The steth volume 6, 2012 issn: 2094-5906
THE STETH VOLUME 6, 2012 ISSN: 2094-5906
Antihelminthic activity of Leucaena glauca (Ipil-ipil) seed and leaf extract in
an Ascaridae model
Student Researchers:Jeanne Janiza B. Delgado, Ed G. Lacsamana,
Raizel S. Macatangay,Reyshelle Ann B. Marquez &
Charrize Franchesca R. Miranda
Faculty Researchers: Redencion B. Reyes, RMT& Reby A. Cabanela, RMT
Abstract - Intestinal parasitism remains to be one of the most common
infections among children despite the increasing awareness on concepts of hygiene. Commercial medicines abound in the treatment of different types of parasitic infections, however, there is still a continuous search for alternative herbal medicine since they are safer to use and possess less toxicity. Among different herbal plants, certain studies revealed that Leucaena spp. have hypoglycemic, anti-diabetic, antimicrobial and antihelminthic properties. This study was carried out to evaluate the antiheminthic activity of Leucaena glauca (Ipil-ipil) seed and leaf extract in an Ascaridae model. Aqueous seed and leaf extracts of L. glauca was tested against Ascaris suum in vitro. Eggs and adult worms were exposed in 5 increasing concentrations of Ipil-ipil seed and leaf extracts. Results of this study revealed an ED50 of 105 mg/ml leaf extract concentration and 47 mg/ml seed extract concentration in egg hatch test. While an ED50 of 102 mg/ml concentration of leaf extract and 96 mg/ml concentration of seed extract in adult motility assay was also obtained. Mann-Whitney U test showed a significant difference (sig. value of 0.037) in the distribution of unfertilized eggs of seed and leaf extracts. It also revealed the similar distribution (sig. value of 0.114) of immotile worms in adult motility test on both extracts. Furthermore, this study presented a dose-dependent antihelminthic activity of Ipil-ipil which provides a new and potential cure against intestinal parasitism.
Keywords - parasitism, Leucaena spp., nematode, antimicrobial,
Ascaridae
INTRODUCTION
Parasitism is a relationship among two organisms in which one
organism, the parasite, is dependent on the other, the host. It oftentimes involves a highly specific relationship which is associated with metabolic dependence of the parasite to its host. Infections caused by parasites usually happen for a long time and may result to death of its host (Gunn and Pitt, 2012).
In the Philippines, according to the Department of Health (DOH, 2011),
parasitism persist because of poverty, poor sanitation and hygiene practices especially in areas where there is low economic and human development scale (Agbakoba, 2009). There are three major causes of intestinal parasitism in the Philippines namely: Ascaris lumbricoides, Trichiuris trichiura and Hookworm.
THE STETH VOLUME 6, 2012 ISSN: 2094-5906
These worms are collectively known as soil-transmitted helminths (Balolong, 2011).
A. lumbricoides also known as roundworm, is one of the six worms
classified by Linnaeus (Ridley, 2012) that is most prevalent parasitic helminthes among humans (Araujo, 2006) that might cause serious adverse effects like malnutrition particularly in children (Huang, 2008).
A variety of drugs, such as Albendazole and Mebendazole, are already
available against these parasites. However, medicinal plants that were used as raw materials in producing new drugs, like Atropa belladonna and Eucalyptus globules, are constantly increasing since these plants have innate active components which are used to treat diseases (Okigbo et al., 2008). Such properties are based on the antimicrobial, antioxidant and antipyretic effects of chemicals within the plant (Adesokan et al., 2008). According to Soetan and Aiyelaagbe (2009), research about these plants is considered as one of the leading fields of research worldwide.
Among the different studied plants is Leucaena glauca (Ipil- Ipil),
commonly known as Leucaena leucocephala. It is a herbal plant under the family of Mimosaceae, comprising the tropical and subtropical trees and shrubs and is usually seen in the plains of India (Khare, 2007). It is an essential foliage crop particularly on resource-limited farms in tropical countries (Paengkoum and Traiyakun, 2011). This plant also played a significant role in fodder supply, soil fertility improvement and fuel wood production in many parts of Africa (Dzowela and Otsyina, 2008). Its leaves revealed that it can also be an alternative source of protein in swamp buffaloes (Cherdthong et al., 2011), Thai Brahman cattle (Jetana et al., 2011), goats (Paengkoum, 2010) and laying hens (Atawodi et al., 2008).
