Slide
Heart Disease after Radiotherapy
for Breast Cancer
University of Oxford
620 names of EBCTCG collaborators in local and systemic therapy trials,
listed alphabetically by institution, then name. Lancet 2011; 378: 771-84.
ACETBC, Tokyo, Japan O Abe, R Abe, K Enomoto, K Kikuchi, H Koyama, H Masuda, Y Nomura,
German Breast Cancer Study Group (BMFT), Freiburg, Germany G Bastert, H Rauschecker,
S Anderson, G Bass, A Brown (deceased), J Bryant (deceased), J Costantino, J Dignam,
Y Ohashi, K Sakai, K Sugimachi, M Toi, T Tominaga, J Uchino, M Yoshida. Addenbrooke's Hospital,
R Sauer, W Sauerbrei, A Schauer, M Schumacher. German Breast Group (GBG),
B Fisher, C Geyer, E P Mamounas, S Paik, C Redmond, S Swain, L Wickerham, N Wolmark.
Cambridge, UK J L Haybittle. Anglo-Celtic Cooperative Oncology Group, UK C F Leonard. ARCOSEIN
NeuIsenburg, Germany J U Blohmer, S D Costa, H Eidtmann, B Gerber, C Jackisch, S Loibl,
Nolvadex Adjuvant Trial Organisation, London, UK M Baum, I M Jackson (deceased),
Group, France G Calais, P Geraud. ATLAS Trial Collaborative Study Group, Oxford, UK V Collett,
G von Minckwitz. Ghent University Hospital, Belgium A de Schryver, L Vakaet. GIVIO
M K Palmer. North Central Cancer Treatment Group, Mayo Clinic, Rochester, MN, USA
C Davies, A Delmestri, J Sayer. Auckland Breast Cancer Study Group, New Zealand V J Harvey,
Interdisciplinary Group for Cancer Care Evaluation, Chieti, Italy M Belfiglio, A Nicolucci,
E Perez, J N Ingle, V J Suman. North Sweden Breast Cancer Group, Umeå, Sweden
I M Holdaway, R G Kay, B H Mason. Australian New Zealand Breast Cancer Trials Group, Sydney,
F Pellegrini, M C Pirozzoli, M Sacco, M Valentini. Glasgow Victoria Infirmary, UK
N O Bengtsson, S Emdin, H Jonsson. North-West Oncology Group (GONO), Italy L Del Mastro,
Australia J F Forbes, N Wilcken. Austrian Breast Cancer Study Group, Vienna, Austria R Bartsch,
C S McArdle, D C Smith, S Stallard. Groote Schuur Hospital, Cape Town, South Africa
M Venturini. North-Western British Surgeons, Manchester, UK J P Lythgoe, R Swindell.
P Dubsky, C Fesl, H Fohler, M Gnant, R Greil, R Jakesz, A Lang, G Luschin-Ebengreuth, C Marth,
D M Dent, C A Gudgeon, A Hacking, E Murray, E Panieri, ID Werner. Grupo Español de
Northwick Park Hospital, London, UK M Kissin. Norwegian Breast Cancer Group, Oslo,
B Mlineritsch, H Samonigg, C F Singer, G G Steger, H Stoger. Beatson Oncology Centre, Glasgow,
Investigación en Cáncer de Mama (GEICAM), Spain E Carrasco, M Martin, M A Segui.
Norway B Erikstein, E Hannisdal, A B Jacobsen, J E Varhaug. Norwegian Radium Hospital,
UK P Canney, H M A Yosef. Belgian Adjuvant Breast Cancer Project, Liège, Belgium C Focan. Berlin-
Gruppo Oncologico Clinico Cooperativo del Nord Est, Aviano, Italy E Galligioni. Gruppo
Oslo, Norway B Erikstein, S Gundersen, M Hauer-Jensen, H Host, A B Jacobsen, R Nissen-
Buch Akademie der Wissenschaften, Germany U Peek. Birmingham General Hospital, UK
Oncologico Dell'Italia Meridionale (GOIM), Rome, Italy M Lopez. Guadalajara Hospital de
Meyer. Nottingham City Hospital, UK R W Blamey, A K Mitchell, D A L Morgan, J F R
G D Oates, J Powell. Bordeaux Institut Bergonié, France M Durand, L Mauriac. Bordet Institute,
