Isolation of the highly pathogenic and zoonotic agent burkholderia pseudomallei from a pet green iguana in prague, czech republic
Elschner et al. BMC Veterinary Research 2014, 10:283http://www.biomedcentral.com/1746-6148/10/283
Isolation of the highly pathogenic and zoonoticagent Burkholderia pseudomallei from a pet greenIguana in Prague, Czech Republic
Mandy C Elschner1*, Jan Hnizdo2, Ivonne Stamm3, Hosny El-Adawy1, Katja Mertens1 and Falk Melzer1
Background: Melioidosis caused by Burkholderia (B.) pseudomallei is an endemic zoonotic disease mainly reportedfrom northern Australia and Southeast Asia. In Europe, cases of human melioidosis have been reported only frompatients travelling to endemic regions. Besides humans, B. pseudomallei has a very broad host range in domesticand wild animals. There are some reports about importation of B. pseudomallei-infected animals from endemicareas into Europe. The present report describes the first case of B. pseudomallei infection of a pet iguana in Europe.
Case presentation: In a 5-year-old pet Iguana iguana living in a private household in Prague, Czech Republic,B. pseudomallei was isolated from pus of an abscess. The isolate VB976100 was identified by Vitek®2, MALDI-TOFmass spectrometry and polymerase chain reaction as B. pseudomallei. The molecular typing resulted in multi-locussequence type 436 hitherto, which has been found only once worldwide in a B. pseudomallei strain isolated in theUSA and originating from Guatemala. The identification as internal transcribed spacer type G indicates a closerelatedness to strains mainly isolated in the Western Hemisphere. These findings support the hypothesis that theiguana became infected in this region or in a breeding facility through contact to other infected animals.
Conclusions: The present case highlights the risk of importation of the highly pathogenic and zoonoticB. pseudomallei into non-endemic regions through animal trade. Therefore, veterinarians treating animals from theseareas and physicians examining patients owning such animals should include melioidosis in differential diagnosiswhenever specific symptoms appear. Furthermore, veterinary authorities responsible for supervision of traders andpet shops should be aware of this risk of zoonotic transmission.
Keywords: Melioidosis, Burkholderia pseudomallei, Zoonoses, Iguana
melioidosis have only reported from patients who had
Burkholderia (B.) pseudomallei is the causative agent of
been travelling to endemic regions The gram-
melioidosis, an endemic disease mainly reported from
negative bacterium belongs to biohazard risk group 3
northern Australia and Southeast Asia. In this area the
agents, is listed as biowarfare agent, and has to be proc-
disease has emerged as an important cause of morbidity
essed under biosafety level 3 conditions. The disease is
and mortality during the last 25 years Endemic and
mainly acquired environmentally by percutaneous infec-
sporadic cases of melioidosis are also reported from
tion, ingestion, or inhalation. The incubation period of
countries of South America, North America, Oceania, as
melioidosis can vary between one day and 62 years, de-
well as Aruba, Guadeloupe, Guam, Haiti, Martinique,
pending on the route of infection and infection dose [.
and Puerto Rico. Increasing numbers of cases are reported
Clinical studies have shown that pneumonia is the pre-
from Africa []. However, in Europe cases of human
dominant clinical manifestation, followed by skin and softtissue infections, as well as acute suppurative parotitis, es-
* Correspondence:
pecially in pediatric cases, and prostatitis in males . One
1Friedrich-Loeffler-Institut, Federal Research Institute for Animal Health,
predominant risk factor is diabetes mellitus, as shown by
Institute of Bacterial Infections and Zoonoses, Naumburger Strasse 96a,
studies in Australia and Thailand, where up to 60% of the
07743 Jena, GermanyFull list of author information is available at the end of the article
melioidosis patients are diabetic, mainly type 2 .
2014 Elschner et al.; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the CreativeCommons Attribution License which permits unrestricted use, distribution, andreproduction in any medium, provided the original work is properly credited. The Creative Commons Public DomainDedication waiver applies to the data made available in this article,unless otherwise stated.

