional
inhibited when a cue instructing subjects to stop an impending response was pre-
volume were subsequently applied to all of the 240 functional image volumes for
sented on some trials the beginning of each trial a fixation cross appeared for
each participant. The normalized functional images were then smoothed with a
850 ms. On Go trials, a target stimulus (a green airplane) was presented for 800 ms.
12-mm full width at half-maximum Gaussian filter
On Stop trials, a Stop signal (a man holding a Stop signal in his hands) replaced the
airplane with equal probability 250, 350, or 450 ms after the airplane appeared and
2.8. Independent component analysis
subjects had to inhibit their response. The task lasted 6.11 min and consisted of six
experimental blocks, three of which were Go blocks (G) and three of which were
fMRI time series from all participants for the response inhibition task were ana-
Stop blocks (S), and there were 7 resting blocks (F) of 10 s fixation each. Each exper-
lysed using a group ICA algorithm (GIFT v1.3h;
imental block had 30 trials and lasted 49.5 s. The experimental and fixation blocks
The fMRI time series data for all participants were concatenated, then subjected
were pseudo-randomly interspersed as follows: (F) G (F) S (F) S (F) G (F) S (F) G (F).
to two principal component analysis data reduction stages data under-
We adopted this 70/30 proportion of trials in both the Go and Stop blocks (e.g., 70%
went a final ICA rotation using Infomax that produced 37 maximally independent
Stop trials in Stop block and 70% of Go trials in Go block) so that subjects would not
components number of components to estimate was determined using the
habituate to fixed trial presentation within a certain block
minimum description length criteria the ICA-derived group solution, data
for each participant were then back-reconstructed that individual participant
2.6. MRI protocols
variability was retained for hypothesis testing. For each component, this back-
reconstruction method produced a spatial map representing brain regions within
Gradient-echo echo-planar functional imaging and structural acquisitions were
each component "network", and a time course of BOLD signal change across the
performed with a 3.0 T whole body scanner (Signa, General Electric Medical System,
fMRI paradigm. Group analyses of spatial maps determined differences in degree of
Milwaukee, WI) at the MR Center within the UIC Hospital. To minimize head motion
regional functional connectivity, while analyses of time-course information allowed
we restricted the participants' head with foam cushions. T2*-weighted functional
us to determine whether or not study groups engaged each network during the fMRI
images were acquired with a gradient-echo echo-planar sequence (TR = 2500 ms,
TE = 25 ms, flip angle = 90◦, FOV 20 × 20 cm2, 64 × 64 matrix, 3.125 × 3.125 mm in
A systematic process was used on the 37 independent components to identify
plane resolution, 5-mm slice thickness, 1-mm gap, 25 slices). Anatomical images
those that would be retained for further analysis. The correlation of each compo-
were also acquired in the axial plane (three-dimensional spoiled gradient recalled,
nent's spatial map with a priori probabilistic maps of gray matter, white matter,
1.5 mm thick contiguous axial slices) and were later co-registered with the func-
and cerebral spinal fluid (CSF) within MNI space (templates provided in SPM5)
tional data. The experiment run consisted of 240 time points including a 5 s rest
was calculated for all components. After discarding components with an r-squared
session at the beginning that was collected to allow for T1 effects to stabilize. These
value >0.025 with CSF or white matter, or with low correlation to gray matter that
initial two images were not included in the analyses
could be an artifact, 28 components were retained. This step primarily identified and
excluded obvious signal artifacts (e.g., head motion, cardiac inflow pulsatile motion).
2.7. fMRI image processing and motion correction
We then discarded components not engaged by the fMRI task. To assess task engage-
ment, component time courses were parameterized using multiple regression to
FIASCO software (Functional Imaging Analysis Software – Computational Olio)
provide association coefficients (-weights) between component time courses and
used to implement 3D motion estimation and correction, removal of slow
an overall condition model of the response inhibition task (i.e., one condition model
signal drift, and identification of images with artifacts such as high shot noise or
for both Stop and Go blocks). One sample t-tests against zero were carried out on
displacement that cannot be readily corrected by motion correction algorithms.
the  weights (pooled across groups) to determine if the evidence for task engage-
We excluded from the analyses individual volumes from the time series if head
ment was greater than zero (i.e., whether or not the network was engaged by the
M.N. Pavuluri et al. / Behavioural Brain Research 226 (2012) 493–503
Influence of pharmacotherapy on response inhibition.
Region of interest
Co-ordinates x, y, z in MNI
Results in the evaluative affective circuit
HC > PBD at baseline (pre-treatment)
Risperidone > HC changes over treatment (Post- vs. pre-treatment)
L inferior frontal gyrus
R inferior frontal gyrus
R medial frontal gyrus
L medial frontal gyrus
Divalproex > HC changes over treatment (Post- vs. pre-treatment)
L inferior frontal gyrus
R inferior frontal gyrus
R medial frontal gyrus
L medial frontal gyrus
Results in the reactive affective circuit
HC > PBD at baseline (pre-treatment)
−23, −4, −19
Risperidone > HC changes over treatment (Post- vs. pre-treatment)
−23, −4, −19
Divalproex > HC changes over treatment (Post- vs. pre-treatment)
L = Left; R = Right; ACC = Anterior cingulate cortex; HC = Healthy controls, PBD = Pediatric bipolar disorder.
task). Only 14 components were significantly (p < 0.05) associated with the overall-
2.11. Whole-brain ANOVAS and region-of-interest (ROI) analyses of functional
condition model and were retained. Estimates were then derived for Stop and Go
blocks separately to investigate the effects of task set on brain networks. To identify
and visualize which brain regions were significantly engaged in each component,
As described above, ICA produced a spatial map for each participant × fMRI
individual participants' spatial maps were entered into an SPM5 voxel-wise one-
sessions (i.e., pre- vs. post-treatment) that depicted the voxel-wise strength of
sample t-test for each component. Component spatial structure was visualized by
functional connectivity. These were examined in a series of SPM5 2 × 2 (pre- vs.
overlaying these results on axial slices of representative brain anatomy. Significance
post-treatment session by group) ANOVA models that contrasted PBD drug effects
was evaluated using p < 0.05 family-wise error rate correction for the whole brain
of divalproex vs. healthy controls, risperidone vs. healthy controls, and finally
risperidone vs. divalproex. The defined ROIs from the EAC and RAC networks
used two-tailed ˛ = 0.05, corrected for the number of ROIs searched (ROI masks
2.9. Clinical effects of treatment and behavioral analyses
Participants' YMRS and CDRS-R values were analysed with 2 × 3 time (pre- vs.
2.12. Correlations of ROI-derived functional connectivity values and clinical
post-trial) by group (risperidone, divalproex, HC) Analyses of variance (ANOVA).
