Getting more out of blood gas

Evolution of Blood Gas Analysis -
Focusing on the Source of Impaired O2
Supply to the Tissue
Ellis Jacobs, Ph.D, DABCC, FACB
Associate Professor of Pathology, NYU School of Medicine
Director of Pathology, Coler-Goldwater Hospital and Nursing
Why measure blood gases Overview of acid-base disturbances Use of the Acid- Base Chart
Full value of the pO2 assessment via
Oxygen uptake, Oxygen transport, Oxygen release
Why a measured saturation is the best Assessment of tissue perfusion - Lactate
The traditional picture
Traditionally, pO
been the sole parameter
used for evaluation of patient
transport
oxygenation
The traditional picture
Traditionally, pO
been the sole parameter
used for evaluation of patient
transport
For a complete evaluation of
the oxygen status, it is
necessary to consider lactate
and all parameters involved
in oxygen uptake, transport,
oxygenation
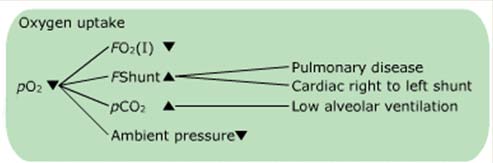
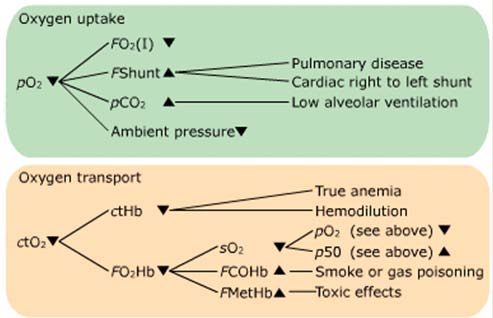
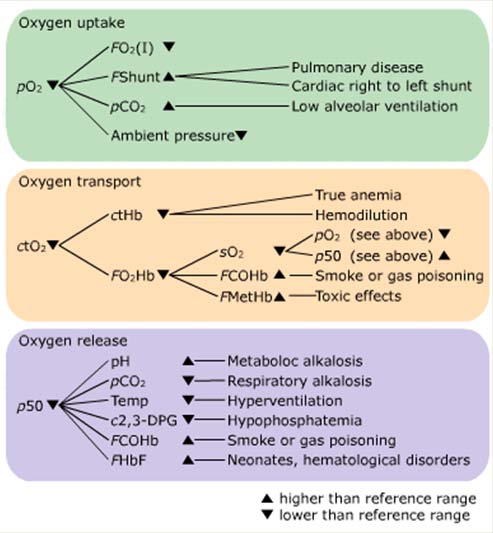
Example of a flowchart
[Adapted from different textbooks and Siggaard-Andersen, O et al. Oxygen status of arterial and mixed venous blood. Crit Care Med. 1995
Jul;23(7):1284-93.
Phase one: Oxygen uptake
pO2(a) – the key parameter
pO2(a) is the key
parameter for evaluation of
oxygen uptake in the lung
When the pO2(a) is low, the
supply of oxygen to cells
might be compromised
Conditions affecting pO2(a)
The amount of oxygen FO2(I) available
The degree of intra- and extrapulmonary shunting FShunt
Hypercapnia, high blood pCO2
The ambient pressure p(amp)
FO2(I) – fraction of inspired oxygen
Oxygen diffuses from the
alveoli into the blood
The higher the oxygen
content of the air, the
higher pO2(a)
Breathing room air equals
an FO2(I) of 21 %
A patient breathing
supplemental oxygen may
have a pO2(a) as high as
400 mmHg (and the oxygen
saturation is normal)
Evaluation of PO2 in Adult, Neonatal, and Geriatric
Patients Breathing Room Air
Arterial PO2 (mmHg) Condition above 80
Normal for adult (< 60 y)
above 70
Adequate for age > 70 y
above 60
Adequate for age > 80 y
Normal neonatal at 5 min
Normal neonatal at 1-5
40 to 60/70/80
Moderate to mild
hypoxemia
Severe hypoxemia
Evaluating Arterial Oxygenation in Patients Breathing
Lowest FI-O2 (%) Acceptable PO2 (mmHg) 30 150 40 200 50 250 80 400 100 500
Patients with a lower PO may be assumed to be hypoxic on room
Estimated FI-O2 of Air When Breathing 100% Oxygen
from Nasal Cannula
For each L/min of oxygen flow, add 4% to the estimated FI-O of air in the room, usually 21%.
