Untitled
Journal of Medical Microbiology (2011), 60, 612–618
Chemical composition and antifungal activity of theessential oils of Lavandula viridis L'He´r.
Mo´nica Zuzarte,1,2 Maria Jose´ Gonc¸alves,1 Carlos Cavaleiro,1Jorge Canhoto,2 Luı´s Vale-Silva,3 Maria Joa˜o Silva,3 Euge´nia Pinto3and Lı´gia Salgueiro1
1Center of Pharmaceutical Studies, Faculty of Pharmacy, Health Science Campus, University of
Lı´gia Salgueiro
Coimbra, Azinhaga de S. Comba, 3000-354 Coimbra, Portugal
2Center of Pharmaceutical Studies, Department of Life Sciences, University of Coimbra, Ap. 3046,
3001-401 Coimbra, Portugal
3Microbiology Service/CEQUIMED, Faculty of Pharmacy, University of Porto, Rua Anı´bal Cunha
164, 4050-047 Porto, Portugal
In the present work we report for what we believe to be the first time the antifungal activity andmechanism of action of the essential oils of Lavandula viridis from Portugal. The essential oilswere isolated by hydrodistillation and analysed by GC and GC/MS. The MIC and the minimallethal concentration (MLC) of the essential oil and its major compounds were determined againstseveral pathogenic fungi. The influence of subinhibitory concentrations of the essential oil on thedimorphic transition in Candida albicans was also studied, as well as propidium iodide and FUN-1staining of Candida albicans cells by flow cytometry following short treatments with the essentialoil. The oils were characterized by a high content of oxygen-containing monoterpenes, with1,8-cineole being the main constituent. Monoterpene hydrocarbons were present at lowerconcentrations. According to the determined MIC and MLC values, the dermatophytes andCryptococcus neoformans were the most sensitive fungi (MIC and MLC values ranging from 0.32to 0.64 ml ml"1), followed by Candida species (at 0.64–2.5 ml ml"1). For most of these strains,MICs were equivalent to MLCs, indicating a fungicidal effect of the essential oil. The oil wasfurther shown to completely inhibit filamentation in Candida albicans at concentrations well belowthe respective MICs (as low as MIC/16). Flow cytometry results suggested a mechanism of actionultimately leading to cytoplasmic membrane disruption and cell death. Our results show thatL. viridis essential oils may be useful in the clinical treatment of fungal diseases, particularly
Received 31 October 2010
dermatophytosis and candidosis, although clinical trials are required to evaluate the practical
Accepted 19 January 2011
relevance of our in vitro research.
In recent years, research on aromatic plants, andparticularly their essential oils, has attracted many
Over the last few decades, there has been an increase in the
investigators. Essential oils have traditionally been used
number of serious human infections in immunocompro-
for centuries for their antifungal properties
mised patients caused by fungi The
More recently, several studies have confirmed the
range of severity of these infections is a consequence of the
huge potential of these natural products as antifungal
host reaction to the metabolic products produced by fungi,
the virulence of the infecting strain, the site of infection
and also environmental factors Nowadays,
Therefore, it is not surprising that essential oils are one of
the increasing impact of these infections, the limitations
the most promising groups of natural products for the
encountered in their treatment (e.g. resistance, side-effects
development of broad-spectrum, safer and cheaper anti-
and high toxicity) and the rising overprescription and
fungal agents.
overuse of conventional antifungals all stimulate a search for
The genus Lavandula provides valuable essential oils
alternative natural drugs.
mainly for the food (flavouring), perfumery and cosmeticindustries, and is also very popular in aromatherapy.
Abbreviations: MLC, minimal lethal concentration; PI, propidium iodide.
However, many other applications can be foreseen, as
027748 G 2011 SGM
Printed in Great Britain
Antifungal activity of Lavandula viridis oils
suggested in several reports on the biological activity of this
strains from the American Type Culture Collection (Candida albicans
genus. Lavandula oils have been reported to have sedative
ATCC 10231, Candida tropicalis ATCC 13803 and Candida
and antispasmodic properties
parapsilopsis ATCC 90018); one Cryptococcus neoformans type strainfrom the Coleccio´n Espano˜la de Cultivos Tipo (Cryptococcus neofor-
as well as acaricidal ),
mans CECT 1078); one Aspergillus clinical strain isolated from
antibacterial (e.g.
bronchial secretions (Aspergillus flavus F44) and two Aspergillus type
), antifungal (e.g.
strains from the American Type Culture Collection (Aspergillus niger
) and antioxidant activities.
