Untitled
The Biologic Basis for Libido
James G. Pfaus, PhD*, and Lisa A.Scepkowski, MA
arousal, desire, reward, and inhibition. In turn, these
*Center for Studies in Behavioral Neurobiology,
aspects of sexual function feed back on mechanisms of
Department of Psychology, Concordia University,
motivation, either to increase (as in the case of arousal,
7141 Sherbrooke West, Montréal QC H4B 1R6, Canada.
desire, or reward) or decrease (as in the case of reward or
inhibition) the expression of sexual interest or libido
Current Sexual Health Reports 2005, Current Science Inc. ISSN 1548-3584
(Fig. 1). Delineating the neural mechanisms that underlie
Copyright 2005 by Current Science Inc.
these aspects of sexual function has been the focus ofrecent research in both animals and humans.
Libido refers to a fluctuating state of sexual motivation in all organisms. Sexual motivation is altered by internal factors,
such as circulating steroid hormone levels and feedback from
Physiologic sexual arousal in all animals can be defined as
sexual stimulation; external factors, such as the presence of
increased autonomic activation that prepares the body for
sexually relevant incentives; and by the cognitive processing
sexual activity. This includes parasympathetic blood flow
of these factors that provides variations in sexual arousability
to genital and erectile tissues and sympathetic blood flow
and expectation of sexual reward. Libido thus reflects
from the heart to striated and smooth muscles that partici-
constant fluctuations in sexual arousal, desire, reward, and
pate in sexual responses (eg, increased breathing rate, heart
inhibition. Recent advances in neurochemical detection,
rate, pupil dilation). Sexual arousal also includes a central
pharmacologic analyses, and brain imaging, have helped
component that increases the psychologic preparedness to
identify neuroanatomic and neurochemical systems that reg-
respond to sexual incentives.
ulate these four aspects of sexual function. Another impor-
Increases in general sympathetic outflow produce
tant factor is the activation of central monoamine and
increases in libido. This can occur following the use of psy-
neuropeptide systems that link incentive motivation, reward,
chomotor stimulant drugs [1] or the ingestion of herbal
and inhibition together with autonomic pathways that detect
"aphrodisiacs" that contain psychoactive alkaloids or other
and relay sexual arousal. The activation of these systems by
substances that stimulate the autonomic nervous system
steroid hormones, and modulation by expectancy of sexual
[2]. However, these putative increases in libido are most
reward, are critical features of the neural "state" in which
likely to occur in sexually specific situations, indicating an
reactivity to sexual incentives is altered.
interaction between autonomic activation and the centralprocessing of sexual incentives in the immediate environ-ment. High sympathetic activation is an important ante-
cedent of premature ejaculation [3], which is often
Libido has always been associated with sexual motivation.
characterized by "high" libido in anticipation of sexual
Indeed, the Latin root refers specifically to sexual lust, a
activity. In women, situations such as acute exercise or
term that conjures images of highly motivated behavior.
exposure to stimuli that arouse a sympathetic response can
Libido is observed in the strength of desire and response
produce increases in physiologic sexual arousal. However,
toward a sexual incentive. Therefore, it can be regarded as a
although vaginal pulse amplitude in response to visual
conscious reflection of sexual motivation, which we define
erotica can be increased following exercise [4] or ephedrine
here as the energizing force that generates our level of
[5], this does not translate into an increase in subjective
sexual interest at any given time. It drives our sexual fanta-
sexual arousal. Thus, general stimulation of sympathetic
sies; compels us to seek out and evaluate sexual incentives;
outflow appears to make individuals more aroused in gen-
regulates our levels of sexual arousal and desire; and
eral and may increase libido if the situation contains
enables us to masturbate, copulate, or engage in other
appropriate sexual cues.
forms of sex play. Sexual motivation is often viewed as aninternal process built upon neuroendocrine mechanisms,
such as alterations in brain neurochemical function set
Erection is stimulated in hypogonadal men and castrated
forth by steroid hormone actions. It is, however, also mod-
male rats by androgens [6]. Treatments that enhance penile
ulated by experiences and expectations; learned patterns of
erection in nonhypogonadal men with erectile dysfunction
behavior; and underlying neural activity related to sexual
also enhance penile erection in gonadally intact male rats.