Example: What is the estimated FIO of the air
being inhaled by a person receiving 2 L/min oxygen from a nasal cannula?
Goals of Oxygen Therapy
Treat hypoxemia Decrease work of breathing
Hyperventilation typical response to
Decrease myocardial work
Increased cardiac output is a mechanism to
compensate for hypoxemia.
FShunt is the fraction of venous blood not
oxygenated when passing the pulmonary capillaries
Examples of different types of shunt
Intrapulmonary respiratory
Intrapulmonary circulatory
• By some called true shunt
• Also called ventilation-
• Incomplete oxygenation in
• Heart defects allowing
perfusion disturbance
venous blood from left
• Incomplete oxygenation in
• Insufficient blood perfusion
chamber of heart to enter
• Lung diseases with
inflammation or edema that
causes the membranes to
FShunt – measured vs calculated
Shunt is calculated with values
from simultaneously drawn
arterial and mixed venous
The mixed venous sample must
be drawn from the pulmonary
artery, as indicated in the
A simpler and faster way to
estimate FShunt is from a single
Assuming that the arterio-venous
difference is normal, i.e.
extraction of 5.1 mL O2 per dL
Hypercapnia, high pCO2
Strong hypercapnia significantly decreases alveolar pO2,
a condition known as hypoventilatory hypoxemia
The hypoxemia develops because the alveolar gas
equation dictates a fall in pO2(a);
pO2(A) = pO2(air) – pCO2(A)/RQ
At any given barometric pressure, any increase in
alveolar pCO2 (caused by hypoventilation) leads to a fall
in alveolar pO2 and therefore also in arterial pO2
Oxygen uptake – a recap
The amount of oxygen FO2(I) available
The degree of intra- and extrapulmonary shunting FShunt
Hypercapnia, high blood pCO2
The ambient pressure p(amp)
Phase two: Oxygen transport
ctO2 – the key parameter
Oxygen content, ctO2 is the
key parameter for evaluating
the capacity for oxygen
When ctO2 is low, the oxygen
delivery to the tissue cells
may be compromised
Does ctO2/pO2 correlate?
A multicenter study on
10079 blood samples [1]
ctO2/pO2 correlation
ctO2 is almost
independent of pO2, so full
information is needed
E.g. pO2 of 60 mmHg (8
kPa ) corresponds to a
ctO2 of 4.8 – 24.2 mL/dL
[1] Gøthgen IH et al. Variations in the hemoglobin-oxygen dissociation curve in 10079 arterial blood samples. Scand J Clin Lab Invest
1990; 50, Suppl. 203:87-90
The blood's oxygen content, ctO2, is the sum of
Oxygen bound to hemoglobin and Physically dissolved oxygen
98% of oxygen is carried by hemoglobin The remaining 2% is dissolved in a gas form ctO normal range 18.8-22.3 mL/dL
ctO = sO × ctHb × (1 – FCOHb – FMetHb) + αO × pO
α is the solubility coefficient of oxygen in blood
Conditions affecting ctO2
The concentration of hemoglobin ctHb
The fraction of oxygenated hemoglobin FO2Hb
The arterial oxygen saturation sO2
The presence of dyshemoglobins FCOHb and FMetHb
Improving ctO2
The oxygen content can be improved by the variable
factors in the equation
ctO = sO × ctHb × (1 – FCOHb – FMetHb) + αO × pO
Types of hemoglobin
Total hemoglobin
Reduced hemoglobin
Carboxyhemoglobin
tHb is defined as the sum of
HHb+O2Hb+COHb+MetHb
COHb and MetHb are called
dyshemoglobins because
they are incapable of oxygen
Hemoglobin consists of 4
identical subunits
Each subunit contains an
Each iron can bind to one
oxygen molecule, O2
Oxygen binding is
Typical reference range is
Carboxyhemoglobin
Causes of raised COHb:
Increased endogeneous
production of CO
Breathing air polluted with
CO (carbon-monooixde
CO's affinity to Hb is 210
times higher than that of O2
The blood turns cherry-red,
but is not always evident
COHb is normally less than
1-2 % but in heavy
smokers up to 10 %
Endogeneous increase in COHb
Hemolytic condition leads to heme catabolism and thus
increased production of CO [1]
Hemolysis induced increase in COHb can be up to 4 %
but 8.3 % is also reported [2]
Slight increase in COHb is also a feature of a
inflammatory disease, and is thus also seen in critically ill
[1] Higgins C. Causes and clinical significance of increased carboxyheomoglobin. www.acutecaretesting.org . Oct 2005.