Leucaena spp. is rich with crude protein (25-35%) and some other
alimentary contents (Ghosh and Bandyopadhyay, 2007). Its seeds and leaves contain 32.16% and 28.75% crude protein, 39.53% and 36.22% carbohydrates, and 55.76% and 51.024% organic carbon respectively (Aijaz et al.).
Though these plants have rich in chemical contents, it also contains toxic
substances like mimosine and tannin. The latter reduces digestibility of proteins which results in marked low metabolizable energy (ME) value of Leucaena leaf meal in poultry while mimosine and its metabolites are the main obstruction which blocks the usage of the plant as animal feed (Bandyopadhyay, 2007).
Mimosine provides 14.8% to overall nitrogen content of Leucaena seeds.
Next only to immature tender leaves, the seeds have higher concentration compared to other parts of the Ipil-Ipil (Chanchay and Poosaran, 2009). The bark and leaves also contain 16.3% and 3% tannin respectively. Furthermore, the leaves also include quercitrin (0.08%). Beta and alpha-aminopropionic acid is
THE STETH VOLUME 6, 2012 ISSN: 2094-5906
reported to be identical with mimosine (from Mimosa pudica). Stachyose is also reported to be present in the seeds (Khare, 2007).
A study on the antihelminthic activity of Leuceana leucocephala seed
(Ademola, Akanbi and Idowu, 2005) and leaf (Adama et al., 2012) extract against Haemonchus contortus ova and larvae has been the major interest of researchers. According to Adama et al. (2012), Leuceana leucocephala extract inhibited the egg hatching and larvae development of H. contortus although the inhibition efficiency is only more evident on eggs than on larvaes.
Given these studies, the researchers aimed to further determine the
nematocidal activity of Leucaena glauca using an Ascaris-swine model (Hall et
al., 2011). Ascaris suum, the comon roundworm of pigs and its life cycle is similar
to that of Ascaris lumbricoides (Araujo, 2006). Its genome is rich in peptidase
linked to the penetration and degradation of host tissues. Therefore, this genome
provides a comprehensive resource to the scientific community and underpins
the development of new interventions such as drugs, vaccines and diagnostic
tests against ascariasis and other nematodiases (Hall et al., 2011).
MATERIALS AND METHODS
Plant Materials
Leaves and seeds of Ipil-ipil were collected from Agoncillo, Batangas.
Voucher specimens of the plant were deposited to the Herbarium of the
University of Philippines for authentication.
Parasites
Mature worms of Ascaris suum were collected freshly from slaughtered
pigs in the abattoirs in Batangas City. The female parasites were selected and
dissected in a mortar and pestle to liberate the eggs. The eggs were washed with
distilled water and were collected using a 20 µm sieve (Adama et al., 2012).
Preparation of Baldwin and Moyle Solution (Austria and Villapando, 2006)
One hundred fifty one (151) grams of sodium chloride (NaCl), 24.8
grams potassium chloride (KCl), 13.4 grams calcium chloride (CaCl2), and 20.4
grams of magnesium chloride (MgCl2) were dissolved in two liters of distilled
water. Another 27.2 grams of potassium phosphoric acid (KH2PO4) was also
dissolved in one liter of distilled water. One volume of KH2PO4 and four volumes
of NaCl-KCl-CaCl2-MgCl2 were mixed and diluted with 35 volumes of distilled
water. The pH was adjusted to 7.2 with 1M sodium bicarbonate.
Preparation of Extracts
THE STETH VOLUME 6, 2012 ISSN: 2094-5906
Aqueous extracts were prepared using hot water extraction method
(Chauhan et al., 2008). The 2.5 g of each dry seed and leaf powder were boiled
in 100 ml of distilled water with constant stirring for 30 minutes. The solution was
allowed to cool down and was filtered through muslin gauze. Filtrate was
centrifuged at 3500-5000 rpm for 15 minutes. The supernatant was filtered again
using filter paper and stored at 4°C for future use. The procedure was repeated
using 5, 10, 15, and 20 grams of dry seed and leaf powder in 100 ml of distilled
water to yield the concentrations 25mg/ml, 50mg/ml, 100mg/ml, 150mg/ml, and
200mg/ml respectively.
