Untitled
Optimal viral strategies for bypassing RNA silencing
Guillermo Rodrigo, Javier Carrera, Alfonso Jaramillo and Santiago F. Elena
J. R. Soc. Interface
, 257-268 first published online 23 June 2010
Email alerting service
Receive free email alerts when new articles cite this article - sign up in the box at the topright-hand corner of the article or click
J. R. Soc. Interface
J. R. Soc. Interface (2011) 8, 257–268
Published online 23 June 2010
Optimal viral strategies for bypassing
Guillermo Rodrigo1, Javier Carrera1,2, Alfonso Jaramillo3,4
and Santiago F. Elena1,5,*
1Instituto de Biologı´a Molecular y Celular de Plantas, Consejo Superior de Investigaciones
Cientı´ficas-Universidad Polite´cnica de Valencia, Campus UPV CPI 8E, Ingeniero Fausto
Elio s/n, 46022 Valencia, Spain
2ITACA, Universidad Polite´cnica de Valencia, Camino de Vera s/n, 46022 Valencia, Spain
3E´cole Polytechnique, Route de Saclay, 91128 Palaiseau Cedex, France
4Epigenomics Project, Genopole-Universite´ d'E´vry Val d'Essonne-CNRS UPS3201,
Batiment Geneavenir 6, 5 Rue Henri Desbrue res, 91030 E
´ vry Cedex, France
5Santa Fe Institute, 1399 Hyde Park Road, Santa Fe, NM 87501, USA
The RNA silencing pathway constitutes a defence mechanism highly conserved in eukaryotes,especially in plants, where the underlying working principle relies on the repressive actiontriggered by the intracellular presence of double-stranded RNAs. This immune system per-forms a post-transcriptional suppression of aberrant mRNAs or viral RNAs by smallinterfering RNAs (siRNAs) that are directed towards their target in a sequence-specificmanner. However, viruses have evolved strategies to escape from silencing surveillancewhile promoting their own replication. Several viruses encode suppressor proteins that inter-act with different elements of the RNA silencing pathway and block it. The differentsuppressors are not phylogenetically nor structurally related and also differ in their mechan-ism of action. Here, we adopt a model-driven forward-engineering approach to understand theevolution of suppressor proteins and, in particular, why viral suppressors preferentially targetsome components of the silencing pathway. We analysed three strategies characterized bydifferent design principles: replication in the absence of a suppressor, suppressors targetingthe first protein component of the pathway and suppressors targeting the siRNAs. Our resultsshed light on the question of whether a virus must opt for devoting more time into transcrip-tion or into translation and on which would be the optimal step of the silencing pathway to betargeted by suppressors. In addition, we discussed the evolutionary implications of suchdesigning principles.
Keywords: RNA silencing; silencing suppression; systems and synthetic biology;
transcription – translation tradeoff; virus evolution; virus – host interaction
The underlying working principle of RNA silencing
relies on the repressive action triggered by the intra-
RNA viruses are difficult to control and eliminate
cellular presence of double-stranded RNAs (dsRNAs)
because of their rapid evolution. This high evolvabil-
[]. In the case of single-stranded RNA (ssRNA)
ity is a consequence of their high mutation rates,
viruses, dsRNAs are by-products of genome replication
large population size and short generation times
mediated by virus-encoded RNA-dependent RNA
] that confer them an astonishing ability to
polymerases (RdRps). During viral genome replication,
explore genotypic space. Indeed, RNA viruses typi-
the dsRNA intermediates become the target of the
first component of the silencing pathway, DICER, a
higher than their DNA hosts []. Eukaryotic organ-
type-III RNase that degrades these dsRNAs into
isms have developed a sequence-specific mechanism
units of 21 – 24 nucleotides called small interfering
to modulate gene expression based on RNA interfer-
RNAs (siRNAs; ]). Subsequently, the cellular
RNA-induced silencing complex (RISC), which con-
Caenorhabditis elegans ] and later on in many
tains the argonaute (AGO) endonuclease loads
other eukaryotes, including plants and mammals
the antisense siRNAs, resulting in an active form.
Likewise, this molecular mechanism is able to
Using the antisense siRNA as a guide, AGO cleaves
silence viral or aberrant genes.
the target viral ssRNA []. Furthermore, in a second-ary cycle of amplification, the host's RNA-dependent
*Author for correspondence ).
RNA polymerase VI (RDR6) uses siRNAs as primers,
Received 19 May 2010Accepted 3 June 2010
This journal is q 2010 The Royal Society
Bypassing RNA silencing
G. Rodrigo et al.
together with partially degraded ssRNAs, to produce
on the outcome of the interaction. On the other
hand, although many kinetic models of intracellular
DICER, a process known as transitivity ]. siRNAs
growth have been proposed for different viruses,
systemically move from cell-to-cell, immunizing new
none of them specifically incorporates the silencing
cells against infection ]. Given the properties of
response (e.g. [– ]). In this work, we present the
the RNA silencing pathway (specificity and amplifica-
first model that incorporates the interaction of differ-
tion), it represents a sort of innate immune system for
ent suppressor proteins with components of the
silencing pathway. We perform a dynamical analysis
Not surprisingly, viruses have evolved strategies to
and show the time course of viral RNA accumulation
actively evade the RNA silencing surveillance while
under a wide set of parameter states. We also show
promoting their own replication ]. Many viruses
phase diagrams for different combinations of par-
encode a suppressor protein (viral suppressor of
ameters and focus our discussion on the behaviour
RNA silencing or VSR) that interacts with elements
of the system for different viral replication and trans-
of the silencing pathway blocking it [– ]. The
lation rates in the presence/absence of different
targets of these VSRs within the RNA silencing
suppressor strategies. These analyses allow us to
rationalize why different viruses may opt for different
siRNA, RISC or the systemic signal [For
strategies in their investment into producing new gen-
example, the helper component-protease (HC-Pro)
omes (i.e. transcription via antigenomic strains) or
encoded by the Potyvirus works as suppressor by
into producing large amounts of protein from a few
sequestering siRNAs [– ]. This binding prevents
initial sense genomes (i.e. translation). Such models
the incorporation of siRNAs into the RISC. Further-
more, by also binding plant endogenous micro-RNAs
design principles of viral systems.