Participants' reaction time and accuracy were examined using a series of 2 × 2 × 3
ANOVAs; block (Go vs. Stop) by time (pre- vs. post-trial) by group (risperidone,
To investigate the effect of changes in brain regions on the clinical indices, cor-
divalproex, HC). In accordance with signal detection theory, sensitivity (d-prime)
relations were conducted within each drug treatment group between the areas that
and criterion bias (c-bias) measures were computed from participants' hit (correct
showed significant changes in the ANOVAs and the changes in YMRS and CDRS-
Go) and false alarm (incorrect Stop) rates. The d-prime score represents participants'
R scores. In all, eight correlations were conducted: for the risperidone group, the
ability to adequately detect whether or not a signal is present – in this case, it is a
YMRS and CDRS-R scores were correlated with the connectivity in the left insula
proxy for their ability inhibit or reverse an already in progress response in response
after treatment and the change in connectivity from pre- to post-treatment, and in
to the Stop signal. The c-bias score represents participants' overall tendency to
the divalproex group, the two clinical indices were each correlated with post and
respond, with higher positive values indicating a greater likelihood to respond to
change in connectivity in the left BA25, given that those were the areas that changed
any trial. These measures were examined using separate 2 × 2 × 3 ANOVAs: block
in each group.
type (Go vs. Stop) by session (pre- vs. post-trial) by group (risperidone, divalproex,
2.13. Correlations of brain and clinical data to behavioral indices
2.10. Identification of task-engaged networks affected by treatment
To investigate the effect of changes in brain regions on the participants' behav-
ioral performance, similar correlations were conducted within each drug treatment
Given that the focus of this study was to examine differential changes between
group between the areas that showed significant changes in the ANOVAs and the
medication groups in the engagement of brain circuits during the response inhi-
changes in sensitivity (d-prime) and response threshold (c-bias) scores. In all, eight
bition task over the course of treatment, with the HC group serving as a control
additional correlations were conducted: for the risperidone group, the d-prime and
for practice effects and development, our analyses focused on two networks that
c-bias scores were correlated with the connectivity in the left insula after treatment
showed a significant group by time interaction. ANOVA compared the beta weights
and the change in connectivity from pre- to post-treatment, and in the divalproex
between groups in SPSS using group (risperidone, divalproex, HC) as the between-
group, the two behavioral indices were each correlated with post and change in
subjects factor, and time (pre vs. post) as the within-subjects factor. Because the Stop
connectivity in the left BA25, given that those were the areas that changed in each
blocks and Go blocks likely engaged different, albeit related cognitive processes,
group. Finally, four correlations between the changes in clinical data and the changes
separate ANOVAs were conducted for Stop and Go blocks (
in behavioral data were also conducted, but no significant results were found
M.N. Pavuluri et al. / Behavioural Brain Research 226 (2012) 493–503
3. Results
divalproex group showed greater connectivity across treatment,
relative to HC, in the left subgenual ACC (BA25) (t(23) = 0.98,
Sample demographic and clinical data are summarized in
p = 0.05), and the left insula (t(23) = 1.84, p =
Comorbid diagnosis included separation anxiety disorder
When compared directly with each other, the risperidone group,
(n = 1) and conduct disorder (n = 1) in risperidone group, and gen-
relative to the divalproex group, showed a trend toward greater
eralized anxiety disorder (n = 1) and a history of substance abuse
functional connectivity in the left insula ROI (t(20) = 1.91, p = 0.07).
(n = 1) in divalproex group.
In the RAC, risperidone group showed decreased change in func-
tional connectivity in the left amygdala, relative to HC (t(23) = 2.47,
3.1. Behavioral results
p = 0.01) Similarly, the divalproex group showed
decreased change in functional connectivity, relative to HC, in the
Patient groups and HC did not differ in reaction time (RT) at
right amygdala (t(23) = 2.44, p = 0.01) With regards
baseline or in degree of change with time between groups, and all
to occipital cortex, relative to HC, the risperidone group showed an
subjects slowed down with time. Accuracy did not differ between
increase in the right middle occipital gyrus (t(23) = 2.96, p = 0.003)
groups in the Go blocks or the Stop blocks and did not change with
and the divalproex group showed an increase in functional connec-
tivity in the right superior occipital gyrus (t(23) = 2.14, p = 0.02).
Patient groups and HC did not show any significant differ-
ences on the 2 × 2 × 3 block type by session by group ANOVA for
3.3. Correlations of ROI values and clinical indices
d-prime scores, except for a significant difference between the
groups' overall d-prime scores, F(2,30) = 3.59, p = 0.040. Post hoc
For the risperidone group, there was a significant correlation
tests revealed that although the two patient groups did not dif-
between the change in YMRS scores and the level of functional con-
fer from each other, they both showed significantly less sensitivity
nectivity of the insula after treatment (r(9) = −0.629, p = 0.038). For
overall than the HC. All groups, however, did show increases in
the divalproex group, there was a significant correlation between
sensitivity on the Go blocks – albeit nonsignificantly – and the
the change in functional connectivity in the left subgenual ACC
HC showed an increase on the Stop Blocks, whereas the patients
between sessions and the decrease in CDRS-R score (r(9) = −0.713,
showed slight decreases. In addition, although there was a signifi-
p = 0.014). No other correlations between clinical indices and activ-
cant difference in sensitivity between the HC and the two patient
ity changes in these ROIs were found.
groups in the first session, F(2,30) = 5.07, p = 0.013, there was no
such difference in the second session, F(2,30) = 1.95, p = 0.160. Thus
3.4. Correlations of ROI values and behavioral indices
there is some evidence that the PBD group became slightly better
at the task, relative to HC, before and after medication treatment.
For the risperidone group, there was a significant correla-
The criterion bias scores suggest a possible mechanism for these
tion between the change in participants' response threshold and
slight increases in sensitivity. All of the participants shifted from
the level of functional connectivity of the insula after treatment
a more liberal responding threshold (higher c-bias) in the first
(r(9) = 0.84, p = 0.009), as well as a trend toward a correlation
session to a more conservative one (lower c-bias) in the second
between participants' threshold change and the change in left
session, F(2,30) = 9.65, p = 0.004, with no other effects of group or
insula connectivity (r(9) = −0.690, p = 0.05). For the divalproex
block type. Taken together, the behavioral results indicate that
group, none of the correlations were significant.