Egg Hatch Test (EHT)
The EHT was performed using Coles et al. (1992). The 100 µl of fresh
macerated eggs was placed in Eppendorf tubes. The tubes were then submitted
to different treatments consisting of 5 extract concentrations (25mg/ml, 50mg/ml,
100mg/ml, 150mg/ml, and 200mg/ml of each extract plant). Distilled water was
used as a negative control. Three replicates for each concentration of extract and
control were performed. These tubes were incubated under humidified condition
at ambient temperature (27°C) for 48 hours. Afterhand, three drops of Lugol s
iodine solution were added to each well to stop further hatching. All the
unhatched eggs (percentage of inhibition) and those in the first stage larvae in
each well were counted under a brightfield microscope (Olympus Model BX43). A
coulter counter was used to ensure that 100 eggs were counted.
Adult Motility Assay
Ten live worms in each disposable container were suspended in Baldwin
and Moyle solution (BaMS). These worms were exposed to different treatments composed of 5 extract concentrations (25mg/ml, 50mg/ml, 100mg/ml, 150mg/ml, and 200mg/ml of each extract plant) in separate containers, in triplicates, at room temperature (25-30°C). The negative control container was added with excess BaMS in order to possess uniform volume (Iqbal et al., 2011).
The worms were observed after 24 hours. Finally, the treated and
negative control worms were exposed to heated inoculating loops to fully validate
for motility. Immotile worms were assessed as dead and the number of live and
dead worms in each container was counted and recorded (Derrain et al., 2012).
Statistical Analysis
Data from EHT and adult motility assay were computed using one-way
analysis of variance (ANOVA). The means of extract concentrations were submitted to the non-parametric test of Mann-Whitney U and Kruskall-Wallis. All analyses were made with the Statistical Package for Social Sciences (SPSS) version 18 for Windows at a significance level of 5%. For EHT and Adult motility assay, effective dose (ED50) and Lethal Concentration (LC50) was calculated as the concentration of extracts producing 50% effect and inhibition of eggs
THE STETH VOLUME 6, 2012 ISSN: 2094-5906
hatching and motility by probit-analysis. ED50 and LC50 were known to have the
same value since the tests used the number of incubation time.
RESULTS AND DISCUSSION
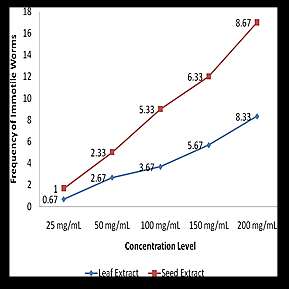
Figure 1 presents the average number of immotile worms out of 10 live
worms using different Ipil-ipil leaf and seed extract concentrations. For the leaf extract, the average numbers of immotile worms were 0.67, 2.67, 3.67, 5.67 and 8.33 in 25mg/ml, 50mg/ml, 100mg/ml, 150mg/ml and 200mg/ml leaf concentration respectively. On the other hand, the seed extract showed the average numbers of 1, 2.33, 5.33, 6.33 and 8.67 in 25mg/ml, 50mg/ml, 100mg/ml, 150mg/ml and 200mg/ml leaf concentration respectively. These results denote that the inhibition of Ipil-ipil leaf extract to the motility of adult worms follow a concentration-dependent manner. This is similar to the study of Iqbal et al. (2011) which presents the dose dependent antihelminthic effect of the herbal formulation of T. ammi seeds, flowers of C. procera, and leaves of A. indica and N. tabacum.