and controlling the expression of other genes, HC-Pro may interfere the expression of DICER proteins[reducing the degradation of dsRNAs and,thus, favouring potyvirus replication. Similarly, the
Nodavirus B2 suppressor also sequesters siRNAs[The Tombusviridae P19 and Cucumovirus 2b
We have constructed a mathematical model based on
suppressors interfere with the systemic spread of
the 24 nucleotide siRNAs produced by DCL3 [
Some suppressors act on the RISC, either avoiding
positive-sense RNA virus that encodes for a single
the upload of siRNAs into AGO, like the Clostero-
polyprotein that is processed into mature peptides,
virus P21 [by binding to AGO1 and avoiding
as is the case for picorna-like viruses (e.g. poliovirus,
its interaction with other proteins required to assem-
hepatitis C virus, foot-and-mouth disease virus and
the potyviruses, which are the largest and more impor-
Tombusvirus [by inhibiting the RISC activity
tant family of plant viruses). The model involves the
after its maturation, like the Begomovirus AC4
following molecular species: genomic and antigenomic
[or by targeting AGO for degradation, as it is
ssRNA (Sþ and S2, respectively), dsRNA (D), anti-
the case for Polerovirus P0 protein ] press).
sense siRNA (I ), viral proteins ( p), virions (V ),
It has also been recently shown that the V2 suppres-
primed ssRNA (S*) and secondary dsRNA (D*).
sor of Geminivirus competes with SGS3, a key
Three different viral proteins are considered, the non-
component of the secondary cycle of siRNA amplifi-
structural replicase and VSR and the structural CP.
cation, in binding dsRNAs and thus interferes with
Their corresponding relative abundances are p, q and
transitivity [Finally, the CP of some carmo-
1 2 p 2 q, respectively. This constraint is biologically
viruses ] and the P14 of Aureusvirus ] can
relevant for picornaviruses as all proteins are self-pro-
also bind long dsRNAs, resulting in the protection
cessed from a single polyprotein and, thus, their
of the intermediaries of replication from DICER
relative abundances remain constant during infection.
activity. Accordingly, VSRs have been divided into
In addition, the model accounts for several cellular
three families [(i) those enhancing within-cell
components: the ribosomes (Z ), the RDR6 polymerase
virus accumulation, (ii) those essential for cell-to-
involved in transitivity (Y ), DICER-like proteins (C )
cell movement but dispensable on virus accumulation
and the inactivated and activated RISC (R and R*,
in single cells, and (iii) those that facilitate virus
respectively). We assume that at the beginning of
infection, a single viral ssRNA genome is present,
symptoms but are not essential for viral replication
which in our particular model must be genomic.
and cell-to-cell movement.
Notice that genomic strands are those that encode
The first mathematical models of the RNA silen-
for proteins, whereas antigenomic strands are comp-
cing pathway focused on aberrant cellular mRNA as
lementary and, for simplification, we will assume are
triggers of the silencing response – More
not coding. To accommodate negative-sense RNA
recent models consider viral RNAs as triggers of the
viruses into the model, the equations can be straight-
response and focused on the spread of viruses in
plants ]. However, on the one hand, these
encapsidated and cleaved by RISC) changing the
studies did not analyse in detail the possible effect
initial conditions. For retroviruses or DNA viruses,
that different viral suppressor strategies may have
the model must be conveniently modified.
J. R. Soc. Interface (2011)
Bypassing RNA silencing
G. Rodrigo et al.
Table 1. Values for the kinetic parameters used in the model. Other non-kinetic model parameters are p ¼ q ¼ 0.4, v ¼ 0.1,n ¼ 2n* ¼ 10, f ¼ 0.01, s ¼ 0.1 and k ¼ 10k ¼
30. The amounts of cellular resources are Z ¼ 105, Y ¼ 105, C0
104 molecules. In the case of a virus encoding a VSR, the corresponding binding constant (GC, GI or GR) takes the value of G.
The cell volume is assumed � 10213 l, then 1 nM � 100 molecules.
value in the literature
10 h21 for HCV []
228 h21 in vitro for E. coli []
25 h21 in vitro for Drosophila melanogaster
�100 M21 h21 for nucleation []
�105 M21 h21 for elongation []
0.06 h21 for HCV
225 nM in vitro for TBSV
8 nM in vitro for D. melanogaster
260 nM in vitro []
335 nM in vitro for E. coli []
�1000 molecules [,]
10 – 1000 nM in vitro p19, p21 and HC-Pro []
The model is constructed following a generalized
enzyme kinetics scheme where both substrates and
enzymes are limited in the medium [and there
are competitions between different enzymes for the
same substrate and different substrates for the same
enzyme [This gives a highly coupled formulation.
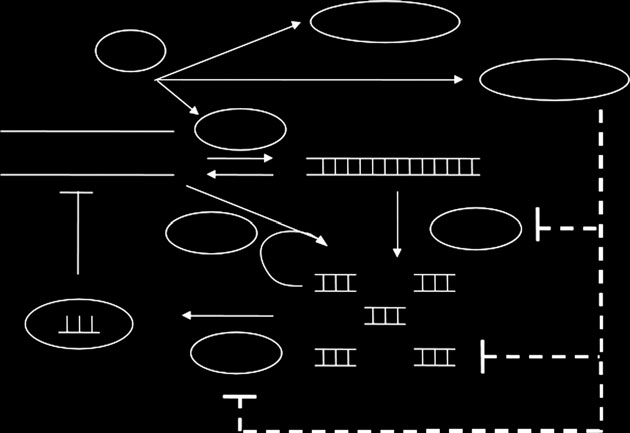
In , we show the scheme of the RNA silencing
pathway, and the kinetic parameters are shown in, with parameter values taken from differentsources.
Viral replication is a process involving multiple
reactions aiming to bypass the defence systems ofthe cell. The RNA replication rates (J ), for both
Figure 1. Schematic of the RNA silencing pathway and itsinteraction with viral replication. RNA viruses encode for
replicase, suppressors of silencing (VSR) and coat proteins.
Three types of suppressors are considered in the scheme: sup-
pressors of DICER (I), sequesters of siRNA (II) and
suppressors of RISC (III).
where a is the maximum replication rate per mol-
ð1 � p � qÞP k0
ecule of ssRNA, KP, KR, KZ and KC are the
binding constants for the replicase, the activated
RISC, the ribosomes and the CP, respectively. The
affinity of the replicase for the antigenomic strands
is incorporated into the model by the parameter v.
If v ¼ 1, then the RdRp has the same affinity for
both strains, whereas v . 1 would imply a larger
affinity for the antigenomic strain. By doing so, we
geometric (v ¼ 1) to the stamping machine one
J. R. Soc. Interface (2011)
Bypassing RNA silencing
G. Rodrigo et al.
A molecule of dsRNA can be separated into
immature virions at a rate given by
two ssRNA molecules of complementary polarityat a first-order rate with a constant parameter b
lSþ½ð1 � p � qÞP=KC�k0
Jencapsidation ¼
dissociation ¼ bD
In addition, genomic ssRNAs are translated into
where l is the maximum assembly rate and k0 , k is
viral proteins with rate
the number of CP monomers associated to the imma-ture virions, Vimmature. Then, virions are produced at
immature½ð1 � p � qÞP =KC�k
f1 þ Vimmature=KC þ ½ðð1 � p � qÞP=KC�k0gk=k0
ð1 � p � qÞP k0
where g is the maximum rate to produce virions, andk is the number of CPs necessary to complete amature virion. All species are thermodynamically
where m is the maximum translation rate per molecule
degraded at rates kS (ssRNAs), kD (dsRNAs), kI
of genomic ssRNA.