ATCC 16404 and Aspergillus fumigatus ATCC 46645); three
More recently, application as a biopesticide has also been
(Epidermophyton floccosum FF9, Trichophyton mentagrophytes FF7and Microsporum canis FF1) and four dermatophyte type strains from
Lavandula viridis L'He´r. is a highly aromatic shrub
the Coleccio´n Espano˜la de Cultivos Tipo (Trichophyton mentagro-
endemic to the south Iberian Peninsula. It is commonly
phytes var. interdigitale CECT 2958, Trichophyton rubrum CECT 2794,
known as green or white lavender due to its white flowers
Trichophyton verrucosum CECT 2992 and Microsporum gypseumCECT 2908). All strains were stored in Sabouraud dextrose broth with
and green floral bracts, which are very distinct from those
20 % glycerol at 280 uC and subcultured on Sabouraud dextrose agar
of the other lavenders. Dried leaves of L. viridis are used
(SDA) or potato dextrose agar (PDA) before each test, to ensure
with medical applications in Madeira, Portugal
optimal growth conditions and purity.
Antifungal activity. Broth macrodilution methods based on the
As part of our ongoing study on the valorization of
Clinical and Laboratory Standards Institute (CLSI) reference proto-
Portuguese lavenders, we now report the chemical
cols M27-A3 and M38-A2 for yeasts and
composition, antifungal activity and mechanism of action
filamentous fungi, respectively, were used to determine MICs of the
of L. viridis essential oils. As far as we know, this is the first
essential oils and their major constituents. A macrodilution rather
report on the antifungal activity of this species.
than a microdilution design was used to allow the use of glass testtubes, thus avoiding the interaction of the essential oil with the plasticpolymer material of the 96-well microtitre plates. Briefly, inoculumsuspensions were prepared at appropriate densities in RPMI 1640
broth (with L-glutamine, without bicarbonate, and with the pHindicator phenol red) from SDA or PDA cultures and distributed into
Plant material. Aerial parts of two samples of L. viridis were
12675 mm glass test tubes. Inoculum densities were confirmed by
collected from field-growing plants in the flowering stage in the south
viability counts on SDA. Serial twofold dilutions of the oil were
of Portugal (A, Barranco do Velho region; B, Salir region). Voucher
prepared in DMSO and added to the cell suspensions in order to
specimens were deposited at the herbarium of the Department of Life
obtain test concentrations ranging from 0.08 to 20.0 ml ml21 (final
Sciences of the University of Coimbra (COI).
DMSO concentrations never exceeded 2 %, v/v). Oil-free growthcontrols, as well as sterility and DMSO control tubes, were also
Essential oil isolation and analysis. The essential oils from air-
included. The test tubes were incubated aerobically at 35 uC for 48 h/
dried plant material were isolated by hydrodistillation for 3 h, using a
72 h (Candida and Aspergillus species/Cryptococcus neoformans) and
Clevenger-type apparatus according to the European Pharmacopoeia
at 30 uC for 7 days (dermatophytes). MIC values were determined as
The oils were preserved in a sealed vial at
the lowest concentration of the oil causing full growth inhibition.
4 uC. Oil analyses were carried out by both GC and GC/MS using
Quality control was performed by testing fluconazole and amphoter-
fused silica capillary columns with two different stationary phases
icin B with the reference strains Candida parapsilopsis ATCC 22019
(SPB-1 and SupelcoWax-10) as previously reported
and Candida krusei ATCC 6258 and the results were within the
predetermined limits. To measure minimal lethal concentrations
The volatile compounds were identified by both their retention
(MLCs), 20 ml samples were taken from each negative tube plus the
indices and their mass spectra. Retention indices, calculated by linear
first tube showing growth (to serve as a growth control) after MIC
interpolation relative to retention times of a series of n-alkanes, were
reading to SDA plates and incubated at 35 uC for 48 h/72 h (Candida
compared with those of authenticated samples from the database of
and Aspergillus species/Cryptococcus neoformans) or at 30 uC for
the Laboratory of Pharmacognosy, Faculty of Pharmacy, University of
7 days (dermatophytes). MLC values were determined as the lowest
Coimbra. Mass spectra were compared with reference spectra from a
concentration of the oil causing fungal death. All experiments were
home-made library or from literature data
performed in triplicate and repeated whenever the results of each
Relative amounts of individual components were
triplicate did not agree. A range of values is presented when different
calculated based on GC peak areas without flame ionization detector
results were obtained.
