jcd xvii.1.covers
Journal of
THE INTERNATIONAL JOURNAL OF APPLIED DENTAL RESEARCH
Volume XXII
Robert C. Emling, EdD
Caren M. Barnes, RDH, MS
Annerose Borutta, Prof.Dr.med.habil.
Robert L. Boyd, DDS, MEd
Kenneth H. Burrell, DDS, MS
Mark E. Cohen, PhD
David Drake, MS. PhD
Subgingival Delivery of Oral Debriding Agents:
Heinz Duschner, Prof.Dr.
William Michael Edgar, PhD, DDSc, FDSRCS
A Proof of Concept
Denise Estafan, DDS, MS
Stuart L. Fischman, DMD
Tanya Dunlap, PhD Duane C. Keller, DMD
Rosa Helena Miranda Grande, DDS, PhD
Perio Protect LLC
John J. Hefferren, PhD
St. Louis, MO, USA
Elliot V. Hersh, DMD, PhD
Mark E. Jensen, DDS, PhD
Milton V. Marshall, PhD
Carl J. Kleber, MSD, PhD
University of Texas Health Science Center at Houston
Israel Kleinberg, DDS, PhD, DSc
Houston, TX, USA
Karl F. Leinfelder, DDS, MS
J. William Costerton, PhD
Jonathan Mann, DMD, MSc
Kenneth Markowitz, DDS
Allegheny General Hospital and Allegheny-Singer Research Institute
Milton V. Marshall, PhD, DABT
Pittsburgh, PA, USA
Pier Francesco Porciani, MD, MScD
Christoph Schaudinn, PhD
Howard M. Proskin, PhD
Mark S. Putt, MSD, PhD
Robert Koch Institute
Bruce R. Schemehorn, MS
Warren Scherer, DDS
Betty Sindelar, PT, PhD John R. Cotton, PhD
Jon B. Suzuki, DDS, PhD, MBA
Jason M. Tanzer, DMD, PhD
Norman Tinanoff, DDS, MS
Athens, OH, USA
Henry O. Trowbridge, DDS, PhD
Richard I. Vogel, DMD
James S. Wefel, PhD
Anthony E. Winston, BSc
Wayne T. Wozniak, PhD
Stefan Zimmer, Prof. Dr. med dent.
Stephen M. Siegel
The Journal of Clinical Dentistry (ISSN 0895-8831) is published by Professional Audience Communications, Inc., P.O. Box 243, Yardley, PA 19067.
POSTMASTER; Send address changes to P. O. Box 243, Yardley, PA 19067.
Copyright 2011 by the YES Group, Inc. All rights reserved. No part of this publication may be reproduced without written permission from the publisher.
Subgingival Delivery of Oral Debriding Agents: A Proof of Concept
Tanya Dunlap, PhD Duane C. Keller, DMD
Perio Protect LLC
St. Louis, MO, USA
Milton V. Marshall, PhD
University of Texas Health Science Center at Houston
Houston, TX, USA
J. William Costerton, PhD
Allegheny General Hospital and Allegheny-Singer Research Institute
Pittsburgh, PA, USA
Christoph Schaudinn, PhD
Robert Koch Institute
Betty Sindelar, PT, PhD John R. Cotton, PhD
Athens, OH, USA
•
Objective: This study is a proof of concept to determine the efficacy of a custom-fabricated tray in placing antimicrobial and debriding
agents in the periodontal pockets of persons with active gingival infections. Localized subgingival delivery of antimicrobial andantibiotic agents is routinely employed as adjunctive therapy for the treatment and management of periopathogens associated withperiodontal disease. Because these delivery techniques often face time constraints and impose temporary restrictions on patient brush-ing and flossing, a custom-formed prescription dental tray can be used to deliver and maintain medications in periodontal pockets between office visits and without brushing or flossing restrictions. The ability of this tray to maintain sufficient concentrations of medi -cation in the periodontal pockets to have a therapeutic effect is evaluated here with theoretical modeling and practical application.
•
Methods: Hydrogen peroxide is an oral debriding agent and oral wound cleanser with antimicrobial properties. The debriding effect
of 1.7% hydrogen peroxide gel was tested
in vitro on
Streptococcus mutans biofilm using glass carriers for collection. Diffusionmodeling tested the potential of the customized tray to place hydrogen peroxide gel into the sulcus in the presence of crevicularfluid flow. Changes in periodontal microflora with scanning electron microscopy analysis of
in vivo paper point site sampling wereanalyzed before and after a thin ribbon of 1.7% hydrogen peroxide gel (approximately 0.7 gm) and a subtherapeutic dose (three drops)of Vibramycin® (50 mg/5 ml) were placed via Perio Trays® into periodontal pockets, ranging from 4–8 mm at daily prescribed inter -vals for two to five weeks.
•
Results: In vitro results indicate that 1.7% hydrogen peroxide gel breaks down the exopolysaccharide slime and cell walls of
S. mutans,
and begins to debride the cells from glass carriers within 10 minutes. Diffusion modeling indicates that hydrogen peroxide can pene -trate into the deeper pockets (9 mm), but also its concentration in these deep pockets will increase over wearing time in the absenceof degradation by peroxidases and catalase. Site sampling data confirm diffusion modeling results, with evidence that medicationdelivered with the prescription tray reduced subgingival bacterial loads and enhanced healing of corresponding oral tissues.
•
Conclusion: The prescription Perio Tray effectively placed medication in the gingival sulcus. Mathematical modeling indicated
Perio Tray placement of hydrogen peroxide gel in periodontal pockets with depths up to 9 mm over 15 minutes treatment time wastheoretically possible. Pathology reports reveal reductions in subgingival bacterial loads and improvements in pretreatment pocketdepths of up to 8 mm after 1.7% hydrogen peroxide and Vibramycin Syrup were prescribed for use with the Perio Tray. The
in vitroanalysis indicating that hydrogen peroxide is the active and effective oral debriding agent needs to be confirmed with additionalstudies.
