Sitegrabber.cloudapp.net
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 1 of 25
IN THE UNITED STATES DISTRICT COURT
FOR THE EASTERN DISTRICT OF PENNSYLVANIA
SOUTHEASTERN PENNSYLVANIA
TRANSPORTATION AUTHORITY,
individually and on behalf of all others
similarly situated,
CLASS ACTION COMPLAINT
GILEAD SCIENCES, INC.,
JURY TRIAL DEMANDED
This case involves a drug manufacturer's attempt to exploit the patent
laws by selectively charging exorbitant prices for its life-saving Hepatitis-C drug,
Sovaldi® (sofosbuvir tablets) ("Sovaldi"). As explained herein, Defendant Gilead
Sciences, Inc.'s ("Gilead" or "Defendant") limited rights as a patent holder do not
translate into a license to price gouge consumers, state and federal health and
welfare programs, and other third party payers under the extraordinary
circumstances presented here.
Sovaldi is, by all accounts, a remarkably effective drug. It is the first
drug approved by the Food and Drug Administration ("FDA") for certain types of
Hepatitis-C infections that does not need to be injected. It can cure about 90 percent
of the patients who have the most common form of Hepatitis-C in three to six
months, and can do so with relatively minor side effects compared to other
treatments. It is also a very lucrative drug: it has already accounted for $5.7 billion
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 2 of 25
in sales in the first half of 2014 alone, which is about half of all of Gilead's revenue.
An image of a 400 mg Sovaldi tablet and bottle they are contained in is below.
In the United States, the cost of a standard 12-week regimen of Sovaldi
treatment costs approximately $84,000, or $1,000 per pill. This is in sharp contrast
to the prices at which Sovaldi is being made available by Gilead in other countries.
For example, it is estimated that the cost of a Sovaldi treatment in Egypt is only
$900, or about 99% below the U.S. price. Additionally, Gilead has recently
announced that it had reached new licensing agreements with seven generic drug
companies to manufacture and sell generic sofosbuvir – the active ingredient in
Sovaldi – in 91 developing countries at deeply discounted prices. Certain large
federal agencies, such as the Bureau of Prisons, have also received significant
discounts on Sovaldi. This obvious paradox is being investigated by the Senate
Finance Committee, which has questioned whether the market for Sovaldi "is
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 3 of 25
working efficiently and rationally," and whether "payors of health care….can carry
Gilead's price gouging has had at least two detrimental consequences
in this country. It has, obviously, resulted in the consumers and entities that have
purchased Sovaldi paying significant prices for the drug. It has also effectively
priced some consumers and government programs alike out of the Sovaldi market,
thereby preventing needed recipients from obtaining this critical drug. Notably,
there have been reports that this pricing scheme has had a disproportionately high
impact on minorities and those in lower income brackets (demographics that have
had historically higher incidents of Hepatitis-C infections). The average annual
income of Hepatitis-C patients is $23,000, which suggests that many of these
patients likely receive their health care coverage from government programs. But
even state Medicaid programs have been limiting their approval of Sovaldi for only
the sickest of patients – an approach which Gilead itself discourages.
As discussed below, however, Gilead's monopoly on Sovaldi is
questionable, as (a) its patents are currently being challenged in infringement
actions, and (b) the sale of Sovaldi infringes on the patents of others.
Even if it is ultimately determined that Gilead actually has a legal
monopoly, Gilead is not authorized by the patent laws (or otherwise) to abuse its
purported monopoly on Sovaldi by charging discriminatory prices that apparently
have no rational basis other than to inflate the company's bottom line. The
biotechnology company that initially conducted the research and development which
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 4 of 25
ultimately led to the creation of Sovaldi (and which was then acquired by Gilead)
had estimated that a regimen of the drug would be sold for $36,000 per treatment in
the United Sates. Gilead is now charging nearly two-and-a-half times that amount.
And unlike other specialty drugs that come with comparable hefty price tags – but
only affect a small number of patients – there are several million Americans living
with Hepatitis-C that could benefit from this drug. If Gilead's conduct is left
unchecked, many of these patients will never get access to this drug and, in those
cases where they do, third party payors like Plaintiff will continue to pay exorbitant
prices for Sovaldi.