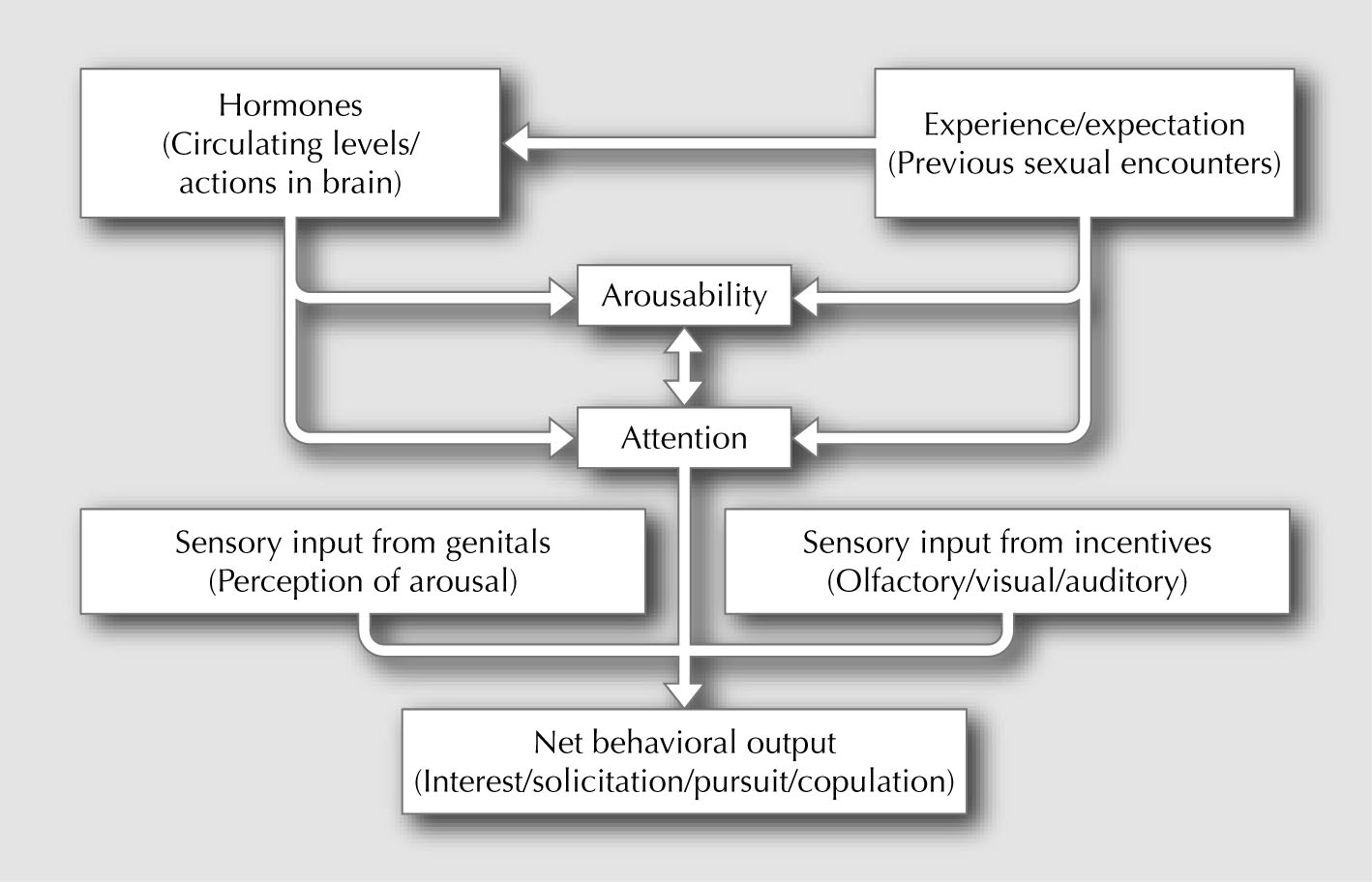
Androgen Deficiency
Figure 1. Hypothetical relationship of
experience, hormonal activation, arousability,
attention, and stimulus processing from
genital sensations and external incentives
on libido. Note that excitatory and inhibitory
feedback can occur anywhere in this flow
chart to strengthen or reduce responding.
Such feedback provides moment-to-moment
modulation of libido.
Examples of these treatments include phosphodiesterase
claustrum, hypothalamus, and amygdala [13–17,18•].
type 5 (PDE5) inhibitors, such as sildenafil, tadalafil, and
However, the last two structures are activated more in
vardenafil; dopamine receptor agonists, such as apomor-
men than in women viewing the same sexual stimuli
phine; melanocortin agonists, such as PT-141, prostaglandin
[17,18•]. Activation of the inferior extrastriate cortex,
E1, oxytocin; α2 receptor agonists, such as yohimbine, ida-
inferolateral prefrontal cortex, hypothalamus, and mid-
zoxan, and imiloxan; and vasodilators that act through
brain was correlated with subjective sexual arousal in
nitric oxide substrates, such as nitroglycerine, sodium nitro-
men after viewing an erotic film [15,17,18•], whereas
prusside, and linsidomine [6,7]. It is presumed that these
activation of the parietal cortex alone in men viewing
compounds exert their erectogenic actions in the autonomic
nervous system, although some of the drugs, such as apo-
sexual arousal [16]. However, men with hypoactive sexual
morphine, oxytocin, and the α2 receptor agonists, could
desire disorder (HSDD) display an abnormal activation
exert actions centrally. In fact, apomorphine can induce
of the medial orbitofrontal cortex, a region previously
erectile responses in male rats after infusions to the medial
implicated in the inhibitory control of motivated behav-
preoptic area (mPOA) of the anterior hypothalamus [8].