(J Clin Dent 2011;22:149–158)
in tooth loss.4,5 A local periodontal inflammatory response may
Periodontal disease is the host response to oral biofilm microb ial
also adversely affect the host systemic immune response and
"triggers"1-3 that can result in localized tissue inflammation, gin-
general health.6-8 For clinical practitioners, these localized and
gival ulcerations with bleeding, tissue destruction, and bone loss
systemically adverse effects are underscored by the prevalence
leading to deep periodontal pocket formation that can culminate
of periodontal disease and the insufficiencies inherent in current
The Journal of Clinical Dentistry
Vol. XXII, No. 5
treatment methods, of which scaling and root planning (SRP) is
When surgery is not required or after it has been performed,
the accepted gold standard in non-surgical treatment. The bene -
adjunctive chemical therapies can enhance treatment out-
fits of SRP9,10 are well recognized, but significant limitations
comes.10,11 Recently, new attention has been paid to peroxide usage
occur with SRP, including mechanical inability to remove all
as a viable subgingival1,31-34 and supragingival35 antibiofilm
bacterial cells, resulting in biofilm regeneration that requires
agent. Aqueous ≤ 3% hydrogen peroxide is a known oral de-
repetitive mechanical procedures and the risks of bacteremia
briding agent and wound cleanser.36 It has been formulated in
associated with mechanical debridement and scaling. Faced with
mouthrinses, dentifrices, and gels for topical application, most
these limiting situations, practitioners employ adjunctive thera-
commonly for tooth whitening.37,38 Researchers are also inter-
pies11 or surgery.
ested in the disinfectant properties of hydrogen peroxide. The hy-
Periodontitis is a persistent inflammatory response to bacter-
droxyl radical formed from hydrogen peroxide decomposition,
ial growth in slime-enclosed communities that, like all classic
especially in the presence of iron (Fe3+), has been shown to kill
biofilms, resists clearance by host defenses and systemic anti -
99.99% of oral periopathogens and 99.999%
Streptococcus
biotic therapy.1,4,12,13 The ability of biofilms to persist in spite of
mutans (
S. mutans) bacteria within three minutes.39
activated host responses lies at the root of their persistence.
One problem with chemotherapeutic treatment is the delivery
Physical biofilm debridement has developed as the gold standard
and maintenance of peroxides in the sulcus. The sulcus is a
in the treatment of biofilm infections as it is in dentistry with
unique space for chemotherapeutic treatment modalities because
SRP, but it is impossible to eliminate all bacteria in the biofilm
it is accessible topically, but the salient problem of overcoming
with SRP and recolonization can occur.14-16
gingival crevicular fluid flow tends to limit chemical contact in
Mechanical debridement can have the specific limitation of
the gingival space. The most effective topical administration of
stim ulating biofilm regeneration. In one study, mechanical
peroxides for biofilm management appears to be tray delivery of
removal of 50% of the initial biofilm resulted in a four-fold
a gel formulation.33,35,40 If peroxides can debride subgingival
increase in biofilm growth. Subsequent 75% removal of the re-
planktonic cells of the biofilm and significantly reduce the pe-
growth resulted in a three-fold increase over that present from the
ripheral elements of biofilms, the peroxides may shift biofilm
first regrowth under magni fic ation analysis.17 A biofilm that is
communities into a defensive growth mode, limiting their ability
mechanically disturbed can thus increase its reproductive capa-
to reproduce or trigger inflammation.
bilities in response to the physical forces used to perturb it.
This study, to evaluate the potential of a custom-fabricated
Given the limitations with mechanical procedures, adjunctive
dental tray to retain medication in the sulcus a sufficient amount
antibiotic therapies are employed to improve treatment out-
of time for the medication to have a therapeutic effect, has three
comes.18,19 Antibiotics effectively kill individual planktonic cells
distinct parts. The first shows
in vitro debridement results of
and some of the peripheral, actively dividing bacteria in the
1.7% hydrogen peroxide gel. The second is a theoretical exercise
biofilm, but have little effect on the dormant core enclosed in the
evaluating the potential for prescription Perio Tray® (Perio Protect,
protective slime matrix.20,21 In order for the antibiotics to work
LLC, St. Louis, MO, USA) delivery of 1.7% hydrogen peroxide
more effectively, the matrix has to be removed and the dormant
gel into the sulcus against the force of crevicular fluid flow. The
core stimulated; however, when the matrix is mechanically de-
third evaluates the practical application and efficacy of using cus-
brided, the remaining biofilm cells are stimulated, prompting rapid
tomized trays for localized subgingival delivery of medication
regeneration until the biofilm returns to a protected stable popu-
based on pathology reports documenting
in vivo subgingival
lation stasis for which additional SRP is often necessary in a cycli-
biofilm changes after medication is placed into periodontal pock-
cal fashion.22 Therefore, most patients with chronic or aggressive
ets via prescription Perio Trays.
periodontitis have SRP performed every three to six months.
Risks of bacteremia associated with mechanical debridement
Material and Methods
and scaling23,24 increase with repetitive use of SRP. For most
In Vitro Debridement with 1.7% Hydrogen Peroxide Gel
healthy adults, the host immune system is capable of managing
In vitro assessments were conducted to confirm the debriding
the inflammatory response induced by bacteremia during perio -
action of 1.7% hydrogen peroxide gel (Dakota Pharmacy, Bis-
dontal procedures, but for the millions of immunocompromised
marck, ND, USA) on oral biofilm using the LIVE/DEAD® sys-
individuals and adults with diabetes, cardiovascular disease,
tem developed by Molecular Probes (Invitrogen, Carlsbad, CA,
joint replacements, and other inflammatory illnesses, an increase
USA).41 The assessment of viability involves staining prepara-
in the chronic systemic inflammatory burden may pose additional
tions with propidium iodide, which penetrates the compromised
health risks.25 Given these potential risks, it would be beneficial
bacterial wall of dead bacteria and binds to their DNA so the cells
to have a treatment modality that could reduce localized perio -
appear in a rich red color. Bacteria with intact cell walls exclude
dontal inflammation before mechanical debridement, and thus
propidium iodide, and are stained green by the Syto 9 counter
decrease the possibility of bacteremia.
stain. Bacteria that are injured with partially compromised cell
For some cases, surgery is necessary. When pathogenic bac-
walls stain an orange color. The viability of bacteria is deter-
teria are capable of penetrating phagocytic and non-phagocytic
mined by assessing the proportion of stained red, orange/yellow,
cells, they evolve to survive within the host cells,26-28 which can
and green bacteria, recorded at the moment at which the popu-
result in the development of host granulomatous tissue.29,30 In
lation was stained.
these cases, the only appropriate therapy is surgical removal of
S. mutans (strain UA 159) were inoculated in Brain Heart Infusion
the internally infected tissues.
(OXOID LTD., Basingstoke, UK) with 2% sucrose (Bethesda
Vol. XXII, No. 5
The Journal of Clinical Dentistry
Research Laboratories, Gaithersburg, MD, USA), into MatTek
These morphotypes were counted in each biofilm colony at three
glass bottom microwell plates (MatTek Corporation Ashland,
standard areas of 100 µm2 and averaged. The results were multi -
MA, USA), which were incubated for 24 hours at 37oC, 5% CO
plied by the colony area and height. The sum for every morpho -
of an orbital shaker. After 24 hours under a laminar hood, me-
type over all colonies yielded the total amount of bacteria per
dia from each plate were removed and new media were replaced.
paper point.
Plates were incubated for another 24 hours under similar condi-
After initial SEM analysis of paper points, the periodontal
tions to form a mature biofilm on the third day.
pockets were treated with 1.7% hydrogen peroxide gel and a sub-
The plates were aseptically removed from the incubator, and
clinical dose (three drops per tray) of Vibramycin® Syrup (50
exposed to the 1.7% hydrogen peroxide gel and to gel with all
mg/5 ml, Pfizer, New York, NY, USA), topically placed via pre-
excipients except hydrogen peroxide for five or 10 minutes prior
scription Perio Trays. Paper point biofilm sampling was repeated
to rinsing with sterile phosphate buffered saline (BioWhittaker/
two to five weeks after daily Perio Tray delivery of medication.