(siRNAs) and kP (the rest of proteins or protein
The process of RNA silencing is initiated when
DICER cleaves dsRNA into siRNAs. The rates describ-
The effect exerted by different VSRs on DICER,
ing this process are given by the following set of
RISC and RDR6 can be conveniently modelled by the
following three equations, respectively:
0ð1 þ fqP =GRÞ
1 þ ðD þ D� þ C Þ=K
0ð1 þ fqP =GYÞ >
where d and KD are the catalytic and binding con-
stants of DICER, respectively. Afterwards, the RISCis activated by uploading the antisense siRNAs pro-
where C0, R0 and Y0 are the corresponding amounts
of each protein in the cell, which are assumed to be
in large excess, and GC, GR and GY are the bindingcoefficients of the corresponding VSR to their sub-strate
respectively. The parameter f determines the effi-
1 þ ðI þ RÞ=KI
ciency at which the suppressor precludes the activityof its target. For example, in the equation for
where r and KI are the catalytic and binding
DICER, an f ¼ 0.01 means that even at saturating
the activation of the RISC, it is now capable
DICER molecules will still be active. To account for
of directing the cleavage of the viral ssRNA with
the suppression on siRNA, we modify JRISC and
introduce a new equation to model the sequestrationof siRNAs.
1 þ ðI þ RÞ=K
ð1 � p � qÞP k0
1 þ ðI þ qPÞ=KI þ R=KI
where r and c are, respectively, the rates at which
the RISC and the suppressor attach to the siRNA
and GI, the binding affinity of the suppressor forthe siRNAs.
where y is the catalytic constant of RNA cleavage.
After defining all the relevant rate equations, it
CPs are pre-assembled
with ssRNA to produce
J. R. Soc. Interface (2011)
Bypassing RNA silencing
G. Rodrigo et al.
differential equations describing the dynamics of the
The vector of steady states is given by F(y1) ¼ 0,
which serves to calculate the asymptotic behaviour of
the system through the eigenvalues of its Jacobian
dissociation þ J �
1). The behaviour can change significantly
by modifying pivotal parameters of the system. Thus,
the construction of bifurcation diagrams is a useful
encapsidation � kSS þ;
tool for evaluating the behaviour regimes under differ-
ent conditions, and also to build up a sensitivity
dissociation � J �
analysis of the parameters of the system.
We show that the trivial solution of the system (i.e.
translation � kPP ;
silenced virus) is stable. The Jacobian matrix evaluated
1 ¼ 0 is given by
dissociation � kDD;
Jsuppression � kII ;
d0 ¼ dC0/KD, r0 ¼ rR0/KI, m0 ¼
mZ/KZ, a00 ¼ a0 þ kS, d00 ¼ d0 þ b þ kD and r00 ¼ r0 þ
I. This Jacobian has five negative real eigenvalues
encapsidation � Jvirion � kPVimmature >
(2a00, 2r00, 2d00, 2k
S and 2kP) that represent an
asymptotically stable solution of the system. Three of
them have multiplicity greater than one. The system
also has a second non-trivial solution in which the
virus beats the silencing response and replicates and
where the stoichiometric parameters n, n* and s
accumulates in the cell. Although we have verified
represent, respectively, the number of siRNAs pro-
numerically the existence of this non-trivial solution
on the full model, without lost of generality, the stab-
siRNAs produced in the secondary cycle of amplifi-
ility analysis for this second solution can be done
analytically by simplifying the system (2.11) as
secondary siRNA amplification to the degradation
of dsRNA relative to the primary siRNAs.
The full model in the Matlab format is available in
the electronic supplementary material.
3. STABILITY ANALYSIS
¼ ndD � yR�S � k
The system (2.11) can be rewritten in a vectorial formas dy/dt ¼ F(y) ¼ VJ(y) 2 Jy, where V is the
where the non-trivial steady state is the solution of S
matrix of stoichiometric coefficients, J(y) the vector
(b 2 d)/(b þ d) ¼ kS þ nday S2/(b þ d)
(y S þ kP).
of production rates and J a diagonal matrix with the
The characteristic polynomial is 2X3 þ tX2 2 hX 2
vector of degradation rates. The initial condition for
the molecular species involved in the system (y0)
the trace of the Jacobian matrix, h ¼ (2aS þ y R þ
depends on the nature of the virus (i.e. the infectious
kS)(b þ dþ yS þ kP) þ (y S þ kP)
(b þ d) 2 4abS 2
particle containing a genomic or an antigenomic RNA
y 2RS is the trace of its adjoint matrix and D ¼
strand). Here, we have considered for our analyses
[4abS 2 (2aS þ y R þ kS) (b þ d)] (y S þ kP) 2 2nad
viruses encapsidating genomic RNAs and therefore all
y S2 þ y 2RS(b þ d) its determinant. By applying the
the elements in y0 are zero except for Sþ ¼ 1. In case
Routh – Hurwitz stability criterion, the system will be
of negative-sense RNA viruses, the initial condition
stable when t , 0, D , 0 and ht , D. Henceforth, by
would be S2 ¼ 1. Accordingly, we construct an initial
taking the appropriate kinetic parameters that meet
value problem to obtain the dynamics of the system.
these three conditions, the system is characterized by
J. R. Soc. Interface (2011)
Bypassing RNA silencing
G. Rodrigo et al.
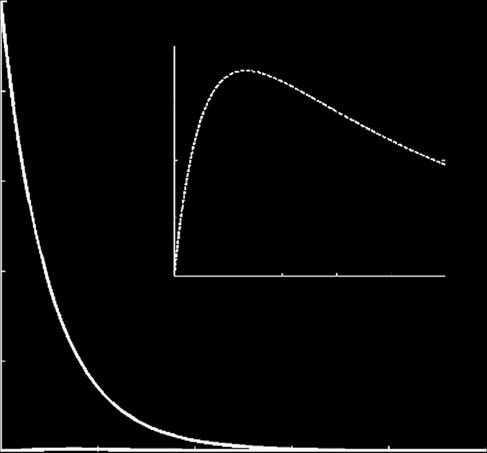
Figure 2. Dynamics of viral infection for different initial conditions. (a) The starting condition of the simulation is a single viralgenome; this results in the virus being silenced. (b) The starting condition is that 10 viral genomes infect the cell; this high mul-tiplicity of infection results in exponential viral replication after a period of latency of 1 day required to reach a threshold level ofRdRps. This successful infection happens even in the absence of a VSR. The parameters values are those shown in
bistability and, therefore, the initial condition is pivotal
In fact, this can be rationalized because viral RdRps
to determine the outcome of the process.
compete with ribosomes and with the activated RISCfor genomic strands, whereas they do not compete forantigenomic strands. In addition, high replication
rates also allow the virus to escape from the silencingmachinery and to minimize the effect of non-specific
4.1. Virus replication in the absence of a VSR
thermodynamic degradation (b).
