Mccordresearch.com
Pediatr Infect Dis J, 2001;20:104–7
Copyright 2001 by Lippincott Williams & Wilkins, Inc.
Printed in U.S.A.
Safety of ofloxacin otic and other ototopical
treatments in animal models and in humans
GEORGE A. GATES, MD
To assess the safety of topical agents in the
terna.4 Their consensus is based on the failure of trial
middle ear, animal studies were reviewed. Com-
evidence to prove that systemic antibiotics improve
pared with aminoglycoside-containing prepara-
outcome compared with ototopical preparations. The
tions, which caused significant loss of hair cells
guidelines stress that topical antibiotics are first line
in the basal turn of the cochlea, ofloxacin caused
treatment for these conditions and that, with consider-
no loss of hair cells, even at concentrations
ation of the bactericidal activity and risk-benefit of the
higher than used clinically. Moreover auditory
available pharmacologic choices, the most effective
brainstem testing revealed no change in auditory
nontoxic option should be selected.
thresholds in the ofloxacin-treated animals,
This article provides a review of the pathophysiology
whereas neomycin-treated animals showed sub-
of drug-induced ototoxicity and the relative risk asso-
stantial threshold shifts.
ciated with different ototopical drugs. It will also at-
In human studies, use of topical ofloxacin 0.3%
tempt to describe the safety experience to date with the
was not associated with any change in hearing.
topical fluoroquinolone antibiotic, ofloxacin otic, which
Topical ofloxacin has no demonstrable adverse
in early reports appears to cause little or no ototoxicity.
effects on middle ear or cochlear function.
CONCERNS REGARDING OTOTOPICAL
In the United States more than 750 000 children each
year receive tympanostomy tubes for chronic otitis me-
Today ototopical medications are the principal treat-
dia.1 The objective benefits of this intervention in terms of
ment for otorrhea caused by external otitis, chronic
improved hearing and decreased recurrent middle ear
suppurative otitis media and acute otitis media associ-
infections are well established,2 and a recent report
ated with a perforation or tympanostomy tube. Treat-
documented an improved quality of life for treated chil-
ment of otorrhea with topical agents is confounded by
dren as well.3 However, the surgical procedure is not
concerns about ototoxicity, which has been a substan-
without risk, and resultant infection marked by otorrhea
tial problem with aminoglycoside-containing prepara-
is relatively common. One-third of patients receiving
tions. Local and systemic uses of aminoglycosides have
tympanostomy tubes develop acute otitis media with
been reported to cause auditory and vestibular toxicity,
otorrhea after surgery, and virtually 100% of those in
including diminished hearing and vertigo. When ad-
whom the tube remains in place for more than 1 year will
ministered to patients with otitis media with a tym-
experience at least one episode of infection.
panic membrane perforation, topical drugs may enter
A wide range of antibiotics currently are used to
the middle ear and cause mucosal or tympanic mem-
treat otorrhea, but reversible and irreversible tinnitus
brane damage. Furthermore if the drug passes into the
and hearing loss have been reported as potential tox-
inner ear via the round window, it may cause cochlear
icities associated with acute intoxication or long term
and vestibular degeneration, ultimately leading to sig-
administration of many of these drugs. To best avoid
nificant hearing loss and imbalance.
these complications, a panel of experts convened by the
Potential mechanisms for drug-related ototoxicity
American Academy of Otolaryngology-Head and Neck
have been evaluated in guinea pig models. In these
Surgery recently presented a set of consensus guide-
animal systems, administration of antibiotic drops to
lines for antibiotic use in chronic suppurative otitis
the middle ear resulted in histologic damage to co-
media, tympanostomy tube otorrhea and otitis ex-
chlear hair cells, with the most severe damage occur-ring at the basal turn of the cochlea.5 On auditorybrainstem response testing the histologic changes
From the Department of Otolaryngology-Head and Neck Sur-
noted with Cortisporin and gentamicin were associated
gery, Department of Epidemiology, University of Washington,and Virginia Merrill Bloedel Hearing Research Center, Seattle,
with high frequency hearing loss. In humans the oto-
toxicity of gentamicin is administered intratympani-
Key words: Ototoxicity, otitis externa, acute otitis media,
cally to disable the vestibular system in patients suf-
tympanostomy tubes, chronic suppurative otitis media.