ior, relative to control subjects [19•]. In male and female
Psychogenic erections can be stimulated in men by
rats, nearly identical regions of the brain are activated by
exposure to visual sexual stimuli. The ease with which men
copulatory stimulation, including ejaculation and vagi-
achieve or maintain erection in response to erotic cues can
nocervical stimulation. A subset of those regions (nucleus
be taken as an index of libido, and latency to, and duration
accumbens, hypothalamus, amygdala) is activated by
of, full erection can be measured. A recent study of both
exposure of male rats to sexually arousing estrous odors
healthy men and those with erectile dysfunction found
or neutral odors paired with sexual reward [20].
that the melanocortin agonist PT-141 induced erections inhealthy men and enhanced erection in response to visual
sexual stimuli in men with erectile dysfunction [9]. "Non-
Relative to our understanding of the mechanisms under-
contact" erections in rats can be provoked by exposure to
lying penile erection, far less is known about the activation
sexually receptive females or vaginal estrous secretions
of physiologic or psychogenic sexual arousal in females.
[10]. Such erections are potentiated by androgens, by drugs
The nitric oxide-cyclic guanosine monophosphate pathway
that stimulate nitric oxide release in the paraventricular
appears to be critical for vaginal blood flow, as it is for
hypothalamus, or by dopamine release in the mPOA [11].
penile blood flow. Treatment with androgens facilitates
Conversely, dopamine receptor antagonists, such as halo-
vaginal nitric oxide synthase activity, along with vaginal
peridol, reduce both physiologic and subjective sexual
smooth muscle relaxation [21]. However, studies testing
arousal in men [1] and inhibit erections in male rats [12].
the efficacy of PDE5 inhibitors to increase vaginal blood
Brain activation during the presentation of visual
flow or pulse amplitude on their own, or to augment
sexual stimuli has been studied using positron emission
genital responses during the presentation of visual sexual
tomography (PET) and functional MRI. These studies
stimuli, have generated conflicting results. These results
have found common regions of activation in men and
range from no appreciable effects, to increases in subjective
women, including the anterior cingulate cortex, medial
arousal, to increases in genital arousal without correspond-
prefrontal cortex, ventral striatum/nucleus accumbens,
ing increases in subjective arousal [22]. Recently, a signifi-
The Biologic Basis for Libido • Pfaus and Scepkowski
cant positive effect of sildenafil was shown on both
occur in control animals that receive either no association
subjective arousal and perception of genital arousal in
of the odor with reward or that receive random association
women with arousal disorder and lower vaginal pulse
of the odor with reward and non-reward [31]. Indeed, we
amplitude [23]. Part of the problem with conducting
have found selective activation of both oxytocin- and
studies of female arousal is the high degree of variability in
GnRH-containing neurons by the odors in males and
physiologic and subjective responses. This may reflect
females, respectively, indicating that systems for sex and
several variables, including differences in placement of a
reproduction are being activated selectively. Finally, the
vaginal plethysmograph, exposure to different types of
melanocortin receptor agonist PT-141 increases rates of
sexual stimuli, and the phase of the ovulatory cycle in
solicitation in female rats primed with estrogen and
which women are tested.
progesterone, or estrogen alone [32•]. In preliminary stud-ies, we found that the dopamine receptor agonist apomor-phine also increases rates of solicitation in females primed
with estrogen alone. To the extent that solicitation in
Sexual desire has been extremely difficult to define. No
female rats and anticipatory psychomotor stimulation in
agreed-upon definition exists except that inferred from the
male rats are analogies of "sexual desire," the activation of
definition of HSDD in the Diagnostic and Statistical Manual
these two neurochemical systems in the brain may form an
of Mental Disorders (DSM-IV-TR) [24]. HSDD is defined as a
important part of the pathway that mediates libido. Inter-
condition in which "desire for and fantasy about sexual
estingly, estradiol increases both dopamine and melano-
activity are chronically or recurrently deficient or absent." By
cortin synthesis in hypothalamic and limbic structures,
converse logic, sexual desire would be the presence of desire
and androgens activate nitric oxide pathways that facilitate
for, and fantasy about, sexual activity. Desire can be viewed
dopamine release. The increase in female-initiated sexual
as distinct from arousal in animals and humans, with desire
activity around the time of ovulation [33], and the increase
constituting a psychologic interest in sex and behaviors that
in anticipatory sexual activity in males, may be primed by
reflect such interest. Despite the fact that desire and arousal
the activation of these two systems by steroid hormones.