response factor correction.
Mechanism of action
Pure and reference compounds. Authentic samples of 1,8-cineole
Germ tube inhibition assay.
(Merck; 99.5 % purity),
Cell suspensions from overnight SDA
a-pinene (Fluka; 99.0 % purity), linalool
(Aldrich; 99.0 % purity) and camphor (Extrasynthese) were used.
cultures of Candida albicans strains ATCC 10231, D5 and M1 wereprepared in NYP medium [N-acetylglucosamine (Sigma; 1023 mol
Fluconazole was kindly provided by Pfizer as the pure powder and
l21), Yeast Nitrogen Base (Difco; 3.35 g l21), proline (Fluka; 1023
amphotericin B was from Sigma (80.0 % purity).
mol l21) and NaCl (4.5 g l21), pH 6.7±0.1] and adjusted to obtain a density of 1.0±0.26106 c.f.u. ml21. The
Fungal strains. The antifungal activity of the essential oil of sample
essential oil was diluted in DMSO and added in 10 ml volumes to
A was evaluated against yeasts and filamentous fungi: four clinical
990 ml of the yeast suspensions (final DMSO concentration of 1 %, v/
Candida strains isolated from recurrent cases of vulvovaginal and oral
v), obtaining a series of subinhibitory concentrations (as low as 1/64
candidosis (Candida albicans D5, Candida albicans M1, Candida
of the MIC). The samples were incubated for 3 h at 37 uC without
krusei H9 and Candida guilliermondii MAT23); three Candida type
agitation and 100 cells from each sample were then counted in a
M. Zuzarte and others
haemocytometer. The percentage of germ tubes was determined as the
comparison to the drug-free controls. Results are presented as
number of cells showing hyphae at least as long as the diameter of the
means±SD of at least three replicate experiments.
blastospore. Cells showing a constriction at the point of connection ofthe hypha to the mother cell, typical for pseudohyphae, were excluded.
The results are presented as means±standard deviation (SD) of three
RESULTS AND DISCUSSION
Flow cytometry. Yeast suspensions were prepared in PBS solution
Chemical compositions of the essential oils
with 2 % (w/v) D-glucose from overnight SDA cultures of Candida
The essential oils were obtained in yields ranging from 0.7
albicans ATCC 10231 at 35 uC and adjusted, using a haemocytometer,
to 1.2 % (v/w). A total of 51 compounds were identified,
to a final density of 2.0±0.26106 c.f.u. ml21. Serial twofold dilutionsof the essential oil (final concentrations of 0.64–10.0 ml ml21) and a
representing 93.2 % (sample A) and 95.3 % (sample B) of
single solution of amphotericin B at 2 mg ml21 (four times the
the total volatile oils. The oils were characterized by high
respective MIC of 0.5 mg ml21) in PBS with 2 % (w/v) D-glucose were
contents of oxygen-containing monoterpenes (69.5 and
added to the cell suspensions and the mixtures were incubated at
75.7 %), followed by monoterpene hydrocarbons (17.1 and
35 uC in a humid atmosphere without agitation for 30 min, 4 h or
15.5 %). The main constituents of the oils were 1,8-cineole
24 h. Drug-free control tubes were included in each experiment. After
(34.5 % and 42.2 %), camphor (13.4 %), a-pinene (9.0 %)
this period, the cells were washed in PBS and resuspended in 500 ml
and linalool (7.9 and 6.7 %). Sesquiterpenic compounds
PBS with 2 % (w/v) D-glucose for FUN-1 (Invitrogen) staining andPBS for propidium iodide (PI; Sigma) staining. Five microlitres of the
attained only 4.8 and 2.3 %.