Lonza Walkersville, MD, USA) and exposure to the LIVE/DEAD®
The accuracy of the SEM image analysis approach can be only
BacLite™ (Invitrogen, Carlsbad, CA, USA) staining procedure.
described as a rough approximation whose exactness is not more
A control series was similarly prepared, and all manipulations
than one order of magnitude. Therefore, only significant changes
were conducted to prevent the removal of biofilm by mechanical
forces for comparison. Following the staining procedure, thestained plates were examined using a Leica TCS-SP2 confocal
scanning laser microscope (CSLM).
In Vitro Debridement with 1.7% Hydrogen Peroxide Gel
When
S. mutans biofilms generated
in vitro were examined by
the LIVE/DEAD technique, without gel treatment, the majority
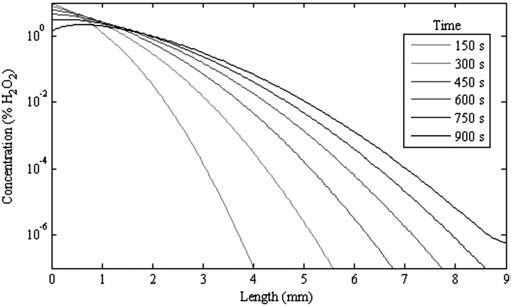
The modeling tests the theory that the prescription tray system
of the millions of bacterial cells in these coherent and luxuriant
results in a concentration of hydrogen peroxide (
c) delivered and
biofilms were alive (green) with uncompromised bacterial walls
maintained in the gingival sulcus or periodontal pocket during the
(Figure 1). In the micrograph, intact bacterial cells, approxi-
period of treatment. A simple mass transport model was used to
mately 0.61 µm in diameter, are seen to be embedded in an exo -
estimate the ability of hydrogen peroxide to penetrate the perio -
polysaccharide (EPS) matrix.
dontal pocket over time as a function of distance.
In Vivo Subgingival Effects of Medication
Delivered with the Perio Tray
A retrospective review of pathology reports from a private
general dental clinic identified records from four patient (threemen, one woman, age range 33–71 years) who had selectedtreatment with the custom-fabricated Perio Tray for delivery ofmedication before SRP, either because of previous mechanicalperiodontal treatment failure or because they refused SRP treat-ment. Because of their treatment status, the patients were askedto consent to biofilm samplings as a diagnostic adjunct to theirplan of care. Sterile Absorbent Points (#504 Henry Schein Inc.,Melville, NY, USA) were held in place for 10 seconds in 19 totalperiodontal pockets before chemotherapeutic treatment beganwith the Perio Tray, and again two to five weeks after dailytreatment began. None of the patients had had SRP three monthsprior to or during the course of this treatment.
Paper points were prepared for scanning electron microscopy
(SEM) by dehydration in a graded ethanol series, critical pointdried, mounted on a stub, sputter coated with a 20 nm layer ofplatinum, and examined with an XL 30 S, FEG SEM (FEI Com-
Figure 1. Confocal micrograph of untreated control S. mutans
biofilm, showing
pany, Hillsboro, OR, USA) operating at 5 kV in the secondary
large numbers of live (green) cells, with a few membrane-compromised (orange)
electron mode.
All paper points were systematically scanned and documented
S. mutans are a good choice for testing because, in contrast to
with overview, regional, and detail images. The approximate
many subgingival bacteria, this biofilm produces large amounts
dimension of each biofilm colony could be calculated based on
of EPS matrix that act as an additional protection barrier for the
the overview and regional images. Furthermore, the approximate
bacteria, increasing the challenge for matrix decomposition and
number of bacteria/colony/layers was counted, and six basic
debridement.42 In further contrast to subgingival bacteria,
S. mutans
morphotypes were defined (spiral-rods, short-rods [1:2 width:
is a mostly aerobic growing organism, able to handle larger
length], middle-long rods [> 1:2 – < 1:7], long rods [> 1:7],
amounts of the peroxide, which makes it less susceptible to the
flagellated rods, filamentous-rods, and coccus-like bacteria).
debriding action of the 1.7% hydrogen peroxide gel.
The Journal of Clinical Dentistry
Vol. XXII, No. 5
When
S. mutans biofilms were treated for five minutes with
1.7% hydrogen peroxide gel and examined using the LIVE/DEAD technique, bacterial wall integrity of only a small pro-portion of the biofilm cells were compromised, and only a smallproportion of them stained orange to yellow (Figure 2). However,when these biofilms were exposed to the 1.7% peroxide gel for10 minutes, virtually all of the bacterial walls were disrupted(red; Figure 3). Most of the cells disintegrated and released theirDNA as a tangled "skein." It can be concluded that, at some pointbetween five and 10 minutes, the chosen concentration of perox -ide eradicates virtually all of the cells in the
S. mutans biofilm.
Figure 3. Confocal micrograph of a S. mutans
biofilm treated for 10 minutes
with the 1.7% hydrogen peroxide gel. All of the bacteria are degraded (red) and
only a few faint cell profiles remain because the cell walls have been compro-
mised and disintegrated, releasing their DNA as a tangled fibrous mass.
Figure 2. Confocal micrograph of S. mutans
biofilm exposed to the 1.7% hy-
drogen peroxide gel for 5 minutes. Most of the coccoid cells are intact (green).
The number of membrane-compromised cells (yellow) is similar to that seen in
untreated biofilms.
As a control for this experiment, a gel with all excipients
except the active ingredient, hydrogen peroxide, was used on
S. mutans biofilm. Exposure to this gel for 10 minutes left thebiofilm almost completely unaffected (Figure 4) in that verylarge areas showed only living (green) cells. In some small areassome bacterial wells were compromised (orange), but these werein the same proportion seen in untreated biofilms.
These results demonstrate that a 10-minute exposure to a 1.7%
hydrogen peroxide aqueous gel can debride bacterial cell wallswithin a typical dental biofilm. The modeling below evaluates the
Figure 4. Confocal micrograph of S. mutans
biofilm treated for 10 minutes with
potential of hydrogen peroxide gel delivery in the sulcus against
a gel containing 0% hydrogen peroxide. Note the clear predominance of living(green) cells in this control preparation.
gingival crevicular fluid, which occurs in the periodontal pocket.
mm) was used as the width of the cross-sectional area at the
gingival-tooth interface. The depth of this space was con sidered
In modeling the geometry of the area of diffusion, only the mo-
as a range from 4–9 mm, reflecting the variation in pocket- probing
lars of a typical adult mouth were considered. The length of the
depth associated with periodontal disease severity. Because both
cross-sectional area of this space was considered as the average
lingual and buccal surfaces are considered with pocket-probing
length of a molar from mesial to distal. After measuring six molars
depth analyses, two rectangular spaces were included in this
three times each, the average length was determined as 8.88 mm.
modeling to represent both of these surfaces.