20 Noviembre, Mexico A Erazo, J Y Medina. Gunma University, Japan J Horiguchi, H Takei.
Robertson. Oita Prefectural Hospital, Japan H Ueo. Oncofrance, Paris, France M Di Palma, G
Brussels, Belgium A Di Leo, S Dolci, D Larsimont, J M Nogaret, C Philippson, M J Piccart. Bradford
Guy's Hospital, London, UK I S Fentiman, J L Hayward, R D Rubens, D Skilton. Heidelberg
Mathe (deceased), J L Misset. Ontario Clinical Oncology Group, Hamilton, Canada M Levine,
Royal Infirmary, UK M B Masood, D Parker, J J Price. Breast Cancer International Research Group
University I, Germany H Scheurlen. Heidelberg University II, Germany M Kaufmann,
K I Pritchard, T Whelan. Osaka City University, Japan K Morimoto. Osaka National Hospital,
(BCIRG) M A Lindsay, J Mackey, M Martin. Breast Cancer Study Group of the Comprehensive
H C Sohn. Helios Klinikum Berlin-Buch, Germany M Untch. Hellenic Breast Surgeons Society,
Japan K Sawa, Y Takatsuka. Oxford Radcliffe Hospitals NHS Trust, Churchill Hospital, Oxford,
Cancer Centre, Limburg, Netherlands P S G J Hupperets. British Association of Surgical Oncology
Greece U Dafni, C Markopoulos. Hellenic Cooperative Oncology Group, Athens, Greece
UK E Crossley, A Harris, D Talbot, M Taylor. PACS Adjuvant Study Group, France A L Martin,
BASO II Trialists, London, UK T Bates, R W Blamey, U Chetty, I O Ellis, E Mallon, D A L Morgan,
U Dafni, G Fountzilas. Hellenic Oncology Research Group, Greece D Mavroudis. Helsinki
H Roche. Parma Hospital, Italy G Cocconi, B di Blasio. Petrov Research Institute of Oncology,
J Patnick, S Pinder. British Columbia Cancer Agency, Vancouver, Canada I Olivotto, J Ragaz. Cancer
Deaconess Medical Centre, Finland P Klefstrom. Helsinki University, Finland C Blomqvist,
St Petersburg, Russia V Ivanov, R Paltuev, V Semiglazov. Piedmont Oncology Association,
and Leukemia Group B, Washington DC, USA D Berry, G Broadwater, C Cirrincione, H Muss,
T Saarto. Hospital del Mar, Barcelona, Spain M Gallen. Innsbruck University, Austria
Winston-Salem, NC, USA J Brockschmidt, M R Cooper. Pretoria University, South Africa
L Norton, R B Weiss. Cancer Care Ontario, Canada H T Abu-Zahra. Cancer Research Centre of the
R Margreiter. Institut Claudius Regaud, Toulouse, France B de Lafontan, J Mihura, H Roche.
C I Falkson. Royal Marsden NHS Trust, London and Sutton, UK R A'Hern, S Ashley, M
Russian Academy of Medical Sciences, Moscow, Russia S M Portnoj. Cancer Research UK Clinical
Institut Curie, Paris, France B Asselain, R J Salmon, J R Vilcoq. Institut Gustave-Roussy, Paris,
Dowsett, A Makris, T J Powles, I E Smith, J R Yarnold. St George's Hospital, London, UK J C
Trials Unit (CRCTU), NCRI, Birmingham, UK S Bowden, C Brookes, J Dunn, I Fernando, M Lee,
France R Arriagada, C. Bourgier, C Hill, S Koscielny, A Laplanche, M G Le, M Spielmann.
C Poole, D Rea, D Spooner. Cardiff Trialists Group, UK P J Barrett-Lee, R E Mansel, I J Monypenny.
Institute of Cancer Research Clinical Trials and Statistics Unit (ICR-CTSU, NCRI), UK R
St George Hospital, Sydney, Australia L Browne, P Graham. St Luke's Hospital, Dublin, Ireland
Case Western Reserve University, Cleveland, OH, USA N H Gordon. Central Oncology Group,
A'Hern, J Bliss, P Ellis, L Kilburn, J R Yarnold. Integraal Kankercentrum, Amsterdam,
N Corcoran. Sardinia Oncology Hospital A Businico, Cagliari, Sardinia N Deshpande,
Milwaukee, WI, USA H L Davis. Centre for Cancer Prevention, Wolfson Institute of Preventive
Netherlands
L di Martino. SASIB International Trialists, Cape Town, South Africa P Douglas, A Hacking,
Medicine, Queen Mary, University of London, UK J Cuzick. Centre Léon-Bérard, Lyon, France
J Benraadt, M Kooi, A O van de Velde, J A van Dongen, J B Vermorken. International Breast
H Host, A Lindtner, G Notter. Saskatchewan Cancer Foundation, Regina, Canada A J S Bryant,
Y Lehingue, P Romestaing. Centre Paul Lamarque, Montpellier, France J B Dubois. Centre Regional
Cancer Study Group (IBCSG), Bern, Switzerland M Castiglione, A Coates, M Colleoni,
G H Ewing, L A Firth, J L Krushen-Kosloski. Scandinavian Adjuvant Chemotherapy Study Group,
François Baclesse, Caen, France T Delozier, B Griffon, J Mace Lesec'h. Centre René Huguenin,
J Collins, J Forbes, R D Gelber, A Goldhirsch, J Lindtner, K N Price, M M Regan,
Oslo, Norway R Nissen-Meyer. South Sweden Breast Cancer Group, Lund, H Anderson,
Paris, St Cloud, France P Rambert. Centro Oncologico, Trieste, Italy G Mustacchi. Charles
C M Rudenstam, H J Senn, B Thuerlimann. International Collaborative Cancer Group,
F Killander, P Malmstrom, L Ryden. South-East Sweden Breast Cancer Group, Linköping,
University in Prague, First Faculty of Medicine, Department of Oncology of the First Faculty of
Charing Cross Hospital, London, UK J M Bliss, C E D Chilvers, R C Coombes, E Hall, M Marty.
Sweden L-G Arnesson, J Carstensen, M Dufmats, H Fohlin, B Nordenskjold, M Soderberg.
Medicine and General Teaching Hospital, Czech Republic L Petruzelka, O Pribylova. Cheltenham
International Drug Development Institute, Louvain-la-Neuve, Belgium M Buyse.