We have studied the viral replication dynamics by using
One question that arises here is whether a tradeoff
the mathematical model presented in the previous sec-
between replication and translation exists. Upon
tion. First, we considered the case of RNA viruses
uncoating and the strictly necessary first event of trans-
that do not encode suppressor proteins. In we
lation, a viral genome can be directed either to
show several time-course evolutions of the system
transcription, and thus increase the concentration of
species (Sþ, S2 and P) for two different sets of initial
RNA, or to translation, and thus increase the concen-
conditions. When the multiplicity of infection is low
tration of viral proteins (in this case only replicase
(one single viral Sþ genome per cell) and for the typical
and coat). In c, we analysed such tradeoff by
parameter values shown in , we show that the
considering the binding affinities to positive strands of
population is extinguished (a), after a transient
replicase (KP) and ribosomes (KZ). We showed that in
where the concentration of P reaches a maximum. The
the absence of a silencing suppressor, silencing is the
model predicts that in this situation, the amount of
outcome favoured when translation is more frequent
antigenomic strains S2 produced is meaningless and
than transcription (KP , KZ). Accordingly, the best
its dynamics is dominated by the degradation term in
strategy for a virus to bypass the RNA silencing
the system of equations (2.11).
response in the absence of a suppressor protein would
However, the virus can bypass the silencing mechan-
be to increase the affinity of its RNA to the replicase
ism if the multiplicity of infection just increases to Sþ ¼
rather than to optimize its binding affinity to the ribo-
10 molecules b). In this case, after a latency
period of about 1 day, viral proteins reach a critical con-
efficiency, a virus will produce more copies of its
centration and promote further exponential replication.
genome up to the point in which the cleavage by
Analytically, the latency period can be estimated when
DICER would no longer control the accumulation of
viral genomes. shows, as expected, that the
P. In all these simulations, the condition
Sþ . S2 holds, in excellent agreement with the obser-
higher the catalytic constants for transcription and
vation of an excess of sense siRNAs for positive-sense
translation, the higher the chances for a successful
viral genomes [The effect of further increasing the
viral replication.
multiplicity of infection is to reduce the latency period(data not shown).
4.2. Virus replication dynamics in presence of a
We performed several sensitivity analyses to study
VSR that acts on DICER
the regions in parameter space in which viral replicationoccurs (non-trivial solution) or for which viral silencing
Many, if not all, viruses encode proteins capable of
takes place (trivial solution). We found that the higher
interacting with the cell molecular machinery. The sup-
the affinity for the negative strand (lower v), the wider
pression mechanism is often a protein – protein or
is the parameter space for viral replication ).
RNA – protein interaction resulting in a sequestration
J. R. Soc. Interface (2011)

Bypassing RNA silencing
G. Rodrigo et al.
m = 10 m = 20 m = 50
κS (h–1)
KP = 5×10
KP (molec)
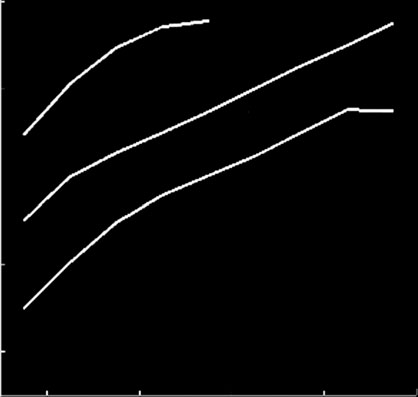
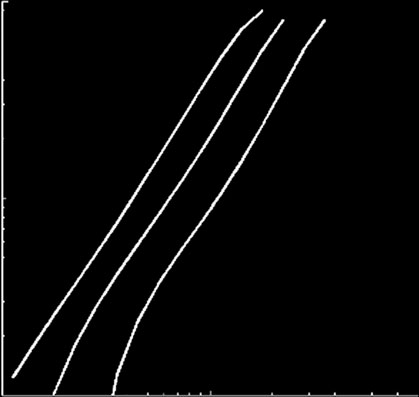
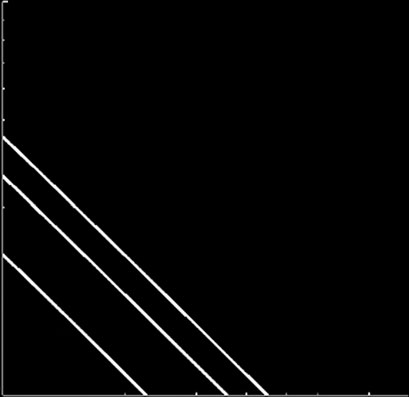
Figure 3. Phase diagrams identify different viral strategies. (a) The effect of the catalytic constant of DICER cleavage (d) in thereplication rate (a) and the differential affinity of RdRps for positive and negative strands (v). (b) The relationship between aand the ssRNA degradation rate (kS) for different values of the translation rate (m). (c) The sensitivity of the binding constants ofribosomes (KZ) and replicases (KP) to a. (d ) The effect of KP on m and a. Rep means viral replication bypassing silencing, and Silviral extinction by silencing.
or blockage of one of the many molecules involved in
necessary for completing a virion as a function of the
the silencing pathway that allows the virus to escape
cellular amounts of DICER (C0). For low amounts of
from silencing surveillance. Our general model can be
DICER, TV is insensitive to variation in GC. In
used to analyse and study the effect of various suppres-
addition, an increase in the number of DICER mol-
sors encoded by different viruses. To analyse the effect
ecules per cell does not have any effect on TV for
of a suppressor, we considered the virus replication
suppressors with weak affinity. However, if GC increases
speed as a characteristic scoring function. This speed
(moving rightwards in the ordinates axis in ),
can be easily computed as the inverse of the time
then the time to produce virions significantly grows
taken to produce mature virions (TV). In , we
up and becomes infinity (indicating viral silencing) for
plot 1/TV versus KZ and KP for the case of a VSR oper-
high amounts of DICER molecules present in the cell
ating over DICER. We found that such a suppressor
at the time of infection.
enhances the speed of virus accumulation with respectto a virus without encoding a VSR.