Plaintiff, on behalf of itself and those similarly situated, brings this
action to stop this unconscionable and unfair conduct, and to secure appropriate
recoveries for consumers and third party payors who, like Plaintiff, have been
victimized by Gilead's price gouging scheme. Plaintiff seeks appropriate relief for
unjust enrichment, for violations of the federal antitrust laws and section 1557(a) of
the Patient Protection and Affordable Care Act, and for breach of the duty of good
faith and fair dealing as an intended third-party beneficiary.
JURISDICTION AND VENUE
This Court has subject matter jurisdiction over this action pursuant to
28 U.S.C. § 1332 because the aggregate amount in controversy exceeds $5,000,000,
exclusive of interest and cost, and because members of the class are citizens of
states other than Defendant. The Court also has federal question subject matter
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 5 of 25
jurisdiction based on the Sherman Act and Affordable Care Act claims asserted
Personal jurisdiction and venue are proper because Defendant
regularly transacts business within this District.
Plaintiff, the Southeastern Pennsylvania Transportation Authority
("SEPTA" or "Plaintiff") is a regional transportation authority that operates various
forms of public transit, serving Bucks, Chester, Delaware, Montgomery, and
Philadelphia counties in Pennsylvania. SEPTA is headquartered at 1234 Market
Street, Philadelphia, Pennsylvania. SEPTA maintains an employee health and
welfare benefit plan pursuant to which it reimburses and pays for certain of its
employees' prescription drug purchases. Plaintiff has paid in excess of $2.4 million
for Sovaldi before the end of 2014 for its members, and has been injured as a result
of Defendant's conduct described herein.
Defendant Gilead is a corporation organized under the laws of
Delaware, having a principal place of business located at 222 Lakeside Drive in
Foster City, California. It has issued securities that are publicly traded on the
NASDAQ exchange under the symbol "GILD." According to its most recent form
10K filed with the Securities and Exchange Commission ("SEC"), the company
recognized over $11.2 billion in revenue for the year ended December 31, 2013, and
over $3 billion in profits.
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 6 of 25
FACTUAL ALLEGATIONS
Gilead and its Limited Sovaldi Patent Rights.
It has been estimated that there are between 2.7 and 5.2 million people
in the United States infected with Hepatitis-C. The virus is transmitted by coming
into contact with infected blood, such as shared needles, a blood transfusion, or
sexual contact. Left untreated, it can lead to cirrhosis or liver cancer, and may
require a liver transplant. Such conditions typically manifest many years after the
initial infection occurs. Since 2007, there have been more deaths in the United
States from Hepatitis-C than from HIV. Prior to Sovaldi, the available treatments
cured only about half of Hepatitis-C patients, and often had debilitating side effects.
Sovaldi is a Hepatitis-C tablet that was originally developed by a
company called Pharmasset, Inc. ("Pharmasset"). In November 2011, Pharmasset
was acquired by Gilead. According to a press release issued by Gilead at the time, it
acquired Pharmasset for $137 per share in cash, putting the value of the transaction
at approximately $11 billion. Pursuant to the merger, Gilead acquired Pharmasset's
assets, including its patent portfolio associated with the development of Sovaldi.
A New Drug Application for Sovaldi was filed with the FDA on April 8,
2013. As noted above, the active ingredient in Sovaldi tablets is
sofosbuvir. Sofosbuvir is a nucleotide analog that acts to inhibit the replication of
the Hepatitis-C virus.
On October 25, 2013, an FDA committee voted to support approval of
Sovaldi for treatment of Hepatitis-C genotypes 1 and 4 in combination with
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 7 of 25
pegylated interferon and ribavirin, and for treatment of Hepatitis-C genotypes 2 and
3 in combination with ribavirin.1
Leading up to the anticipated approval for the commercial sale of
Sovaldi, Kevin Young and Executive Vice President at Gilead, said on an October
29, 2013 investors conference call that "we feel that our commercial launch plans are
where they should be to bring sofosbuvir responsibly to specialists and their patients
upon regulatory approval."
The FDA approved the sale of Sovaldi on or around December 6, 2013.