[2] Necheles T, Rai U, Valaes T. The role of hemolysis in neonatal hyperbilirubinemia as reflected in carboxyhemoglobin values. Acta
Paediatr Scand. 1976; 65: 361-67
[3] Morimatsu H, Takahashi T, Maeshima K et al. Increased heme catabolism in critically ill patients: Correlation among exhaled carbon
monoxide, arterial carboxyhemoglobin and serum bilirubin IX {alpha} concentrations. Am J Physiol Lung Cell Mol Physiol. (EPub) 2005 Aug
12th doi:/0.1152/ajplung.00031.2005
COHb intoxication
COHb intoxication may be deliberate or accidential In the US is accounts for 40,000 ED visits and between 5
and 6,000 death a year (2004) [1]
Sources of CO – common [2]
Fire, motor-vehicle exhaust and faulty domestic heating
Less commonly, gas ovens, paraffin (kerosene) heaters and
even charcoal briquettes
[1] Kao L. Nanagas K. Carbon monoxide poisoning. Emerg Clin N Amer 2004; 22: 985-1018
[2] Higgins C. Causes and clinical significance of increased carboxyheomoglobin. www.acutecaretesting.org . Oct 2005.
Relationship COHb
CO conc. in
inspired air
in blood %
Examples of typical symptoms
No appreciable effect except shortness of
breath on vigorous exertion, possible
tightness across forehead
Shortness of breath on moderate exertion,
occasional headache
Headache, easily fatigued, judgement
disturbed, dizziness, dimness of vision
Headache, confusion, fainting, collapse
Unconsciousness, convulsions, respiratory
failure, death if exposure continues
Immediately fatal
[1] Higgins C. Causes and clinical significance of increased carboxyheomoglobin. www.acutecaretesting.org . Oct 2005.
Clinical cases - Carboxyhemoglobin
Read three interesting case stories in "Causes and
clinical significance of increased carboxyheomoglobin"
by Chris Higgins on www.acutecaretesting.org
Methemoglobin is formed
when blood is exposed to
oxidizing agents, oxidizing
the iron atom: Fe2+ ⇒ Fe3+
MetHb has a very low
The blood typically turns
Causes for increased methemoglobin
Inherited – very seldom Acquired – more frequent Acquired methemoglobinemia occurs when hemoglobin is
oxidized in a rate faster by which methemopglobin is
Drugs or toxins that may cause methemoglobinemia
Acetanilide, p-aminosalicylic acid, amyl nitrate, aniline, benzocaine,
cetacaine, chloroquinone, clorfazimine, dapsone, hydroxylamine, isobutyl
nitrite, lidocaine, mafenide acetate, menadione, metoclopramide,
naphthoquinone, nitric oxide, nitrobezene, nitroethane, nitrofurane,
nitroglycerin, nitroprusside, paraquat, phenacitin, phenazopyridine,
prilocaine, primaquine, resorcinol, silver nitrate, sodium nitrate, sodium
nitrite, sodium valproate, sulphonamide anitibiotics, trinitrotoluene
[1] Higgins C. Methemoglobin. www.acutecaretesting.org . Oct 2006.
in blood %
Examples of typical symptoms
Is typically well tolerated and, in an otherwise healthy
individual, is asymptomatic Typically first sign of tissue hypoxia is cyanosis with skin
taking on a classically blue/slate gray appearance.
Symptoms: more profound hypoxia, including increased
heart rate, headache, dizziness and anxiety, accompany
deepening cyanosis as methemoglobin rises above 20 %.
May be associated with increasing breathlessness and
fatigue. Confusion, drowsiness and coma Methemoglobin
Symptoms of methemoglobinemia are generally more severe in a
patient who has some pre-existing condition (e.g. anemia, respiratory
or cardiovascular disease) that compromises oxygenation of tissues.
[1] Higgins C. Methemoglobin. www.acutecaretesting.org . Oct 2006.