Given that a dental probe is able to fit into the periodontal pocket
Prior studies have demonstrated that GCF flow rate increases
when disease is present, the width of a typical dental probe (0.83
with periodontal disease, exhibiting a range of 1.8 to 137.0 µl/h
Vol. XXII, No. 5
The Journal of Clinical Dentistry
with a mean of 45.7 ± 35.7 µl/h.43 The flow rates were indica-
simulated for 15 minutes of treatment. The plot in Figure 5
tive of pocket depths greater than 4 mm, bleeding on probing at
shows the simulation.
greater than 40% of the sites, and clinical attachment levelsgreater than 4.5 mm. These characteristics are consistent with the"typical patient" receiving treatment with the prescription trays.44For subsequent calculations, the flow rate was converted to afluid velocity (v) by dividing the flow rate (Q) by the cross- sectional area (A) or
v = Q / A
This resulted in a velocity of 0.861 x 10-3 mm/s.
Reducing the problem to one dimension, the distance along the
tooth root is considered the positive x direction, with the originat 0 mm pocket probing depth. The governing equation for thisdiffusion problem is then
Figure 5. The simulated results show the concentration of hydrogen peroxide
into the pocket increases with the length of time the peroxide remains applied.
where c is the concentration of hydrogen peroxide as a functionof distance x (pocket depth) and time t.45 The velocity (v) flows
This analysis indicates hydrogen peroxide held in the Perio
out of the tooth, and the constant D is the coefficient of diffusion.
Trays can diffuse into the periodontal pockets over time. Even
From prior studies, the coefficient of diffusion for a 10% solution
with a relatively large GCF flow, the diffusion enters 9 mm deep
of hydrogen peroxide was given as 1.48 cm2/day.46 Although the
pockets within the 15-minute time period studied here. Thus,
treating concentration of hydrogen peroxide is lower (1.7%), the
throughout the time that a patient would wear the prescription Pe-
10% diffusion coefficient is a reasonable approximation for the
rio Tray, the concentration of hydrogen peroxide in the deeper
treatment concentration in this exercise. The first term on the right
pockets improves, indicating that even with GCF there is an in-
side of the equation reflects the process of convection where
creasing concentration in the deeper areas.
fluid flow lowers the concentration of hydrogen peroxide, whilethe second term describes hydrogen peroxide diffusion against the
In Vivo Subgingival Effects of Medication
concentration gradient. If the fluid velocity drops to 0, the above
Delivered with the Perio Tray
equation reduces to Fick's law of diffusion.
Tables I–IV detail microbial descriptions provided by SEM
In order to solve the equation, initial and boundary conditions
analysis in pathology reports for 19 total periodontal pockets
had to be assumed: no concentration of hydrogen peroxide is pre-
from four patients. Examples of SEMs from the pathology re-
sent in the pocket at time = 0; the concentration of hydrogen per-
ports are presented in Figures 6–9, and they are representative of
oxide in the tray at x = 0 is c ; and no hydrogen peroxide leaves
the results seen from each sampling site before and after treat-
the base of the periodontal pocket, assumed to be x = 9 for this
ment with 1.7% hydrogen peroxide gel and three drops of Vibra -
problem (this is considered a no flux condition at x = 9). In ad-
mycin Syrup delivered via the Perio Tray.
dition, the concentration of hydrogen peroxide in the tray c was
Patient 1 had Type II periodontal disease on arrival at the
modeled as a decreasing amount to account for the change in
clinic. Pathology reports record sites analyzed evidenced bleed-
degradation and outflow. Because of the changing hydrogen
ing on probing before treatment, and absence of bleeding on
peroxide concentration in the tray, no steady state will be
probing after four times daily use of the Perio Tray subgingival
placement of medication for five weeks. Pocket probing depth
The time-dependent solution was coded into Matlab (Version
decreased 1–2 mm during this time, and one of three sites had
7.4; Natick, MA, USA) using finite difference analogues, and
no bacteria recovered after five weeks of treatment. Patient 2 had
Retrospective Compilation of Microbiological Data from Pathology Reports for Male Patient with Type 2 Periodontal Disease
Patient 1—Male, Periodontal Disease Type II—Treatment Duration 5 Weeks, 4 Day, 15 Minutes
Microbial Description Based on Scanning Electron Micrograph
Extensive presence of biofilm ( 5 107 bacteria) with notable high percentage of Treponema-like morphotypes.
Multilayered biofilm ( 5 105 bacteria) composed primarily of short rods, long rods and coccus-like morphotypes.
Relatively little amount of biofilm ( 5 104 bacteria) dominated by Coccus-like morphotypes.
No bacteria could be found on the entire paper point, only large numbers of eukaryotic cells.
Abundant biofilm ( 5 107 bacteria) in which coccus-like bacteria and rods of different length were found.
Polymicrobial biofilm ( 5 105 bacteria) with predominance of filamentous-like morphotypes, as formed by many Actinomyces species.
The Journal of Clinical Dentistry
Vol. XXII, No. 5
Retrospective Compilation of Microbiological Data from Pathology Reports for Female Patient with Type 1 Periodontal Disease
Patient 2— Female, Periodontal Disease Type I—Treatment Duration 5 Weeks, 2
Microbial Description Based on Scanning Electron Micrograph
Dense, multi-layered biofilm ( 1 106 bacteria) in which only Treponema-like bacteria and rods of different
length were found.
Only eukaryotic cells found; no evidence of bacteria.
Great number of Treponema-like bacteria in a very densely composed biofilm ( 1 105 bacteria).
Many eukaryotic cells of different types; no evidence of bacteria.
Patchy groups of bacteria ( 1 104 bacteria) together with eukaryotic cells.
Many large eukaryotic cells; no evidence of bacteria.
Poly-microbial biofilm ( 5 106 bacteria) consisting to a large extent of Treponema-like morphotypes.
Few spots with filamentous and coccus-like bacteria biofilm ( 5 104 bacteria) together with many eukaryotic cells.
Table III
Retrospective Compilation of Microbiological Data from Pathology Reports for Male Patient with Type 4 Periodontal Disease
Patient 3—Male, Periodontal Disease Type IV—Treatment Duration 2 Weeks, 6 Day, 15 Minutes
Microbial Description Based on Scanning Electron Micrograph
Multi-layered biofilm (5 106 bacteria) with a high number of Treponema-like morphotypes.
Only very sparse biofilm patches (1 103 bacteria) and many eukaryotic cells.
Biofilm (1 107 bacteria) with bacteria partly embedded in extracellular matrix.
Multi-layered biofilm ( 1 106 bacteria) predominantly composed of mid-long rods.
Poly-microbial biofilm (1 107 bacteria) with characteristic long rods.