The Orange Book currently lists five patents associated with Sovaldi, the last of
which expires December 11, 2030. The Orange Book is a database maintained by the
FDA that identifies, inter alia, patents that may be applicable to prescription drugs.
According to the Orange Book, the "Exclusivity Expiration" date for Sovaldi is
December 6, 2018.
Sovaldi is not the only Hepatitis C drug sold by Gilead at excessive
prices. On or around October 10, 2014, it received FDA approval to sell an even more
expensive drug, Harvoni. Unlike Sovaldi, which must be taken in conjunction with
another drug, Harvoni is a complete, one-a-day pill that can be taken alone. It
reportedly costs in excess of $94,000 for a 12 week regimen. Unlike Sovaldi, Harvoni
has only been approved for the main subtype of hepatitis, genotype 1. According to
the Orange Book, there are ten patents associated with Harvoni, the last of which
expires on December 11, 2030.
1 Hepatitis-C is comprised of six different genotypes. Genotype 1 is the most predominant genotype in the United States, followed by genotype 2 and 3.
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 8 of 25
The Sovaldi Price Disparity.
On September 15, 2014, Gilead issued a press release revealing license
agreements that it reached with seven generic drug manufacturers providing for the
manufacture of the generic form of Sovaldi. These arrangements reportedly provide
for the generic drug manufacturers to receive a "complete technology transfer" of the
Gilead manufacturing process of Sovaldi so that cheaper, generic versions of the
drug can be sold in 91 developing countries. The licensees can set their own prices
for the drug and will pay a 7% royalty back to Gilead.
While rolling out its self-congratulatory marketing campaign about
how the company is making this lifesaving drug available in third world countries,
Gilead has been simultaneously gouging its U.S.-based consumers and third party
payors of the drug. As noted above, Sovaldi can cost $1,000 per pill, making the total
cost of a standard treatment approximately $84,000.
A handful of large purchasers of Sovaldi in the U.S. have been able to
successfully negotiate discounts on the drug. For example, it has been reported that
the Federal Bureau of Prisons (which houses about 9% of the nation's inmates)
receives a 44% discount on Sovaldi. The U.S. Department of Veterans Affairs
reportedly receives a similar discount. However, state prison systems (which house
approximately 58% of U.S. inmates) generally do not have access to these discounts.
Similarly, the federal government is prohibited from negotiating lower prices for
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 9 of 25
State Medicaid programs have been similarly affected. For example, it
has been reported that the Illinois Medicaid program has indicated that Hepatitis-C
patients would need to meet "25 criteria" to qualify for Sovaldi. Other states, such as
Pennsylvania, are limiting their approval for purchases of Sovaldi to only the sickest
patients. For its part, however, Gilead has reportedly encouraged the early use of
Sovaldi because doing so "'yields better health and economic outcomes compared with
later initiation,' by reducing such complications as cancer and the ‘downstream costs
associated with advancing [liver] disease.'"
Gilead's pricing makes early use economically impossible. It was
reported in March 2014 that two Medicaid patients in Pennsylvania had applied for
Sovaldi in the previous month, and were not approved by the state's Office of
Medical Assistance Program. Other states are apparently reviewing applications for
Medicaid coverage of the drug on a case-by-case basis.
On October 28, 2014, the National Association of Medicaid Directors
("NAMD") sent a letter to eight members of Congress discussing the unique
challenges that the state Medicaid programs were facing related to Sovaldi. The
NAMD is a bipartisan, non-profit organization that represents the Medicaid
Directors in fifty states. The NAMD letter notes that "most people living with a
chronic hepatitis C infection are unaware of their infection status." It is anticipated
that the number of Americans with confirmed Hepatitis C infections will increase as
additional testing is conducted, per recommendations by the Centers for Disease
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 10 of 25
Control and Prevention ("CDC"). As indicated in the letter, this will only add to the
significant financial strain already placed on the Medicaid system:
The challenge Sovaldi and other new hepatitis C medications pose for the
Medicaid program is the intersection of a high-cost therapy and a potentially
large population eligible for the therapy. To date, several states have reported that their first quarter 2014 prescription drug expenditures for hepatitis C
treatments has doubled or tripled compared to their entire 2013 spending,…
The Senate Finance Committee Investigation.