are separate processes, desire may be informed or confirmedby the presence of autonomic or central arousal. In fact,many women and men regard sexual desire and arousal as
parts of one another, despite their distinct definitions
An emerging idea from animal studies is that desire is
[25,26]. Thus, desire as it is expressed physically in
linked to an expectation of reward and that such expecta-
conscious goal-directed behavior, most closely resembles
tion fluctuates over time given the actual level of reward
the "lust" of libido.
experienced. Sexual reward is inferred in animals by the
Desire can be inferred in animals by their willingness
strength of operant or instrumental responding toward a
to work for sexual reinforcers or in behavior that reflects
particular sexual reinforcer and by the strength of copula-
the anticipation of sexual activity [27]. Several lines of
tory responding (ie, behaviors that typically denote desire).
evidence link the desire for sex to the activation of brain
Contextual factors, such as settings, are also important
dopamine systems. Microdialysis studies have shown that
components of positive sexual experiences for both men
dopamine release in the mPOA and nucleus accumbens
and women. Recent work using the conditioned place pref-
increases in male rats in response to both conditioned and
erence (CPP) paradigm has been particularly useful in
unconditioned incentive cues that predict sexual reward
delineating the behaviors and neurochemical systems
[28]. Dopamine receptor antagonists injected peripherally
necessary for sexual reward in rats [34,35]. For male rats,
or centrally to these regions disrupt anticipatory
ejaculation is critical in the formation of CPP, whereas for
conditioned excitement [29]. Lesions of the basolateral
female rats, the ability to control the initiation and rate of
amygdala (a region that sends glutamate afferents to the
copulation (pacing) is critical. If one distinctive side of a
nucleus accumbens) decrease operant responding for sec-
CPP apparatus is paired with a rewarding sexual experience
ondary sexual reinforcers. This decrease can be reversed by
and the other side is paired with a less rewarding sexual
infusions of amphetamine to the nucleus accumbens [30].
experience (eg, copulation but not ejaculation in males;
Conditioned partner preferences in rats occur when a
nonpaced copulation in females), both male and female
neutral stimulus (eg, almond odor) is paired with a sexual
rats will spend significantly more time in the side associ-
reward. In male and female rats, we have shown that pre-
ated with reward. This indicates a preference for contextual
sentation of the conditioned stimulus alone induces anti-
cues associated with reward. Systemic administration of
cipatory psychomotor stimulation and activates brain
the opioid receptor antagonist naloxone during rewarded
regions associated with incentive motivation and attention
training trials blocks the induction of sexual CPP in both
(eg, nucleus accumbens, ventral tegmentum), sexual
males and females. This suggests that the release of endo-
behavior (mPOA, basolateral and medial amygdala), and
genous opioids is a critical factor in the sexual reward
reproductive processes (supraoptic and paraventricular
induced by ejaculation in males and pacing in females.
nuclei of the hypothalamus). Such activation does not
Interestingly, treatment with dopamine receptor antago-
Androgen Deficiency
nists does not block the induction of sexual CPP, indicat-
neuronal markers typically used in rat brain sections (eg,
ing that dopamine activation is not a necessary component
induction of nuclear Fos protein). It is possible that small
of sexual reward [36]. However, dopamine is required for
hypothalamic regions may still have been activated but
animals to display conditioned appetitive responses and
undetected. In women with complete spinal cord injury who
may be necessary for smaller, more appetitive types of
still experienced orgasm from masturbation, fMRI revealed
reward when animals attempt to gain access to sex partners
an activation of hypothalamic structures including the
and solicit sex. Systemic administration of opioid agonists
paraventricular nucleus; the medial amygdala; the anterior
disrupts the initiation of sexual behavior in both male and
cingulate; the frontal, parietal, and insular cortices; and the
female rats [37], and opioid agonists infused directly into
cerebellum. Because of the spinal damage, it was concluded
the mPOA have similar inhibitory effects in male rats.
that the stimulation of orgasm traveled through the Vagus
Dopamine release decreases abruptly in the nucleus
nerve to activate the brain.
accumbens and in the mPOA when male rats ejaculate,and the incentive salience of females is diminished duringthe absolute refractory period. The decrease in dopamine
Sexual Inhibition
release in the nucleus accumbens may be due to an activa-
Sexual inhibition can be induced by stressful life events or
tion of serotonin release in the lateral hypothalamus by
after high sexual rewards (ie, during a refractory period in
ejaculation [38]. Lesion studies suggest that the nucleus
which reproductive capacity needs to be regenerated before
accumbens plays an excitatory role in sexual arousal,
resumption of copulation) [47]. In either case, the activa-
whereas the lateral hypothalamus plays an inhibitory role
tion of inhibitory pathways for sexual arousal and desire
in sexual arousal but an excitatory role in the regulation of
generates a state of reduced libido.
ejaculation [39].