Clinical cases - Methemoglobin
Read three interesting case stories in "Methemoglobin"
by Chris Higgins on www.acutecaretesting.org
A 84-year-old man had undergone a left hemicolectomy for
bowel torsion. After 10 days he became hypotensive,
tachypneic, oliguric, progressively acidotic, and anemic. Also,
the patient had passed bloody stools
ctO normal range: 18.8-22.3 mL/dL
1) With a FO (I) of 0.6 a blood
2) After bicarbonate and blood had
been administered i.v.
– pH = 7.25
– pCO = 29 mmHg
– pCO2 = 24 mmHg
– pO = 169 mmHg
– pO2 = 169 mmHg
– ctHb = 4.2 g/dL
– ctHb = 7.8 g/dL
– sO = 98 %
– sO2 = 98 %
– ctO = 6.08 mL/dL
– ctO2 = 10.8 mL/dL
This case is not a real life case – it is made for illustration purposes only
Oxygen transport – a recap
The concentration of hemoglobin ctHb
The fraction of oxygenated hemoglobin FO2Hb
The arterial oxygen saturation sO2
The presence of dyshemoglobins FCOHb and FMetHb
Phase three: Oxygen release
Conditions affecting release
Oxygen release depends
The arterial and end-
capillary oxygen tensions
The hemoglobin-oxygen
affinity expressed by the
p50 value
p50 is the key parameter
for evaluation of oxygen
release from hemoglobin
Conditions affecting p50
The hemoglobin-oxygen affinity is expressed by the
oxygen dissociation curve (ODC), the position of which is
expressed by the p50 value
As illustrated in the flowchart, several conditions can
affect the p50 value
p50 and the ODC curve
The p50 is the oxygen tension at half
saturation (sO2 = 50 %) and reflects the
affinity of hemoglobin for oxygen
Different factors affect the position of the
ODC, and p50 express the position of the
Typical reference range: 25-29 mmHg
Conditions affecting position of ODC
Can p50 be read from the ODC curve? [1]
If sO2 = 90 % then pO2 = 29-137
mmHg (4–18 kPa)
If pO2 = 60 mmHg (8 kPa) then sO2
Conclusion: Need information about
p50 via measurement of the factors
affecting ODC (MetHb, COHb etc)
[1] Gøthgen IH et al. Variations in the hemoglobin-oxygen dissociation curve in 10079 arterial blood samples. Scand J Clin Lab Invest
1990; 50, Suppl. 203:87-90
Oxygen release – a recap
The hemoglobin-oxygen affinity is expressed by the
oxygen dissociation curve (ODC), the position of which is
expressed by the p50 value
As illustrated in the flowchart, several conditions can
affect the p50 value
Some cases using the Flowchart
75-year-old woman Suffering from anemia, probably due to an ulcer What to do? Some of the results from the lab showed
pH = 7.40 (7.35-7.45)
sO = 97 % (95-99)
pCO = 40 mmHg (35-48)
FMetHb =0.005 (.002-.008)
pO = 98 mmHg (83-108)
FCOHb =0.005 (0.0 – 0.008)
FO (I) = 0.21
ctHb = 9.0 g/dL (12.0-17.5)
p50 = 25.5 mmHg (24-28)
ctO = 8.8 mg/dL (18.8-22.3)
This case is not a real life case – it is made for illustration purposes only
pO 98 mmHg
ctO 8.8 mg/dL
p50 25.5 mmHg
ctHb 9.0 g/dL
No DysHb
True anemia
This case is not a real life case – it is made for illustration purposes only
40-year-old man Exposed to smoke from a fire Some of the test results showed
pH = 7.400 (7.35-7.45)
sO = 97 % (95-99)
pCO = 40 mmHg (35-48)
FMetHb =0.005 (0.002-0.008)
pO = 98 mmHg (83-108)
FCOHb =0.300 (0.0-0.008)
FO (I) = 0.21
ctHb = 14.5 g/dL (12.0-17.5)
p50 = 26.3 mmHg (24-28)
ctO = 16.6 mL/dL (18.8-22.2)
This case is not a real life case – it is made for illustration purposes only
pO 98 mmHg
ctO 16.6 mg/dL
p50 26.3 mmHg
ctHb 14.5 g/dL
COHb 30%
CO poisoning
This case is not a real life case – it is made for illustration purposes only
15-year-old boy Severe asthmatic attack Some of the test results showed
pH = 7.350 (7.35-7.45)
sO = 80 % (95-99)
pCO = 35 mmHg (35-48)
FMetHb =0.005 (0.002-0.008)
pO = 60 mmHg (83-108)
FCOHb =0.005 (0.0-0.008)
FO (I) = 0.21
ctHb = 14.5 g/dL (12.0-17.5)
p50 = 37 mmHg (24-28)
ctO = 15.8 mL/dL (18.8-22.3)