4.3. The effect of suppressing downstream steps
To further analyse the suppressor strategy of manip-
of the silencing pathway
ulating DICER, we constructed a phase diagrambetween the catalytic constant of cleavage by DICER
Next, we sought the effect of VSRs operating down-
stream in the silencing pathway. Surprisingly, we
a). We found that the effect of the suppressor
found that suppressors affecting at other levels of the
is only significant beyond a threshold level of GC (in
pathway (e.g. sequestering siRNAs, interfering with
this case 7000 molecules). In other words, if the affinity
RISC or with RDR6) did not enlarge the parameter
of the suppressor is not high enough, it only represents a
space in which the virus successfully replicates within
cost for the virus because it cannot help in its replica-
a single cell (data not shown). This result suggests
tion. b shows the effect that the binding
that only by suppressing DICER, the first bottleneck
affinity of the suppressor for DICER has on the time
to replication imposed by the system, viruses could
J. R. Soc. Interface (2011)
Bypassing RNA silencing
G. Rodrigo et al.
suppressor sensitivity
GC (molec)
KZ (molec)
KP (molec)
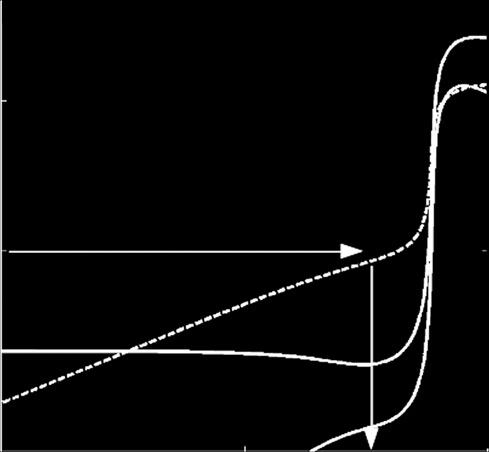
Figure 4. Virus replication speed (computed as the inverse of
the time to form a mature virion, TV) versus the binding con-
stants of ribosomes (K
C = 8 × 10
Z) and replicases (KP), with GC
molecules. (a) Virus without a VSR. (b) Virus encoding a sup-
pressor that blocks DICER. The benefit associated with
C0 = 5 × 103
carrying such a suppressor is evaluated as the differencebetween both surfaces and is indicated by the dashed lineand the arrow. The other parameters take the values shown
widen the parameter region, resulting in successfulreplication. Hence, the question is why other types of
GC (molec)
VSRs, such as siRNA sequesters, have evolved? Our
Figure 5. (a) Phase diagram to analyse the suppressor effect
negative result suggests that the RNA silencing mode
on DICER between d and G
of action cannot be rationalized by only looking into a
C, with a ¼ 20 h21 for different
values of m. (b) Time to form one virion (TV) versus the sup-
single cell but that a more complex situation in which
pressor constant of DICER, with a ¼m ¼ 20 h21 for different
cell-to-cell effects may contribute should be considered.
values of C0 (in molecules). The other parameters take the
This leads us to consider the role of the space to analyse
values shown in . Rep means viral replication bypassing
such mechanism.
silencing, and Sil viral extinction by silencing.
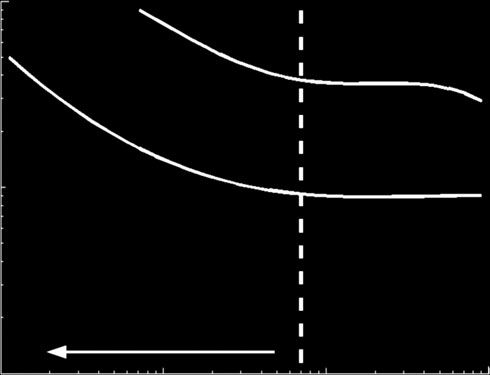
In a, we plot the relative amount of accumu-
lated siRNAs (normalized by the amount or siRNAproduced in the absence of a VSR, I/IG!1), in the pres-
ence of two suppression strategies. For illustrativepurposes, we have chosen the successful operation
We have presented a deterministic model of the inter-
over DICER described in the previous section and one
play between viral replication and the RNA silencing
based on sequestering siRNAs. By increasing the affi-
pathway. For the sake of biological realism, we modelled
nity for the corresponding target molecule (moving
a particular type of virus, the picorna-like. By doing so,
rightwards on the ordinate axis) to the maximum
the model pays the cost of reduced generality and the
conclusions may not be applicable to viruses with
DICER reduces the concentration of siRNA around
other genomic architectures such as negative-sense
two orders of magnitude. However, the strategy based
RNA, retroviruses or DNA viruses. Although our results
on sequestering siRNAs is far less efficient since at the
have been performed for positive-sense RNA viruses,
strongest affinity it only reduces the accumulation of
the model can also be used to study negative-sense
virus-derived siRNAs by one order of magnitude.
viruses with minor changes in some rates and the initial
However, the transfer of siRNAs from infected to
conditions. Readers interested in exploring the inter-
neighbouring healthy cells, which allows the peripheral
play between the silencing pathway and any of these
cells to activate the RISC in the absence of viral infec-
viruses must necessarily look at this article as the start-
tion, has the expected effect b). In the
ing point for developing their own models. Nonetheless,
absence of triggering siRNAs, infection progresses
our approximation has allowed us to study and compare
with the time delay already described above. However,
different viral suppression strategies. We have shown
if the cell has been already activated, the virus is not
that the RNA silencing pathway allows a large variety
able to overcome the cleavage by the RISC and runs
of behaviours, suggesting multiple potential evolution-
to extinction.
ary trajectories for RNA viruses. Future models will
J. R. Soc. Interface (2011)
Bypassing RNA silencing
G. Rodrigo et al.
because one may expect more replication to generate
more dsRNA and, therefore, to strength the silencingresponse and, likewise, more translation to producemore suppressor protein. It can be argued that, afterthe very initial burst of translation from the infecting
genomic sequence resulting in a few viral proteins, the
I/I G
optimal strategy involves synthesizing antigenomic
strands and using them as templates for producing alarge excess of genomic strands (i.e. using a stampingmachine replication strategy) without diverting theminto translation. If replication is fast enough, this repli-cative strategy works even in the absence of a
suppressor protein: a positive feedback is establishedsuch that the replication overcomes the capacity of
the available DICER molecules to keep virus replication
under control. Once a significant amount of genomic
strands has been produced, then translation may take
place. If translation results in a VSR protein, then a
synergistic effect between fast transcription and trans-lation appears, resulting in successful viral replication.
Among many possibilities, we have focused on three
viral strategies. The first one, consisting of blockingDICER, turns out to be the most efficient promoting
viral replication. This result is somehow logical from
an optimal design perspective. By hitting the first bot-
tleneck in the pathway, the virus ensures its own
R*(0) = 10
replication. Hitting downstream steps would allowDICER to still exert partial control on virus replication.