Activation of opioid and serotonin release during sexual
Activation of oxytocin and vasopressin pathways by
reward is associated with inhibition of ongoing sexual
sexual reward may be a critical component of future social
behavior. This has been studied in male rats following sex-
bonding. Monogamous prairie voles bond with their first
ual exhaustion. Male rats allowed to copulate to sexual
sex partner for life and share parental duties [40]. Polyga-
exhaustion with multiple ejaculations do not respond to
mous montane voles do not and neither do rats. Monoga-
female solicitations for a period of 24 to 72 hours. This inhi-
mous bonding in female prairie voles can be disrupted by
bition can be reversed by the 5-HT1A agonist 8-OH-DPAT
injections of an oxytocin antagonist, whereas bonding in
(an autoreceptor agonist that inhibits serotonin release), the
male prairie voles is disrupted by injections of a vaso-
α2 receptor agonist yohimbine, and the opioid receptor
pressin antagonist [41]. Male prairie voles have a greater
antagonist naloxone [48]. Thus, blockade of opioid or sero-
density of the vasopressin type 1a (V1a) receptor in the
tonin transmission, or activation of parasympathetic path-
ventral pallidum compared with male montane voles, and
ways involved in erection, can overcome the state of
viral gene transfection of the V1a receptor to the ventral
inhibition induced by sexual exhaustion. Activation of
pallidum of male montane voles renders them behavior-
opioid transmission by stress may also play a role in sexual
ally monogamous [42••]. Polygamous male and female
inhibition. Male rats find novel environments stressful. In
rats can be conditioned to display a partner preference
fact, males that are not desensitized to the environment in
based on odors or other cues associated with sexual reward
which they have their first sexual experiences often will
[43,44], and we have recently found that such cues activate
never copulate. Pre-exposure to the environment, or treat-
oxytocin and vasopressin neurons, in addition to dopam-
ment with naloxone, increases the proportion of males that
ine release. Thus, a consequence of early sexual reward is
copulate on their first trial [49]. Interestingly, sexually naïve
bonding to cues that predict the reward, cues that become
males sensitized to amphetamine do not show inhibition
highly arousing and desired. In humans, this process may
during their first exposure to females in a novel environ-
play an important role in the formation of preferences for
ment, despite the drug exposure happening weeks before
cues that we find attractive at a distance.
[50]. Although sexually experienced males show signs of
Brain imaging studies have also been conducted in men
fear (eg, freezing) in novel environments, they do not show
and women during manual genital stimulation to orgasm
subsequent sexual inhibition if a receptive female is placed
[45•,46•]. In men stimulated to ejaculation, PET revealed an
into the environment. Together, these data suggest that sen-
increased activation of the cerebellum and midbrain regions,
sitized dopamine systems, produced either by sexual experi-
including the ventral tegmental area, zona incerta, sub-
ence, amphetamine preexposure, or blockade of opioid
parafascicular nucleus, intralaminar thalamus, lateral puta-
transmission, can overcome the stress-induced inhibition of
men, and claustrum. No increased activation was observed in
sexual responding in males.
hypothalamic regions, and decreased activation wasobserved in the amygdala and surrounding entorhinalcortex. Most of these regions are activated by ejaculation in
male rats, although the general activation patterns offered by
Libido reflects our level of sexual interest at any given time.
PET do not have the fine-grained spatial resolution of the
It is determined by the interaction of neural systems that
The Biologic Basis for Libido • Pfaus and Scepkowski
underlie sexual arousal, desire, reward, and inhibition, pro-
Sachs BD: Contextual approaches to the physiology and
cesses that are highly influenced by steroid hormone
classification of erectile function, erectile dysfunction, and
sexual arousal. Neurosci Biobehav Rev 2000, 24:541–560.
actions. This is especially true for brain dopamine systems
Pehek EA, Thompson JT, Eaton RC, et al.: Apomorphine and
that modulate attention toward external sexual incentives
haloperidol, but not domperidone, affect penile reflexes
and help generate appropriate motor responses. The neuro-
in rats. Pharmacol Biochem Behav 1988, 31:201–208.
anatomical and neurochemical mechanisms that influence
Stoleru S, Gregoire MC, Gerard D, et al.: Neuroanatomical
correlates of visually evoked sexual arousal in human males.
this process, and are influenced by it, are only beginning to
Arch Sex Behav 1999, 28:1–21.