South-Eastern Cancer Study Group and Alabama Breast Cancer Project, Birmingham, AL, USA
General Hospital, UK J R Owen. Chemo N0 Trial Group, Germany N Harbeck, F Janicke, C Meisner,
International TABLE Study Group, Berlin, Germany K Possinger, P Schmid, M Untch,
J T Carpenter. Southampton Oncology Centre, UK N Murray, G T Royle, P D Simmonds.
M Schmitt, C Thomssen. Chicago University, IL, USA P Meier. Chinese Academy of Medical
D Wallwiener. ISD Cancer Clinical Trials Team (incorporating the former Scottish Cancer
Southwest Oncology Group, San Antonio, TX, USA K Albain, W Barlow, J Crowley, D Hayes,
Sciences, Beijing, People's Republic of China (in collaboration with the Oxford CTSU) Y Shan,
Therapy Network), Edinburgh, UK L Foster, W D George, H J Stewart, P Stroner. Israel
J Gralow, S Green, G Hortobagyi, R Livingston, S Martino, C K Osborne, P M Ravdin.
Y F Shao, X Wang, D B Zhao (CTSU: Z M Chen, H C Pan). Christie Hospital and Holt Radium
NSABC, Tel Aviv, Israel R Borovik, H Hayat, M J Inbar, E Robinson. Istituto Nazionale per la
Stockholm Breast Cancer Study Group, Sweden J Adolfsson, J Bergh, T Bondesson, F
Institute, Manchester, UK A Howell, R Swindell. Clinical Trial Service Unit, Oxford, UK (ie, EBCTCG
Ricerca sul Cancro, Genova, Italy P Bruzzi, L Del Mastro, P Pronzato, M R Sertoli,
Secretariat) J A Burrett, M Clarke, R Collins, C Correa, D Cutter, S Darby, C Davies, K Davies,
M Venturini. Istituto Nazionale per lo Studio e la Cura dei Tumori, Milan, Italy T Camerini,
K Dahlberg, T Fornander, I Fredriksson, J Frisell, E Goransson, M Iiristo, U Johansson,
A Delmestri, P Elphinstone, V Evans, L Gettins, J Godwin, R Gray, C Gregory, D Hermans, C Hicks,
G De Palo, M G Di Mauro, F Formelli, P Valagussa. Istituto Oncologico Romagnolo, Forli,
E Lenner, L Lofgren, P Nikolaidis, L Perbeck, S Rotstein, K Sandelin, L Skoog, G Svane, E af
S James, A Kerr, E MacKinnon, M Lay, P McGale, T McHugh, R Peto, J Sayer, C Taylor, Y Wang.
Italy D Amadori. Italian Cooperative Chemo-Radio-Surgical Group, Bologna, Italy A Martoni,
Trampe, C Wadstrom. Swiss Group for Clinical Cancer Research (SAKK), Bern, and OSAKO,
Coimbra Instituto de Oncologia, Portugal J Albano, C F de Oliveira, H Gervasio, J Gordilho.
F Pannuti. Italian Oncology Group for Clinical Research (GOIRC), Parma, Italy R Camisa,
St Gallen, Switzerland M Castiglione, A Goldhirsch, R Maibach, H J Senn, B Thurlimann.
Copenhagen Radium Centre, Denmark H Johansen, H T Mouridsen. Dana-Farber Cancer Institute,
G Cocconi, A Colozza, R Passalacqua. Japan Clinical Oncology Group– Breast Cancer Study
Tampere University Hospital, Finland M Hakama, K Holli, J Isola, K Rouhento, R Saaristo.
Boston, MA, USA R S Gelman, J R Harris, D Hayes, C Henderson, C L Shapiro, E Winer. Danish
Group, Matsuyama, Japan K Aogi, S Takashima. Japanese Foundation for Multidisciplinary
Tel Aviv University, Israel H Brenner, A Hercbergs. The High-Dose Chemotherapy for Breast
Breast Cancer Cooperative Group, Copenhagen, Denmark P Christiansen, B Ejlertsen, M Ewertz,
Treatment of Cancer, Tokyo, Japan O Abe, T Ikeda, K Inokuchi, K Kikuchi, K Sawa. Kawasaki
Cancer Study Group (PEGASE), France A L Martin, H Roche. Tokyo Cancer Institute Hospital,
M-B Jensen, S Moller, H T Mouridsen. Danish Cancer Registry, Copenhagen, Denmark
Medical School, Japan H Sonoo. Krakow Institute of Oncology, Poland S Korzeniowski,
Japan M Yoshimoto. Toronto-Edmonton Breast Cancer Study Group, Canada A H G Paterson,
B Carstensen, T Palshof. Düsseldorf University, Germany H J Trampisch. Dutch Working Party for
J Skolyszewski. Kumamoto University Group, Japan M Ogawa, J Yamashita. Leiden
K I Pritchard. Toronto Princess Margaret Hospital, Canada A Fyles, J W Meakin, T Panzarella,
Autologous Bone Marrow Transplant in Solid Tumours, Amsterdam & Groningen, Netherlands
University Medical Center, Netherlands E Bastiaannet, C J H van de Velde, W van de Water,
K I Pritchard. Tunis Institut Salah Azaiz, Tunisia J Bahi. UK Multicentre Cancer Chemotherapy
O Dalesio, E G E de Vries, S Rodenhuis, H van Tinteren. Eastern Cooperative Oncology Group,
J G H van Nes. Leuven Akademisch Ziekenhuis, Gasthuisberg, Belgium R Christiaens,
Study Group, London, UK M Reid, M Spittle. UK/ANZ DCIS Trial H Bishop, N J Bundred,
Boston, MA, USA R L Comis, N E Davidson, R Gray, N Robert, G Sledge, L J Solin, J A Sparano,
P Neven, R Paridaens, W Van den Bogaert. Ludwig-Maximilians University, Munich,
J Cuzick, I O Ellis, I S Fentiman, J F Forbes, S Forsyth, W D George, S E Pinder, I Sestak.