both patients' and controls were able to strategically adjust their
response criteria in the second session to attempt to improve their
performance above the first session, but that HC were slightly
more successful than patients in actually doing so. (A table and
additional detail that summarizes the behavioral data is available
There are four key findings in this study. First, the ICA meth-
two hypothesized functional circuits
engaged during response inhibition, the "appraising" EAC (bilateral
inferior frontal gyrus, middle frontal gyrus, ACC, middle temporal
3.2. fMRI whole brain analyses results
gyrus, insulae, striatum) and the "automatic" RAC (bilateral occip-
ital lobe, parietal lobe, and amygdala). Second, ROI analysis within
Only two networks survived our selection process that identi-
the EAC and RAC confirmed our hypothesis that medications altered
fied non-artifactual, task-associated components with evidence for
the functional connectivity. Patients, relative to HC and within EAC,
significant effects of treatment in the PBD group. The first compo-
showed greater engagement of left insula with risperidone and left
nent (henceforth known as the Evaluative Affective Circuit (EAC))
subgenual ACC with divalproex. Third, as predicted, reduction in
showed functionally-integrated activity in the bilateral inferior
manic symptoms correlated with increased connectivity of insula
frontal gyrus, middle frontal gyrus, anterior cingulate cortex, mid-
within EAC with risperidone. The reduction in depressive symp-
dle temporal gyrus, insulae, and striatum (FDR p < 0.001,
toms correlated with increased left subgenual engagement with
The second component (henceforth known as the Reactive Affec-
divalproex. Fourth, amygdala functional connectivity did not alter
tive Circuit (RAC)) showed functionally-integrated activity in the
with either of the medications. This could be a trait abnormality in
bilateral occipital lobe, parietal lobe, and amygdala (FDR p < 0.001,
patients where prefrontal effects, rather than subcortical activity
The increase or decrease in activity within the networks sig-
changes, are seen during treatment, during motor inhibition. An
nifies their connectivity within the component in all subjects, PBD
alternate explanation may be that the amygdala activity reduces
and HC included, during the task performance.
with treatment, but relative to HC, it remains high.
Next, the change in ROI within the EAC and RAC were examined
Both groups engaged in the behavioral strategy of becoming
to determine the change in connectivity among these networks
more conservative in their responses, but were only slightly suc-
within each patient group in response to risperidone or dival-
cessful in translating this strategy into improved performance to
proex, relative to HC. In the EAC, the risperidone group showed
perform the task in the second session than the patients were, but
greater increase in functional connectivity across treatment, rel-
both groups showed a similar shift in strategy. Thus, these results
ative to HC, in the left and (t(23) = 3.22, p = 0.003) and the right
suggest that as patients' mood symptoms abated with treatment,
insula (t (23) = 1.90, p = 0.07) (Also in the EAC, the
they were able to employ the same response strategy as HC.
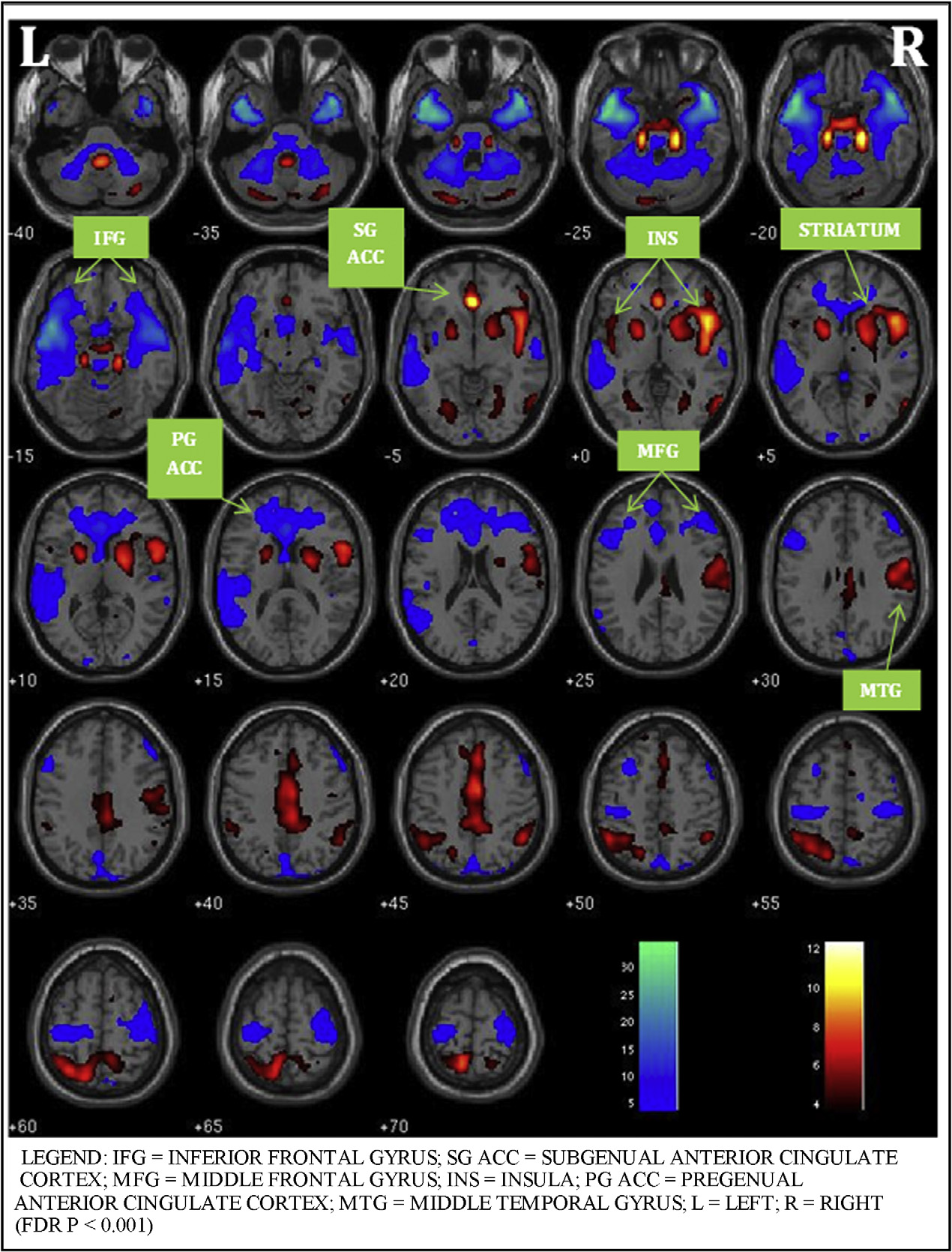
M.N. Pavuluri et al. / Behavioural Brain Research 226 (2012) 493–503
Fig. 1. Evaluative affective circuit during response inhibition.