Elschner et al. BMC Veterinary Research 2014, 10:283
Besides humans, B. pseudomallei has a very broad host
Zophobas morio larvae purchased from different breeders.
range. In domestic animals it is most commonly re-
In 2011, the animal developed an abscess underneath the
ported in cattle, goats and swine However, sporadic
left eye, which was transected and treated antibiotically
cases or small outbreaks have been reported in monkeys,
without microbiological investigation. In 2013, the animal
gibbons, orangutans, kangaroos, wallabies, deer, buffaloes,
was admitted to the Animal Clinic, Bila Hora, Prague,
cows, camels, llamas, zebras, koalas, dogs, cats, horses,
Czech Republic, where again an abscess was diagnosed,
mules, parrots, rats, hamsters, rabbits, guinea pigs, ground
this time at the root of the tongue. After surgical interven-
squirrels, seals, dolphins, crocodiles ]. Very recently, B.
tion and antibiotic treatment by marbofloxacine the
pseudomallei infections in two pet iguanas in California
animal recovered. One year later, in 2014 the owners pre-
were reported ]. In animals, acute and chronical form of
sented the animal again to the clinic because of a week-
melioidosis is seen. Common symptoms in animals in-
long anorexia. The clinical investigation resulted again in
clude anorexia, pyrexia, coughing, skin dehydration and
an encapsulated abscess located at the root of the tongue.
This time the abscess was sampled for microbiologicalanalysis. As soon as the first suspicion of a B. pseudomallei
Case presentation
infection was announced, the veterinary authority was in-
The female Iguana iguana was purchased by private
formed, and it mandated the immediate euthanization and
owners as a young animal from a pet shop in Prague in
incineration of the animal. One veterinary assistant was
2009. It was kept alone in a terrarium, but was also allowed
bitten by the iguana when he tried to fix the maxilla for
to move freely outside the cage in the flat (Figure . The
sampling of the abscess by the veterinarian. The skin was
owners had a very close and tender contact to the animal,
injured although he wore gloves. Finally, the wound was
such as hand feeding and kissing. Usually the iguana was
cured and the assistant was prophylactically treated with
fed with special pellet formulation for iguanas, fresh
amoxicillin-clavulanate for 10 days in a clinic specialized
green salad, vegetables and fruits, and once a month by
for infectious diseases. Also the owners, not showing any
Figure 1 The infected animal: 5-year-old female green iguana (Iguana iguana).
Elschner et al. BMC Veterinary Research 2014, 10:283
clinical signs, were referred to a physician for medical
Germany) with very high score values of >2.600 corre-
examination and received the same treatment. Serological
sponding to highly probable species identification.
investigations were not induced in the contact persons,
For confirmation of B. pseudomallei and further char-
because the physicians could not find a laboratory provid-
acterization, the culture was sent to the Federal Research
ing this analysis. Unfortunately, no information about
Institute for Animal Health, Jena, Germany. The isolate
follow up examinations of the contact persons is available.
VB976100 was cultured under biosafety level 3 condi-tions and the typical shape of colonies was seen on
Isolation, identification, and molecular characterisation of
Ashdown-Agar after 72 h (Figure
Molecular identification was performed by real-time
For microbiological diagnosis, the pus from the abscess
PCR detecting B. mallei/pseudomallei-specific sequences
was submitted on a swab with Amies transport medium
of the fliC gene and B. pseudomallei-specific se-
(D&D Laborservice, Henningsdorf, Germany) to the Vet
quences of the orf2 gene, which belongs to the type III
Med Labor GmbH, Ludwigsburg, Germany. The sample
secretion system .