Frequency distribution of immotile worms in relationship with extract
THE STETH VOLUME 6, 2012 ISSN: 2094-5906
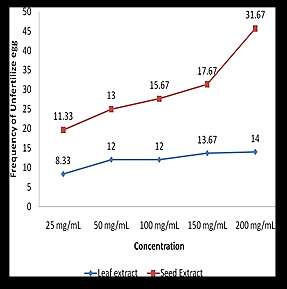
Frequency distribution of unhatched eggs in relationship with extract
Figure 2 shows the frequency of EHT using different Ipil-ipil leaf and
seed extract concentrations. This test indicates the average number of unfertilized egg out of 100 eggs counted in every extract concentration. For the leaf extract, the average numbers of unhatched eggs were 8.33, 12, 12, 13.67, and 14 in 25mg/ml, 50mg/ml, 100mg/ml, 150mg/ml and 200mg/ml leaf concentration respectively. While the seed extract concentration of 25mg/ml, 50mg/ml, 100mg/ml, 150mg/ml and 200mg/ml showed 11.33, 13, 15.67, 17.67, and 31.67 average numbers of unfertilized eggs respectively. These results revealed that the number of unfertilized eggs increases in a minimal amount as the concentration increases. This is related to the study of Adama et al. (2012) that shows inhibitory effects of L. leucocephala and Gliricidia sepcium leaf extract on Haemonchus contortus ova. This is also similar to the study of Ademola, Akanbi and Idowu (2005) which shows the nematocidal activity of L. leucocephala seed extract against sheep nematodes in a dose-dependent manner by chromatographic fraction.
Kruskal Wallis Test
Null Hypothesis
Decision
The distribution of unfertilized eggs is the
same across categories of concentrations
The distribution of dead worms is the same
across categories of concentrations
Table 1 illustrates the significant difference (P-value <0.05) between
distribution of unhatched eggs and immotile worms and the different
THE STETH VOLUME 6, 2012 ISSN: 2094-5906
concentration used. Kruskal Wallis test revealed the statistically insignificant values of 0.285 and 0.071 in EHT and adult motility assay respectively. Both of these results showed that the distribution of unfertilized eggs and immotile worms do not vary in any category of Ipil-ipil concentration. This is contradictory to the study of Peter and Deogracious (2006) which showed that the ascaridal effect of T. riparia, C. papaya, C. occidentalis and M. foetida leaf extracts increases with the increasing concentration and incubation time.
Mann-Whitney U test presented the significant values of 0.037 and 0.114
in EHT and adult motility assay respectively. These results indicated that the statistically significant difference between the seed and leaf extract was only observed on egg hatch test. This is parallel to the calculated ED50 (Table 3) of EHT which are 105 mg/ml and 47 mg/ml concentration of leaf and seed extract respectively. While the ED50 (Table 3) of 102 and 96mg/ml were observed in the adult motility assay. These concentrations would entice 50% of eggs not to hatch and 50% of the adult worms to be immotile. This also present that the seed extract is better in EHT (ED50= 47mg/ml) since it could already inhibit 50% of egg hatching in a minimal amount of concentration as compared to the leaf extract (ED50= 105mg/ml). These results correspond to the study of Ademola, Akanbi and Idowu (2005) which observed the rich fractions of alkaloids on Leucaena seeds. This also supports the study of Soetan et al. (2011) which showed that the Parkia biglobosa seed extract have more antihelminthic potential on nematode eggs than the leaf extract.
Mann-Whitney U test
Null Hypothesis
Sig. Value
Decision
The distribution of
unfertilized eggs is the same
across categories of extract
The distribution of dead
worms is the same across
categories of extract
Table 2 presents the significant d
ifference (P-value <0.05) between the
distribution of unhatched eggs and immotile worms and the kind of extract used (seed or leaf). The effective and lethal dose is presented in Table 3.
THE STETH VOLUME 6, 2012 ISSN: 2094-5906
Effective Dose 50 and Lethal Dose 50
Adult Motility Assay
CONCLUSION
The results of this study showed the anthelminthic activity of Ipil-ipil seed
and leaf extracts against Ascaris suum. These are quite similar to the reported ovicial and larvicidal effect of Leucaena leucocephala seed extract against Haemonchus contortus ova (Adama et al., 2012) as well as the effect of leaf extract against gastrointestinal sheep nematodes (Ademola, Akanbi and Idowu, 2005) which presents the increase number of efficacy as the concentration increases.