4.1. ICA reveals EAC and RAC engagement in PBD during response
networks were significantly engaged differently in patients, rela-
tive to HC. On tasks where performance might be challenging and
elicit emotional reactions to success or failures, demanding the
We were able to map two distinct functional networks, the EAC
need to modulate responses in the context of performing an ardu-
and the RAC, in PBD and HC. Interestingly, these putative emotional
ous task, evaluative higher cortical regions have come into play
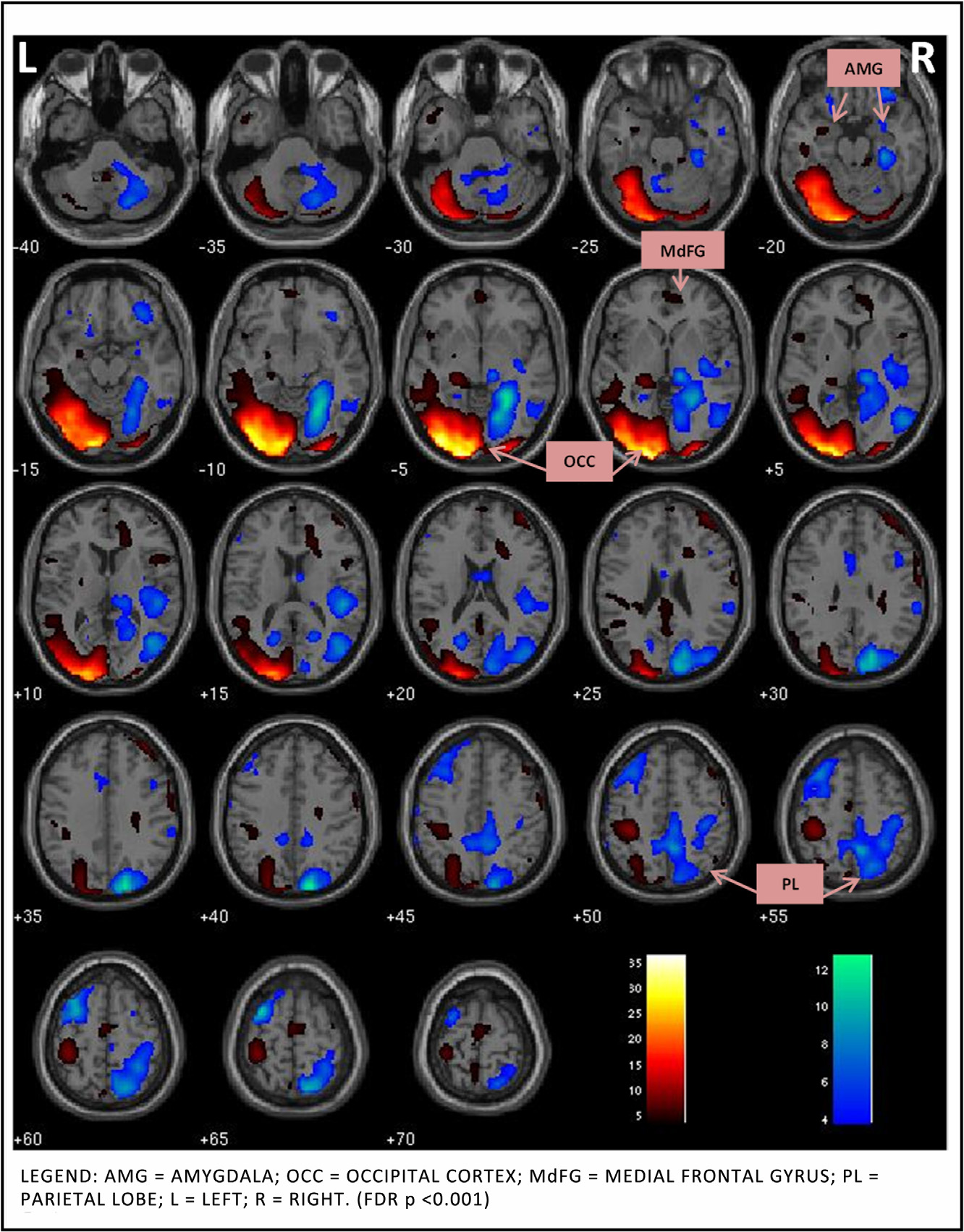
M.N. Pavuluri et al. / Behavioural Brain Research 226 (2012) 493–503
Fig. 2. Reactive affective circuit during response inhibition.
is now well established that inferior frontal gyrus engages
of insula, ACC, and temporo-striatal regions a
in affective and response inhibition control, has
liaison between affective and cognitive regions of decision con-
strong connectivity to the "cognitive" middle frontal gyrus
trol, error correction, and emotional moderation, as in this study.
These cortical regions of EAC work in concert with opercular regions
For example, in previous work, the pregenual cingulate-insular
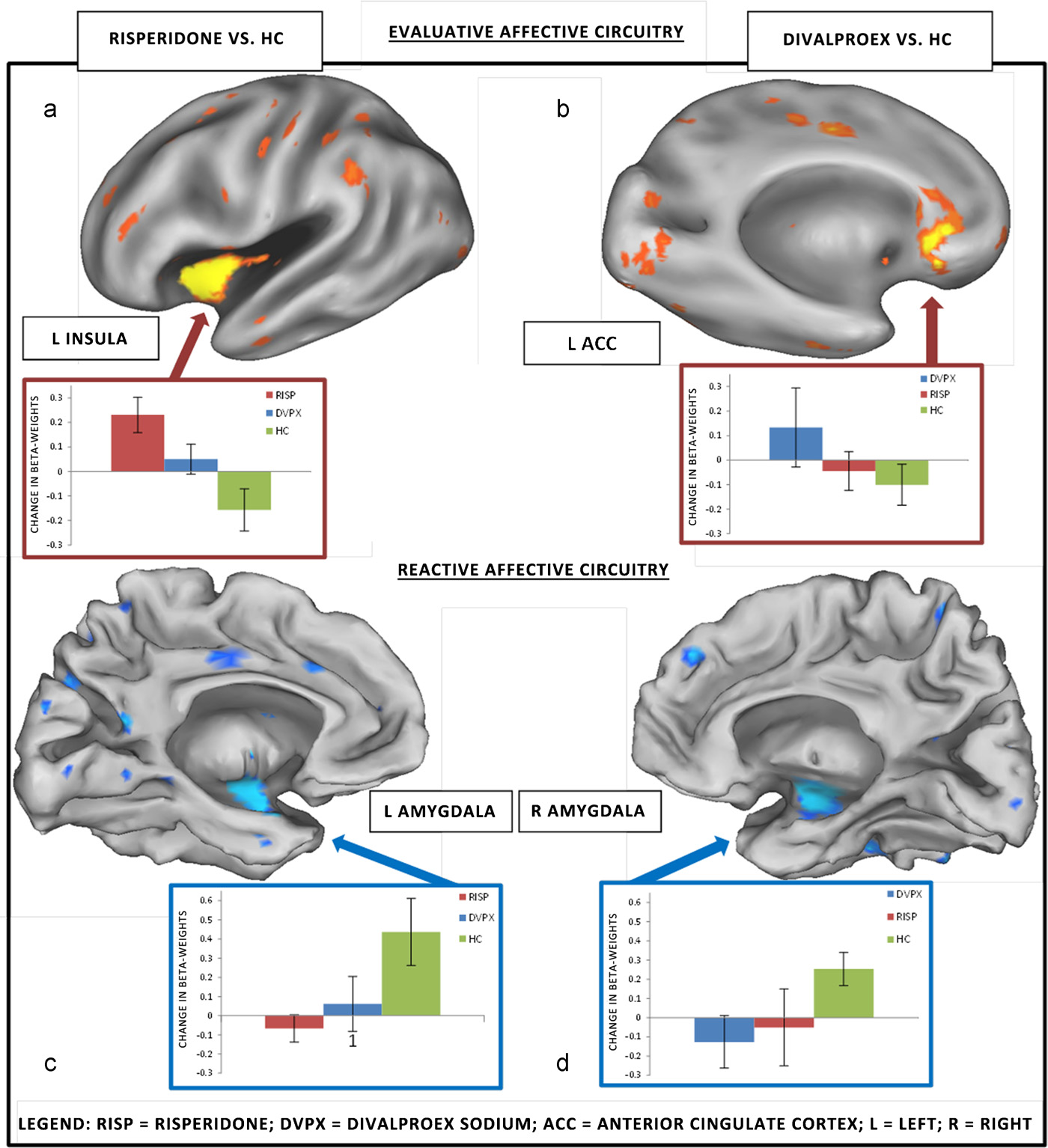
M.N. Pavuluri et al. / Behavioural Brain Research 226 (2012) 493–503
Fig. 3. Region of interest within functional circuits: medication effects over treatment period.