be understood. Likewise, studies concerning the nature of
Redoute J, Stoleru S, Gregoire MC, et al.: Brain processing of
sexual reward; its translation into pleasure, bonding, and
visual sexual stimuli in human males. Hum Brain Mapp 2000,
11:162–177.
sexual inhibition; and the mechanisms that underlie them,
Bocher M, Chisin R, Parag Y, et al.: Cerebral activation associ-
have only begun. We have understood libido for centuries at
ated with sexual arousal in response to a pornographic clip:
a behavioral level. Studying its biologic basis will help us
a 15O-H2O PET study in heterosexual men. Neuroimage 2001,
14:105–117.
identify mechanisms of sexual function and dysfunction,
Karama S, Lecours AR, Leroux JM, et al.: Areas of brain
and it will perhaps allow us to better understand how func-
activation in males and females during viewing of erotic
tion and dysfunction, and also desire and inhibition, are
film clips. Hum Brain Mapp 2002, 16:1–13.
integrated into the experience of all individuals.
Mouras H, Stoleru S, Bittoun J, et al.: Brain processing of
visual sexual stimuli in healthy men: a functional magnetic
resonance imaging study. Neuroimage 2003, 20:855–869.
18.• Hamann S, Herman RA, Nolan CL, Wallen K: Men and
women differ in amygdala response to visual sexual stimuli.
Nat Neurosci 2004, 7:411–416.
Research from JGP's laboratory reported here was sup-
This study used fMRI to examine whether patterns of brain activation
ported by grants from the Canadian Institutes of Health
differ between men and women viewing the same visual sexual
Research, Natural Sciences and Engineering Research
stimuli. Although men and women showed similar activation of a majority of brain regions, men had greater activation of the hypothal-
Council of Canada, and FCAR du Québec.
amus and amygdala relative to women overall and equal activation as
LAS is currently at the Center for Anxiety and Related
women who reported increased sexual arousal after the presentation.
Disorders, Department of Psychology, Boston University,
Because men are reported to have a greater sensitivity to visual sexual stimuli compared to women, the activation of these structures may
648 Beacon Street, 6th Floor, Boston, MA 02215, USA.
mediate the sex difference.
19.• Stoleru S, Redoute J, Costes N, et al.: Brain processing of visual
sexual stimuli in men with hypoactive sexual desire disorder.
Psychiatry Res 2003, 124:67–86.
This study used PET to examine differences in brain activation patterns
References and Recommended Reading
between sexually healthy men and those with a diagnosis of HSDD
Papers of particular interest, published recently,
while viewing visual sexual stimuli of graded stimulus intensity. A statisti-
have been highlighted as:
cal mapping procedure was used to determine regions that were activated
and deactivated between groups. Activation was found in frontal, soma-tosensory, and parietal cortices and in the cingulate gyrus in control sub-
Of major importance
jects compared to men with HSDD. However, prolonged activation of
Crenshaw TL, Goldberg JP: Sexual Pharmacology.
medial orbitofrontal cortex occurred in men with HSDD relative to con-
New York: Norton; 1996.
trols. Because the orbitofrontal cortex is involved in the general inhibi-tion of motivation, these results have important implications for the
Miller RA: The Magical and Ritual Use of Aphrodisiacs.
neural basis of sexual arousal and inhibition.
Rochester, VT: Destiny Books; 1993.
Pfaus JG, Heeb MM: Implications of immediate-early gene
Rowland DL, Cooper SE, Slob AK: Genital and psychoaffective
induction in the brain following sexual stimulation of
response to erotic stimulation in sexually functional and
female and male rodents. Brain Res Bull 1997, 44:397–407.
dysfunctional men. J Abnorm Psychol 1996, 105:194–203.
Traish AM, Kim NN, Munarriz R, et al.: Biochemical and
Meston CM, Gorzalka BB: The effects of immediate, delayed,
physiological mechanisms of female genital sexual arousal.
and residual sympathetic activation on sexual arousal in
Arch Sex Behav 2002, 31:393–400.
women. Behav Res Ther 1996, 34:143–148.
Scepkowski LA, Georgescu M, Pfaus JG: Neuroendocrine
Meston CM, Heiman JR: Ephedrine-activated physiological
factors in sexual desire and motivation. In Female Sexual
sexual arousal in women. Arch Gen Psychiatry 1998, 55:652–656.
Dysfunction. Edited by Goldstein I, Meston C, Davis K, Traish A.
Meisel RL, Sachs BD: The physiology of male sexual behavior.
London: Parthenon, In press.
In The Physiology of Reproduction, Second Edition. Edited by
Basson R, Brotto LA: Sexual psychophysiology and effects
Knobil E, Neill JD. New York: Raven Press; 1994:3–105.
of sildenafil citrate in oestrogenised women with acquired
McKenna K: Central nervous system pathways involved in the
genital arousal disorder and impaired orgasm: a randomised
control of penile erection. Annu Rev Sex Behav 1999, 10:157–183.
controlled trial. Br J Obst Gyn 2003, 110:1014–1024.