FUN-1 and PI solutions in DMSO and PBS, respectively, were added
In a previous study carried out by
to the cell suspensions to obtain final concentrations of 0.5 mM FUN-
some individual samples of L. viridis from the south of
1 and 1.0 mg PI ml21. FUN-1-stained cells were incubated for a
Portugal and Spain were analysed. The chemical composi-
further 20 min at 35 uC, away from incident light, while PI-stainedsamples were read after about 10 min at room temperature.
tion of these samples was very similar to that of our
Unstained cell suspensions were included as autofluorescence
collective samples, 1,8-cineole being the major component
controls. Flow cytometry was performed using a FACSCalibur
in all samples. This fact points to a very high homogeneity
(Becton Dickinson Biosciences) flow cytometer with a 488 nm blue
in the composition of the essential oils of L. viridis from
argon laser emitting at 15 mW and the results were analysed using
Portugal and Spain.
CellQuest Pro Software (Becton Dickinson). Intrinsic parameters(forward and side scatter, for relative cell size and complexityanalysis) and fluorescence in the FL2 channel (log yellow/orange
Antifungal activity of the essential oils
fluorescence, bandpass filter 585/42 nm) for FUN-1 and the FL3channel (log red fluorescence, longpass filter .650 nm) for PI were
The essential oil was used to evaluate the antifungal activity
acquired and recorded for a minimum of 7500 events per sample
against several pathogenic strains involved in human
using logarithmic scales. Markers (M1) were adjusted to include a
diseases. Various degrees of inhibition were registered
maximum of 5 % of the cells in monoparametric histograms of the
against all the fungi tested
fluorescence intensity of control samples (see for examples) andkept unchanged through the analysis of the remaining samples to
The highest antifungal activity was observed against
quantify the percentages of cells showing altered fluorescence in
dermatophyte strains and Cryptococcus neoformans, with
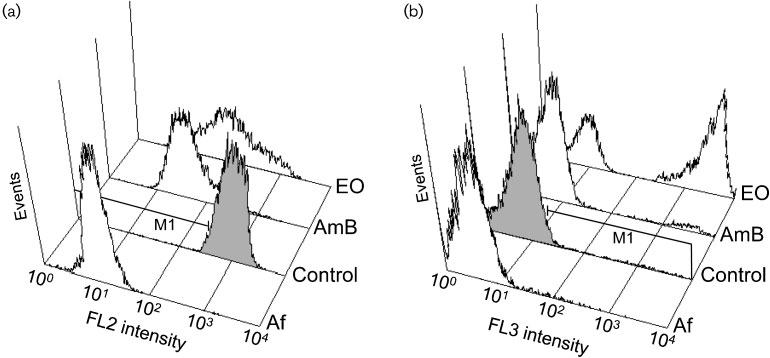
Fig. 1. Flow cytometry histograms showing fluorescence intensity versus number of events (Candida albicans ATCC 10231cells) in relative units. (a) Orange fluorescence (FL2 channel) intensity of samples stained with FUN-1. (b) Red fluorescence(FL3 channel) intensity of samples stained with PI. Af, Autofluorescence of unstained cells; control, untreated cells; AmB, cellstreated with amphotericin B at 2.0 mg ml"1; EO, cells treated with the essential oil of L. viridis at 10.0 ml ml"1.
Journal of Medical Microbiology 60
Table 1. Antifungal activity (MIC and MLC) of the essential oil of Lavandula viridis (sample A) for Candida, dermatophyte and Aspergillus strains
Results were obtained from three independent experiments performed in duplicate. When different MIC values were obtained, a range of values is presented. NT, Not tested.
Candida albicans ATCC 10231
Candida albicans D5
Candida albicans M1
Candida tropicalis ATCC 13803
Candida krusei H9
Candida guilliermondii MAT23
Candida parapsilopsis ATCC
Cryptococcus neoformans CECT
var. interdigitale CECT 2958
Trichophyton rubrum CECT
Trichophyton verrucosum CECT
Microsporum canis FF1
Microsporum gypseum CECT
Epidermophyton floccosum FF9
Aspergillus niger ATCC16404
Aspergillus fumigatus ATCC
Aspergillus flavus F44
*MIC and MLC were determined by a macrodilution method and expressed in ml ml21 (v/v).