The Senate's Committee on Finance has recently commenced an
investigation into Gilead's pricing practices. A July 11, 2014 letter to Gilead's CEO
from Senators Chuck Grassley (R-IA) and Ron Wyden (D-OR) notes that Sovaldi's
"pricing has raised serious questions about the extent to which the market for this
drug is working efficiently and rationally." The letter notes that the cost of a Sovaldi
treatment regimen in Egypt (the country with the highest Hepatitis-C presence in
the world) is around $900, which is 99% less than what it costs in the U.S. The
letter cites statistics about how the government could very well end up spending
several billion dollars on this drug through Medicare, Medicaid and other federal
Significantly, the Senators' letter observes that the high price charged
for Sovaldi "appears to be higher than expected given the costs of development, and
production and the steep discounts in other countries." The Senate Finance
Committee has requested that Gilead produce numerous categories of documents,
largely concerning the Pharmasset 2011 merger,2 and the price at which
2 Pharmasset had said in its SEC filings before the merger that it expected to sell
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 11 of 25
Pharmasset had anticipated selling Sovaldi. A spokesperson for Gilead said at the
time that the company received the Senators' letter and "will cooperate with their
request." The company's most recent Form 10-Q filed with the SEC on November 5,
2014 states that it is "cooperating with the inquiries."
Profits from Sovaldi Sales.
Gilead's price gouging has been a remarkable financial success for the
company and its shareholders. Gilead's $2.3 billion in worldwide Sovaldi sales in the
first quarter of 2014 apparently set a record for the sale of a drug during its first full
quarter on the market. Approximately $2.1 billion of these sales were in the United
States. Second quarter 2014 sales of Sovaldi climbed to $3.4 billion and third
quarter sales totaled $2.8 billion, bringing the nine month total to an astounding
Gilead's exorbitant pricing practices for Sovaldi have placed Hepatitis-
C patients – and the third party payors like Plaintiff who shoulder most of these
prescription drug costs – in an impossible position: either (a) expend substantial
amounts for Sovaldi, thereby depleting health and welfare funds and negatively
impacting their continued viability, or (b) fail to provide or limit coverage for
Sovaldi, effectively denying plan beneficiaries access to this highly effective drug.
Indeed, some insurance companies do not even have a choice, as they are often
required to provide coverage for drugs like Sovaldi that are highly effective and have
no alternative equivalents.
the drug in the United States for $36,000. The Senators' letter asks for information about this figure.
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 12 of 25
Gilead has attempted to justify these exorbitant prices by predicting
that Sovaldi has the potential to save the health care system money by avoiding
other costly Hepitatis-C treatments. John F. Milligan, the Chief Operating Officer of
Gilead, said on an analysts' conference call in April 2014 that "the value of a cure, I
tend to think, is underestimated in terms of the overall advantage that the health
care system receives from it…" In another call in July 2014, Mr. Milligan said that
the price is "an outlier because we are curing people of a horrible disease in a very
rapid time frame." Mr. Milligan stated on this July call that over 70,000 people in
the United States have been treated with Sovaldi-containing regimens.
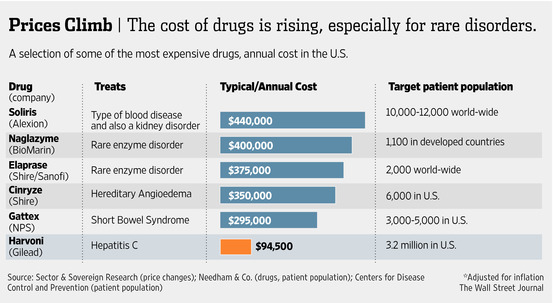
To be sure, Sovaldi is not the only extremely expensive prescription
drug on the market. However, these other drugs, referred to as orphan drugs, are
designed to treat rare diseases in a relatively small population of patients. A Wall
Street Journal article dated October 10, 2014 contained the diagram on the
following page which illustrates this point:
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 13 of 25
As noted above and depicted in the chart, there are several million
people in the United States who are infected with Hepatitis-C. Indeed, during an
October 28, 2014 conference call with investors, Patrick O'Brien (VP of Investor
Relations at Gilead) said that "approximately 100,000 patients have been treated
with Sovaldi in the United States….This represents a fraction of the estimated 185
million people in the world suffering from [Hepatitis-C], who have the potential to
benefit from sofosbuvir-based regimens."