Biofilm ( 1 105 bacteria) with different rod morphologies, coccus-like bacteria and filamentous bacteria.
Biofilm (5 105 bacteria) in which Treponema-like morphotypes contributed 50% of all bacteria.
Dense layer of eukaryotic cells; no evidence of bacteria.
Large, multi-layered biofilm (5 108 bacteria) with fusiform bacteria and Treponema-like morphotypes.
Large number of eukaryotic cells; no evidence of bacteria.
Biofilm (5 107 bacteria) in which fusiform bacteria and short rods were frequently found as well as coccus-like
Eukaryotic cells; no evidence of bacteria.
Retrospective Compilation of Microbiological Data from Pathology Reports for Male Patient with Type 4 Periodontal Disease
Patient 4—Male, Periodontal Disease Type IV—Treatment Duration 2 Weeks, 6 Day, 15 Minutes
Microbial Description Based on Scanning Electron Micrograph
Biofilm ( 1 107 bacteria) with large numbers of long and short rods, as well as Treponema-like bacteria.
Many eukaryotic cells; no evidence of bacteria.
Multi-layered biofilm ( 5 106 bacteria) in which Treponema-like morphotypes predominated.
Biofilm ( 5 104 bacteria) composed of a majority of Treponema-like bacteria.
Patches of biofilm ( 1 106 bacteria) with great diversity of rod-shaped bacteria (short, middle and long).
Biofilm amount decreased ( 1 104 bacteria), while the same morphotypes were observed.
Densely composed biofilm ( 1 106 bacteria) with all types of rod-like morphotypes.
Eukaryotic cells; no evidence of bacteria.
Biofilm ( 1 106 bacteria) embedded in its extracellular matrix.
Few patches of biofilm ( 5 103 bacteria) consisting of rod shaped bacteria with varying length.
Densely embedded biofilm ( 1 107 bacteria) revealing large numbers of Treponema-like morphotypes.
Eukaryotic cells and small biofilm colonies ( 5 103 bacteria) with rod shaped morphology.
Type I periodontal disease and was treated for 10 minutes twice
after five weeks. Patient 3 had Type IV periodontal disease on ar-
daily for five weeks; pocket probing depth decreased 2–3 mm at
rival at the clinic, and was treated for 15 minutes six times daily
the sites analyzed, no bleeding on probing was seen at any site,
for two weeks; no bleeding on probing was seen, and pocket
and no bacteria were recovered from three of four sites sampled
probing depth decreased 1–5 mm at the sites analyzed after two


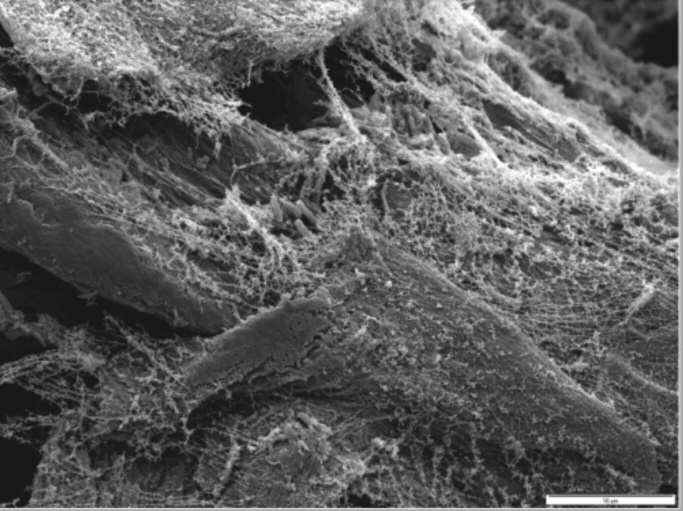
Vol. XXII, No. 5
The Journal of Clinical Dentistry
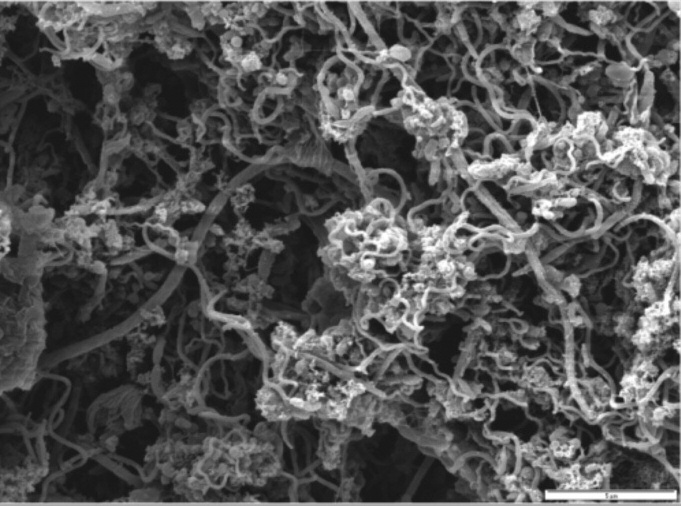
Figure 6a. Patient 1 SEM from paper point sample 24 mb before treatment
Figure 6b. Patient 1 SEM from paper point sample 24 mb after five weeks' daily
indicates predominance of Treponema-like morphotypes.
treatment shows multi-layered biofilm with short rods, long rods, and coccus-likemorphotypes.
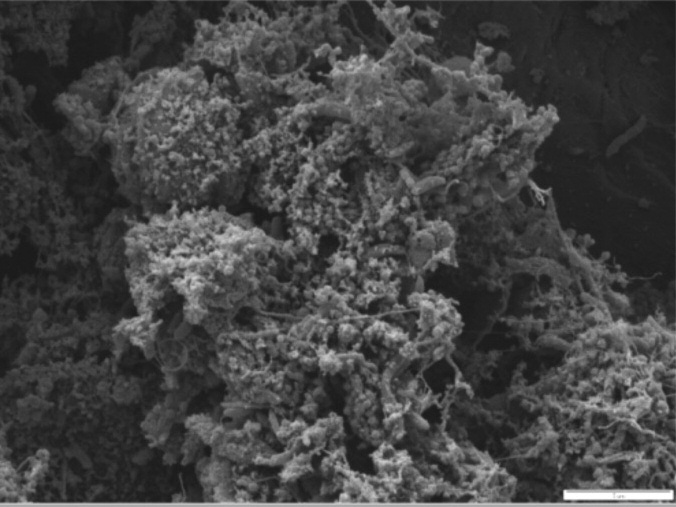
Figure 7a. Patient 2 SEM from paper point sample 14 mb before treatment
indicates dense multi-layered biofilm with Treponema-like bacteria and rods of
Figure 7b. Patient 2 SEM from paper point sample 14 mb after five weeks' daily
treatment. No bacteria were found.