Antihelminthic activity of Ipil-ipil is bound to the presence of alkaloids,
flavanoids and tannin (Ademola, Akanb and Idowu, 2005). However, this study showed that there is no significant difference (sig. value of 0.114) between the seed and leaf extract on adult motility test. However, there is a significant difference (sig. value of 0.037) between the seed and leaf extract concentration on the egg hatch test. According to Adama et al. (2012), these findings may be due to the active substances present in the extracts that would cross more easily in the shell of eggs than the cuticles of larva. It was also found out that the seeds are more ovicidal than the leaf extract, as assessed in the ED50 of 47 mg/ml. This result was probably because Ipil-ipil seeds have higher concentration of mimosine, an active alkaloid but toxic component which results to impaired growth and alopecia on ruminants (Bandyopadhyay and Ghosh, 2007), compared to other parts of the Ipil-Ipil (Chanchay and Poosaran, 2009). According to Ademola, Akanbi and Idowu (2005), the most active fraction of the Leucaena seed are polyphenols, namely flavonoids and tannin, which in the absence of alkaloids, means that the most potent anthelminthic principles of the seed can be obtained without the risk of mimosine toxicity.
The parasite used in the study was chosen due to its considerable
similarity in protein profile and morphology with A. suum (Alba et al., 2009). Based from the findings of the present study, it is safe to assumed that Ipil-ipil seed and leaf extract may also have probable effects on the most common human infecting nematode, A lumbricoides. The researchers therefore conclude that Ipil-ipil seed and leaf extract can be used as an antihelminthic agent in regulated concentration due to the acceptable results on the selected parasite.
THE STETH VOLUME 6, 2012 ISSN: 2094-5906
REFERENCES
Abdul-Hamid, H., Jamaludin, K., Nor Yuziah, M. Y., Rahim, S., & Wan-Mohd-
Nazri, W. A. R. (2011). Strand properties of Leucaena leucocephala (Lam.) de wit wood. African Journal of Agricultural Research Vol. 6(22), pp. 5181-5191.
Adama, K., Amadou, T., Gaston, B.M., Hamidou, T.H., Isidore, G.B., Man, N. et.
al. (2012).In vitro anthelmintic activity of Leucaena leucocephala (Lam.) de wit. (Mimosaceae) and Gliricidia sepium (Jacq.) kunth ex steud (Fabaceae) leave extracts on Haemonchus contortus ova and larvae. Journal of Chemical and Pharmaceutical Research, 4(1).303-309.
Ademola, I.O., Akanbi, A.I. & Idowu, S.O. (2005).Comparative nematocidaL
activity of chromatographic fractions of Leucaena leucocephala seed against gastrointestinal sheep nematodes.Pharmaceutical Biology, 43(7).599-604.
Aderibigbe, S. A., Adetunji O. A., & Odeniyi M. A. (2011). Antimicrobial and
pharmaceutical properties of the seed oil of Leucaena leucocephala (Lam.) de wit (Leguminosae).Afr. J. Biomed. Res. 14 (January 2011); 63 -6.
Afza, N., Kalhoro, M.A., Khan, R.A., & Nwar, M.A. (2007).Physico-chemical and
toxicological studies of different parts of Leucaena leucocephala.Pakistan Journal of Pharmacology Vol.24, No.2, pp.13-16.
Agbakoba, N.R., Anaghalu, I.C., Chukwuma, M.C., Ekejindu, I.M., Ezeagwuna,
D.A., & Nwosu, D.C. (2009).The prevalence and risk factors of geohelminth infections among primary school children in Ebenebe Town, Anambra State, Nigeria.Middle-East Journal of Scientific Research 4 (3): 211-215.
Aiyelaagbe, O.O. & Soetan, K.O. (2009).The need for bioactivity-safety
evaluation and conservation of medicinal plants- a review.Journal of Medicinal Plants Research, 3(5).324-328.
Aldovino, Mabelle R., Derain, Rachelle G., Holgado, Ma. Cristine B., Lescano,
Joy Ever H., Magbuhos, Arrian Marie S., Villanueva Jenessa Camille P., and Dumaoal, Oliver Shane R.(2012). Parasiticidal activity of Brassica oleracea var. Botrytis (cauliflower) on an Ascaridae model.Undergraduate Thesis. Lyceum of the Philippines Universty Batangas,Batangas City.
Alvarado, M.A., Badii, M.H., Foroughbakch, R., Guzman-Lucio, M.A., Ramirez,
R. et. al. (2007). Nutrients, mineral and volatile fatty acids content in four Leucaena species and the hybrid k743. Journal of Animal and Veterinary Advances, 6(9).1083-1087.