was cultured on MacConkey agar (BioMerieux, Nürtin-
Multi-locus sequence typing (MLST), the predominant
gen, Germany), tryptic soy agar with 5% sheep blood,
method for molecular subtyping of B. pseudomallei was
chocolate agar (both BD, Heidelberg, Germany) and in-
performed by comparison of 7 housekeeping genes
cubated at 36°C under atmosphere with 5% CO2 for
The isolate was typed as sequence type (ST) 436 by the
24 hours, and afterwards for 24 hours in ambient atmos-
B. pseudomallei database of MLST.Net and data
phere. Additionally, brain heart infusion enrichment
have been deposited there. This ST has been only
broth was inoculated and sub-cultured for 24 hours on
isolated in California from a patient originating from
the same agar media as above. Heavy growth of metallic
Guatemala in 2012 ]. However, for an isolate of un-
gleaming navel forming colonies was visible in pure
known origin, MLST alone is not adequate for determin-
culture after 48-h incubation on all culture media. The
ation of the geographic origin Therefore, we
isolate VB976100 could also be grown at 42°C, but not
performed the 16S-32S internal transcribed spacer (ITS)
at 4°C. It showed a positive Oxidase reaction (VWR,
typing For identification of the ITS type, the
Darmstadt, Germany) and was tested negative for cata-
obtained sequence data were aligned to known ITS
lase (Sigma-Aldrich Chemie GmbH, Munich, Germany).
types A, B, C, D, E, and G, available data at GenBank
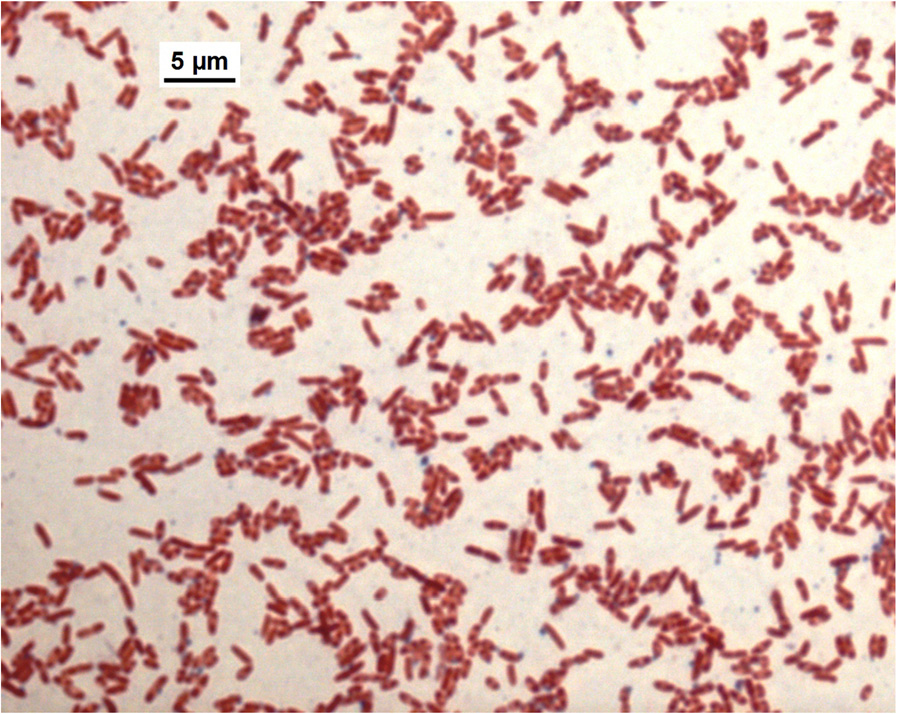
Short gram-negative rods with bipolar "safety pin" ap-
(FJ981703-F981726) using the Software Geneious 6.1.8.
pearance were visible after Gram staining (Figure
(Biomatters Development Team). Our B. pseudomallei
Identification with Vitek®2 (BioMerieux) resulted in B.
isolate VB976100 was typed as ITS type G (613 base
pseudomallei with 95% probability, corresponding to a
pairs), and the ITS sequence was annotated and submitted
very good validity of identification. The same identification
to GenBank under the accession number KP069478.
result was obtained with MALDI-TOF mass spectrometry(Biotyper Microflex LT, Bruker Daltonics GmbH, Bremen,
ConclusionsImport of B. pseudomallei infected animals from en-demic areas has been previously reported in sheep, goatsand pigs from Aruba In the 1970s a B. pseudomal-lei outbreak was reported in the zoo of Paris, and the in-fection was spread to other zoos and equestrian clubs.
In the course of this outbreak, termed "l'affaire du jardindes plantes", two individuals died. Imported horses fromIran or an imported panda from China were assumed assources of infection
B. pseudomallei infections in green iguanas were
already detected in California, USA, in 2007 and 2012.