Reprints not available.
fering from vertigo caused by Menie re's disease. In this
Vol. 20, No. 1, January, 2001
THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
setting gentamicin results in hearing loss in about 30%of cases. Other topical antibiotic agents, such as neo-mycin, consistently cause substantially greater preva-lence and degree of loss and must be avoided in peoplewith a perforated tympanic membrane and normalmiddle ear mucosa. In inflamed middle ears the risk oftoxicity is reduced but not eliminated. Because of thisrisk continuing effort has been directed at identifyingnonototoxic antibiotic medications.
OFLOXACIN OTIC: ANIMAL EVIDENCE OF
SAFETY
The fluoroquinolone antibiotics have proved to be
effective antimicrobials against the pathogens most
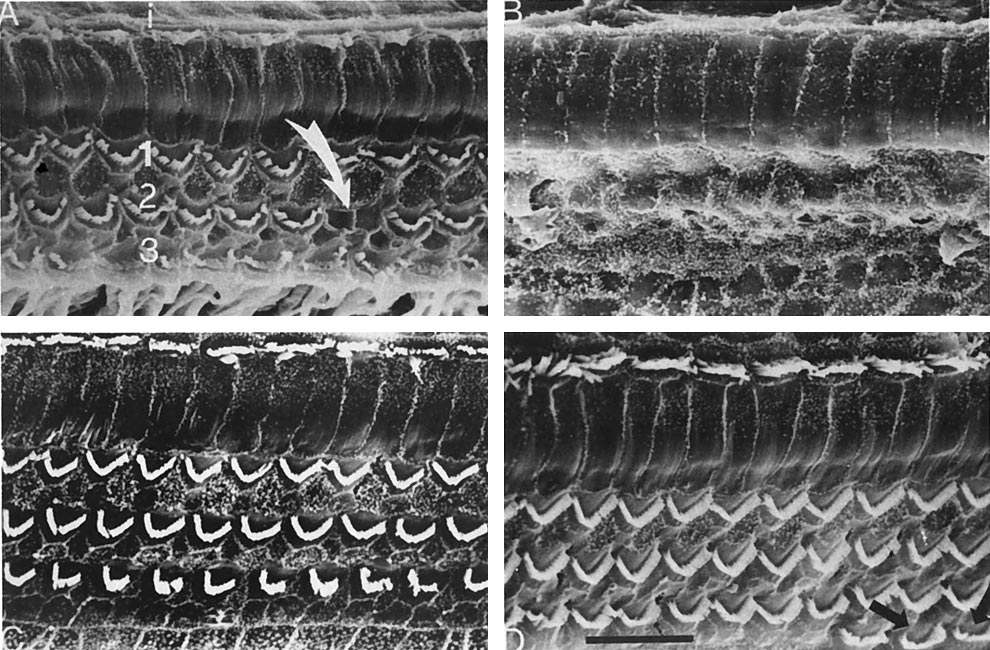
FIG. 1. Ototoxicity of topical otomicrobial agents in guinea pig
models. Reproduced with permission from Barlow et al.5
frequently responsible for otorrhea,6, 7 and in earlyreports they appear to be essentially nonototoxic.8–10In particular ofloxacin otic has not been associatedwith cochlear or middle ear toxicity in animal models,8while providing excellent activity against Staphylococ-cus aureus, methicillin-resistant S. aureus and Pseudo-monas aeruginosa.7
Barlow et al.5 at the University of Washington ex-
amined the effect of ofloxacin otic solution on thestructure and function of the middle ear, ossicles andcochlea of albino Hartley guinea pigs. The investigatorscompared the toxicity of 1% ofloxacin otic solution,which is a dose three times the concentration containedin the preparation available for clinical use, Cortis-porin otic solution, 0.3% gentamicin ophthalmic solu-tion and benzalkonium chloride (0.026% and 0.05%)
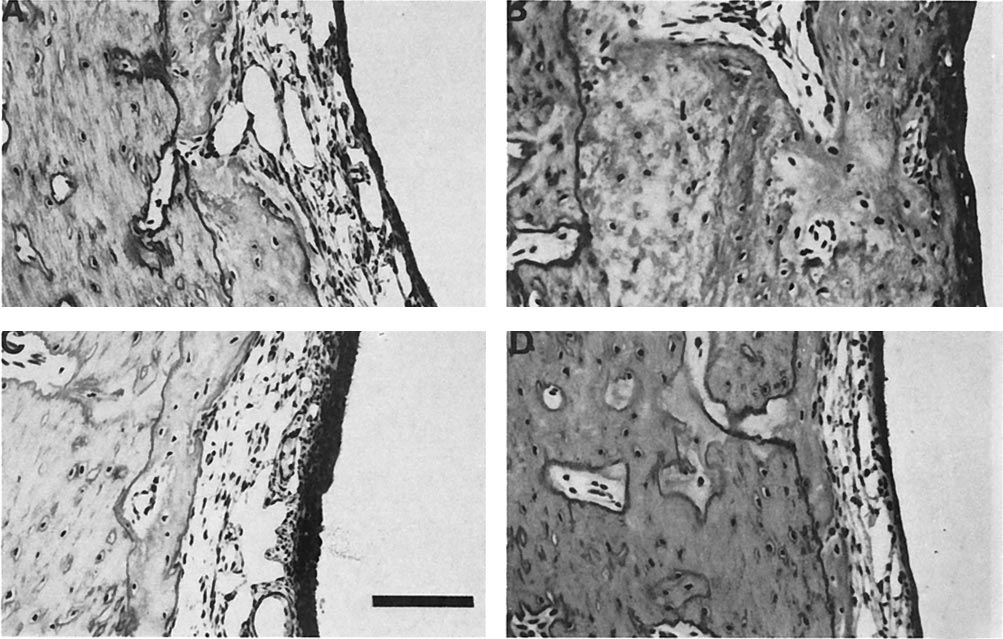
FIG. 2. Cross-section of guinea pig middle ear mucosa exposed
to gentamicin (A), Cortisporin (B), benzalkonium 0.05% (C), and
when administered to the middle ear of the guinea pigs
ofloxacin otic 1.0% (D). Reproduced with permission from Barlow
for 7 days. Surface preparation light microscopy and
scanning electron microscopy were used to evaluatetoxicity to the hair cells of the organ of Corti, whichamplify incoming signals for normal hearing. Figure 1
trol, the finding indicates the potential for ototoxicity
illustrates the results, revealing minimal (1%) cochlear
that must be considered when prescribing these drugs.