As the president of a major group of insurers and other third party
payors has been quoted as saying, multiplying Sovaldi's "price by three million
people…can torpedo the whole health insurance system." Express Scripts, the
largest pharmacy benefit manager in the United States, has estimated that states
would have to pay $55 billion to treat all of their Medicaid patients and prison
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 14 of 25
inmates, calling the drug "a tax on all Americans." Such exorbitant pricing cannot be
rationally justified by any research and development costs associated with
developing Sovaldi, nor by predictions of future healthcare cost-avoidance.
Gilead's Sovaldi Patents May Not Even Be Valid.
While Gilead's price gouging conduct described herein cannot be
justified by the patent laws, it is noteworthy that the validity and applicability of
some of the Sovaldi patents are currently being challenged. Indeed, Gilead's most
recent Form 10-Q acknowledges that while it owns patents "that claim sofosbuvir as
a chemical entity and its metabolites," they do "not necessarily guarantee our right
to practice the patented technology or commercialize the patented product." Several
of the ongoing cases related to Gilead's purported patents concerning Sovaldi are
summarized below.
Gilead Sciences, Inc. v. Merck & Co, Inc. et al, No. 5:13-cv-04057-BLF (N.D. Cal.)
On August 30, 2013, Gilead filed a declaratory judgment action in the
United States District Court for the Northern District of California against Merck
and related entities, seeking a declaratory judgment that its sale of Sovaldi would
not infringe upon two patents to which the defendants were assigned – U.S. Patent
7,105,499 and 8,481,712. These two patents are entitled "Nucleoside Derivatives as
Inhibitors of RNA-Dependent RNA Viral Polymerase." According to Gilead, those
two patents cover compounds which do not include, but which may relate to,
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 15 of 25
Gilead's action was filed shortly after Merck's director of corporate
licensing had sent a letter to Gilead offering it a license related its two patents.
Merck has responded to Gilead's lawsuit by filing an answer and counter-claims
seeking, among other things, a declaration that Gilead's commercial sale of Sovaldi
would infringe on these two patents. Gilead has filed an answer to those counter-
claims. The parties are conducting discovery in that case, which is scheduled to be
concluded by October 15, 2015. Trial is scheduled to begin on March 7, 2016.
In describing this action in its SEC filings, Gilead has stated that "[i]f
the court determines that Merck's patents are valid and that we have infringed
those claims, we may be required to obtain a license from and pay royalties to Merck
to commercialize sofosbuvir."
Idenix Pharmaceuticals, Inc., et al. v. Gilead Sciences, Inc., et al., No. 1:13-cv-01987-LPS (D. Del.)
On December 1, 2013, a biopharmaceutical company that focuses on
developing drugs to treat viral disease, Idenix Pharmaceuticals, Inc. and others filed
a patent infringement action in the United States District Court for the District of
Delaware related to Gilead's then-pending FDA approval to sell sofosbuvir. The
plaintiffs in that lawsuit are alleging that (a) Gilead's sale of sofosbuvir will infringe
one of their patents (U.S. Patent No. 7,608,600), and (b) one of Gilead's patents that
purportedly covers sofosbuvir – U.S. Patent No. 8,415,322 – is invalid because those
plaintiffs have an earlier-filed patent with priority.
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 16 of 25
Gilead filed an answer with counter-claims on February 6, 2014. This
case is also ongoing. The court has reserved trial dates between October and
December of 2016.
Idenix Pharmaceuticals, Inc., et al. v. Gilead Sciences, Inc., et al.,
No. 1:13-cv-13052 (D. Mass.)
Also on December 1, 2013, Idenix and others filed a second, related
declaratory judgment action against Gilead in the United States District Court for
the District of Massachusetts.3 In the Massachusetts case, Idenix alleged that
Gilead infringed on two more of its patents: U.S. Patent Nos. 6,914,054 and
7,608,597. These two patents are titled "Methods and Compositions for Treating
Hepatitis C Virus," and relate to 2' – methyl nucleosides that hinder the replication
of Hepatitis C in the human body.