The other three strategies explored, sequestering
siRNA, blocking the RISC and disrupting the second-
ary amplification via RDR6, have been less efficient in
promoting intracellular virus accumulation, althoughthey may gain some benefit when looking at cell-to-
Figure 6. (a) Amount of siRNA (relative to the amount accu-
cell movement. This finding is in good agreement with
mulated without a viral suppressor of RNA silencing) versus
the observation that Cucumovirus 2b and Tombusvirus
the suppressor constant (G) on DICER or siRNA. (b) Viral
RNA dynamics in a cell which has not been immunized by
receiving siRNA from neighbouring cells (R*(0) ¼ 0) and ina cell that has received a small input of siRNA from an
accumulation ].
infected neighbour cell (R*(0) ¼ 10 molecules). The par-
Although mathematically convenient, it may be a
ameters take the values shown in expect a ¼ 50 h21.
biological oversimplification to assume that suppressorsact at a single stage of the silencing pathway. Evidenceexists showing that VSRs may well simultaneously
account for different viral genomic organizations and
operate at diverse stages of the pathway. For example,
for inherent stochastic effects associated with small
the potyviral HC-Pro sequesters siRNAs but also affects
numbers of molecules ]. The model presented here
the expression of plant genes, including the dcl-like
differs from other models of the interaction between
genes encoding for the different DICER proteins in Ara-
virus and the host silencing response [in which
bidopsis thaliana [or by reducing the 30
here we have explored the role played by different
methylation of siRNAs, making them sensitive to oli-
suppressors of RNA silencing. We have demonstrated
and shown in that the system has two stable
Another example of multiple actions is the Polerovirus
steady states (replication and silencing) and, thus, the
P0 that interferes with the silencing pathway at least
initial condition of the system (i.e. the initial amount
at two levels: binding siRNAs and avoiding the for-
of ssRNA in the cell) is important to determine its
mation of the activated AGO complex and labelling it
dynamics. Likewise, the higher the initial amount of
for degradation ([press). Also, a virus may
viral RNA, the higher the zone for exponential viral
carry more than one VSR, as seems to be the case for
replication in the parameter space. This suggests that
some tombusviruses (P19 and CP).
increasing the multiplicity of infection is a possible
We have also found that in certain regions of par-
strategy for virus to escape from the control of RNA
ameter space, a virus would be capable of replicating
even in the absence of a VSR. The plant subviral patho-
We have shown that in the presence of an active
gens known as viroids do not encode for any protein at
silencing response, it is to the benefit of the virus to
all and are still capable of replication in susceptible
invest into a transcriptional strategy rather than in
hosts ], despite the fact that their RNA molecules
translation. This may be somehow counterintuitive
are targets of DICER [It has been suggested that
J. R. Soc. Interface (2011)
Bypassing RNA silencing
G. Rodrigo et al.
viroids may evade silencing because of their highly com-
4 Fire, A., Xu, S., Mongomery, M. K., Kostas, S. A., Driver,
plex and packed secondary structure [Other
S. E. & Mello, C. C. 1998 Potent and specific genetic inter-
strategies viruses may use for avoiding silencing consist
ference by double-stranded RNA in Caenorhabditis
in replicating within spherules in the endoplasmic reti-
elegans. Nature 391, 806 – 811. ()
culum membrane ], where they remain inaccessible
5 Vaucheret, H., Beclin, C. & Fagard, M. 2001 Post-tran-
scriptional gene silencing in plants. J. Cell Sci. 14,
3083 – 3091.
Although we have modelled the effect of VSRs on
6 Elbashir, S. M., Harborth, J., Lendeckel, W., Yalcin, A.,
DICER as a direct protein – protein interaction, VSRs
Weber, K. & Tuschl, T. 2001 Duplexes of 21-nucleotide
can also interfere with DICER activity by protecting
RNAs mediate RNA interference in cultured mammalian
the dsRNA as it is produced as reported for CP [
cells. Nature 411, 494 – 498.
This particular activity would be easily incorporated
7 Hamilton, A. J. & Baulcombe, D. C. 1999 A species of
into our mathematical framework in two simple ways.
small antisense RNA in posttranscriptional gene silencing
First, by treating the binding affinity of DICER for
in plants. Science 286, 950 – 952. (
D) as a decreasing function of CP concen-
tration. Second, by defining a new molecular species
8 Vermeulen, A., Behlen, L., Reynolds, A., Wolfson, A.,
Marshall, W. S., Karpilow, J. & Khvorova, A. 2005 The
for the complex dsRNA/CP and writing down the cor-
contributions of dsRNA structure to DICER specificity
responding rates and adding a new differential equation
and efficiency. RNA 11, 674 – 682.
in equation (2.11).
In conclusion, we have shown that from a system
9 Bohmert, K., Camus, I., Bellini, C., Bouchez, D., Caboche,
design perspective, the best strategy that a virus may
M. & Benning, C. 1998 AGO1 defines a novel locus of Ara-
take to ensure its replication in the presence of the anti-
bidopsis controlling leaf development. EMBO J. 17, 170 –
viral response mediated by RNA silencing would be to
(i) replicate fast and by producing an excess of genomic
10 Rand, T., Petersen, S., Du, F. & Wang, X. 2005 Argo-
strands, (ii) encode for a VSR that interacts with the
naute2 cleaves the anti-guide strand of siRNA during
DICER protein, and (iii) exert some control on the mul-
RISC activation. Cell 123, 621 – 629. (
tiplicity of infection, ensuring that multiple genomes
11 Voinnet, O., Vain, P., Angell, S. & Baulcombe, D. C. 1998
infect each cell. Obviously, evolution is not a perfect
Systemic spread of sequence specific transgene RNA
designer and viruses have acquired suppressor proteins
degradation is initiated by localized introduction of ecto-
that target at different steps of the silencing pathway.
pic promoter less DNA. Cell 95, 177 – 187.
Understanding the exact mechanisms by which these
VSRs operate will allow us to develop better models
12 Himber, C., Dunoyer, P., Moissiard, G., Ritzenthaler, C. &
and to increase our ability to predict the outcome of
Voinnet, O. 2003 Transitivity-dependent and -independent
the virus – host interaction. Furthermore, VSRs have
cell-to-cell movement of RNA silencing. EMBO J. 22,
clear biotechnological potential as they can be used to
maximize the expression of transgenes ]. Designing
13 Lecellier, C. H. & Voinnet, O. 2004 RNA silencing: no
optimal suppressors would benefit from the knowledge
mercy for viruses? Immunol. Rev. 1998, 285 – 303.
14 Ding, S. & Voinnet, O. 2007 Antiviral immunity directed
advanced in this article.
by small RNAs. Cell 130, 413 – 426. (
This work was supported by the Spanish Ministerio de
Ciencia e Innovacio´n grants BFU2009-06993 to S.F.E. and
15 Li, F. & Ding, S. W. 2006 Virus counterdefenses: diverse
strategies for evading the RNA-silencing immunity.