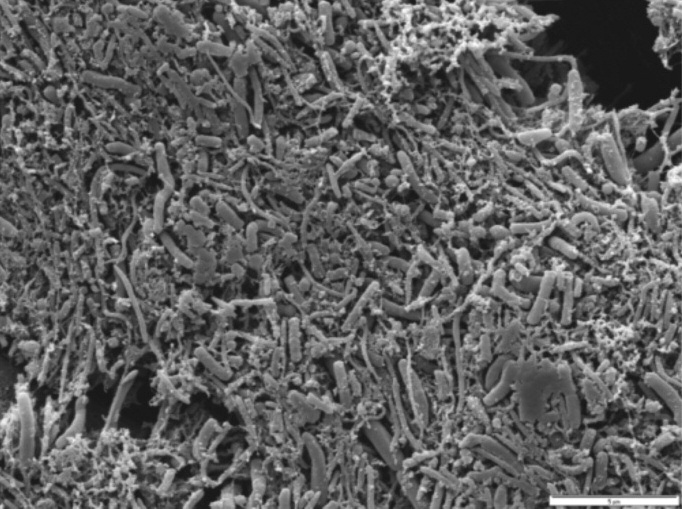
Figure 8a. Patient 3 SEM from paper point sample 31 mb before treatment in-
Figure 8b. Patient 3 SEM from paper point sample 31 mb after two weeks' daily
dicates multi-layered biofilm with high number of Treponema-like morphotypes.
treatment indicates sparse biofilm and eukaryotic cells.
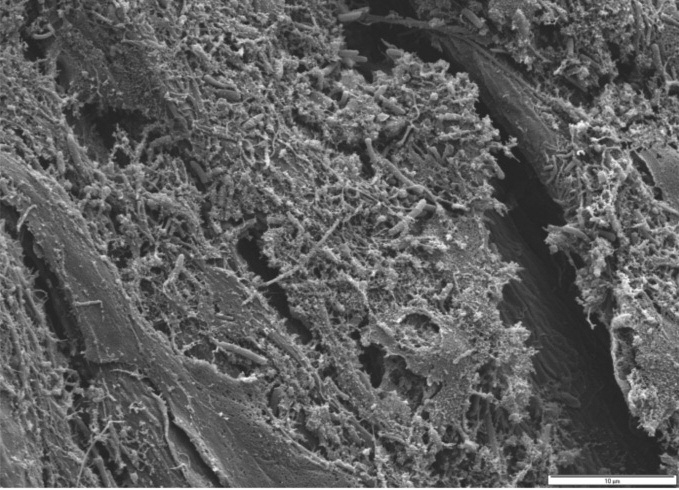
The Journal of Clinical Dentistry
Vol. XXII, No. 5
Figure 9a. Patient 4 SEM from paper point sample 21 ml before treatment in-
Figure 9b. Patient 4 SEM from paper point sample 21 ml after two weeks'
dicates biofilm composed of rod-like morphotypes.
daily treatment indicates presence of eukaryotic cells and no bacteria.
weeks of Perio Tray delivery of medication. Additionally, no bac-
reports were reviewed here self-reportedly did not strictly com-
teria were recovered from paper points at three of five sites af-
ply with treatment protocols; they missed one or more of the
ter two weeks of treatment. Patient 4 had Type IV periodontal
daily recommended treatments, or did not sustain treatment for
disease at the time of treatment, and no bleeding on probing at
the recommended 10 or 15 minutes. Nevertheless, the pathology
three of six sites was seen after two weeks of six times daily use
reports establish in vivo evidence of subgingival biofilm de-
of medication in the Perio Tray for 15 minutes. Pocket probing
bridement after Perio Tray placement of medication, even with-
depth decreased 0–5 mm during this time; two treated sites had
out strictly controlled conditions.
no bacteria present after treatment. In all cases after treatment
Direct visualization with SEM in these pathology reports differs
when bacteria were observed on paper points, the recovery was
from DNA analysis primarily in that standard DNA- Polymerase
reduced compared to the initial sampling.
Chain Reaction tests (commercially available with OralDNA® Labs,Brentwood, TN, USA and Hain Diagnostics, LLC, Midland, TX,
USA) evaluate a relatively small number of selected bacterial
The mathematical diffusion modeling indicates hydrogen per-
species, whereas SEM offers an indiscriminative view of the entire
oxide can effectively be placed into deep periodontal pockets
biofilm. Neither procedure can discriminate between living and
(> 6 mm) with Perio Trays, and that concentration of hydrogen
dead bacteria.
peroxide increases over time in the absence of degrading en-
In addition to the evidence of biofilm suppression, an overview
zymes like catalase or peroxidase. Even with a relatively large
of the pathology reports indicates that the prescription tray deliv-
GCF flow, the medication theoretically diffuses into 9 mm deep
ery of 1.7% hydrogen peroxide and three drops of Vibra mycin
pockets within the 15-minute time period evaluated.
helped reduce pocket depths and bleeding on probing, a confir-
Hydrogen peroxide (1.7%) was chosen as the treatment agent
mation that the prescribed solutions were held in place long enough
for this diffusion modeling exercise because in prior studies,
for the medication to have a therapeutic effect. Yet because the re-
aqueous solutions of hydrogen peroxide (> 1%) have been shown
ductions were not uniform, the tray delivery of prescribed solutions
to decrease plaque and gingivitis indices,47,48 to have antimicro-
may only be suggested as an adjunct to a compre hensive treatment
bial effects on bacteria associated with periodontal disease,49,50
plan implemented under the supervision of a dentist. In clinical
and to enhance wound healing after gingival surgery.37 In addi-
practice, use of the prescription tray delivery of medication is of-
tion, the biofilm potential technique1,51 provides case study evi-
ten followed by full mouth debridement, site- specific scaling as
dence of subgingival biofilm suppression in 6 mm pockets after
needed, site-specific surgery when needed, and prophylaxis.
Perio Tray delivery (20 minutes, four times a day for five days)
While the in vitro results indicate that hydrogen peroxide is
of 1.5% hydrogen peroxide (Peroxyl®, Colgate-Palmolive Com-
effective on oral biofilms, their debriding action on in vivo sub-
pany, New York, NY, USA) alone, and then in combination with
gingival biofilms cannot be confirmed with the pathology reports
three drops of Sumycin® Syrup (125 mg/5ml, Par Pharmaceuti-
because hydrogen peroxide and Vibramycin were both used.
cals, Woodcliff Lake, NJ, USA) for two five-day periods.34,52
Tetracyclines are commonly prescribed in the course of treatment
While the debriding effects of hydrogen peroxide on oral
for periodontal disease.55-59 For these patients, the sorbitol-based
biofilm were confirmed with the in vitro study presented here and
Vibramycin was prescribed for topical delivery for its anti-
the previously published case study, the strictly controlled clin-
inflammatory properties. Further testing needs to be completed
ical environment of the previously published case study does not
to confirm the specific therapeutic effects of individual solutions
take into consideration patient compliance with a patient tray
used with the prescription tray system.
delivery system. Real-world patients often do not adhere to pre-
Additional testing is also needed to confirm the most appropri-
scribed treatment regimens,53,54 and the patients whose pathology
ate course of treatment with individual medications. If medication
Vol. XXII, No. 5
The Journal of Clinical Dentistry
delivered subgingivally with the Perio Tray can decrease biofilm
ment protocol: a blinded, randomized clinical trial. J Clin Periodontol 2010;
populations, reduce pocket depths, and eliminate bleeding before
mechanical debridement, then the risks of bacteremia with me-
16. Resposo S, Tobler J, Alfant B, Gollwitzer J, Walker C, Shaddox L. Poster
presentation: Differences between Biofilm Growth Before and After Perio -
chanical debridement may also be reduced, as may the extent and
dontal Therapy. AADR 37th Annual Meeting. April 3, 2008. Abstract avail-
scope of necessary mechanical debriding procedures.