D C Tormey, W Wood. Edinburgh Breast Unit, UK D Cameron, U Chetty, J M Dixon, P Forrest,
Germany S Braun, W Janni. Marseille Laboratoire de Cancérologie Biologique APM, France
UK/Asia Collaborative Breast Cancer Group, London, UK G P Deutsch, R Gray, D L W Kwong,
W Jack, I Kunkler. Elim Hospital, Hamburg, Germany J Rossbach. Erasmus MC/Daniel den Hoed
P Martin, S Romain. Medical University Vienna – General Hospital - Department of
V R Pai, R Peto, F Senanayake. University and Istituto Nazionale per la Ricerca sul Cancro,
Cancer Center, Rotterdam, Netherlands J G M Klijn, A D Treurniet-Donker, W L J van Putten.
Obstetrics and Gynaecology and Department of Medicine I, Vienna, Austria M Janauer,
Genoa, Italy on behalf of GROCTA trialists F Boccardo, A Rubagotti. University College
European Institute of Oncology, Milan, Italy N Rotmensz, U Veronesi, G Viale. European
M Seifert, P Sevelda, C C Zielinski. Memorial Sloan-Kettering Cancer Center, New York, NY,
London, UK M Baum, S Forsyth, A Hackshaw, J Houghton, J Ledermann, K Monson, JS Tobias.
Organization for Research and Treatment of Cancer, Brussels, Belgium H Bartelink, N Bijker,
USA T Hakes, C A Hudis, L Norton, R Wittes. Metaxas Memorial Cancer Hospital, Athens,
University Federico II, Naples, Italy C Carlomagno, M De Laurentiis, S De Placido.
J Bogaerts, F Cardoso, T Cufer, J P Julien, E Rutgers, C J H van de Velde. Evanston Hospital, IL, USA
Greece G Giokas, D Kondylis, B Lissaios. Mexican National Medical Center, Mexico City,
University of Edinburgh, UK L Williams. University of Michigan, USA D Hayes, L J Pierce.
M P Cunningham. Finnish Breast Cancer Group, Finland R Huovinen, H Joensuu. Fondazione
Mexico R de la Huerta, M G Sainz. National Cancer Institute, Bethesda, MD, USA R Altemus,
University of Texas MD Anderson Cancer Center, Houston, TX, USA K Broglio, A U Buzdar.
Maugeri Pavia, Italy A Costa, C Tinterri. Fondazione Michelangelo, Milan, Italy G Bonadonna,
K Camphausen, K Cowan, D Danforth, A Lichter, M Lippman, J O'Shaughnessy, L J Pierce,
University of Wisconsin, USA R R Love. Uppsala-Örebro Breast Cancer Study Group, Sweden
L Gianni, P Valagussa. Fox Chase Cancer Center, Philadelphia, PA, USA L J Goldstein. French
S Steinberg, D Venzon, J A Zujewski. National Cancer Institute of Bari, Italy C D'Amico,
J Ahlgren, H Garmo, L Holmberg, G Liljegren, H Lindman, F Warnberg. US Oncology, Houston,
Adjuvant Study Group (GFEA), Guyancourt, France J Bonneterre, P Fargeot, P Fumoleau,
M Lioce, A Paradiso. NCIC Clinical Trials Group, Kingston, Ontario, Canada J-A W Chapman,
USA L Asmar, S E Jones. West German Study Group (WSG), Germany O Gluz, N Harbeck,
P Kerbrat, E Luporsi, M Namer. German Adjuvant Breast Group (GABG), Frankfurt, Germany
K Gelmon, P E Goss, M N Levine, R Meyer, W Parulekar, J L Pater, K I Pritchard,
C Liedtke, U Nitz. West of Scotland Breast Trial Group, Glasgow, UK A Litton. West Sweden
W Eiermann, J Hilfrich, W Jonat, M Kaufmann, R Kreienberg, M Schumacher.
L E Shepherd, D Tu, T Whelan. National Kyushu Cancer Center, Japan Y Nomura, S Ohno.
Breast Cancer Study Group, Gothenburg, Sweden A Wallgren, P Karlsson, B K Linderholm.
National Surgical Adjuvant Breast and Bowel Project (NSABP), Pittsburgh, PA, USA
Western Cancer Study Group, Torrance, CA, USA R T Chlebowski. Würzburg University,
Germany H Caffier.