(BioModularH2), FP7-ICT-043338 (Bactocom), FP7-KBBE-
503 – 531.
212894 (Tarpol), the Structural Funds of the European
Regional Development Fund, the ATIGE-Genopole and the
16 Brigneti, G., Voinnet, O., Li, W. X., Ji, L. H., Ding, S. W. &
Foundation pour la Recherche Medicale grants (all to A.J.).
Baulcombe, D. C. 1998 Viral pathogenicity determinants are
J.C, G.R. and A.J. also acknowledge the HPC-Europa
suppressors of transgene silencing in Nicotiana benthamiana.
programme (RII3-CT-2003-506079). G.R. was supported by
EMBO J. 17, 6739–6746. )
a graduate fellowship from the Generalitat Valenciana and
17 Baulcombe, D. 2004 RNA silencing in plants. Nature 431,
an EMBO Short-term fellowship. S.F.E. also acknowledges
support from the Santa Fe Institute.
18 Kasschau, K. D., Xie, Z., Allen, E., Llave, C., Chapman,
E. J., Krizan, K. A. & Carrington, J. C. 2003 P1/HC-Pro, a viral suppressor of RNA silencing, interfereswith Arabidopsis development and miRNA function.
Dev.
205 – 217.
1 Domingo, E. & Holland, J. J. 1997 RNA virus mutations
19 Moissiard, G. & Voinnet, O. 2004 Viral suppression of
and fitness for survival. Annu. Rev. Microbiol. 51, 151 –
RNA silencing in plants. Mol. Plant Pathol. 5, 71 – 82.
2 Elena, S. F. & Sanjua´n, R. 2007 Virus evolution: insights
20 Dı´az-Pendo´n, J. A. & Ding, S. W. 2008 Direct and indirect
from an experimental approach. Annu. Rev. Ecol. Evol.
roles of viral suppressors of RNA silencing in pathogenesis.
Syst. 38, 27 – 52.
Annu. Rev. Phytopathol. 46, 303 – 326.
3 Drake, J. W. & Holland, J. J. 1999 Mutation rates among
21 Mallory, A. C., Reinhart, B. J., Bartel, D., Vance, V. B. &
RNA viruses. Proc. Natl Acad. Sci. USA 96, 13 910 –
Bowman, L. H. 2002 A viral suppressor of RNA silencing
differentially regulates the accumulation of short interfering
J. R. Soc. Interface (2011)
Bypassing RNA silencing
G. Rodrigo et al.
RNAs and microRNAs in tobacco. Proc. Natl Acad. Sci.
amplification limits accidental self-directed reactions.
USA 99, 15 228 – 15 233. (
Proc. Natl. Acad. Sci. USA 100, 11 511 – 11 516. (
22 Chapman, E. J., Prokhnevsky, A. I., Gopinath, K., Dolja,
V. & Carrington, J. C. 2004 Viral RNA silencing suppres-
37 Raab, R. M. & Stephanopoulos, G. 2004 Dynamics of gene
sors inhibit the microRNA pathway at an intermediate
silencing by RNA interference. Biotechnol. Bioeng. 88,
step. Genes Dev. 18, 1179 – 1186. (
38 Groenenboom, M. A. C., Mare´e, A. F. M. & Hogeweg, P.
23 Dunoyer, P., Lecellier, C. H., Parizotto, E. A., Himber, C.
2005 The RNA silencing pathway: the bits and pieces
& Voinnet, O. 2004 Probing the microRNA and small
that matter. PLoS Comp. Biol. 1, e21.
interfering RNA pathways with virus-encoded suppressors
of RNA silencing. Plant Cell 16, 1235 – 1250.
39 Groenenboom, M. & Hogeweg, P. 2008 The dynamics and
efficacy of antiviral RNA silencing: a model study. BMC
24 Lakatos, L., Szittya, G., Silhavy, D. & Burgyan, J. 2004
Syst. Biol. 2, 28.
Molecular mechanism of RNA silencing suppression
40 Groenenboom, M. & Hogeweg, P. 2008 RNA silencing can
mediated by p19 protein of tombusviruses. EMBO J. 23,
explain chlorotic infection patterns on plant leaves. BMC
Syst. Biol. 2, 105. ()
25 Mlotshwa, S. et al. 2005 Ectopic DICERLIKE1 expression
41 Endy, D., Kong, D. & Yin, J. 1996 Intracellular kinetics of
in P1/HC-Pro Arabidopsis rescues phenotypic anomalies
a growing virus: a genetically structured simulation for
but not defects in microRNA and silencing pathways.
bacteriophage T7. Biotechnol. Bioeng. 55, 375 – 389.
Plant Cell 17, 2873 – 2885. )
26 Deleris, A., Gallego-Bartolome, J., Bao, J., Kasschau,
K. D., Carrington, J. C. & Voinnet, O. 2006 Hierarchical
42 Reddy, B. & Yin, J. 1999 Quantitative intracellular kin-
action and inhibition of plant DICER-like proteins in anti-
etics of HIV type 1. AIDS Res. Hum. Retrovir. 15, 273 –
viral defense. Science 313, 68 – 71. (
43 Srivastava, R., You, L., Summers, J. & Yinn, J. 2002 Sto-
27 Qi, Y., Zhong, X., Itaya, A. & Ding, B. 2004 Dissecting RNA
chastic vs. deterministic modeling of intracellular viral
silencing in protoplasts uncovers novel effects of viral sup-
kinetics. J. Theor. Biol. 218, 309 – 321. (
pressors on the silencing pathway at the cellular level.
Nucleic Acids Res. 32, e179.
44 Sidorenko, Y. & Reichl, U. 2004 Structured model of influ-
28 Peremyslov, V. V., Hagiwara, Y. & Dolja, V. V. 1999
enza virus replication in MDCK cells. Biotechnol. Bioeng.
of a plant virus. Proc. Natl Acad. Sci. USA 96, 14 771 –
45 Lim, K., Lang, V., Lam, T. & Yin, J. 2006 Model-based
design of growth-attenuated viruses. PLoS Comput. Biol.
29 Azevedo, J. et al. 2010 Argonaute quenching and global
changes in Dicer homeostasis caused by a pathogen-
46 Dahari, H., Ribeiro, R. M., Rice, C. M. & Perelson, A. S.
encoded GW repeat protein. Genes Develop. 24, 904 –
2007 Mathematical modeling of subgenomic hepatitis C
virus replication in Huh-7 cells. J. Virol. 81, 750 – 760.