On June 30, 2014, U.S. District Court Judge Denise J. Casper issued a
Memorandum and Order that granted a motion filed by Gilead to have this case
transferred to the District of Delaware. Judge Casper noted that Idenix's case in
Massachusetts was filed approximately 30 minutes after its action was commenced
in Delaware. She also found that the two cases concern the same patented product
(sofosbuvir) and involve the pharmaceutical products designed to treat Hepatitis C
and relate to 2' –methyl nucleosides that hinder its replication in the human body.
Accordingly, the Massachusetts action was transferred to Delaware, where it was
related to the District of Delaware action.
3 Idenix has explained that it simultaneously filed these two cases in different courts
because they involve patents "from different patent families, with different priority dates, different specifications, and different claim terms,…"
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 17 of 25
Gilead has stated in its SEC filings that it believes "Idenix's patents
are invalid and would not be infringed by our commercialization of sofosbuvir and
that we have the sole right to commercialize sofosbuvir. However, if the court
disagrees with our view and determines that these patents are infringed, we may be
required to obtain a license from and pay royalties to Idenix to commercialize
sofosbuvir." It also noted that Merck acquired Idenix on June 9, 2014, which Gilead
noted may be significant because "Merck has greater resources than Idenix and may
therefore choose to fund the litigation at higher levels than Idenix."
CLASS ACTION ALLEGATIONS
Plaintiff brings this action individually, and on behalf of a class,
pursuant to FED. R. CIV. P. 23(a), 23(b)(2), and/or 23(b)(3). Specifically, Plaintiff
seeks to represent the following class:
All persons or entities in the United States and its territories who were
harmed as a result of the excessive pricing of Sovaldi by (a) purchasing or paying for some or all of the purchase price for Sovaldi, for
consumption by themselves, their families, or their members, employees, insureds, participants, or beneficiaries, other than for resale, or (b) being
prevented from obtaining a needed Sovaldi regimen.4
Numerosity: The Class is so numerous that joinder of all members is
impracticable. While the exact number and identities of individual Class Members
are unknown at this time, such information being in the sole possession of
Defendant and obtainable by Plaintiff only through the discovery process, Plaintiff
believes that there are thousands of class members. As noted above, Gilead has
estimated that there have been over 70,000 treatments of Sovaldi in the United
4 Plaintiffs reserve the right to modify this class definition.
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 18 of 25
States, and there are millions more Americans living with Hepatitis-C who have
sought or may in the future seek to purchase Sovaldi.
Existence and Predominance of Common Questions of Fact and Law:
Common questions or law and fact exist as to all Class Members. These questions
predominate over the questions affecting individual Class Members. These common
legal and factual questions include, but are not limited to:
o Whether Gilead has engaged in price gouging.
o Whether Gilead has violated the statutes asserted herein.
o Whether Gilead has been unjustly enriched (and if so, in what amount).
o Whether Gilead's pricing practices can be justified or defended through its
patents or otherwise.
o Whether Gilead should be ordered to cease this unconscionable conduct,
or otherwise modify it.
o The extent and measurement of classwide damages, and nature of other
appropriate relief.
Typicality: The claims of Plaintiff are typical of the claims of the Class
in that Plaintiff, like all Class Members, paid exorbitant prices for Sovaldi.
Furthermore, the facts related to Gilead's misconduct are common to all Class
Members and represent a common thread resulting in injury to all Class Members.
Adequacy: Plaintiff will fairly and adequately protect the interests of
the Class Members. Plaintiff has retained attorneys experienced in the prosecution
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 19 of 25
of class actions, including healthcare, antitrust and consumer protection matters,
and Plaintiff and its counsel intend to prosecute this action vigorously.