Lancet 2011; 378:771
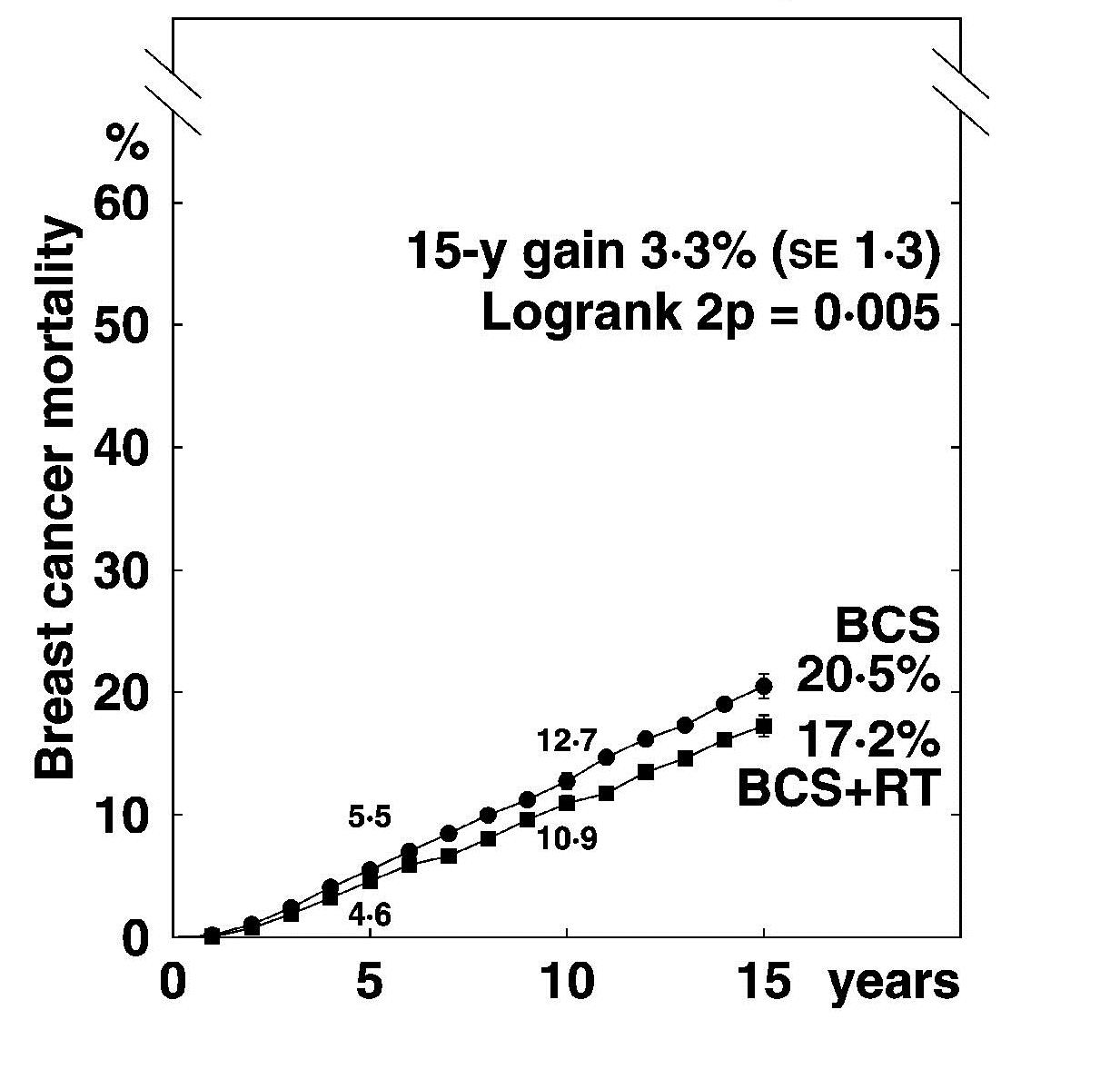
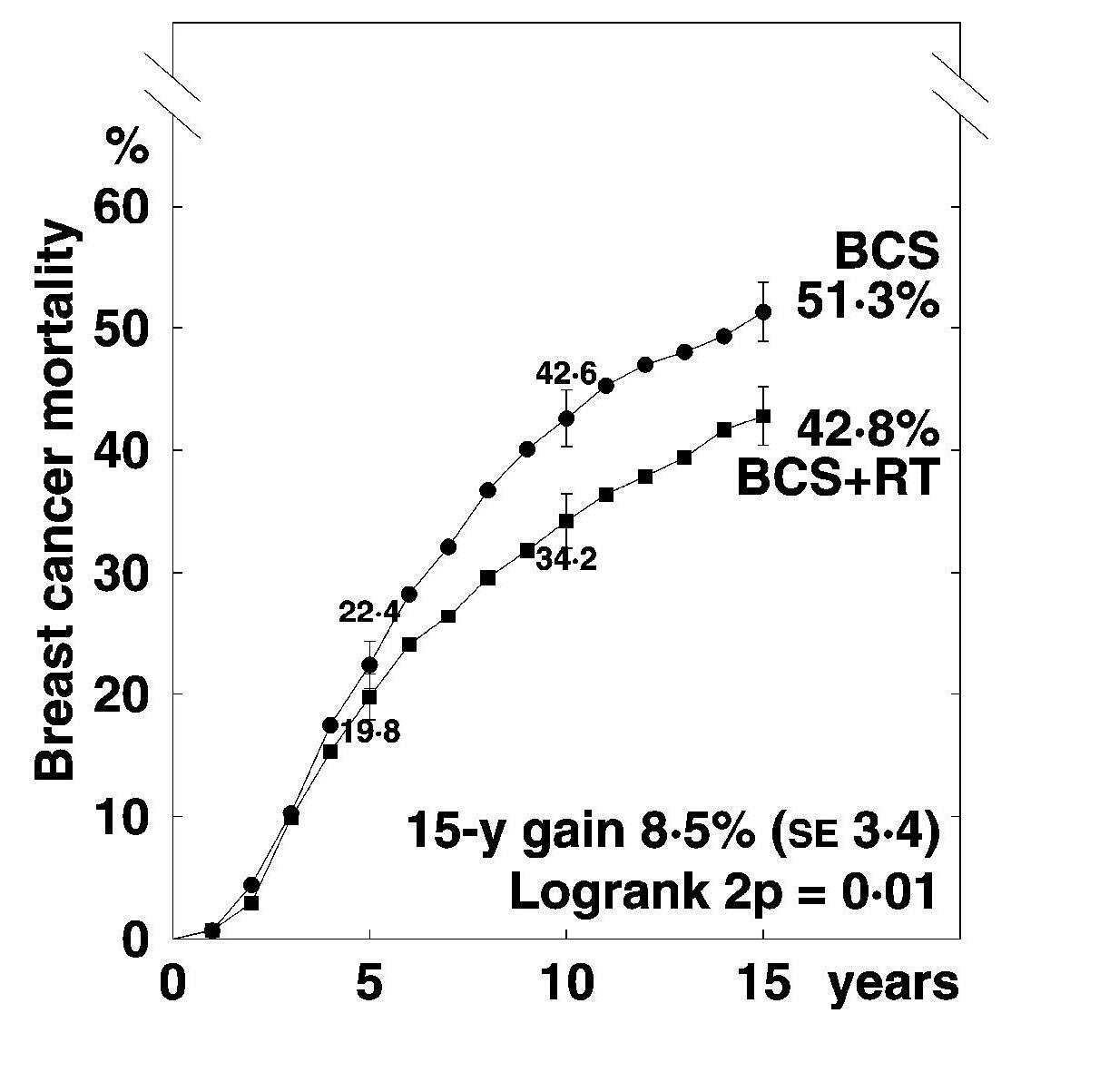
Breast cancer mortality in 17 randomised trials of
radiotherapy (RT) after breast conserving surgery (BCS)
1050 pN+ women 7287 pN0 women
EBCTCG. Lancet 2011; 378:1707
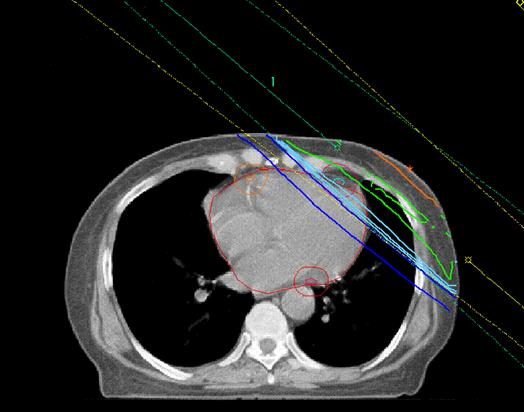
Left tangential pair Co60
Left anterior descending
Circumflex coronary artery
Beam energy Typical dose
Mean dose (Gy)
arrangement
Tangential pair 6MV
Taylor et al , IJROBP 2007

Irradiating heart
Shielding breast
Need to balance absolute benefit and absolute risk
To produce clinically useful results on the absolute risk of radiotherapy related heart disease we need:
• Data on large numbers of women
• Data on non-fatal as well as fatal events
• More detailed studies:
– Medical history at time of cancer diagnosis
– Information on dose of radiation to the heart
Cardiac mortality in 300 000 breast cancer patients in the US SEER
cancer registries: mortality ratios, irradiated vs. not (95 % CIs)
Calendar year
Certified cause of death: heart disease
of diagnosis
Breast conserving
Mastectomy
All women
All years
0.53 (0.48-0.58)
1.41 (1.34-1.48)
1.03 (0.98-1.07)
– 308 861 US women with breast cancer 1973-2001 followed until 1 Jan 2002 –-12,000 deaths from heart disease – 37% irradiated – Analyses stratified for age, year of diagnosis, time since diagnosis and race
McGale & Darby Int J Epidemiol 2008; 37:518-523
Cardiac mortality in 300 000 breast cancer patients in the US SEER
cancer registries: mortality ratios, irradiated vs. not (95 % CIs)