THE STETH VOLUME 6, 2012 ISSN: 2094-5906
Araujo, C.A., Enobe, C.S., Macedo, M.S., Martins, M.A., Macedo-Soares, M. F. &
Perini, A. (2006). Early stages of Ascaris suum induce airway inflammation and hyperreactivity in a mouse model. Parasite Immunology, 28.453-461.
Arigbede, O.M., Fayemi, P.O., Isah, O.A., Jegede, A.V., Muchenje, V., & Onwuka,
C.F.I. (2011). Effects of mimosine and tannin toxicity on rabbits fed processed Leucaena leucocephala (Lam) de wit. leaves. African Journal of Agricultural Research Vol. 6(17), pp. 4081-4085.
Atawodi, J.C., Atawodi, S.E., Mari, D. & Yahaya, Y. (2008). Assessment of
Leucaena leucocephala leaves as feed supplement in laying hens. African Journal of Biotechnology, 7.317-321.
Austria,Honeyln M. And Villapando, Hermia Mae D.(2006). Anthelminthic
Activities in Vitro of the Crude Alcoholic Seed ectracts of Cucumis sativus(Cucumber) and Cucurbita maxima(squash) in Ascaris suum (Pig roundworm). Undergraduate Thesis. Batangas State University Main Campus,Batangas City.
Balolong Jr, E., Carabin, H., Joseph, L., McGarvey, S.T., Olveda, R. & Tarafder,
M.R. (2011).Assessing the impact of misclassification error on an epidemiological association between two helminthic infections.Neglected Tropical Diseases, 5.e995.
Bandyopadhyay, S. & Ghosh, M.K. (2007).Mimosine toxicity- a problem of
Leucaena feeding in ruminants.Asian Journal of Animal and Veterinary Advances, 2(2).63-73.
Bowman, D., Butkus, M.A., Hughes, K.T., Jenkins, M.B., Labare, M.P. & Liotta,
J.L. (2011).Inactivation of Ascaris suum by short-chain fatty acids.Applied and Environmental Microbiology, 77(1).363-366.
Cantacessi, C., Chen, N., Gasser, R.B., Huang,C., Lin, R., Loukas, A. et. al.
(2008).Genomic-bioinformatics analysis of transcripts enriched in the third-stage larva of the parasitic nematode Ascaris suum.Neglected Tropical Diseases, 2.e246.
Castro, M., Grageola, F., Lemus, C. & Ly, J. (2007). Ileal and rectal digestability
of nutrients in diets based on Leucaena (Leucaena leucocephala (Lam.) de wit) for pigs.influence of the inclusion of Zeolite. Journal of Animal and Veterinary Advances, 6(12).1371-1376.
Chanchay, N., & Poosaran, N. (2009).The reduction of mimosine and tannin
contents in leaves of Leucaena leucocephala.As. J. Food Ag-Ind. 2009, Special Issue, S137-S144.
THE STETH VOLUME 6, 2012 ISSN: 2094-5906
Chen, W.C., Hsieh, M.H., Hsu, Y.C., Hsu, Y.K., Lin, Y.Y., Yang, J.F., et al. (2011).
Intestinal parasitic infections in foreigners detected by stool examination in Taiwan.The Open Infectious Diseases Journal, 2011, 5, 135-141.
Cherdthong, A., Kang, S., Pakdee, P., & Wanapat, M. (2011). Supplemental
energy influenced on Leucaena leucocephala leaf meal in swamp buffaloes. Journal of Animal and Veterinary Advances, 10(17).2225-2233.
Chew, Y.L., Goh, J.K., Lim, Y.Y., Ling Chan, E.W., Stanslas, J. & Tan, P.L. (2011).
Assessment of phytochemical content, polyphenolic composition, antioxidant and antibacterial activities of Leguminosae medicinal plants in Peninsular Malaysia.BMC
Complementary
Alternative
11:12.Retrieved from http://www.biomedcentral.com/1472-6882/11/12.