Hull EM, Eaton RC, Markowski VP, et al.: Opposite influence
American Psychiatric Association: Diagnostic and Statistical
of medial preoptic D1 and D2 receptors on genital reflexes:
Manual of Psychiatric Disorders IV-TR (Text Revision).
implications for copulation. Life Sci 1992, 51:1705–1713.
Washington, DC: APA Press; 2000.
Diamond LE, Earle DC, Rosen RC, et al.: Double-blind, placebo-
Basson R: Female sexual response: the role of drugs in the
controlled evaluation of the safety, pharmacokinetic properties,
management of sexual dysfunction. Obst Gyn 2001, 98:350–353.
and pharmacodynamic effects of intranasal PT-141, a melano-
Toledano RR, Pfaus JG: The sexual arousal and desire inventory:
cortin receptor agonist, in healthy males and patients with mild-
a multidimensional scale to assess the subjective experience of
to-moderate erectile dysfunction. Int J Impot Res 2004, 16:51–59.
sexual arousal and desire. Arch Sex Behav 2005, In press.
Sachs BD, Akasofu K, Citron JH, et al.: Noncontact stimulation
Pfaus JG, Kippin TE, Coria-Avila G: What can animal models tell us
from estrous females evokes penile erection in rats. Physiol
about human sexual function? Annu Rev Sex Res 2003, 14:1–63.
Behav 1994, 55:1073–1079.
Androgen Deficiency
Blackburn JR, Pfaus JG, Phillips AG: Dopamine functions
Kippin TE, Talianakis S, Schattmann L, et al.: Olfactory
in appetitive and defensive behaviours. Prog Neurobiol 1992,
conditioning of sexual behavior in the male rat. J Comp
Psychol 1998, 112:389–399.
Pfaus JG, Phillips AG: Role of dopamine in anticipatory and
Coria-Avila G, Ouimet AJ, Pacheco P, et al.: Olfactory
consummatory aspects of sexual behavior in the male rat.
conditioned partner preference in the female rat.
Behav Neurosci 1991, 105:727–743.
Behav Neurosci 2005, In press.
Everitt BJ: Sexual motivation: a neural and behavioral analysis of
45.• Holstege G, Georgiadis JR, Paans AM, et al.: Brain activation
the mechanisms underlying appetitive and copulatory responses
during human male ejaculation. J Neurosci 2003, 23:9185–9193.
of male rats. Neurosci Biobehav Rev 1990, 14:217–232.
This study used PET to examine the pattern of brain activation during
Kippin TE, Cain SW, Pfaus JG: Estrous odors and sexually
ejaculation in men given manual stimulation of the penis. To control
conditioned neutral odors activate separate neural pathways
for facial motor artifacts during orgasm, subjects were instructed to
in the male rat. Neurosci 2003, 117:971–979.
make orgasm-like facial grimaces before the study, and the activated
32.• Pfaus JG, Shadiack A, Van Soest T, et al.: Selective facilitation
regional blood flow patterns in the brain associated with those gri-
of sexual solicitation in the female rat by a melanocortin
maces was subtracted from the activation during the study. Ejacula-
agonist. Proc Natl Acad Sci USA 2004, 101:10201–10204.
tion induced neocortical activation only on the right side, but
In this study, we reported that systemic administration of PT-141,
bilateral activation was observed in the lateral putamen and claus-
a selective melanocortin receptor agonist, to female rats produced a
trum; intralaminar thalamus; in midbrain regions such as the ventral
selective facilitation of solicitation. However, the drug did not alter
tegmental area, central tegmental field, zona incerta, and subparafas-
lordosis, locomotion, or CPP, suggesting that the drug produces a
cicular nucleus; and in the cerebellum. This pattern of activation is
specific effect on measures of sexual desire rather than copulation or
nearly identical to that observed in male rats after ejaculation. How-
sexual reward.
ever, unlike in the rat, decreased activation in the amygdala and
Stanislaw H, Rice FJ: Correlation between sexual desire and men-
entorhinal cortex was observed in men. Perhaps the most interesting
strual cycle characteristics. Arch Sex Behav 1988, 17:499–508.
finding was the massive activation of the cerebellum, a structure only recently thought to participate in emotional processing.