Calendar year
Certified cause of death: heart disease
of diagnosis
Breast conserving
Mastectomy
All women
All years
0.53 (0.48-0.58)
1.41 (1.34-1.48)
1.03 (0.98-1.07)
– 308 861 US women with breast cancer 1973-2001 followed until 1 Jan 2002 –-12,000 deaths from heart disease – 37% irradiated – Analyses stratified for age, year of diagnosis, time since diagnosis and race
McGale & Darby Int J Epidemiol 2008; 37:518-523
***** WARNING *****
The comparison of irradiated and unirradiated women outside the context of a randomised
trial does not provide information about the risk of radiation-related heart disease
Breast and nodal radiotherapy fields
SEER data: Left versus right-sided breast cancer:
subsequent mortality ratios by radiotherapy status
Darby et al, Lancet Oncology 2005; 6:557-65
To produce clinically useful results on the absolute risk of radiotherapy related heart disease we need:
• Data on large numbers of women
• Data on non-fatal as well as fatal events
• More detailed studies:
– Medical history at time of cancer diagnosis
– Information on dose of radiation to the heart
Supported by funding to the Oxford University Clinical Trial Service Unit from Cancer Research UK, the British Heart Foundation, and the UK Medical Research Council and by grants from the European Commission (FI6R-012796), the UK Department of Health (RRX 108), the British Heart Foundation Centre for Research Excellence (CRE RE/08/004, to DC),
and the Oxford National Institute for Health Research Biomedical Research Centre (to KR).