cortex elements of the EAC network described in this study have
connectivity between visual association cortex to the amygdale
been linked to cognitive set maintenance they increase
the occipito-temporal inferior longitudinal fasciculus
in response to errors during ongoing performance of a similar
the high level of frustration associated with having to
Go/No-Go response inhibition task suggests that impair-
inhibit responses in this study, amygdala was engaged on the left
ment of processing due to abnormal integration of insular cortex
side The amygdala is found to play a key role in the inter-
might disrupt these executive control abilities, with inability to
action of emotion and cognition such as emotion's influence on
monitor inhibition in the light of manic symptoms. The RAC is a
attention and perception findings support the notion of
posterior affective circuit that also emerged during response inhi-
emotional impulsivity this early automatic
bition task performance. The functional connectivity that emerged
response is proportional to the attention directed to the stimuli
in RAC is in line with our previous findings in support of direct
have yet to establish the sequence of signal pathways
M.N. Pavuluri et al. / Behavioural Brain Research 226 (2012) 493–503
to determine whether EAC activity is preceded by that in RAC or
4.2.3. Risperidone and divalproex fail to dampen amygdala
connectivity within RAC
The amygdala showed significantly increased functional con-
nectivity within RAC, at baseline that failed to change with
4.2. Medication-specific effects on functional connectivity
treatment. There is consistent evidence from functional studies
in PBD showing increased amygdala activity remained
While biomarkers of medication outcome and prognosis are an
hyperactive, relative to HC, regardless of treatment for mania
ideal goal, given the preliminary nature and the complexity of our
and during euthymic illness state addition to functional
studies, it may be optimal to provide a bio-signature based on a
neuroimaging abnormalities in the PFC and amygdala, structural
cluster of biological findings that maps any given drug's operation.
neuroimaging studies indicate smaller amygdala volumes in PBD
Also, findings on fMRI studies of drug mechanism must always
patients relative to HC, contrasts with adult stud-
be interpreted based on the paradigm chosen to probe a specific
ies that report larger normal volumes.
domain, the illness status, and the class of drug.
Larger amygdala in adult studies in fact been
hypothesized to result from hypertrophy due to chronic and exces-
4.2.1. Risperidone increases insular engagement within EAC
sive activation in manic patients While findings of altered
Our main finding with risperidone is of critical importance as
size do not necessarily imply intrinsic primary abnormalities in the
risperidone is often administered to address aggression and neg-
amygdala, they may correlate with functional abnormalities in the
ative reactivity within acute mania parallel, insula
amygdala such as increased connectivity in response to the emo-
increased activation while processing negative emotions in healthy
tional and cognitive challenges posed by the task (e.g. frustration,
humans with increased serotonin neurotransmission well
effortful inhibition of responses), regardless of dysfunction in PFC
as in PBD the insula showed hypometabolism
input to the amygdala. Given the central role that the amygdala
during omission errors on an attentional task in euthymic bipolar
plays in arousal and emotional reactivity its role in the
adults a finding that aligns with structural studies showing
emotion-cognition interface is meaningful that amygdala
decreased grey matter in left insula in patients and their rela-
did not alter its task-engagement following treatment with either
tives Risperidone appears to engage this critical region in
medication in PBD as was seen in controls. The change in amyg-
the EAC to a greater extent, likely by the virtue of its serotonin-
dala connectivity found in HC might be the result of the increase in
dopamine antagonism, due to greater serotonin HT 1A receptors
automaticity due to task familiarity in the RAC and decreased need
in the insula emotion and attention during task
performance. The increased functional connectivity of insula in the
EAC with risperidone treatment and its correlation with change
4.3. Clinical symptoms influence response inhibition
in manic symptoms underscores the mechanism behind the effect
of risperidone in mania. However, given that HT 1A receptors are
The novel findings are that both risperidone and divalproex
abundantly present in hippocampus and amygdala, these results of
groups showed greater connectivity in two distinct regions in EAC,
insular hyperconnectivity must be interpreted as potentially spe-
relative to HC, and that both of these regions correlated signifi-
cific to risperidone and during behavior inhibition. Alternatively,
cantly and differentially with the reduction in manic and depressive
risperidone administered in a larger sample may illustrate a more
symptoms, respectively. The increased engagement of insula in the
wide spread and significantly increased engagement of these other
EAC after treatment with risperidone illustrates the alterations in
regions that are rich in HT 1A receptors.
neural connectivity underlying the reduction in manic symptoms.