DMIC and MLC were determined by a macrodilution method and expressed in mg ml21 (w/v).
M. Zuzarte and others
MIC and MLC values ranging from 0.32 to 0.64 ml ml21.
inhibition of cell metabolism after short incubation periods
For Candida strains, MIC and MLC values ranged from
with the oil at concentrations starting from the respective
0.64 to 2.5 ml ml21. The oil was less effective against
MIC The dye FUN-1 freely permeates fungal
Aspergillus strains The higher susceptibility of
plasma membranes into the cell and is distributed in the
dermatophytes has also been reported for other essential
cytoplasm as a bright diffuse green/yellow stain. In normal
fungal cells, the dye is metabolized into orange/red
cylindrical intravacuolar structures. However, in cells withimpaired metabolism, this change does not occur and FUN-1
For most of the dermatophytes, Cryptococcus neoformans
remains in the cytoplasm in a diffuse pattern, indicating a
and Candida strains, the MIC was equivalent to the MLC,
disorder in cell metabolism . This change
indicating a clear fungicidal effect of L. viridis essential oil.
was detected by a reduction of orange fluorescence (FL2
The major constituents of the oil (1,8-cineole, camphor, a-
channel) in cells exposed to the essential oil in comparison to
pinene and linalool) were also assayed individually for their
untreated controls (and . To observe PI staining of
antifungal activity. 1,8-Cineole and camphor displayed the
the test cells, on the other hand, a 4 h incubation with a
lowest antifungal activity against all strains but a-pinene
concentration of the oil at least two log2 dilutions above the
proved to be a very active compound, particularly against
MIC was required ). The nucleic acid binding
dermatophyte strains Since the essential oils are
fluorescent probe PI penetrates only dead cells showing
complex mixtures of several compounds, it is difficult to
severe membrane lesions . The
attribute their biological activity to a particular constituent.
observed asymmetry between metabolic inhibition and cell
Usually, major compounds are the ones responsible for the
death shows that cells clearly become metabolically inactive
antifungal activity of the essential oils. However, some
in the presence of the essential oil of L. viridis before it leads
studies show that minor components may have a crucial
to cell death, thus appearing to exclude a potential
role in the biological activity of the oils
mechanism of antifungal action relying on primary leakage
Our results seem to indicate that the activity of L.
of cytoplasmic contents due to direct damage to cell
viridis essential oil is mainly due to the presence of a-
membranes. It is worth pointing out that under the same
pinene in the oil.
experimental conditions, the reference fungicidal drugamphotericin B tested at a concentration two log2 dilutionsabove the respective MIC did not lead to PI staining .
Mechanism of action of the essential oil
After 24 h, however, over 90 % of the cells presented positive
The essential oil was also found to inhibit filamentation in
PI staining with amphotericin B treatment (data not shown).
the tested Candida albicans strains at concentrations of
The mechanism of action of essential oils remains
0.08–0.16 ml ml21, well below the corresponding MICs
somewhat controversial. While some studies suggest that
This marked difference between MICs and
the compounds may penetrate the micro-organism and
filamentation-inhibiting concentrations seems to suggest
react with active sites of enzymes and/or interfere with
that different mechanisms may be responsible for these two
cellular metabolism, most evidence supports direct disrup-
biological activities. This finding is particularly relevant
tion of cellular membranes and concentration-dependent
considering the fact that filamentation has long been
pro-oxidant cytotoxic effects
shown to be essential for virulence in Candida albicans
Concerning antifungal activity specifically, the mechanism
In fact, inhibition of the dimorphic
of action of the oils seems to involve penetration through
transition alone has been suggested to be sufficient to treat
cell walls and direct damage to both cytoplasmic and
disseminated candidosis, thus proving to be a good target
mitochondrial membranes This leads
mechanism in the development of novel antifungal agents
to changes in permeability leading to leakage and
Additionally, flow cytometry analyses
ultimately resulting in cell death
after FUN-1 staining have revealed a dose-dependent
Bearing this knowledge in mind, the present results for the
Table 2. Percentage of germ tubes after treatment of three Candida albicans strains with subinhibitory concentrations of theessential oil of L. viridis for 3 h in a filamentation-inducing medium at 37 6C
Results are presented as mean (±SD) values of three independent experiments. Concentration is in ml ml21 (v/v).