The bacteria were isolated from the abscess, as well asfrom the animal housing In both cases the isolateswere typed as ST 518 and ITS type G. The authorssuggested that the animals had been infected in CentralAmerica, where most imported iguanas in the USA origin-ate from. Furthermore, these cases already highlighted the
Figure 2 Gram-stain of B pseudomallei isolate VB976100, phase
possible risk of human infections because the infection in
contrast microscopy with a 100 x oil immersion objective
the iguanas was not curable in spite of refractory antibiotic
(Microscope Leica DM4000B).
treatment. In one of these cases after the incubation
Elschner et al. BMC Veterinary Research 2014, 10:283
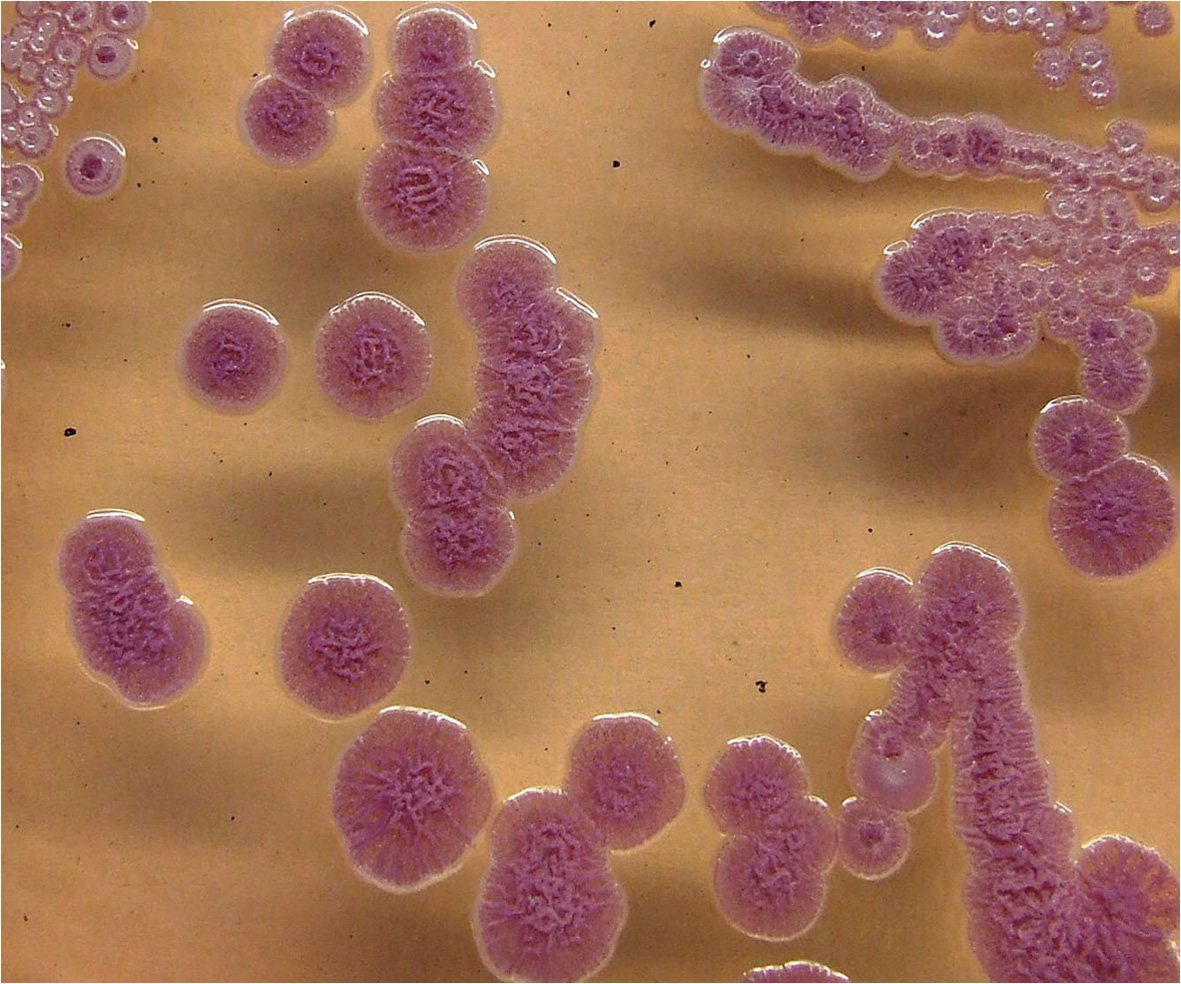
Figure 3 Colonies of B. pseudomallei isolate VB976100 on Ashdown agar showing the typical pink crinkled colonies after 72 h incubation.