Left-sided versus right-sided breast cancer: heart disease incidence ratios
in women given adjuvant radiotherapy for breast cancer
Number of
Incidence ratio,
category
events left/right left vs. right (95% CI)
Ischaemic heart disease
1.18 (1.07-1.30)
Hypertensive heart disease
1.11 (0.91-1.35)
Pulmonary embolism
1.08 (0.86-1.35)
Pericarditis
1.61 (1.06-2.43)
Valvular heart disease
1.54 (1.11-2.13)
Other rheumatic heart disease
0.82 (0.11-5.90)
Acute endocarditis
1.07 (0.39-2.97)
Myocardial disease
0.99 (0.63-1.57)
Conduction disorders & arrythmias
0.94 (0.82-1.07)
Cardiac arrest
1.28 (0.78-2.12)
Heart failure
0.95 (0.81-1.11)
Other & ill defined heart disease
0.78 (0.48-1.26)
All heart disease
2275/2016
1.08 (1.02-1.15)
Analyses based on first diagnosis of any type of heart disease after diagnosis of breast cancer.
*Results similar considering any diagnosis of acute myocardial infarction (rather than just first diagnosis):
McGale et al, Radiother Onc 2011; 100: 167-75
To produce clinically useful results on the absolute risk of radiotherapy related heart disease we need:
• Data on large numbers of women
• Data on non-fatal as well as fatal events
• More detailed studies:
– Medical history at time of cancer diagnosis
– Information on dose of radiation to the heart
Supported by funding to the Oxford University Clinical Trial Service Unit from Cancer Research UK, the British Heart Foundation, and the UK Medical Research Council and by grants from the European Commission (FI6R-012796), the UK Department of Health (RRX 108), the British Heart Foundation Centre for Research Excellence (CRE RE/08/004, to DC),
and the Oxford National Institute for Health Research Biomedical Research Centre (to KR).
Population-based case-control study of
major coronary events (MCEs)
• Population: Women irradiated for breast cancer
(Denmark: 1977-2006, Stockholm: 1958-2002)
• 963 MCEs (ie, incident MI, coronary revascularisation, or IHD death)
• 1205 controls matched for country, age, calendar
period and time since (ie with no MCE at least until the time since cancer diagnosis of the case MCE)
• Information on tumor characteristics and medical
history at time of bc diagnosis
• Cardiology records sought for all cases to verify
NEJM 2013; 368:987-98
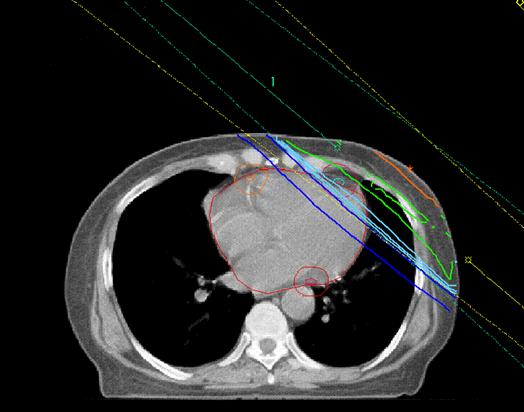
Left tangential pair Co60
Left anterior descending
Circumflex coronary artery
• Detailed dose-plan for each woman reconstructed on modern planning system • 97 x 2 different regimens reconstructed using CT-scan for patient with typical anatomy • Mean physical dose to whole heart estimated from dose-volume histogram • LAD coronary artery and mean EQD2 also estimated
Taylor et al, Rad Onc 2011
Distribution of mean heart doses in case-control study of MCEs
after breast cancer RT
Mean heart dose (Gy)
NEJM 2013; 368:987-98
Case-control study of MCEs in women irradiated for breast cancer
Method of analysis
Data stratified by country, age at breast cancer diagnosis, year of breast cancer
diagnosis, years from breast cancer diagnosis to first subsequent MCE (cases) or index date (controls) (all in 5-year groups) and presence of a cardiac risk factor.
Proportional increase in the MCE rate per Gy modelled as:
Bs (1+ KX)
X is cardiac radiation dose in Gy
K is the percentage increase in MCE rate per Gy
Bs is the stratum-specific MCE rate for women with no RT
Estimation K using logistic regression conditional on stratum totals.
NEJM 2013; 368:987-98
Radiation Associated Cardiac Events (RACE)
Dose-response relationship for major coronary events
7.4% increase per Gy
(95% CI 3-14; p=0.0001)
NEJM 2013; 368:987-98
Association between cardiac risk factors and
subsequent rate of major coronary events
in women irradiated for breast cancer
Rate ratio*
Circulatory disease
History of diabetes
History of COPD
Body mass index (kg/m2)
Analgesic medication
Any cardiac risk factor
<0.00001
*Estimated by logistic regression after stratification for country, age at cancer diagnosis, year of cancer diagnosis, and years from cancer diagnosis to first subsequent MCE (cases) or index date (controls)
† Category listed first is baseline
NEJM 2013; 368:987-98
Percentage increase in MCE rate per Gy mean heart
dose for women with and without cardiac risk factors
at time of breast cancer diagnosis
% increase
in MCE rate
difference
Cardiac risk factor
7.4 (2.0-20.3)
7.4 (1.2-20.1)
*Estimated by regression after stratification for country, age at cancer diagnosis, year of cancer diagnosis, and years from cancer diagnosis to first subsequent MCE (cases) or index date (controls)
NEJM 2013; 368:987-98
Percentage increase in rate of major coronary
events (MCEs) per Gy mean dose to whole
heart by years since exposure to radiation.