This case is not a real life case – it is made for illustration purposes only
pO 60 mmHg
ctO 15.8 mg/dL
p50 37 mmHg
pCO 35 mmHg
This case is not a real life case – it is made for illustration purposes only
Oxygen saturation, sO2
cO Hb + cHHb
sO2 is defined as
The percentage of oxygenated hemoglobin in relation to the
amount of hemoglobin capable of carrying oxygen
Typical reference interval 95-99 %
High sO2:
Indicates that there is sufficient utilization of actual oxygen
transport capacity
Low sO2:
Indicates that the patient can likely benefit from supplemental
No information about tHb, COHb, MetHb, ventilation or
O2-release to tissue
3 different ways to get sO2
1. BG analyzer with CO-OX:
Measured by the CO-oximeter Golden standard
2. BG analyzer without CO-OX:
cO Hb + cHHb
Calculated from a pO2(a)
via the ODC curve
3. Pulse oximeters
BGA without CO-OX
CALCULATED sO2 dependents on
Available information (parameters) Algorithm applied by manufacturer
Correlation of pO2 and sO2 in real life [1]
If sO2 = 90% then pO2 = 29-137 mmHg (4 – 18 kPa) If pO2 = 60 mmHg (8 kPa) then sO2 = 70-99% At pO2 = 45 mmHg (6 kPa) and
pH = 7.25, then sO2 = 80 % pH = 7.40, then sO2 = 88 %
[1] Gøthgen IH, Siggaard-Andersen O, Kokholm G. "Variations in the hemoglobin-oxygen dissociation curve in 10079 arterial blood
samples" By. Scand J Clin Lab Invest 1990; 50, Suppl. 203:87-90
Why measured over calculated sO2
Several studies are supporting the importance of using a
measured sO2 and not calculated
CLSI [1]: "Clinically significant errors can result from
incorporation of such an estimated value for sO2 in further
calculations such as shunt fraction"
Breuer [2]: "No calculation mode can be performed with
constant accuracy and reliability when covering a wide
range of acid-base values. If sO2 values are used for further
calculations, e.g. for determination of cardiac output,
measured values are preferred"
[1] Blood gas and pH analysis and related measurements: Approved Guidelines, National Committee for Clinical Laboratory Standards
C46-A2, 29; 2009
[2] Breuer HWM et al. Oxygen saturation calculation procedures: a critical analysis os six equations or the determination of oxygen
saturation. Intensive Care Med 1989; 15: 385-89
A reliable sO2 (and pO2) matters
Hypoxemia - severe
Hypoxemia –moderate
Hypoxemia - mild
Normoxemia
10.6 kPa/80 mmHg
Normoxemia
13.3 kPa/100 mmHg
Hyperoxemia
16.0 kPa/120 mmHg
Hyperoxemia - marked
20.0kPa/150 mmHg
SpO2 Reflects the utilization of the current oxygen transport
Continuous monitoring Noninvasive method Easy and convenient 37 out of 42 pulse oximeters companies reported best
analytical performance as 1SD of +/- 2 % [1, 2]
[1] From as accessed September 2010,
[2 as accessed in 2007
Pulse oximeters in the ICU
Reputation: 90'ies studies conclude like these:
"We conclude that the accuracy of the tested nine pulse oximeters does not enable
precise absolute measurements, specially at lower oxygen saturation ranges" [1]
"Infants with acute cardiorespiratory problems, pulse oximetry unreliably reflects
pO2(a), but may be useful in detecting clinical deterioration [2]
A 2010 publication [3]
"The accuracy of pulse oximetry to estimate arterial oxygen saturation in critically ill
patients has yielded mixed results. Both the degree of inaccuracy, or bias, and its
direction has been inconsistent"…"analysis demonstrated that hypoxemia (sO2(a) <
90) significantly affected pulse oximeter accuracy. The mean difference was 4.9 % in
hypoxemic patients and 1.89 % in non-hypoxemic patients (p < 0.004). In 50 %
(11/22) of cases in which SpO2 was in the 90-93 % range the sO2(a) was <90 % ".
A 2012 publication [4]
"Despite its accepted utility, it is not a substitute for arterial blood gas monitoring as
it provides no information about the ventilatory status and has several other
limitations".