The increase in the functional connectivity in subgenual ACC pro-
vides insight into the mechanisms of the traditional mood stabilizer
4.2.2. Divalproex increases subgenual ACC engagement within
divalproex, which additionally addresses depressive symptoms
subsyndromal depressive symptoms commonly
Within the EAC, left subgenual ACC showed greater functional
coexist with pediatric mania. Depressive symptoms paired with
connectivity in divalproex patients, relative to HC. This finding
expending attentional resources during response inhibition task
is consistent with the underactive subgenual ACC in untreated
performance would lead to "emotional impulsivity" in PBD,
patients with adult bipolar disorder while performing an atten-
especially because cognitive conflicts require effortful processing
tional task key region modulates autonomic responses
and, therefore, can be aversive and evoke negative emotions
and neurotransmission in animals Indeed, stimulation of
If the patients were dealing with mood symptoms, they could have
subgenual ACC ameliorated symptoms in adult depression
interfered with their ability to implement a more conservative
and perfusion was normalized with treatment in the same region
response strategy similar to that found by the HC. Thus, our study
Subgenual ACC also exhibited decreased grey matter in
is significant in exhibiting the effects of risperidone and divalproex
adult mood disorders where there was reduction in glia
during motor inhibition, with significant reductions in manic and
Further, we were able to show that subgenual ACC engagement
depressive mood symptoms and engagement of the EAC through
significantly correlated with reduced depressive symptoms in the
insula and subgenual ACC, respectively, to moderate patients' emo-
current study, although we failed to show such correlation with
tional impulsivity.
manic symptoms. Finally, while divalproex engaged subgenual ACC
A major strength of this study is that the ICA method is a data-
greater than HC, risperidone did not differ from divalproex when
driven (i.e. model-blind) technique to measure dynamic changes
we compared the two patient groups. These results are in line
in brain network circuits working in concert, rather than isolated
with our findings from a previous study of risperidone illustrating
changes in activity. A weakness of this study is the relatively small
increased subgenual ACC activity during a cognitive control task
sample size, although couched in a strong study design. The use
performed under emotional challenge alternate explana-
of a block design for the task also did not permit us to compare
tion for this lack of difference on direct comparison between the
the networks that were engaged for Stop vs. Go trials or correct
two medications may be due to the fact that the brain circuitry
vs. incorrect trials, unlike some previous studies
engagement may be specific to a mood state than a specific med-
given that that the study is based on a model of the neural circuits
ication. The brain functional signature may be more specific to a
that takes into account overall performance during the response
manic episode vs. a depressive episode or a euthymic state
inhibition task, the fact that the study was a block design is less
M.N. Pavuluri et al. / Behavioural Brain Research 226 (2012) 493–503
relevant. Moreover, given that we found comparable levels of per-
of cognitive and emotional brain systems in pediatric bipolar disorder. J Child
formance between patients and HCs, we avoided the potential
Adolesc Psychopharmacol 2010;20(October (5)):395–406.
[11] Pavuluri MN, Passarotti AM, Mohammed T, Carbray JA, Sweeney JA. Enhanced
confound that large differences in task difficulty between groups
working and verbal memory after lamotrigine treatment in pediatric bipolar
caused the neural changes we observed. We were able to compare
disorder. Bipolar Disord 2010;12(March (2)):213–20.
two types of medications in an unmedicated manic patient sample,
[12] Pavuluri MN, Passarotti AM, Lu LH, Carbray JA, Sweeney JA. Double-blind ran-
domized trial of risperidone versus divalproex in pediatric bipolar disorder:
using monotherapy, with a normative HC sample for comparison. It
fMRI outcomes. Psychiatry Res 2011;193(July (1)):28–37.
is compelling to see that previous findings can now be incorporated
[13] Passarotti AM, Sweeney JA, Pavuluri MN. Fronto-limbic dysfunction in mania
into more sophisticated circuit-level models that serve as potential
pre-treatment and persistent amygdala over-activity post-treatment in pedi-
bio-signatures of treatment outcome. These results are beginning
atric bipolar disorder. Psychopharmacology 2011;216(August (4)):485–99.
[14] Passarotti AM, Sweeney JA, Pavuluri MN. Differential engagement of cognitive
to inform why clinicians would need to combine two classes of
and affective neural systems in pediatric bipolar disorder and attention deficit
medications because they exhibit complementary mechanisms of
hyperactivity disorder. J Int Neuropsychol Soc 2010;16(January (1)):106–17.
action within specific functional neural circuits.
[15] Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of incidental and
directed facial emotion processing in adolescents and adults. Sog Cogn Affect
Neurosci 2009;4(January (4)):387–98.
[16] Passarotti AM, Pavuluri MN. Brain functional domains inform therapeutic
interventions in attention-deficit/hyperactivity disorder and pediatric bipolar
disorder. Expert Rev Neurother 2011;11(June (6)):897–914.
Behavioral response inhibition in pediatric mania engages two
[17] Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, et al. Neu-
functionally connected circuits, the EAC and the RAC. Risperidone
ral circuitry engaged during unsuccessful motor inhibition in pediatric bipolar
disorder. Am J Psychiatry 2007;164(January (1)):52–60.
works by engaging insula to a greater degree than HC, and dival-
[18] Singh MK, Chang KD, Mazaika P, Garrett A, Adleman N, Kelley R, et al. Neural
proex works by engaging subgenual ACC relative to HC within the
correlates of response inhibition in pediatric bipolar disorder. J Child Adolesc
EAC. Reduction in manic symptoms for the risperidone group and
[19] Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. Enhanced prefrontal func-
depressive symptoms in the divalproex group distinctly showed
tion with pharmacotherapy on a response inhibition task in adolescent bipolar
correlations with change in the functional connectivity of EAC.
disorder. J Clin Psychiatry 2010;71(November (11)):1526–34.
However, amygdala activity did not alter with treatment within
[20] Pavuluri MN, Sweeney JA. Integrating functional brain neuroimaging and devel-
opmental cognitive neuroscience in child psychiatry research. J Am Acad Child
RAC with either risperidone or divalproex, suggesting a probable
Adolesc Psychiatry 2008;47(November (11)):1273–88.
trait of dysfunctional emotional reactivity in PBD, relative to HC.
[21] Pavuluri MN, O‘connor MM, Harral EM, Sweeney JA. An fMRI study of the
These patterns of activity contribute to the development of biosig-
interface between affective and cognitive neural circuitry in pediatric bipolar
disorder. Psychiatry Res 2008;162(February (3)):244–55.
natures of treatment response in PBD.
[22] Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to
behaviour. Brain 1995;118(February (Pt 1)):279–306.
[23] Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in
the human brain. Brain 2003;126(September (Pt 9)):2093–107.
[24] Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning
This research was funded by NIH 1 K23 RR018638-01 and NIH-
in the human amygdala. Nature 1998;393(June (6684)):467–70.
[25] Friston K. Beyond phrenology: what can neuroimaging tell us about distributed
circuitry? Annu Rev Neurosci 2002;25:221–50. Epub 2002 March 19.
[26] Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of response inhibi-
tion in pediatric bipolar disorder and attention deficit hyperactivity disorder.
Appendix A. Supplementary data
Psychiatry Res 2010;181(January (1)):36–43.
[27] Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks
Supplementary data associated with this article can be found, in
underlying response inhibition in adolescents and adults. Behav Brain Res
the online version, at
[28] Konarski JZ, Kennedy SH, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, et al.
Predictors of nonresponse to cognitive behavioural therapy or venlafaxine
using glucose metabolism in major depressive disorder. J Psychiatry Neurosci
[29] Pandina G, DelBello M, Kushner S, Van Hove I, Augustyns I, Kusumakar V, et al.