Median time from breast cancer diagnosis to start of RT: 42 days
Estimated by regression after stratification for country, age at breast cancer diagnosis, year of breast cancer diagnosis, and years from breast cancer diagnosis to first subsequent MCE (cases) or index date (controls) and with adjustment for presence of a cardiac risk
χ2 for heterogeneity: 6.7 on 3 df, 2p=0.08
χ2 for trend: 1.7 on 1 df, 2p=0.19
NEJM 2013; 368:987-98
Excess rate ratios (ERRs) for a major coronary event (MCE)
Mean heart dose
Equivalent mean heart dose in 2 Gray fractions
7.4% increase per Gy
7.4% increase per Gy
(95% CI 3-14; p=0.0001)
(95% CI 3-14; p=0.0001)
Doses estimated from dose-volume histograms. Solid lines calculated using dose estimates for individual women. Circles calculated for categories of women. Vertical lines are 95% CIs. Baseline is 0 Gy
NEJM 2013; 368:987-98
Mean doses (Gy) to whole heart and LAD coronary artery
Correlation
with mean
whole heart
Whole heart
EQD2 to whole heart
Doses estimated from dose-volume histograms
NEJM 2013; 368:987-98
Radiation Associated Cardiac Events (RACE)
Dose-response relationship for major coronary events
7.4% increase per Gy
(95% CI 3-14; p=0.0001)
NEJM 2013; 368:987-98
Radiation Associated Cardiac Events (RACE)
Illustrative calculations
for a woman aged 50 at RT
Death from IHD
NEJM 2013; 368:987-98
Radiation Associated Cardiac Events (RACE)
Illustrative calculations
for a woman aged 50 at RT
Incident IHD
NEJM 2013; 368:987-98
Heart dose from breast cancer
Systematic review of studies published 2003 – 2013
Number of
Number of
Mean heart
dose (Gray)
Taylor et al, 2013
Heart dose from breast cancer radiotherapy
Systematic review of studies published 2003 – 2013
Number of
of women heart dose
Left inverse planned
intensity modulated RT (IMRT)
Taylor et al, 2013
• Worldwide 50 million survivors after radiotherapy for
• 10 million survivors after radiotherapy for breast
• 5 million survivors after radiotherapy for left-sided
• Radiotherapy similar to bilateral breast cancer
radiotherapy can be useful in to counteract side-effects of treatment for prostate cancer
• Relationships between heart disease risk and mean
heart dose can be derived from breast cancer patients irradiated in the past
• Proportional risk of a major coronary event increases by
7.4% for each 1 Gy increase in mean heart dose
• Absolute risks higher for those already at increased risk
• No apparent threshold below which there is no risk
• For most women the absolute benefit of breast cancer
radiotherapy far outweighs the absolute risk of heart disease
• Further studies needed: RT after anthracyclines, other
types of heart disease, etc
Source: http://www.melodi-online.eu/doc/Darby_Heart%20disease%20after%20radiotherapy%20in%20breast%20cancer.pdf
Juna Amagara Ministries International Mission Team Juna Amagara Ministries Post Office Box 2384 Glen El yn, IL 60138 630-248-9472 [email protected] Contents: Short Term Mission Trip Participant Information --------------------------- 3 Travel Insurance Sources ------------------------------------------------------- 3 Timeline for Traveler ------------------------------------------------------------ 6 Quick Guide to Rukiga / Runyankole ------------------------------------------ 7 Documents to be Filled out and Submitted to JAM Prior to Departure Application ------------------------------------------------------------------------ 9 Waiver of Liability --------------------------------------------------------------- 11 Personal Covenant ---------------------------------------------------------------- 12 Researchmaster Infection & Immuntiy Laboratory rotations & Reaearch topics IMMUNOLOGY – LABROTATIONS & RESEARCH TOPICS Researchmaster Infection & Immuntiy Laboratory rotations & Reaearch topics Title: (Immuno)pathogenesis of chronic lymphocytic leukemia Workgroupleader: dr. A.W. Langerak T: 010-704 4089 E: [email protected] W: http://www.erasmusmc.nl/immunologie/onderzoek/moleculaireimmunologie/mid/?lang=en Background Chronic lymphocytic leukemia (CLL) is the most frequent type of leukemic proliferation in the Western world. CLL is found in adults and typically associated with age. The majority of CLL cases is of B-cell type, while a minority derives from T lymphocytes (also called T-cell large granular lymphocyte leukemia, T-LGL). Over the last years it has become increasingly clear that CLL is a heterogeneous disease, with a variable clinical course and differences in survival. CLL is an example of a multi-factorial disease, in which both genetic and micro-environmental factors contribute to leukemogenesis. Although in recent years many studies have focused on prognostic markers, there is still no complete picture of the factors that are involved in the (immuno)pathogenesis and that are determining for the prognosis.
|
|