period of around 1.5 years an abscess was diagnosed. In
physician could misdiagnose or overlook the suspicion
the clinical course of the present reported B. pseudomallei
of melioidosis. The utility of a post exposure prophylaxis
infection, the animal developed abscesses at the age of 2, 4
in contact persons should be discussed with the physi-
and 5 years. However, there was no known history of any
cians. Recommendations for prophylaxis and treatment
kind of trauma, which potentially could have triggered the
are available from Bossi et al.
development of abscesses []. Unfortunately, only in
Personal safety can be improved by wearing eye pro-
2014 bacteriological examination was initiated. It is specu-
tection and suitable gloves during sampling and treat-
lative but possible that after an incubation period of 2 years
ment of possibly affected animals. For the serological
the first abscess was caused by a B. pseudomallei infection.
investigations in non-endemic areas IgG ELISA could be
These finding strongly supports the presumption, that in
useful to identify chronically infected persons Since
these animals a long incubation period is seen, bearing a
no standardized commercial tests are available only spe-
risk of long not recognized phase of shedding and here-
cialized laboratories can perform such investigations.
with infection risk for contact persons.
Although we are unable to prove the source of infec-
The iguana was purchased as a young animal in 2009,
tion in the present case, veterinarians involved in treat-
and unfortunately, it was not possible to determine the
ment of animals and competent veterinary authorities
origin of this animal. However, the ST 436 was hitherto
responsible for supervision of traders should be aware of
described for one B. pseudomallei strain, isolated in
this potential risk.
USA, originating from Guatemala On the otherhand, the ITS type G of B. pseudomallei is a less com-
AbbreviationsB: Burkholderia; MLST: Multi-locus sequence typing; ITS: Internal transcribed
mon type in endemic regions, such as Australia and
spacer typing; ST: Sequence type; PCR: Polymerase chain reaction.
Southeast Asia. This type seems to be associated withisolates found only in sporadic melioidosis regions like
Competing interests
Africa and South America These findings sup-
The authors declare that they have no competing interests.
port the hypothesis that the iguana became infected in
Authors' contributions
this region or in a breeding facility through contact to
MCE designed the study, coordinated the investigations, substantially
other infected animals originating from this area.
contributed to analysis and evaluation of ITS sequence data, and wrote themanuscript. JH performed the clinical examination of the iguana, collected
Our report emphasizes the risk of importation of B.
the samples and substantially contributed to the manuscript by drafting the
pseudomallei into non-endemic regions through animal
clinical and epidemiological section. IS performed isolation and bacteriological
trade. It is possible that for patients presenting charac-
identification of the B.pseudomallei isolate and substantially contributed to themanuscript by drafting the bacteriological methodological section. HEA, KM
teristic symptoms of melioidosis but with absolutely no
performed the MLST and ITS, and contributed substantially to evaluation and
history of travelling to endemic regions, the attending
analysis of the sequence data. FM, KM, HEA have been substantially involved in
Elschner et al. BMC Veterinary Research 2014, 10:283
the design of the study and discussion of results. All authors revised the
Sutmoller P, Kraneveld FC, Van Der Schaaf A: Melioidosis (Pseudomalleus)
manuscript critically for technical and scientific content and approved the final
in sheep, goats, and pigs on Aruba (Netherland Antilles). J Am Vet Med Assoc
Dance DA: Melioidosis: the tip of the iceberg? Clin Microbiol Rev 1991,
We are grateful to K. Nitzsche, P. Marten and K. Fischer for excellent technical
White NJ: Melioidosis. Lancet 2003, 361(9370):1715–1722.
