Dnp.ku.dk
The Journal of Neuroscience, October 29, 2014 • 34(44):14769 –14776 •
14769
Acute and Sustained Effects of Methylphenidate on
Cognition and Presynaptic Dopamine Metabolism:
An [18F]FDOPA PET Study
Ina Schabram,1
Karsten Henkel,1
Siamak Mohammadkhani Shali,2
Claudia Dietrich,1
Jo¨rn Schmaljohann,2
Oliver Winz,2
Susanne Prinz,8
Lena Rademacher,1,10
Bernd Neumaier,11
Marc Felzen,5
Yoshitaka Kumakura,4
Paul Cumming,3,9
Felix M. Mottaghy,2,6,7
Gerhard Gru¨nder,1,6
and Ingo Vernaleken1,6
1Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University, 52074 Aachen, Germany, 2Department of Nuclear Medicine,
RWTH Aachen University, 52074 Aachen, Germany, 3Department of Nuclear Medicine, University of Erlangen/Nu¨rnberg, 91054 Erlangen, Germany,
4Department of Nuclear Medicine, Graduate School of Medicine, University of Tokyo, 7-3-1, Hongo, Bunkyo-ku, Tokyo 113-8654 Japan, 5Department of
Anesthesiology, University Hospital of the RWTH Aachen University, 52074 Aachen, Germany, 6Ju¨lich/Aachen Research Alliance, Ju¨lich/Aachen, 52074
Aachen, Germany, 7Department of Nuclear Medicine, Maastricht University Medical Center, 6229 HX Maastricht, The Netherlands, 8Department of
Psychiatry, Psychotherapy and Psychosomatics, University Hospital of Zurich, 8006 Zurich, Switzerland, 9Department of Neuroscience and Pharmacology,
Copenhagen University, 1165 Copenhagen, Denmark, 10Department of Child and Adolescent Psychiatry, University of Marburg, 35039 Marburg, Germany,
and 11Max-Planck-Institute for Neurological Research, 50931 Cologne, Germany
Methylphenidate (MPH) inhibits the reuptake of dopamine and noradrenaline. PET studies with MPH challenge show increased compe-
tition at postsynaptic D -receptors, thus indirectly revealing presynaptic dopamine release. We used [ 18F]fluorodopamine
([ 18F]FDOPA)-PET in conjunction with the inlet– outlet model (IOM) of to investigate acute and long-term
changes in dopamine synthesis capacity and turnover in nigrostriatal fibers of healthy subjects with MPH challenge. Twenty healthy
human females underwent two dynamic [ 18F]FDOPA PET scans (124 min; slow bolus-injection; arterial blood sampling), with one scan
in untreated baseline condition and the other after MPH administration (0.5 mg/kg, p.o.), in randomized order. Subjects underwent
cognitive testing at each PET session. Time activity curves were obtained for ventral putamen and caudate and were analyzed according
to the IOM to obtain the regional net-uptake of [ 18F]FDOPA (K; dopamine synthesis capacity) as well as the [ 18F]fluorodopamine washout
rate (k
, index of dopamine turnover). MPH substantially decreased k
in putamen (⫺
22%; p ⫽
0.003). In the reversed treatment
order group (MPH/no drug), K was increased by 18% at no drug follow-up. The magnitude of K at the no drug baseline correlated with
cognitive parameters. Furthermore, individual k
changes correlated with altered cognitive performance under MPH. [ 18F]FDOPA PET
in combination with the IOM detects an MPH-evoked decrease in striatal dopamine turnover, in accordance with the known acute
pharmacodynamics of MPH. Furthermore, the scan-ordering effect on K suggested that a single MPH challenge persistently increased
striatal dopamine synthesis capacity. Attenuation of dopamine turnover by MPH is linked to enhanced cognitive performance in healthy
females.
Key words: [ 18F]FDOPA PET; cognition; dopamine turnover; long-term effects; methylphenidate; stimulants
ical practice, MPH is treatment of choice in attention deficit/
Methylphenidate (MPH) facilitates dopaminergic transmission
hyperactivity disorder (ADHD). Because of its straightforward
by inhibiting the dopamine reuptake transporter (DAT). In clin-
pharmacodynamic mechanism, MPH has also been used as achallenge in PET investigations inducing increased competitionbetween D2/3-receptor ligands and endogenous dopamine
Received April 16, 2014; revised Sept. 5, 2014; accepted Sept. 11, 2014.
Author contributions: Y.K., P.C., F.M.M., G.G., and I.V. designed research; I.S., K.H., S.M.S., C.D., J.S., O.W., S.P.,
L.R., B.N., M.F., and I.V. performed research; I.S., J.S., and O.W. analyzed data; I.S., K.H., S.P., P.C., and I.V. wrote the
reduction of the ligand binding potential, however, is a surrogate
parameter of the changes in dopamine concentrations and is con-
This work was supported by the German Research Foundation (DFG, VE 466/2-1 and IRTG 1328, International
founded by some biological processes, such as receptor internal-
Research Training Group) and Brain Imaging Facility of the Interdisciplinary Center for Clinical Research (IZKF) at theRWTH Aachen University, Germany. We thank the student assistants for their support.
ization The present approach using
The authors declare no competing financial interests.