[1] Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anoma-
Risperidone for the treatment of acute mania in bipolar youth [poster]. In:
lous prefrontal-subcortical activation in familial pediatric bipolar disorder:
Presented at the 54th annual meeting of the American Academy of Child and
a functional magnetic resonance imaging investigation. Arch Gen Psychiatry
Adolescent Psychiatry (AACAP). 2007.
[30] Monti B, Polazzi E, Contestabile A. Biochemical, molecular and epigenetic mech-
[2] Pavuluri MN, Schenkel LS, Aryal S, Harral EM, Hill SK, Herbener ES, et al. Neu-
anisms of valproic acid neuroprotection. Curr Mol Pharmacol 2009;2(January
rocognitive function in unmedicated manic and medicated euthymic pediatric
bipolar patients. Am J Psychiatry 2006;163(February (2)):286–93.
[31] Kowatch RA, Findling R, Scheffer R, Stanford KE. Placebo controlled trial of dival-
[3] Pavuluri MN, O‘Connor MM, Harral EM, Sweeney JA. Affective neural circuitry
proex versus lithium for bipolar disorder. In: Presented at the Annual Meeting
during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry
of the American Academy of Child and Adolescent Psychiatry. 2007.
[32] Wagner KD, Redden L, Kowatch RA, Wilens TE, Segal S, Chang K, et al. A double-
[4] Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure
blind, randomized, placebo-controlled trial of divalproex extended-release in
EB, et al. Limbic hyperactivation during processing of neutral facial expres-
the treatment of bipolar disorder in children and adolescents. J Am Acad Child
sions in children with bipolar disorder. Proc Natl Acad Sci USA 2006;103(June
Adolesc Psychiatry 2009;48(May (5)):519–32.
[33] Chang KD, Wagner C, Garrett A, Howe M, Reiss A. A preliminary functional
[5] Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, et al.
magnetic resonance imaging study of prefrontal-amygdalar activation changes
Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary
in adolescents with bipolar depression treated with lamotrigine. Bipolar Disord
observations from functional MRI. Am J Psychiatry 2003;160(July (7)):1345–7.
[6] Blumberg HP, Kaufman J, Martin A, Charney DS, Krystal JH, Peterson BS. Sig-
[34] Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-SADS-PL. J Am Acad Child
nificance of adolescent neurodevelopment for the neural circuitry of bipolar
Adolesc Psychiatry 2000;39(October (10)):1208.
disorder. Ann N Y Acad Sci 2004;1021(June):376–83.
[35] Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability,
[7] Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural
validity and sensitivity. Br J Psychiatry 1978;133(November):429–35.
correlates of incidental versus directed emotion processing in pediatric bipolar
[36] Poznanski EO, Mokros HB. Children‘s Depression Rating Scale Revised (CDRS-R).
disorder. J Am Acad Child Adolesc Psychiatry 2009.
Los Angeles, CA: Western Psychological Services; 1995.
[8] Rich BA, Fromm SJ, Berghorst LH, Dickstein DP, Brotman MA, Pine DS, et al.
[37] Eddy WF, Fitzgerald M, Genovese CR, Mockus A, Noll DC. Functional image
Neural connectivity in children with bipolar disorder: impairment in the
analysis software – computational olio. In: Prat A, editor. Proceedings in Com-
face emotion processing circuit. J Child Psychol Psychiatry 2008;49(January
putational Statistics. Heidelberg: Physica-Verlag; 1996. p. 39–49.
[38] Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group infer-
[9] Dickstein DP, Gorrostieta C, Ombao H, Goldberg LD, Brazel AC, Gable CJ, et al.
ences from functional MRI data using independent component analysis. Hum
Fronto-temporal spontaneous resting state functional connectivity in pediatric
Brain Mapp 2001;14(November (3)):140–51.
bipolar disorder. Biol Psychiatry 2010;68(November (9)):839–46.
[39] Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain
[10] Pavuluri MN, Passarotti AM, Parnes SA, Fitzgerald JM, Sweeney JA. A pharma-
networks estimated using ICA at rest and during cognitive tasks. Hum Brain
cological functional magnetic resonance imaging study probing the interface
Mapp 2008;29(July (7)):828–38.
M.N. Pavuluri et al. / Behavioural Brain Research 226 (2012) 493–503
[40] Bell AJ, Sejnowski TJ. An information-maximization approach to blind separa-
[64] Kodaka F, Ito H, Takano H, Takahashi H, Arakawa R, Miyoshi M, et al. Effect of
tion and blind deconvolution. Neural Comput 1995;7(November (6)):1129–59.
risperidone on high-affinity state of dopamine D2 receptors: a PET study with
[41] Li YO, Adali T, Calhoun VD. Estimating the number of independent com-
agonist ligand [11C](R)-2-CH3O-N-n-propylnorapomorphine. Int J Neuropsy-
ponents for functional magnetic resonance imaging data. Hum Brain Mapp
[65] Strakowski SM, Adler CM, Holland SK, Mills N, DelBello MP. A preliminary
[42] Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Compar-
FMRI study of sustained attention in euthymic, unmedicated bipolar disorder.
ison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp
[66] Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in
[43] Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified
mood disorders. CNS Spectr 2008;13(August (8)):663–81.
statistical approach for determining significant signals in images of cerebral
[67] Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C,
activation. Hum Brain Mapp 1996;4(1):58–73.
et al. Deep brain stimulation for treatment-resistant depression. Neuron
[44] Leibenluft E, Rich BA. Pediatric bipolar disorder. Annu Rev Clin Psychol
[68] Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antide-
[45] Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, et al. Map-
pressant drug treatment assessed using PET measures of regional glucose
ping motor inhibition: conjunctive brain activations across different versions
metabolism. Eur Neuropsychopharmacol 2002;12(December (6)):527–44.
of go/no-go and stop tasks. Neuroimage 2001;13(February (2)):250–61.
[69] Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al.
[46] Durston S, Thomas KM, Worden MS, Yang Y, Casey BJ. The effect of preceding
Regional metabolic effects of fluoxetine in major depression: serial changes and
context on inhibition: an event-related fMRI study. Neuroimage 2002;16(June
relationship to clinical response. Biol Psychiatry 2000;48(October (8)):830–43.
[70] Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA,
[47] Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human
et al. Reciprocal limbic-cortical function and negative mood: converging PET
and the macaque ventrolateral prefrontal cortex and corticocortical connection
findings in depression and normal sadness. Am J Psychiatry 1999;156(May
patterns in the monkey. Eur J Neurosci 2002;16(July (2)):291–310.
[48] Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. Func-
[71] Dickstein DP, Milham MP, Nugent AC, Drevets WC, Charney DS, Pine DS, et al.
tional brain networks develop from a "local to distributed" organization. PLoS
Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-
Comput Biol 2009;5(May (5)):e1000381.
based morphometry study. Arch Gen Psychiatry 2005;62(July (7)):734–41.