Superiority: Plaintiff and the Class Members have all suffered and will
continue to suffer harm and damages as a result of Defendant's unlawful and
wrongful conduct. A class action is superior to other available methods for the fair
and efficient adjudication of the controversy. Absent a class action, most Class
Members would likely find the cost of litigating their claims prohibitively high and
would therefore have no effective remedy at law. Because of the relatively small size
of the individual Class Members' claims, it is likely that only a few Class Members
could afford to seek legal redress for Defendant's misconduct. Absent a class action,
Class Members will continue to incur damages, and Defendant's misconduct will
continue without remedy. Class treatment of common questions of law and fact
would also be a superior method to multiple individual actions or piecemeal
litigation in that class treatment will conserve the resources of the courts and the
litigants, and will promote consistency and efficiency of adjudication.
Gilead has acted or refused to act on grounds that apply generally to
the class, so that final injunctive relief or corresponding declaratory relief is
appropriate respecting the class as a whole.
VIOLATIONS ALLEGED
Unjust Enrichment
Plaintiff repeats and incorporates by reference each preceding and
succeeding paragraph as though fully set forth herein.
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 20 of 25
Plaintiff and those similarly situated conferred a benefit upon Gilead
by purchasing the Sovaldi for its members. Gilead realized and appreciated this
Gilead's price gouging was not done in good faith and, under the
circumstances, a reasonable factfinder could conclude that it would be unjust for it
to retain the benefit of those excessive charges. Among other things, the prices
charged by Gilead for Sovaldi were unreasonable, excessive, arbitrary,
discriminatory, inflated, exorbitant and inflated.
As a result of Gilead's price gouging and related unfair conduct, it has
been unjustly enriched at the expense of Plaintiff and the Class, in amounts to be
determined at trial. Under the circumstances, Gilead's acceptance and retention of
these exorbitant charges would be inequitable and unjust.
Plaintiffs do not have an adequate alternative remedy available at law.
Gilead's pricing scheme has no end in sight and – if left unchecked – has the
potential to literally bankrupt segments of the U.S. healthcare system, given the
large number of Americans infected with Hepatitis-C.
For Declaratory and Injunctive Relief Under Section 16 of
the Clayton Act for Defendant's Violations
of Section 2 of the Sherman Act, 15 U.S.C. 2
Plaintiff repeats and incorporates by reference each preceding and
succeeding paragraph as though fully set forth herein.
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 21 of 25
For purposes of this claim, the relevant product market is defined as
the sofosbuvir indications approved by the FDA. The relevant geographic market is
the United States. At all relevant times, Gilead possessed substantial market power
(i.e., monopoly power) in these relevant markets.
Gilead has engaged in the willful acquisition and maintenance of
monopoly power in the market for Sovaldi through its conduct, and not through the
growth or development as a consequence of a superior product, business acumen, or
historic accident.
Gilead has engaged in predatory, exclusionary and/or unfair conduct
with the specific intent to monopolize the market for Sovaldi.
As a direct and proximate result of Gilead's unlawful restraint of
trade and unfair trade practices, Plaintiff and members of the Class were harmed as
described herein.
Plaintiff, pursuant to FED. R. CIV. P. 57 and 28 U.S.C. § 2201(a)
hereby seeks a declaratory judgment that Gilead's conduct as described herein
violates Section 2 of the Sherman Act.
Plaintiff and the Class further seeks equitable and injunctive relief
pursuant to Section 16 of the Clayton Act, 15 U.S.C. § 26, and other applicable
law, to correct for the anticompetitive market effects caused by the unlawful and
unfair conduct of Gilead, and other relief so as to assure that similar anticompetitive
conduct does not occur in the future.
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 22 of 25
Discrimination in violation of section 1557(a) of the Patient
Protection and Affordable Care Act
Plaintiff hereby repeats and incorporates by reference each preceding
and succeeding paragraph as though fully set forth herein.
Gilead meets the qualifications for being a "health program or activity,
any part of which is receiving Federal financial assistance" under section 1557 of the
Affordable Care Act. See 42 U.S.C. § 18116. Upon information and belief, the
federal government provided grants and/or other financial assistance that
contributed to the development of Sovaldi.