Ågmo A, Berenfeld R: Reinforcing properties of ejaculation
in the male rat: the role of opioids and dopamine. Behav
46.• Komisaruk BR, Whipple B, Crawford A, et al.: Brain activation
Neurosci 1990, 104:177–182.
during vaginocervical self-stimulation and orgasm in women
with complete spinal cord injury: fMRI evidence of media-
Paredes RG, Martinez I: Naloxone blocks place preference
tion by the vagus nerves. Brain Res 2004, 1024:77–88.
conditioning after paced mating in female rats. Behav
This study used fMRI to examine the pattern of brain activation in
Neurosci 2001, 115:117–127.
women with complete spinal cord injury at T10 or above who are still
Ågmo A: Sexual motivation— an inquiry into events
capable of achieving orgasm through manual vaginocervical stimula-
determining the occurrence of sexual behavior. Behav Brain
tion. Orgasm activated the regional blood oxygen level-dependent
Res 1999, 105:129–150.
signal intensity in hypothalamic paraventricular nucleus; medial
Pfaus JG, Gorzalka BB: Opioids and sexual behavior.
amygdala; anterior cingulate; frontal, parietal, and insular cortices;
Neurosci Biobehav Rev 1987, 11:1–34.
cerebellum; and brainstem regions such as the nucleus of the solitary
Lorrain DS, Matuszewich L, Friedman RD, Hull EM:
tract—the main projection region of the Vagus nerve. As in women
Extracellular serotonin in the lateral hypothalamic area is
without spinal cord injury, orgasm also induced analgesia measured
increased during the postejaculatory interval and impairs
by tolerance to pressure intensity in the fingers of the nondominant
copulation in male rats. J Neurosci 1997, 17:9361–9366.
hand. These results suggest that vagal afferents carried the orgasm-
Kippin TE, Sotiropoulos V, Badih J, Pfaus JG: Opposing roles
relevant information to the brain and activated both sensory and
of the nucleus accumbens and anterior lateral hypothalamic
emotional systems involved in pleasure and pain modulation.
area in the control of sexual behaviour in the male rat.
Bancroft J, Janssen E: The dual control model of male sexual
Eur J Neurosci 2004, 19:698–704.
response: a theoretical approach to centrally mediated
Carter CS, Witt DM, Thompson EG, Carlstead K: Effects of
erectile dysfunction. Neurosci Biobehav Rev 2000, 24:571–579.
hormonal, sexual, and social history on mating and pair
Rodriguez-Manzo G: Blockade of the establishment of the
bonding in prairie voles. Physiol Behav 1988, 44:691–697.
sexual inhibition resulting from sexual exhaustion by the
Insel TR: A neurobiological basis of social attachment.
Coolidge effect. Behav Brain Res 1999, 100:245–254.
Am J Psychiatry 1997, 154:726–735.
Pfaus JG, Wilkins MF: A novel environment disrupts copula-
42.•• Lim MM, Wang Z, Olazabal DE, et al.: Enhanced partner
tion in sexually naïve but not experienced male rats: reversal
preference in a promiscuous species by manipulating the
with naloxone. Physiol Behav 1995, 57:1045–1049.
expression of a single gene. Nature 2004, 429:754–757.
Fiorino DF, Phillips AG: Facilitation of sexual behavior in
Libido is almost always directed toward sexual incentives in the exter-
male rats following d-amphetamine-induced behavioral
nal world. Even in fantasy, the persons or situations fantasized about
are typically externalized. This study shows just how malleable the sys-tem is. One important difference between the brains of monogamous versus polygamous voles concerns a population of V1a receptors in the ventral pallidum, a region that receives dopamine-modulated input from the nucleus accumbens and controls goal-directed movements. These receptors are greater in density in monogamous voles than in polygamous voles. To examine whether these receptors are important in the display of selective partner preference, the authors constructed an adeno-associated viral vector that contained the V1a receptor gene and infused it to the ventral pallidum of sexually naïve polygamous montane voles. This viral gene transfection was successful—the mon-tane males subsequently displayed both sexual and parental monog-amy, and the V1a receptor populations had grown in density. Thus, relative differences in brain vasopressin transmission can alter patterns of preference and entire mating strategies.
Source: http://psychology.concordia.ca/fac/pfaus/Pfaus-Scepkowski-(2005)-Curr-Sex-Health-Rep.pdf
Speech Enabled GPS Based Navigation System in Hungarian for Blind People on Symbian Based Mobile Devices B. Tóth, G. Németh Budapest University of Technology and Economics, Department of Telecommunications and Media Informatics, Magyar tudósok körútja 2., Budapest, 1117, Hungary Phone: (36)-(1)-4633883, {toth.b, [email protected]}
NCIC Missing Person File Agency Case # Data Collection Entry Guide PRINT or SAVE form before clicking RESET. Table of Contents Categories for Entry into the Missing Person File 2 NCIC INITIAL ENTRY REPORT MEDICAL INFORMATION Authorization to Release Medical Records PERSONAL DESCRIPTORS