C. albicans ATCC 10231
47.5±10.6 (0.04)
42.7±10.5 (0.08)
*Untreated samples including the solvent (1 % DMSO) only.
Journal of Medical Microbiology 60
Antifungal activity of Lavandula viridis oils
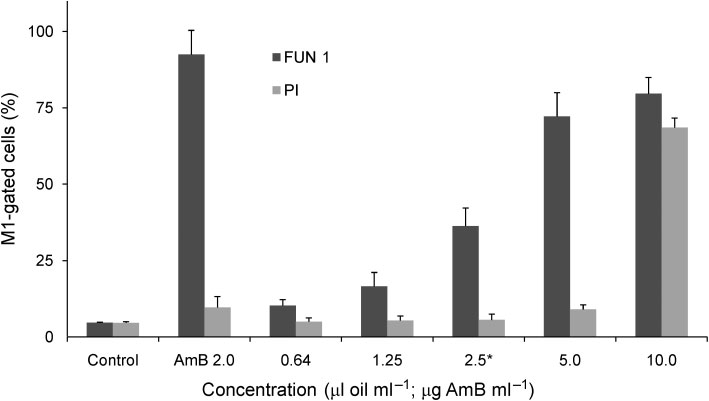
Fig. 2. Percentage (and SD) of M1-gated Candida albicans ATCC 10231 cells, analysed by flow cytometry, after treatmentswith different concentrations of the essential oil of L. viridis in comparison with amphotericin B (AmB) and an untreated control.
Cells were treated with the compounds for 30 min for staining with FUN-1 and 4 h for staining with PI. *MIC of the essential oil.
specific case of Candida albicans treated with the essential
of Lavandula stoechas L. ssp. stoechas essential oils from stem/
oil of L. viridis are consistent with a mechanism of action
leaves and flowers. J Agric Food Chem 54, 4364–4370.
starting from damage to mitochondrial membranes,
Bakkali, F., Averbeck, S., Averbeck, D. & Idaomar, M. (2008).
considering the rapid metabolical arrest appearing earlier
Biological effects of essential oils – a review. Food Chem Toxicol 46,
and in the presence of lower concentrations of the essential
oil than those required to cause cell death. In such a
Cavaleiro, C., Salgueiro, L. R., Miguel, M. G. & Proenc¸a da Cunha, A.
scenario, changes in mitochondrial permeability would
(2004). Analysis by gas chromatography-mass spectrometry of thevolatile components of Teucrium lusitanicum and Teucrium algar-
disturb electron flow in the electron transport chain,
biensis. J Chromatogr A 1033, 187–190.
generating free radicals that proceed to damage essential
Cavaleiro, C., Pinto, E., Gonc¸alves, M. J. & Salgueiro, L. R. (2006).
biomolecules (including lipids, proteins and nucleic acids).
Antifungal activity of Juniperus essential oils against dermatophyte,
Given a high enough concentration and/or exposure time,
Aspergillus and Candida strains. J Appl Microbiol 100, 1333–1338.
the oil may eventually lead to disruption of cytoplasmic
Cavanagh, H. M. A. & Wilkinson, J. M. (2002). Biological activities of
membranes and cell death. Further data are now required
lavender essential oil. Phytother Res 16, 301–308.
to definitively confirm these speculations, however.
CLSI (2008a). Reference Method for Broth Dilution Antifungal
The wide-spectrum antifungal activity and high potency of
Susceptibility Testing of Yeasts; Approved Standard, 3rd edn. M27-A3. Wayne, PA: Clinical and Laboratory Standards Institute.
the oil of L. viridis support further investigations into thedevelopment of this essential oil for clinical use in the
CLSI (2008b). Reference Method for Broth Dilution AntifungalSusceptibility Testing of Filamentous Fungi; Approved Standard, 3rd
management of superficial and/or mucosal fungal infections.
edn. M38-A2. Wayne, PA: Clinical and Laboratory Standards Institute.