17. Palmer RJ, Caldwell DE. A flowcell for the study of plaque removal and
regrowth. J Micro Methods 1995;24:171-82.
Theoretical in vitro and in vivo evidence presented here indi-
18. Darby I. Non-surgical management of periodontal disease. Aust Dent J
cate that the custom-formed prescription Perio Tray can place
19. Krayer JW, Leite RS, Kirkwood KL. Non-surgical chemotherapeutic treat-
and maintain medications in periodontal pockets at a sufficient
ment strategies for the management of periodontal diseases. Dent Clin North
concentration for the medication to have a significant therapeu-
tic effect. In vitro studies established the ability of 1.7% hydro-
20. Tuomanen E, Cozens R, Tosch W, Zak O, Tomasz A. The rate of killing of
gen peroxide to debride the S. mutans biofilms on glass carriers.
Escherichia coli by beta-lactam antibiotics is strictly proportional to the rateof bacterial growth. J Gen Microbio 1986;132:1297-1304.
This is consistent with the action of hydrogen peroxide as an oral
21. Stewart PS. Theoretical aspects of antibiotic diffusion into microbial
debriding agent. Theoretical calculations indicate hydrogen per-
biofilms. Antimicrob Agents Chemother 1996;40:2517-22.
oxide is capable of reaching deep periodontal pockets, even
22. Schara R, Medvescek M, Skaleric U. Periodontal disease and diabetes meta-
against gingival crevicular fluid pressure. These theoretical cal-
bolic control: a full-mouth disinfection approach. J Int Acad Periodontol
culations were confirmed with the analysis of paper points placed
23. Lafaurie GI, Mayorga-Fayad I, Torres MF, Castillo DM, Aya MR, Barón A,
in periodontal pockets before and after 1.7% hydrogen peroxide
Hurtado PA. Periodontopathic microorganisms in peripheric blood after
and a subclinical dose of Vibramycin were delivered subgingi-
scaling and root planing. J Clin Periodontol 2007;34:873-9.
vally with the Perio Tray.
24. Morozumi T, Kubota T, Abe D, Shimizu T, Komatsu Y, Yoshie H. Effects
of irrigation with an antiseptic and oral administration of azithromycin on
Acknowledgment: This work was supported by Perio Protect, LLC.
bacteremia caused by scaling and root planing. J Periodontol 2010;81:1555-
For correspondence with the authors of this paper, contact
25. Fischer MA, Borgnakke WS, Taylor GW. Periodontal disease as a risk
marker in coronary heart disease and chronic kidney disease. Curr OpinNephrol Hypertens 2010;19:519-26.
26. Johnson JD, Chen R, Lenton PA, Zhang G, Hinrichs JE, Rudney JD. Peri-
1. Schaudinn C, Gorur A, Keller D, Sedghizadeh PP, Costerton JW. Perio -
stence of extracrevicular bacterial reservoirs after treatment of aggressive
dontitis. An archetypical biofilm disease. J Am Dent Assoc 2009;140:978-86.
perio dontitis. J Periodontol 2008;79:2305-12.
2. Socransky SS, Haffajee AD. The nature of periodontal diseases. Ann Perio -
27. Tribble GD, Lamont RJ. Bacterial invasion of epithelial cells and spreading
in periodontal tissue. Periodontol 2000 2010;52;68-83.
3. Travis J, Potempa J, Maeda H. Are bacterial proteases pathogenic factors?
28. Dorn BR, Dunn WA, Progulske-Fox A. Invasion of human coronary artery
Trends Microbiol 1995;3:405-7.
cells by periodontal pathogens. Infect Immun 1999;67:5792-8.
4. Rateitschak KH, Rateitschak EM, Wolf E. Farbatlanten der Zahnmedizin 1,
29. Bermudez LE, Goodman J, Petrofsky M. Role of complement receptors in
Parodontolgie. Thieme Verlag, 1984.
uptake of Mycobacterium avium by macrophages in vivo; evidence from
5. Taubman MA, Dawai T. Involvement of T-lymphocytes in periodontal dis-
studies using CD18-deficient mice. Infect Immun 1999;67:4912-6.
ease and in direct and indirect induction of bone resorption. Crit Rev Oral
30. Lamont RJ. Bacterial invasion of host cells. Adv Molecul Cell Microbiol.
Biol Med. 2001;12:125-35.
Cambridge University Press 2004.
6. Costerton J, Keller D. Oral periopathogens and systemic effects. Gen Dent
31. Keller D. How to manage oral biofilm using perio protect as a minimally in-
vasive method for lasting oral health. DPR 2010;44:54-55.
7. Williams RC, Barnett AH, Claffey N, Davis M, Gadsby R, Kellett M, Lip
32. Keller D. Managing periodontal disease in a patient suffering from renal fail-
GY, Thackray S. The potential impact of periodontal disease on general
ure. Dent Today 2008;27:144-7.
health: A consensus view. Curr Med Res Opin 2008;24:1635-43.
33. Keller D, Nguyen LT, Jobe LR, Sindelar BJ. Poster presentation: Prelimi-
8. Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. Microbial
nary data on periodontal disease treatment using topical oxidizing agents.
complexes in subgingival plaque. J Clin Periodontol 1998;25:134-44.
AADR (March 3-6, 2010), Washington DC. Abstract available online:
9. AAP statement on the efficacy of laser in the non-surgical treatment of in-
flammatory periodontal disease. J Periodontol 2011;82:513-14.
34. Schaudinn C, Gorur A, Sedghizadeh P, Costerton J, Keller D. Manipulation
10. AAP statement on the local delivery of sustained or controlled release anti -
of the microbial ecology of the periodontal pocket. World Dent 2010 Feb-
microbials as adjunctive therapy in the treatment of periodontitis. J Perio -
March: 14-18.
35. Lazarchik DA, Haywood VB. Use of tray-applied 10 percent carbamide
11. Apatzidou DA, Kinane DF. Nonsurgical mechanical treatment strategies for
perox ide gels for improving oral health in patients with special-care needs.
periodontal disease. Dent Clin North Am 2010;54:1-12.
J Am Dent Assoc 2010;141:639-46.
12. Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, Forbes
36. 21 CFR Parts 201, 356, and 369. Federal Register, Vol 53. No. 17. Wednes-
M, Greenberg DP, Dice B, Burrows A, Wackym PA, Stoodley P, Post JC,
day, January 27, 1988.
Ehrlich GD, Kerschner JE. Direct detection of bacterial biofilms on the
37. Marshall MV, Cancro LP, Fischman SL. Hydrogen peroxide. A review of its
middle-ear mucosa of children with chronic otitis media. J Am Med Assoc
uses in dentistry. J Periodontol 1995;66:786-96.
38. Gerlach RW, Barker ML, Sagel PA, Ralston CS, McMillan DA. In-use per-
13. Nickel JC, Costerton JW. Bacterial localization in antibiotic-refractory
oxide kinetics of 10% hydrogen peroxide whitening strips. J Clin Dent
chronic bacterial prostatitis. Prostate 1993;23:107-14.
14. Adriaens PA, Adriaens LM. Effects of nonsurgical periodontal therapy on
39. Ikai H, Nakamura K, Shirato M, Kanno T, Iwasawa A, Sasaki K, Niwano
hard and soft tissues. Periodontol 2000 2004;36:121-45.
Y, Kohno M. Photolysis of hydrogen peroxide, an effective disinfection
15. Zijnge V, Meijer HF, Lie MA, Tromp JA, Degener JE, Harmsen HJ, Abbas
system via hydroxyl radical formation. Antimicrob Agents Chemother
F. The recolonization hypothesis in a full-mouth or multiple-session treat-
The Journal of Clinical Dentistry
Vol. XXII, No. 5
40. Gusberti FA, Sampathkumar P, Siegrist BE, Lang NP. Microbiological and
development of bacterial biofilms in human periodontal pockets. FEMS
clinical effects of chlorhexidine digluconate and hydrogen peroxide
Micro biol Lett 2000;191:95-101.
mouthrinses on developing plaque and gingivitis. J Clin Periodontol 1988;
53. Eisen SA, Miller DK, Woodward RS, Spitznagel E, Przybeck TR. The ef-
41. Nocker A, Mazza A, Masson L, Camper AK, Brousseau R. Selective de-
fect of prescribed daily dose frequency on patient medication compliance.
tection of live bacteria combining propidium monoazide sample treatment
Arch Intern Med. 1990;150:1881-4.
with microarray technology. J Microbiol Methods 2009;76:253-61.
54. Cramer JA, Lynch NO, Gaudin AF, Walker M, Cowell W. The effect of dos-
42. Koo H, Xiao J, Klein MI, Jeon JG. Exopolysaccharides produced by Strep-
ing frequency on compliance and persistence with bisphosphonte therapy in
tococcus mutans glucosyltransferases modulate the establishment of mi-
postmenopausal women: a comparison of studies in the United States, the
crocolonies within multispecies biofilms. J Bacteriol 2010;192:3024-32.
United Kindgom, and France. Clin Ther 2006;28:1686-94.
43. Goodson JM. Gingival crevice fluid flow. Periodontol 2000 2003;31:43-54.
55. Gu Y, Lee HM, Sorsa T, Simon SR, Golub LM. Doxycycline [corrected] in-
44. Wentz LE, Blake AM, Keller DC, Sindelar BJ. Initial study of the Perio Pro-
hibits mononuclear cell-mediated connective tissue breakdown. FEMS Im-
tect treatment for periodontal disease. J Dent Res 2006;85(Spec Iss A):1164.
munol Med Microbiol 2010;58:218-25.
45. Treybal RE. Mass-transfer operations. McGraw-Hill, New York, p. 26, 1980.
56. Soory M. A role for non-antimicrobial actions of tetracyclines in combating
46. US Peroxide, LLC. Coefficient of diffusion. 2009. http://www.h2o2.com/
oxidative stress in periodontal and metabolic diseases: a literature review.
technical-library. Accessed 10/29/2010.
Open Dent J 2008;2:5-12.
47. Boyd RL. Effects on gingivitis of daily rinsing with 1.5% H O . J Clin Perio -
57. Payne JB, Golub LM, Stoner JA, Lee HM, Reinhardt RA, Sorsa T, Slepian
MJ. The effect of subantimicrobial-dose-doxycycline periodontal therapy on
48. Gomes DC, Shakun ML, Ripa LW. Effect of rinsing with 1.5% hydrogen per-
serum biomarkers of systemic inflammation: A randomized, double-masked,
oxide solution (Peroxyl) on gingivitis and plaque in handicapped and non-
placebo-controlled clinical trial. J Am Dent Assoc 2011;142:262-73.
handicapped subjects. Clin Prev Dent 1984;6:21-5.
58. Almazin SM, Dziak R, Andreana S, Ciancio SG. The effect of doxycycline
49. Miyasaki KT, Genco RJ, Wilson ME. Antimicrobial properties of hydrogen
hyclate, chlorhexidine gluconate, and minocycline hydrochloride on osteo -
peroxide and sodium bicarbonate individually and in combination against se-
blastic proliferation and differentiation in vitro. J Periodontol 2009;80:999-
lected oral, Gram-negative, facultative bacteria. J Dent Res 1986;65:1142-8.
50. Fletcher RD, Brastins ED, Albers AC, Conway J. The effect of the Keyes
59. Gupta R, Pandit N, Aggarwal S, Verma A. Comparative evaluation of sub-
procedure in vitro on microbial agents associated with periodontal disease.
gingivally delivered 10% doxycycline hyclate and xanthan-based chlorhex-
Quintessence Int 1984;3:329-34.
idine gels in the treatment of chronic periodontitis. J Contemp Dent
51. Wecke J, Dersten T, Madela K. A novel technique for monitoring the
Source: https://www.perioprotect.com/pdf/journal-of-clinical-dentistry-perio-protect.pdf
RESOLUCIÓN 05-03-ARCOTEL-2016 EL DIRECTORIO DE LA AGENCIA DE REGULACIÓN Y CONTROL DE LAS Que, el artículo 226 de la Constitución de la República del Ecuador dispone que: "Las instituciones del Estado, sus organismos, dependencias, las servidoras o servidores públicos y las personas que actúen en virtud de una potestad estatal ejercerán solamente las competencias y facultades que les sean atribuidas en la Constitución y la Ley. Tendrán el deber de coordinar acciones para el cumplimiento de sus fines y hacer
SPINE Volume 28, Number 17, pp 1978–1992©2003, Lippincott Williams & Wilkins, Inc. Muscle Relaxants for Nonspecific Low Back Pain:A Systematic Review Within the Framework of theCochrane Collaboration Maurits W. van Tulder, PhD,*† Tony Touray, MD,* Andrea D. Furlan, MD,‡Sherra Solway, MSc, BSc (PT),‡ and Lex M. Bouter, PhD* Study Design. A systematic review of randomized