By definition, consumers and potential consumers of Sovaldi all have
Hepatitis C, a debilitating disease. According to Stedman's Medical Dictionary,
Hepatitis C is a viral infection that causes a progressive inflammation of the liver –
a major and essential organ. The World Health Organization has described
Hepatitis as "one of the most prevalent and serious infectious conditions in the
world." The CDC has stated that Hepatitis C "is the most common chronic
bloodborne infection in the United States." Hepatitis C reportedly accounts for a
large percentage of cirrhosis, liver failure and liver cancer cases in the United
Given the severity of this chronic condition, Hepatitis C interferes (and
inevitably will interfere) with at least one or more of the following major life
activities: caring for oneself, performing manual tasks, reproducing/procreating,
engaging in sexual relations, and working. Accordingly, persons infected with
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 23 of 25
Hepatitis C have a "disability" within the meaning of section 504 of the
Rehabilitation Act of 1973. See 29 U.S.C. § 794.
Defendant has violated and continues to violate section 1557(a) of the
Affordable Care Act by intentionally causing Plaintiffs to "be excluded from
participation in, be denied the benefits of, or be subjected to discrimination under,
any health program or activity, any part of which is receiving Federal financial
assistance" based on their disability, which is a prohibited ground of
discrimination under section 504 of the Rehabilitation Act.
Plaintiffs have been aggrieved and damaged by this violation of
section 1557 of the Affordable Care Act.
Breach of the Duty of Good Faith and Fair Dealing
As an Intended Third-Party Beneficiary
Plaintiff hereby repeats and incorporates by reference each preceding
and succeeding paragraph as though fully set forth herein.
As a drug manufacturer, Gilead primarily sold Sovaldi directly to
wholesalers and distributors. Upon information and belief, Sovaldi patients and
third-party payors for it were intended third-party beneficiaries of those contracts.
Every contract contains a duty of good faith and fair dealing.
Gilead failed to carry out its contracts with these direct purchasers of
Sovaldi in good faith. Gilead abused any discretion it may have possessed related
to those agreements by charging grossly excessive prices for Sovaldi. Gilead has
Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 24 of 25
arbitrarily, unreasonably, and/or capriciously breached its duty of good faith and
fair dealing by demanding the excessive prices it charged for Sovaldi.
Gilead has been able to leverage its market power and purported
patent rights to successfully coerce these wholesalers to extract excessive
purchase prices from third party-payors and patients alike. Both the wholesalers
and end-consumers (as well as third-party payors) have no other alternative
means to obtain Sovaldi, or any other alternative to treat and cure Hepatitis C.
Because of this, Gilead (and the wholesalers) know that consumers must and will
pay whatever exorbitant price is charged by Gilead, and passed on by the
Alternatively, the wholesalers have acted as de facto agents of
Gilead, serving no realistic market function except to serve as a phantom middle
men in the distribution scheme.
Gilead's conduct breached of its duty of good faith and fair dealing,
and directly and proximately caused Plaintiff and other intended third party
beneficiaries to be injured.
PRAYER FOR RELIEF
WHEREFORE, Plaintiff respectfully requests that the Court:
Issue an order certifying the Class defined above, appointing the
Plaintiff as Class representative, and designating the undersigned firm
as Class Counsel;
Find that Gilead has committed the violations of law alleged herein;

Case 2:14-cv-06978 Document 1 Filed 12/09/14 Page 25 of 25
Source: http://sitegrabber.cloudapp.net/proxy?remoteUrl=http%3A%2F%2Fkeionline.org%2Fsites%2Fdefault%2Ffiles%2Fsovaldi-lawsuit.pdf
CG100649, a Novel Dual-Acting COX-2 and Carbonic Anhydrase Inhibitor: Ascending Single CrystalGenomics, Inc. 6F, 2nd Building of Asan Institute for Life Sciences 388-1, Pungnap-2dong, Songpa-gu Dose and Multi-Dose Pharmacokinetics and Safety Evaluation in Healthy Male Subjects Asan Medical Center, Seoul, 138-736, Korea
Istruzioni per luso Posizionamento e collegamentoReversibilità apertura porte Italiano, 1 Descrizione dellapparecchio, 3Vista d'insieme Accessori, 4 Avvio e utilizzo, 5-6Avviare l'apparecchio Sistema di raffreddamentoUtilizzare al meglio il frigorifero Utilizzare al meglio il congelatore Manutenzione e cura, 7Escludere la corrente elettrica