Council of Europe (1997). European Pharmacopoeia, 3rd edn.
Strasbourg: Council of Europe.
Dadaliog˘lu, I. & Evrendilek, G. A. (2004). Chemical compositions and
This work was supported by CEF/POCI2010/FEDER and by the
antibacterial effects of essential oils of Turkish oregano (Origanum
Portuguese Foundation for Science and Technology (FCT) through a
PhD fellowship to M. R. Z. (SFRH/BD/40218/2007) and a post-
(Lavandula stoechas L.), and fennel (Foeniculum vulgare) on common
doctoral fellowship to L. V.-S. (SFRH/BPD/29112/2006).
foodborne pathogens. J Agric Food Chem 52, 8255–8260.
Ferris, D. G., Nyirjesy, P., Sobel, J. D., Soper, D., Pavletic, A. &Litaker, M. S. (2002). Over-the-counter antifungal drug misuse
associated with patient-diagnosed vulvovaginal candidiasis. ObstetGynecol 99, 419–425.
Adams, R. P. (1995). Identification of Essential Oil Components by Gas
Garcia-Vallejo, M. I. (1992). Aceites esenciales de las Lavandulas
Chromatography/Mass Spectroscopy. Carol Stream, IL: Allured
Ibericas – Ensayo de la quimiotaxonomia. Tesis Doctoral, Universidad
Complutense de Madrid.
Angioni, A., Barra, A., Coroneo, V., Dessi, S. & Cabras, P. (2006).
Chemical composition, seasonal variability, and antifungal activity
Garcı´a-Vallejo, M. C. & Soria, A. C. (2006). Antifeedant effects and
M. Zuzarte and others
chemical composition of essential oils from different populations of
Pina-Vaz, C., Sansonetty, F., Rodrigues, A. G., Costa-Oliveira, S.,
Lavandula luisieri L. Biochem Syst Ecol 34, 609–616.
Tavares, C. & Martinez-de-Oliveira, J. (2001). Cytometric approach
Joulain, D. & Ko¨nig, W. A. (1998). The Atlas of Spectral Data of
for a rapid evaluation of susceptibility of Candida strains to
Sesquiterpene Hydrocarbons. Hamburg: E. B. Verlag.
antifungals. Clin Microbiol Infect 7, 609–618.
Pina-Vaz, C., Gonc¸alves Rodrigues, A., Pinto, E., Costa-de-Oliveira, S.,
Koroch, A. R., Juliani, H. R. & Zygadlo, J. A. (2007). Bioactivity of
Tavares, C., Salgueiro, L. R., Cavaleiro, C., Gonc¸alves, M. J. &
essential oils and their components. In Flavours and Fragrances.
Martinez-de-Oliveira, J. (2004). Antifungal actvity of Thymus oils
Chemistry, Bioprocessing and Sustainability, pp. 87–115. Edited by
and their major compounds. J Eur Acad Dermatol 18, 73–78.
R. G. Berger. Berlin, Heidelberg: Springer-Verlag.
Pinto, E., Pina-Vaz, C., Salgueiro, L., Gonc¸alves, M. J., Costa-de-
Marichal, P., Gorrens, J., Van Cutsem, J. & Vanden Bossche, H.
Oliveira, S., Cavaleiro, C., Palmeira, A., Rodrigues, A. & Martinez-de-
(1986). Culture media for the study of the effects of azole derivatives
Oliveira, J. (2006). Antifungal activity of the essential oil of Thymus
on germ tube formation and hyphal growth of C. albicans. Mykosen
pulegioides on Candida, Aspergillus and dermatophyte species. J Med
29, 76–81.
Microbiol 55, 1367–1373.
Matos, F., Miguel, M. G., Duarte, J., Venaˆncio, F., Moiteiro, C.,
Pinto, E., Vale-Silva, L., Cavaleiro, C. & Salgueiro, L. (2009).
Correia, A. I. D., Figueiredo, A. C., Barroso, J. G. & Pedro, L. G.