Ngauy V, Lemeshev Y, Sadkowski L, Crawford G: Cutaneous melioidosis ina man who was taken as a prisoner of war by the Japanese during
World War II. J Clin Microbiol 2005, 43(2):970–972.
1Friedrich-Loeffler-Institut, Federal Research Institute for Animal Health,
Bossi P, Tegnell A, Baka A, Van Loock F, Hendriks J, Werner A, Maidhof H,
Institute of Bacterial Infections and Zoonoses, Naumburger Strasse 96a,
Gouvras G: Bichat guidelines for the clinical management of glanders
07743 Jena, Germany. 2Animal Clinic, Bílá Hora, Cistovicka 44, 16300 Prague
and melioidosis and bioterrorism-related glanders and melioidosis. Euro
6, Czech Republic. 3Vet Med Labor GmbH, Division of IDEXX Laboratories,
Surveill 2004, 9(12):E17–E18.
Mörikestrasse 28/3, 71636 Ludwigsburg, Germany.
Kekulé AS, Bartling C, Beyer W, Bockemühl J, Diller R, Dobler G, Essbauer S,Finke EJ, Fleischer B, Frangoulidis D, Hagen RM, Henning K, Meyer H,
Received: 20 August 2014 Accepted: 18 November 2014
Neubauer H, Oehme A, Pauli G, Pfeffer M, Rakin A, Schmitz H, SplettstösserWD, Sprague LD, Tomaso H, Wölfel R, Zimmermann P: Mikrobiologisch-infektiologische Qualitätsstandards (MiQ), Hochpathogene Erreger - BiologischeKampfstoffe, vol. 27. München: Elsevier; 2008:84–93.
Limmathurotsakul D, Peacock SJ: Melioidosis: a clinical overview. Br Med Bull
2011, 99:125–139.
Cite this article as: Elschner et al.: Isolation of the highly pathogenic and
Katangwe T, Purcell J, Bar-Zeev N, Denis B, Montgomery J, Alaerts M,
zoonotic agent Burkholderia pseudomallei from a pet green Iguana in
Heyderman RS, Dance DA, Kennedy N, Feasey N, Moxon CA: Human
Prague, Czech Republic. BMC Veterinary Research 2014 10:283.
melioidosis, Malawi, 2011. Emerg Infect Dis 2013, 19(6):981–984.
Aardema H, Luijnenburg EM, Salm EF, Bijlmer HA, Visser CE, Van't Wout JW:Changing epidemiology of melioidosis? a case of acute pulmonarymelioidosis with fatal outcome imported from Brazil. Epidemiol Infect2005, 133(5):871–875.
Cuadros J, Gil H, Miguel JD, Marabe G, Gomez-Herruz TA, Lobo B, Marcos R,Anda P: Case report: melioidosis imported from West Africa to Europe.
Am J Trop Med Hyg 2011, 85(2):282–284.
Cheng AC, Currie BJ: Melioidosis: epidemiology, pathophysiology, andmanagement. Clin Microbiol Rev 2005, 18(2):383–416.
Ouadah A, Zahedi D, Perumal R: Animal melioidosis surveillance in Sabah.
Internet J Vet Med 2006, 2(2).
Sprague LD, Neubauer H: Melioidosis in animals: a review onepizootiology, diagnosis and clinical presentation. J Vet Med B Infect DisVet Public Health 2004, 51(7):305–320.
Zehnder AM, Hawkins MG, Koski MA, Lifland B, Byrne BA, Swanson AA, RoodMP, Gee JE, Elrod MG, Beesley CA, Blaney DD, Ventura J, Hoffmaster AR,Beeler ES: Burkholderia pseudomallei isolates in 2 pet iguanas, California,USA. Emerg Infect Dis 2014, 20(2):304–306.
Galyov EE, Brett PJ, DeShazer D: Molecular insights into Burkholderiapseudomallei and Burkholderia mallei pathogenesis. Annu Rev Microbiol2010, 64:495–517.