Dzowela, B. & Otsyina, R. (2008).Importance of Leucaena in Africa.Leucaena
psyllid: a threat to agroforestry in Africa, 12:24. Retrieved from http://www.fao.org/docrep/008/v5020e/V5020E04.htm#03.1.1
Eme, U.E., Ogbogu, S. & Okigbo, R.N. (2008).Biodiversity and conservation of
medicinal and aromatic plants in Africa.Biotechnology and Molecular Biology Reviews, 3(6).127-134.
Gunn, A., & Pitt, S. J. (2012).Parasitology: an integrated approach. West Sussex,
UK: John Wiley & Sons.
Hall, R.S., Jex, A.R., Li B., Li, Y., Liu, S., Yang, L. et. al. (2011). Ascaris suum
draft genome.Nature, 479.Retrieved November 24, 2011.529-533.
Hassan, R.A. & Radwan H.M. (2010).The lipids and volatile oil constituents of
Leucaena glauca (L.) benth.growing in Egypt and their biological activity. Journal of Applied Sciences Research, 6(5).478-482. http://www.fao.org/docrep/008/v5020e/V5020E04.htm#03.1.1
Iqbal, Z., Khan, M.N., Muhammad, G. & Zaman, M.A. (2011). Anthelimintic
activity of a herbal formulation against gastrointestinal nematodes of sheep. Pakistan Veterinary Journal, 32(x).Retrieved from September 06, 2011.1-5.
Jetana, T., Sophon, S., Usawang, S., & Vongpipatana, C. (2011). Using treated
Leucaena (Leucaena leucocephala) leaves as supplements to Thai Brahman cattle giving a basal diet of rice straw. Journal of Animal and Veterinary Advances 10, 8.1054-1060.
Khare, C.P. (2007). Indian medicinal plants:an illustrated dictionary. New Dehli:
Springer Science.
Paengkoum, P. & Traiyakun, S. (2011). Ruminal and intestinal digestibility of
Leucaena (Leucaena leucocephala) and jack fruit (Artocarpus heterophyllus)
THE STETH VOLUME 6, 2012 ISSN: 2094-5906
foliages using in saccoand three-step techniques. Research Journal of Applied Sciences, 6(2).88-91.
Paengkoum, P. (2010). Effects of Neem (Azadirachta indica) and Leucaena
(Leucaena leucocephala) fodders on digestibility, rumen fermentation and Nitrogen balance of goat fed corn silage. Journal of Animal and Veterinary Advances, 9(5).883-886.
Ridley, J. W. (2012). Parasitology for medical and clinical laboratory
professionals.New York: Delmar Cengage Learning.
Soil-transmitted helminthiasis and other parasitoses.(2011). Department of
Simanjuntak, P., Sumarny, R., & Syamsudin. (2010). Antidiabetic activity of active
fractions of Leucaena leucocephala (lmk) dewit seeds in experiment model. European Journal of Scientific Research Vol.43 No.3 (2010), pp.384-391.
Soetan, K.O., Lasisi, O.T., & Agboluaje, A. K. (2011).Comparative assessment of
in-vitro anthelmintic effects of the aqueous extracts of the seeds and leaves of the African locust bean (Parkia biglobosa) on bovine nematode eggs.Journal of Cell and Animal Biology Vol. 5 (6), pp. 109-112.
Source: http://research.lpubatangas.edu.ph/wp-content/uploads/2014/10/STETH-6.5.pdf
Qué hay detrás de. Productos contra la disfunción eréctil El 55% de los mexicanos mayores de 40años sufren disfunción eréctil. En laactualidad, se ofrecen un sinnúmero deopciones para superar este padecimiento,entre ellas, algunas que han comprobadocientíficamente su seguridad y eficacia. Sinembargo, es necesario denunciar laexistencia de un mercado negro deproductos que engañan y frustran a loshombres que recurren a ellos e, incluso,pueden provocarles serios daños a su salud.
Servicio de publicaciones y difusión científica (SPDC), Universidad de Las Palmas de Gran Canaria, Parque Científico-Tecnológico, Edificio Polivalente II, C/ Practicante Ignacio Rodríguez, s/n Campus Universitario de Tafira 35017 - Las Palmas de Gran Canaria, Spain. Revista de lenguas para fines específicos eISSN: 2340-8561 Journal information, indexing and abstracting details, archives, and instructions for submissions: https://ojsspdc.ulpgc.es/ojs/index.php/LFE/index