30 Vanitharani, R., Chellappan, P., Pita, J. S. & Fauquet, C.
M. 2004 Differential roles of AC2 and AC4 of Cassava
47 Sardanye´s, J., Sole´, R. V. & Elena, S. F. 2009 Replication
Geminiviruses in mediating synergism and suppression of
mode and landscape topology differentially affect RNA
posttranscriptional gene silencing. J. Virol. 78, 9487 –
virus mutational load and robustness. J. Virol. 83, 12
579 – 12 589. )
31 Baumberger, N., Tsai, C. H., Lie, M., Havecker, E. & Baul-
48 DeAngelis, D. L., Goldstein, R. A. & O'Neill, R. V. 1975 A
combe, D. C. 2007 The polerovirus silencing suppressor P0
model for trophic interaction. Ecology 56, 881 – 892.
targets ARGONAUTE proteins for degradation. Curr.
Biol. 17, 1609–1614.
49 MacRae, I. J., Zhou, K. & Doudna, J. A. 2007 Structural
32 Csorba, T., Lo´zsa, R., Hutva´gner, G. & Burgya´n, J. 2010
determinants of RNA recognition and cleavage by
Polerovirus protein P0 prevents the assembly of small
DICER. Nat. Struct. Mol. Biol. 14, 934 – 940. (
RNA containing RISC complexes and leads to degradation
of ARGONAUTE1. Plant J. 62, 463 – 472. (
50 Qi, X., Bao, F. S. & Xie, Z. 2010 Small RNA deep sequen-
cing reveals role for Arabidopsis thaliana RNA-dependent
33 Fukunaga, R. & Doudna, J. A. 2009 dsRNA with 50 over-
RNA polymerases in viral siRNA biogenesis. PLoS ONE 4,
hangs contributes to endogenous and antiviral RNA
silencing pathways in plants. EMBO J. 28, 545 – 555.
51 Gillespie, D. T. 1977 Exact stochastic simulation of
coupled chemical reactions. J. Phys. Chem. 81, 2340 –
34 Meng, C., Chen, J., Peng, J. & Wong, S. M. 2006 Host-
induced avirulence of Hibiscus chlorotic ringspot virus
52 Ebhart, H. A., Thi, E. P., Wang, M. B. & Unrau, P. J.
mutant correlates with reduced gene-silencing suppression
2005 Extensive 30 modification of plant small RNAs is
activity. J. Gen. Virol. 87, 451 – 459.
modulated by helper component-proteinase expression.
Proc. Natl Acad. Sci. USA 102, 13 398 – 13 403.
35 Me´rai, Z., Kere´ncyi, Z., Molna´r, A., Barta, E., Bisztray,
53 Me´rai, Z., Kere´nyi, Z., Kerste´sz, S., Magna, M., Lakatos,
G., Havelda, Z., Burgya´n, J. & Silhavy, D. 2005 Aureus-
L. & Silhavy, D. 2006 Double-stranded RNA binding
virus P14 is an efficient RNA silencing suppressor that
may be a general plant RNA viral strategy to suppress
binds double-stranded RNAs without size specificity.
RNA silencing. J. Virol. 80, 5747 – 5756. (
J. Virol. 79, 7217 – 7226.
54 Daro s, J. A., Elena, S. F. & Flores, R. 2006 Viroids: an
36 Bergstrom, C. T., McKittrick, E. & Antia, R. 2003 Math-
Ariadne's thread into the RNA labyrinth. EMBO Rep.
J. R. Soc. Interface (2011)
Bypassing RNA silencing
G. Rodrigo et al.
55 Di Serio, F., Gisel, A., Navarro, B., Delgado, S., Martı´nez
60 Haley, B. & Zamore, P. D. 2004 Kinetic analysis of the
de Alba, A. E., Donvito, G. & Flores, R. 2009 Deep
RNAi enzyme complex. Nat. Struct. Mol. Biol. 11, 599 –
sequencing of the small RNAs derived from two sympto-
matic variants of a chloroplastic viroid: implications for
61 Endres, D. & Zlotnick, A. 2002 Model-based analysis of
their genesis and for pathogenesis. PLoS ONE 4, e7539.
assembly kinetics for virus capsids or other spherical poly-
mers. Biophys. J. 83, 1217 – 1230.
56 Wang, M. B. et al. 2004 On the role of RNA silencing in
the pathogenicity and evolution of viroids and viral satel-
62 Rana, T. M. 2007 Illuminating the silence: understanding
lites. Proc. Natl Acad. Sci. USA 101, 3275 – 3280. (
the structure and function of small RNAs. Mol. Cell. Biol.
8, 23 – 36.
57 Go´mez, G. & Palla´s, V. 2007 Mature monomeric forms of
63 Rajendran, K. S. & Nagy, P. D. 2006 Kinetics and func-
Hop stunt viroid resist RNA silencing in transgenic plants.
tional studies on interaction between the replicase
Plant J. 51, 1041 – 1049.
proteins of Tomato bushy stunt virus: requirement of
p33: p92 interaction for replicase assembly. Virology 345,
58 Schwartz, M., Chen, J., Janda, M., Sulivan, M., den Boon,
J. & Ahlquist, P. 2002 A positive-strand RNA virus repli-
64 Scheper, G. C., van Kollenburg Dagger, B., Hu, J., Luo,
cation complex parallels form and function of retrovirus
Y., Goss, D. J. & Proud, C. G. 2002 Phosphorylation of
capsids. Mol.
9, 505 – 514.
eukaryotic initiation factor 4E markedly reduces its affi-
nity for capped mRNA. J. Biol. Chem. 277, 3303 – 3309.
59 Sun, W., Jun, E. & Nicholson, A. W. 2001 Intrinsic
65 Lakatos, L. et al. 2006 Small RNA binding is a common
Escherichia coli ribonuclease III lacking the dsRNA-bind-
strategy to suppress RNA silencing by several viral sup-
ing domain. Biochemistry 40, 14 976 – 14 984. (
pressors. EMBO J. 25, 2768 – 2780.
J. R. Soc. Interface (2011)
Source: http://jaramillolab.issb.genopole.fr/download/attachments/589899/Rodrigo-Optimal+viral+strategies+for+bypassing+RNA+silencing.-2011.pdf
Parkinson's Disease Information Sheet 1.4 Parkinson's A a Medication Treatment Options Parkinson's Sinemet® progressive neurological condition which decarboxylase inhibitor carbidopa. Kinson® is is related to a deficit of dopamine as a the generic form of Sinemet®. Madopar® result of degeneration of dopamine
Hair Stylist, Makeup Artist and Cosmetic Tattooist Ph: +61 [0] 412 546 278 GORDANA IS A LEADER IN HAIR AND MAKEUP FOR PROMINENT AUSTRALIAN AND OVERSEAS CELEBRITIESGordana's clients have included Russell Crowe, Cate Blanchett, Zac Effron, Jennifer Love Hewitt and Reece Witherspoon. Music clients have included Sir Paul McCartney, Kate Cebrano and Shirley MacLaine