Antifungal activity of the clove essential oil from Syzygium
(2009). Antioxidant capacity of the essential oils from Lavandula
aromaticum on Candida, Aspergillus and dermatophyte species.
luisieri, L. stoechas subsp. lusitanica, L. stoechas subsp. lusitanica x L.
J Med Microbiol 58, 1454–1462.
luisieri and L. viridis grown in Algarve (Portugal). JEOR 21, 327–336.
Rı´os, J. L. & Recio, M. C. (2005). Medicinal plants and antimicrobial
Millard, P. J., Roth, B. L., Thi, H. P., Yue, S. T. & Haugland, R. P.
activity. J Ethnopharmacol 100, 80–84.
(1997). Development of the FUN-1 family of fluorescent probes forvacuole labeling and viability testing of yeasts. Appl Environ Microbiol
Romani, L. (2007). Immunity to fungi. In New Insights in Medical
63, 2897–2905.
Mycology, pp. 1–18. Edited by K. Kavanagh. Dordrecht: Springer.
Salgueiro, L. R., Pinto, E., Gonc¸alves, M. J., Pina-Vaz, C., Cavaleiro, C.,
Mitchell, A. P. (1998). Dimorphism and virulence in Candida albicans.
Rodrigues, A. G., Palmeira, A., Tavares, C., Costa-de-Oliveira, S.
Curr Opin Microbiol 1, 687–692.
(2004). Chemical composition and
Moon, T., Wilkinson, J. M. & Cavanagh, H. M. A. (2006). Antibacterial
antifungal activity of the essential oil of Thymbra capitata. Planta
activity of essential oils, hydrosols and plant extracts from Australian
Med 70, 572–575.
grown Lavandula spp. Int J Aromatherapy 16, 9–14.
Saville, S. P., Lazzell, A. L., Bryant, A. P., Fretzen, A., Monreal, A.,
Pe´rez-Parra, A., Mun˜oz, P., Guinea, J., Martı´n-Rabada´n, P., Guembe, M.
Solberg, E. O., Monteagudo, C., Lopez-Ribot, J. L. & Milne, G. T.
& Bouza, E. (2009). Is Candida colonization of central vascular
(2006). Inhibition of filamentation can be used to treat disseminated
catheters in non-candidemic, non-neutropenic patients an indication
candidiasis. Antimicrob Agents Chemother 50, 3312–3316.
for antifungals? Intensive Care Med 35, 707–712.
Upson, T. M. & Andrews, S. (2004). The Genus Lavandula. Kew,
Perrucci, S., Macchioni, G., Cioni, P. C., Flamini, G., Morelli, I. &
London: The Royal Botanical Gardens.
Taccini, F. (1996). The activity of volatile compounds from Lavandula
Zuzarte, M., Gonc¸alves, M. J., Cavaleiro, C., Dinis, A. M., Canhoto,
angustifolia against Psoroptes cuniculi. Phytother Res 10, 5–8.
J. M. & Salgueiro, L. R. (2009). Chemical composition and antifungal
Pfaller, M. A., Pappas, P. G. & Wingard, J. R. (2006). Invasive fungal
activity of the essential oils of Lavandula pedunculata (Miller) Cav.
pathogens: current epidemiological trends. Clin Infect Dis 43, S3–S14.
Chem Biodivers 6, 1283–1292.
Journal of Medical Microbiology 60
Source: http://www.naturatrade.eu/assets/journal-of-medical-microbiology2011-vol60.pdf
A Drug Abuse Prevention Guide For Teens Table of Contents Introduction:Substance Abuse Guide For Teens 1 Part One:Today's Drug Problem 2Extent of Problem 2 Drugs of Abuse 3• Cannabis • Heroin • Cocaine 4• Methamphetamine • Prescription Drugs 5• GHB • Ecstasy 6 • LSD • PCP • Ketamine 7• Anabolic Steroids • Inhalants • Over the Counter (OTCs) 8
Parkinson's disease Medicines for Parkinson's disease provide symptomatic relief. No medicine has yet been shown to slow progression of the disease. There are also some medicines that should be avoided. Medicine regimens are individual Avoid interactions with meals Doses, preparations, frequency and timing need to be individualised Food, particularly protein, can interfere with absorption of