inner and outer hair cell loss associated with ofloxacin
In a similar study Black et al. delivered 0.3 and 1.0%
otic exposure, compared with greater (6.5%) loss with
ofloxacin otic solution or 10% neomycin solution twice
gentamicin and significant hair loss (⬎65%) with Cor-
daily to the middle ear of guinea pigs (5/sex/group) for 30
tisporin. Benzalkonium, a vehicle used in the prepara-
days.11 After 1 month of treatment ofloxacin otic had not
tion of the solution forms of ofloxacin otic and genta-
caused any damage to the middle ear mucosa or ossicles.
micin, also caused minimal toxicity.
There was no evidence of change in the auditory brain-
The investigators also evaluated middle ear damage
stem response (which is a functional measure of hearing)
from the three antibiotics using scanning electron
or to cochlear morphology. In addition there was no
microscopy. When the animals were sacrificed, cross-
significant shift from baseline in mean threshold auditory
sections of the middle ear mucosa indicated differential
responsivity when measured at 4, 10 and 20 kHz with
effects of the three drugs (Fig. 2). Gentamicin caused
either of the ofloxacin otic solutions. In contrast 10%
mild mucosal thickening and inflammatory cell infil-
neomycin caused a marked shift, in the range of 35.0 to
tration, whereas Cortisporin produced severe mucosal
47.8 decibels (Table 1), indicating substantial hearing
thickening and inflammatory cell infiltrate. Ofloxacin
loss caused by drug-induced ototoxicity.
otic 1%, on the other hand, caused minimal mucosal
Overall animal studies of ototoxicity during topical
thickening that, in fact, was less than that associated
ofloxacin otic administration have demonstrated a lack
with exposure to the benzalkonium medium. Although
of local irritation, regardless of high concentrations of
the mucosal effect of Cortisporin and gentamicin was
drug achieved locally. There has been no histologic or
not statistically significant compared with saline con-
functional evidence of adverse effects on the mucosa or
THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
Vol. 20, No. 1, January, 2001
TABLE 1. Ototoxicity of topical otomicrobial agents in guinea pigs: auditory brainstem response results (n ⫽ 20)11
Mean Threshold Shift from Baseline (dB) at Frequency of
0.3% ofloxacin otic
1.0% ofloxacin otic
* Positive change (⫹) ⫽ improvement; negative change (⫺) ⫽ worsening.