Ashdown LR: An improved screening technique for the isolation ofPseudomonas pseudomallei from clinical specimens. Pathology 1979,11:293–297.
Tomaso H, Scholz HC, Al Dahouk S, Pitt TL, Treu TM, Neubauer H:Development of 5' nuclease real-time PCR assays for the rapid identificationof the burkholderia mallei//burkholderia pseudomallei complex. Diagn MolPathol 2004, 13(4):247–253.
Novak RT, Glass MB, Gee JE, Gal D, Mayo MJ, Currie BJ, Wilkins PP:Development and evaluation of a real-time PCR assay targeting the typeIII secretion system of Burkholderia pseudomallei. J Clin Microbiol 2006,44(1):85–90.
Godoy D, Randle G, Simpson AJ, Aanensen DM, Pitt TL, Kinoshita R, SprattBG: Multilocus sequence typing and evolutionary relationships among
Submit your next manuscript to BioMed Central
the causative agents of melioidosis and glanders, Burkholderia
and take full advantage of:
pseudomallei and Burkholderia mallei. J Clin Microbiol 2003,41(5):2068–2079.
• Convenient online submission
Aanensen DM, Spratt BG: The multilocus sequence typing network: mlst.
net. Nucleic Acids Res 2005, 33(Web Server issue):W728–W733.
• Thorough peer review
Gee JE, Allender CJ, Tuanyok A, Elrod MG, Hoffmaster AR: Burkholderia
• No space constraints or color figure charges
pseudomallei type G in Western Hemisphere. Emerg Infect Dis 2014,
• Immediate publication on acceptance
Liguori AP, Warrington SD, Ginther JL, Pearson T, Bowers J, Glass MB, Mayo
• Inclusion in PubMed, CAS, Scopus and Google Scholar
M, Wuthiekanun V, Engelthaler D, Peacock SJ, Currie BJ, Wagner DM, Keim P,
• Research which is freely available for redistribution
Tuanyok A: Diversity of 16S-23S rDNA internal transcribed spacer (ITS)reveals phylogenetic relationships in Burkholderia pseudomallei and itsnear-neighbors. PLoS One 2011, 6(12):e29323.
Submit your manuscript at www.biomedcentral.com/submit
Source: http://www.veterinarni-ordinace-praha.cz/news/soubor_263.pdf
SUMMARY OF PRODUCT CHARACTERISTICS TRADE NAME OF THE MEDICINAL PRODUCT Rhinocort Aqua 32 micrograms/dose nasal spray, suspension Rhinocort Aqua 64 micrograms/dose nasal spray, suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One dose (0.05 ml) contains 32 micrograms or 64 micrograms of budesonide.
Safe harbor This presentation may include forward-looking statements that are based on our management's beliefs andassumptions and on information currently available to our management.The inclusion of forward-looking statements should not be regarded as a representation by Cosmo that any ofits plans will be achieved. Actual results may differ materially from those set forth in this presentation due tothe risks and uncertainties inherent in Cosmo's ability to develop and expand its business, successfullycomplete development of its current product candidates and current and future collaborations for thedevelopment and commercialisation of its product candidates and reduce costs (including staff costs), themarket for drugs to treat IBD diseases, Cosmo's anticipated future revenues, capital expenditures and financialresources and other similar statements, may be "forward-looking" and as such involve risks and uncertaintiesand risks related to the collaboration between Partners and Cosmo, including the potential for delays in thedevelopment programs for Budesonide MMX® and Rifamycin SV MMX®. No assurance can be given that theresults anticipated in such forward looking statements will occur. Actual events or results may differ materiallyfrom Cosmo's expectations due to factors which include, but are not limited to, increased competition, Cosmo'sability to finance expansion plans, the results of Cosmo's research and development activities, the success ofCosmo's products, regulatory, legislative and judicial developments or changes in market and/or overalleconomic conditions. Cosmo assumes no responsibility to update forward-looking statements or to adapt themto future events or developments.You are cautioned not to place undue reliance on these forward-looking statements, which speak only as ofthe date hereof, and Cosmo undertakes no obligation to revise or update this presentation.