[1] Würtembe rger G. Accuracy of nine commercially available pulse oximeters in monitoring patients with chronic respiratory insifficiency.
Monaldi Arch Chest Dis 1994; 49: 348-353
[2] Walsh, M. Relationship of pulse oximetry to arterial oxygen tension in infants. Crit Care Med 15; 12: 1102-05.
[3] Wilson et al. The accuracy of pulse oximetry in emergency department patients with severe sepsis and septic shock: a retrospective
cohort study. BMC Emergency Medicine 2010; 10:9
[4] Kipnis, E et al. Monitoring in the Intensive Care . Critical Care Research and Practice, Volume 2012, Article ID 473507,
doi:10.1155/2012/473507
Oxygen saturation - Summary
GOLDEN STANDARD is the oxygen saturation
measured by the CO-oximeter analysis
Other oxygen saturation methods have various
Oxygen saturation does not give information on
oxygen delivery, ventilation, etc.
Does the oxygen get to the tissue?
Lactate is a waste product from anaerobic metabolism
Takes place when there is insufficient oxygen delivery to
Thus lactate is an early sensitive indicator imbalance
between tissue oxygen demand and oxygen supply
Aerobic metabolism
Anaerobic metabolism
Lactate is used….
……as a tool for
Diagnostically, admitting and triaging patients As a marker of tissue hypoperfusion in patients with
circulatory shock
As an index of adequacy of resuscitation after shock As a marker for monitoring resuscitation therapies Prognostically, as a prognostic indicator for patient
From: Bakker J. Increased blood
lactate levels: a marker of.?
When to measure lactate?
When there are signs and symptoms such as
Rapid breathing, nausea, hypotension, hypovolemia and
sweating that suggest the possibility of reduced tissue
oxygenation or an acid/base imbalance
Suspicion of inherited metabolic or mitochondrial disorder.
Data shows that….
Lactic acidosis
Occurs in approximately 1% of hospital admissions[1]. Has a mortality rate greater than 60% and approaches
100% if hypotension also is present [1].
Elevated lactate
Have been demonstrated to be associated with mortality in
both emergency departments and hospitalized patients [2,
[1] Burtis CA, Ashwood ER, Bruns DE. In: Tietz textbook of Clinical Chemistry and molecular diagnostics, 5th edition. St. Louis: Saunders
[2] Dellinger RP, Levy MM, Rhodes A et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and
Septic Shock: 2012. Crit Care Med, 2012; 41: 580-637
[3] Shapiro NI, Howell MD, Talmor D et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann
Emerg Med, 2005; 45; 524-528.
[4] Trzeciak S, Dellinger RP, Chansky ME et al. Serum lactate as a predictor of mortality in patients with infection. Intensive Care Med,
2007; 33; 970-977.
[5] Mikkelsen ME, Miltiades AN, Gaieski DF et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure
and stock. Crit Care Med. 2009; 37; 1670-1677
Surviving sepsis
The surviving sepsis campaign care bundle recommends,
among others, to measure lactate within 3 hours of
If lactate is elevated a second lactate measure could be
completed within 6 hours [1].
www.survivingsepsis.org
[1] Dellinger RP, Levy MM, Rhodes A et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and
Septic Shock: 2012. Crit Care Med, 2012; 41: 580-637
Hyperlactatemia and lactic acidosis
Hyperlactatemia:
Is typically defined as a lactate >2.0 mmol/L Occurs when the rate of lactate release from peripheral
tissue exceeds the rate of lactate removal by liver and
Lactic acidosis
If lactate is > 3-4mmol/L there is increasing risk of
associated acidosis
The combination of hyperlactatemia and acidosis is called
lactic acidosis, which is a disruption of acid/base balance.
Lactic acidosis A and B
Type A (hypoxic)
Inadequate oxygen uptake in the lungs and/or to reduced blood
flow resulting in decreased transport of oxygen
E.g.: Shock from blood loss/sepsis, myocardial, infarction/cardiac
arrest, congestive heart failure, pulmonary edema, severe anemia,
severe hypoxemia , carbon monoxide poisoning
Type B (metabolic)
Conditions that increase the amount of lactate in the blood but are
not related to a decreased availability of oxygen
E.g.: Liver disease, Kidney disease, Diabetic ketoacidosis (DKA),
Leukemia, HIV, glycogen storage diseases ( like glucose-6-
phosphatase deficiency), server infections – both systemic sepsis
and meningitis, strenuous exercise
Drugs and toxins typically represent the most common cause of
type B lactic acidosis
Lactic acidosis and pH
No universal agreement for definition of lactic acidosis [1] Lactic acidosis is the most common cause of metabolic
Lactic acidosis may not necessarily produce acidemia in a
patient as it depends on [1]
Magnitude of hyperlactatemia Buffering capacity of the body Coexistence of other conditions that produce tachypnea and
alkalosis (eg, liver disease, sepsis).