[49] Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks
[72] Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A.
architecture of top-down control. Trends Cogn Sci 2008;12(March (3)):
Reduced amygdalar gray matter volume in familial pediatric bipolar disorder.
J Am Acad Child Adolesc Psychiatry 2005;44(June (6)):565–73.
[50] Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Brain network dynamics during
[73] DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. Magnetic res-
error commission. Hum Brain Mapp 2009;30(January (1)):24–37.
onance imaging analysis of amygdala and other subcortical brain regions in
[51] Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of
adolescents with bipolar disorder. Bipolar Disord 2004;6(February (1)):43–52.
the primate amygdaloid complex. In: Aggleton J, editor. The Amygdala: Neu-
[74] Frazier JA, Ahn MS, DeJong S, Bent EK, Breeze JL, Giuliano AJ. Magnetic reso-
robiological Aspects of Emotion, Memory, and Mental Dysfunction. New York:
nance imaging studies in early-onset bipolar disorder: a critical review. Harv
Wiley-Liss; 1992. p. 1–66.
Rev Psychiatry 2005;13(May (3)):125–40.
[52] Armony JL, Servan-Schreiber D, Cohen JD, LeDoux JE. An anatomically
[75] Altshuler L, Bartzokis G, Grieder T, Curran J, Mintz J. Amygdala enlargement
constrained neural network model of fear conditioning. Behav Neurosci
in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study
demonstrating neuroanatomic specificity. Arch Gen Psychiatry 1998;55:663–4.
[53] Phelps EA. Emotion and cognition: insights from studies of the human amyg-
[76] Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM,
dala. Ann Rev Psychol 2006;57:27–53.
et al. Brain magnetic resonance imaging of structural abnormalities in bipolar
[54] Whalen PJ, Bush G, McNally RJ, Wilhelm S, Mcinerney SC, Jenike MA, et al.
disorder. Arch Gen Psychiatry 1999;56(March (3)):254–60.
The emotional counting stroop paradigm: a functional magnetic resonance
[77] Swayze VW, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC. Subcortical and
imaging probe of the anterior cingulate affective division. Biol Psychiatry
temporal structures in affective disorder and schizophrenia: a magnetic reso-
nance imaging study. Biol Psychiatry 1992;31(February (3)):221–40.
[55] Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of
[78] Strakowski SM, Adler CM, DelBello MP. Volumetric MRI studies of mood dis-
emotional faces requires attention. Proc Natl Acad Sci USA 2002;99(August
orders: do they distinguish unipolar and bipolar disorder? Bipolar Disord
[56] Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry
[79] Brambilla P, Harenski K, Nicoletti M, Sassi RB, Mallinger AG, Frank E, et al. MRI
investigation of temporal lobe structures in bipolar patients. J Psychiatr Res
[57] Biederman J, Mick E, Hammerness P, Harpold T, Aleardi M, Dougherty M, et al.
Open-label, 8-week trial of olanzapine and risperidone for the treatment of
[80] Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, et al. An MRI
bipolar disorder in preschool-age children. Biol Psychiatry 2005;58(October
study of temporal lobe structures in men with bipolar disorder or schizophre-
nia. Biol Psychiatry 2000;48(July (2)):147–62.
[58] Pavuluri MN, Henry DB, Devineni B, Carbray JA, Naylor MW, Janicak PG.
[81] Gorno-Tempini ML, Pradelli S, Serafini M, Pagnoni G, Baraldi P, Porro C, et al.
A pharmacotherapy algorithm for stabilization and maintenance of pedi-
Explicit and incidental facial expression processing: an fMRI study. Neuroimage
atric bipolar disorder. J Am Acad Child Adolesc Psychiatry 2004;43(July (7)):
[82] Critchley H, Daly E, Phillips M, Brammer M, Bullmore E, Williams S, et al. Explicit
[59] Pavuluri MN, Henry DB, Findling RL, Parnes S, Carbray JA, Mohammed T, et al.
and implicit neural mechanisms for processing of social information from facial
Double-blind randomized trial of risperidone versus divalproex in pediatric
expressions: a functional magnetic resonance imaging study. Hum Brain Mapp
bipolar disorder. Bipolar Disord 2010;12(September (6)):593–605.
[60] Takano H, Ito H, Takahashi H, Arakawa R, Okumura M, Kodaka F, et al. Sero-
[83] Keightley ML, Winocur G, Graham SJ, Mayberg HS, Hevenor SJ, Grady CL. An
tonergic neurotransmission in the living human brain: A positron emission
fMRI study investigating cognitive modulation of brain regions associated with
tomography study using [(11)C]dasb and [(11)C]WAY100635 in young healthy
emotional processing of visual stimuli. Neuropsychologia 2003;41(5):585–96.
men. Synapse 2010;(November).
[84] Geller B, Fox LW, Clark KA. Rate and predictors of prepubertal bipolarity during
[61] Pavuluri MN, O'Connor MM, Harral EM, Sweeney JA. Lamotrigine effects on
follow-up of 6- to 12-year-old depressed children. J Am Acad Child Adolesc
neurocognition in pediatric bipolar disorder. [Poster]. Florida: Am College of
Psychiatry 1994;33(May (4)):461–8.
[85] Findling RL, Youngstrom EA, McNamara NK, Stansbrey RJ, Demeter CA, Bedoya
[62] Brooks III JO, Hoblyn JC, Ketter TA. Metabolic evidence of corticolimbic dysreg-
D, et al. Early symptoms of mania and the role of parental risk. Bipolar Disord
ulation in bipolar mania. Psychiatry Res 2010;181(February (2)):136–40.
[63] Matsuo K, Kopecek M, Nicoletti MA, Hatch JP, Watanabe Y, Nery FG, et al. New
[86] Botvinick MM. Conflict monitoring and decision making: reconciling two
structural brain imaging endophenotype in bipolar disorder. Mol Psychiatry
perspectives on anterior cingulate function. Cogn Affect Behav Neurosci
Source: http://jaellis.com/PharmacotherapyAffectiveCircuits.pdf
Clinical Neurophysiology 119 (2008) 842–852 Non-provocative diagnostics of photosensitivity using visual evoked potentials Joost Vermeulen a,1, Stiliyan Kalitzin b,*, Jaime Parra c, Erwin Dekker c, Albert Vossepoel f, Fernando Lopes da Silva d,e a Quantitative Imaging Group, Department of Imaging Science and Technology, Faculty of Applied Sciences, Delft University of Technology,
Assessment and Management of Pain in the Elderly Self-directed learning package for nurses in long-term care. Supporting Implementation of the RNAO BPG Assessment and Management of Pain The Registered Nurses' Association of Ontario (RNAO) and the Nursing Best Practice Guidelines Program would like to acknowledge the following individuals and organizations for their contribution to the development of the educational resource, Assessment and Management of Pain in the Elderly: Self-directed learning package for nurses in long-term care.