Reprinted from Ref. 11 with permission from the presenters.
TABLE 2. Clinical toxicity of ofloxacin otic and oral amoxicillin in children: change from baseline in bone and air conduction
pure tone average11*
Ofloxacin otic (n ⫽ 30)
Amoxicillin oral (n ⫽ 26)
* Change of 10 dB hearing loss is minimum clinically significant change; positive change ⫽ improvement; negative change ⫽ worsening.
* Numbers in parentheses, percent.
† P ⫽ 0.029 vs. ofloxacin otic.
‡ P ⫽ 0.167 vs. ofloxacin otic.
[Note: The number discrepancy is due to the fact that more air conduction tests than bone conduction tests were performed because some of the subjects' bone testing could not be
performed or included.]
ossicles of the middle ear and inner ear. Based on these
Over the course of the study the subjects underwent
data, ofloxacin otic appears to be a safe topical antibi-
standard audiometry and octave interval testing (500
otic for application to the middle ear and is well
to 4000 Hz) before therapy and at test of cure (Visit 4,
tolerated even at concentrations three times that rec-
Days 17 to 20) or failure visits. The target ear was the
ommended for clinical use.
affected ear or the more severely affected ear in sub-jects with bilateral infection; if both ears were equally
CLINICAL SAFETY OF OFLOXACIN OTIC IN
affected the right ear was designated as the target ear.
The primary audiologic endpoint was a 10-dB change
To confirm the lack of ototoxicity observed in animal
in air or bone conduction pure tone average at 500,
models, the hearing of children enrolled in a large,
1000 and 2000 Hz. The change in threshold at 4000 Hz
multicenter, randomized parallel group study was ex-
was also measured. A positive change comprised "im-
amined. The study was designed to compare the safety
provement" and a negative change was termed "wors-
and efficacy of 0.3% ofloxacin otic solution with that of
amoxicillin oral suspension in the treatment of acute
Results of the study are outlined in Table 2. There was
purulent otorrhea in children with tympanostomy
no worsening of bone conduction parameters in either the
tubes. The hearing tests were done on a subpopulation
target or nontarget ear associated with either ofloxacin
of children receiving 10 days of treatment with either
otic or amoxicillin oral suspension therapy. In fact one
0.25 ml of 0.3% ofloxacin otic twice a day (n ⫽ 30) or 40
patient in the ofloxacin otic group experienced a slight
mg/kg/day amoxicillin suspension (n ⫽ 26) drawn from
improvement in the nontarget ear. Air conduction mea-
the total of 474 subjects enrolled in the safety and
sures revealed improvement or no change in 93% of
efficacy trial. These 56 children underwent audiomet-
ofloxacin otic-treated patients and 97% of the amoxicillin-
ric testing to compare the safety of the two treatments
treated subjects. However, more children in the ofloxacin-
on auditory function parameters: bone conduction
treated group (68%) showed improved hearing than in
threshold measures to determine the integrity and
the amoxicillin group (35%), P ⫽ 0.029. These results
function of the inner ear; and air conduction threshold
indicate that ofloxacin otic and oral amoxicillin were
measures to evaluate middle and inner ear function.
equivalent in safety, with a trend toward benefit for
The children involved were ⬎4 years old and had
ofloxacin in air conduction parameters, when adminis-
normal pretreatment sensorineural hearing.
tered to pediatric patients with acute otitis media caused
Vol. 20, No. 1, January, 2001
THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
by tympanostomy tubes. Given the current American
Academy of Otolaryngology-Head and Neck Surgery rec-
1. CDC Survey of Ambulatory Surgery.
ommendation to use topical antibiotics for first line ther-
2. Gates GA, Avery C, Prihoda TJ, Cooper JC Jr. Effectiveness
of adenoidectomy and tympanostomy tubes in the treatment
apy, ofloxacin otic could be considered the agent of choice
of chronic otitis media with effusion. N Engl J Med 1987;317:
in purulent otorrhea through a tympanostomy tube.
3. Rosenfeld RM, Bhaya MH, Bower CM, et al. Impact of
tympanostomy tubes on child quality of life. Arch Otolaryngol
Head Neck Surg 2000;126:585–92.