Thus, hyperlactatemia or lactic acidosis may be
associated with acidemia, a normal pH, or alkalemia [1]
[1] Acutecaretesting Handbook 2013 – Radiometer Medical - in press
[2] Cassaletto J. Differential diagnosis of metabolic acidosis. Emerg Med Clin N Amer, 2005; 23: 771-87.
Lactate and oxygen uptake, transport and release [1]
[1] Adapted from different textbooks and Siggaard-Andersen, O et al. Oxygen status of arterial and mixed venous blood. Crit Care Med.
1995 Jul;23(7):1284-93.
Examples of reference
interval
Short summary
Indicates the acidity or alkalinity of blood. pH is the indispensable measure of
acidemia or alkalemia.
M 35–48 (4.7-6.4)
pCO2 is the carbon dioxide partial pressure in blood. pCO2(a) is a reflection of the
F 32–45 (4.3–6.0)
adequacy of alveolar ventilation in relation to the metabolic state.
3 is standardized with the aim to eliminate effects of the respiratory
component on the HCO3 . HCO3 is classified as the metabolic component of acid-
BE predicts the quantity of acid or alkali to return the plasma in vivo to a normal
pH under standard conditions. BE may help determine whether an acid/base
disturbance is a respiratory, metabolic for mixed metabolic/respiratory problem
Base(Ecf) is independent from changes on pCO2 and is also called "in-vivo base
excess" or "standard base excess" (SBE).
pO2 is the oxygen partial pressure in blood. The pO2(a) is an indicator of the
oxygen uptake in the lungs.
sO2(a) is the percentage of oxygenated hemoglobin in relation to the amount of
hemoglobin capable of carrying oxygen and indicates if there is sufficient
utilization of actual oxygen transport capacity.
M 13.5-17.5 (8.4–10.9) tHb is defined as the sum of HHb+O2Hb+COHb+MetHb. tHb is a measure of the
F 12.0-16.0 (7.4–9.9) potential oxygen-carrying capacity.
ctO2 is the blood's oxygen content and is the sum of oxygen bound to hemoglobin
and physically dissolved oxygen. ctO2 reflects the integrated effects of changes in
the arterial pO2, the effective hemoglobin concentration and the hemoglobin
24–29 (3.2-3.9)
p50 is the oxygen tension at half saturation and reflects the affinity of hemoglobin
MetHb is formed when blood is exposed to certain oxidizing agents. MetHb has a
very low affinity to O2 resulting in decreased oxygen-carrying capacity.
COHb is primarily formed when breathing air polluted with CO. COHb is not
capable of transporting oxygen.
4.5–14.4 (0.5-1.6)
Lactate is a waste product from anaerobic metabolism. Lactate is an early
sensitive indicator imbalance between tissue oxygen demand and oxygen supply.
Sources for Scientific knowledge about acute care testing
Avoid preanalytical errors app
- for smartphones and tablets
- for smartphones
Source: http://www.radiometer.ru/~/media/radiometer/corporate/files/webinar-slides/webinar/webinar-slides/blood_gas_part_2_e_jacobs_050114.pdf
LIVING WELL WITH DEMENTIA IN LEEDS Our local strategy First published draft, June 25th 2012 Open for comment to 30th September 2012 Our vision, values and approach The Dementia Journey - diagram The views of people with dementia, families and carers Dementia-friendly Leeds Prevention and research Early detection and diagnosis
Der transjuguläre intrahepatische portosystemische Shunt Informationen für den Arzt Stand: Juni 2005 Die pathophysiologisch bedeutsamste Folge der Leberzirrhose ist die Erhöhung des beim Gesunden 3-6 mm Hg messenden Pfortaderdruckes. Steigt dieser auf über 12-15 mmHg an, können Komplikationen der portalen Hypertension auftreten, zu denen die Varizenblutung