Based on results of both animal and human studies,
4. Hannley MT, Denneny JC 3rd, Holzer SS. Use of ototopical
administration of ofloxacin 0.3% otic solution appears to be
antibiotics in treating 3 common ear diseases. OtolaryngolHead Neck Surg 2000;122:934 – 40.
a safe and well-tolerated treatment for middle ear infec-
5. Barlow DW, Duckert LG, Kreig CS, Gates GA. Ototoxicity of
tions, including acute otitis media in children with a tym-
topical otomicrobial agents. Acta Otolaryngol (Stockh) 1994;
panostomy tubes. Ofloxacin otic therapy was not associated
6. Rohn GN, Meyerhoff WL, Wright CG. Ototoxicity of topical
with changes to the ossicles in a guinea pig model, nor did it
agents. Otolaryngol Clin North Am 1993;26:747–58.
induce functional or histologic changes in the middle or
7. Ikeda K, Takasaka T. In vitro activity of ototopical drops
inner ear as measured on both microscopic evaluation and
against middle ear pathogens. Am J Otol 1993;14:170 –1.
audiologic brainstem response testing. In contrast hair cell
8. Nobori T, Hanamure Y, Matuzaki T. A study of the influence
damage and more inflammatory cell infiltration and muco-
of ofloxacin on the cochlea after topical administration in to
sal thickening were identified in animals treated with Cor-
the middle ear cavity. Otol Fukuoka 1988;34:1028 –34.
9. Brownlee RE, Hulka GF, Prazma J, Pillsbury HC. Ciprofloxa-
tisporin otic suspension and gentamicin ophthalmic solu-
cin: use as a topical otic preparation. Arch Otolaryngol Head
tion. In the clinical trial setting ofloxacin otic did not
Neck Surg 1992;118:392– 6.
adversely impact on hearing function in children treated for
10. Esposito S, Gioacchino D, Montanaro C. Topical and oral
treatment of chronic otitis media with ciprofloxacin. Arch
acute otitis media related to tympanostomy tube placement.
Otolaryngol Head Neck Surg 1990;116:557–9.
For these reasons this agent can be considered a safe agent
11. Black HE, Schaefer GJ, Dolan DF, et al. Preclinical study of
for use in first line therapy for patients with chronic suppu-
the ototoxic potential of an otic solution of ofloxacin. Posterpresented at the 12th Annual Meeting of the American
rative otitis media and acute otorrhea in children with
Society of Pediatric Otolaryngology, Scottsdale, AZ, May 14
tympanostomy tubes.
to 16, 1997.
Source: http://www.mccordresearch.com/sites/default/files/research/OtitisExterna-Gates.pdf
Antimicrobial Treatment of Andrej AurerDarije PlanËak Periodontal Diseases Department of PeriodontologySchool of Dental MedicineUniversity of Zagreb Acta Stomat Croat2004; 67-72 This paper presents a critical evaluation of the use of systemic antimi- crobial treatment in periodontal disease. Recognizing specific types ofperiodontal infections can significantly influence the choice of antimicro-bial treatment. Therapy should be tailored to differences in antibioticsusceptibility between various periodontal pathogens.
Identifying commercially relevant Echinaceaspecies by AFLP molecular markers Luigi Russi, Chiaraluce Moretti, Lorenzo Raggi, Emidio Albertini, and EgiziaFalistocco Abstract: The rising interest in medicinal plants has brought several species of the genus Echinacea to the attention ofmany scientists. Echinacea angustifolia, E. pallida, and E. purpurea are the most important for their immunological prop-erties, well known and widely used by the native Americans. The three species are easily distinguishable on the basis oftheir morphological characteristics, but it would be difficult, if not impossible, to distinguish them in commercial prepara-tions of ground, dry plant parts of E. purpurea (the most valuable species for chemotherapeutic properties) mixed with theother two species. Species-specific molecular markers could be useful to address this issue. In the present work, usingfresh material collected from cultivated Echinacea spp., AFLP analysis was used to discriminate the three species and todetect species-specific DNA fragments. By using 14 primer combinations it was possible to detect a total of 994 frag-ments, of which 565 were polymorphic. Overall, 89 fragments were unique to E. purpurea, 32 to E. angustifolia, and 26to E. pallida. E+CAC/M+AAT or E+CAC/M+AGC alone provided 13, 9, and 4 or 7, 5, and 5 specific fragments forE. purpurea, E. angustifolia, and E. pallida, respectively. A validation trial to confirm the results was carried out onbulked samples of 23 accessions covering most of the genetic diversity of the three species. The results are discussed interms of practical applications in the field of popular medicine, detecting frauds, and implications for the genus Echina-cea.