[ 18F]fluorodopamine ([ 18F]FDOPA) does not target the estima-
Correspondence should be addressed to Ina Schabram, Department of Psychiatry, Psychotherapy, and Psycho-
tion of dopamine release but mimics the presynaptic synthesis
somatics, RWTH Aachen University Pauwelsstrasse 30, D-52074 Aachen, Germany.
pathway of dopamine. Given that the primary application of
Copyright 2014 the authors 0270-6474/14/3414769-08$15.00/0
MPH (ADHD) is claimed to be characterized by presynaptic dis-
14770 • J. Neurosci., October 29, 2014 • 34(44):14769 –14776
Schabram et al. • [18F]FDOPA PET Study
turbances (e.g.,
values) trend toward accepting a stimulus as a target and is independent
the evaluation of MPH effects by a predominantly
of the overall performance. The first part (d2 test and TMT) of neuro-
presynaptic tracer appears to be reasonable.
psychological testing was performed ⬃1 h before PET scan, whereas for
Until now, [ 18F]FDOPA PET was not used for the evaluation
technical reasons the dsCPT was performed after scan.
of MPH effects; it traces the dopamine synthesis capacity in brain,
MPH challenge. Participants received MPH (Ritalin, Novartis Pharma-
ceuticals) at a dose of 0.5 mg/kg adjusted for body weight either at first
which appears to be less vulnerable for environmental and phar-
(
n ⫽ 6) or second PET scan (
n ⫽ 14). Based upon pharmacokinetics of
macological influences
oral MPH, the drug was administered 2 h before tracer injection
However, most quantitative FDOPA PET studies assume
Because of legal and institutional restrictions, it
irreversible trapping of [ 18F]FDOPA, whereas the inlet– outlet
was not possible to include a placebo drug formulation in the study
model (IOM) of yields the net clearance
design. The medication condition was therefore not blinded.
of [ 18F]FDOPA to brain (
K; ml 䡠 g⫺1min⫺1), accommodating
PET scanning and plasma sampling procedure. Before each PET scan, a
the delayed washout of decarboxylated and deaminated metabo-
pregnancy test was performed. [ 18F]FDOPA scans were recorded with
lites, which is explicitly defined by the rate constant
k
the Siemens ECAT HR⫹ whole-body PET, which has a field of view of
loss (min).
Thus, the [ 18F]FDOPA IOM can reveal acute changes in the do-
16.2 cm in 47 planes, an interplane spacing of 3.375 mm, and an axial
pamine dynamics.
resolution of 5.4 mm FWHM. Decarboxylation of [ 18F]FDOPA in pe-ripheral tissues was inhibited by oral administration of carbidopa (Merck
Using this model, the present investigation intends to monitor
Sharp & Dome, 2 mg/kg body weight), of which two-thirds were given 1 h
the acute and delayed effects of MPH on dopamine turnover
after and one-third before start of the emission recording. After a brief
(
kloss) and dopamine synthesis capacity (
K) as well as respective
attenuation scan, a dynamic emission sequence lasting 124 min began
correlations with cognitive changes. Because DAT inhibition will
upon intravenous injection of [ 18F]FDOPA at a dose of 226 ⫾ 21 MBq
reduce the rate of dopamine reuptake into the cytosolic compart-
(range, 166 –263 MBq) as a slow bolus. Frame length increased progres-
ment where it would be exposed to monoamine oxidase
sively according to the following schedule: 3 ⫻ 20 s; 3 ⫻ 1 min, 3 ⫻ 2 min,
we hypothesize that the
3 ⫻ 3 min, 15 ⫻ 5 min, 3 ⫻ 10 min interval Blood
magnitude of
k
was automatically drawn from a radial arterial catheter (first 10 min),
loss will be reduced. We furthermore expect that
changes in
k
and the radioactivity concentration measured at 1 s intervals with an
loss correlate with enhancement in cognition under
MPH treatment.
online ␥-counter (Allogg ABSS V3) cross-calibrated to the tomograph.
Therefore, two [ 18F]FDOPA PET scans (A: untreated control
Subsequently, a series of 15 arterial blood samples are drawn manually,and their radioactivity concentrations measured using a well counter
condition; B: scan after MPH challenge) were conducted in healthy
(PerkinElmer Wizzard2 gamma-counter). The fractions of untrans-
subjects. Because long-term effects of psychostimulants on dopa-
formed [ 18F]FDOPA and its major plasma metabolite 3-
O-methyl-
mine transmission and sensitization are known
[ 18F]-fluorodopa ([ 18F]OMFD) were measured by reverse-phase high
this study was conducted with two treatment order groups
performance liquid chromatography in plasma
(no drug/MPH and MPH/no drug) to obtain possible regulatory
extracts prepared from arterial blood samples. The continuous plasma
effects of a single MPH dose on presynaptic dopamine metabolism.
fractions of [ 18F]FDOPA and OMFD were calculated by interpolation ofbiexponential functions fitted to the measured fractions, and the two
Materials and Methods
input functions calculated by multiplication with the total blood curve
Subjects. Twenty healthy, nonsmoking female subjects 21–28 years of age
(mean ⫾ SD, 24.0 ⫾ 1.9 years) were included in the study, which had
PET data analysis. Emission images were reconstructed by filtered
been approved by the Research and Ethics Committees of the University
back projection with a 4 mm Hanning filter. The dynamic sequence was
Hospital of RWTH Aachen University. All subjects provided written
frame-wise corrected for head motion, using an interframe rigid-body
informed consent. Exclusion criteria for the volunteers included current
transformation implemented in PMOD (Version 3.4, PMOD Technol-
neurological, psychiatric, or systemic disease, pregnancy (13 of 20 sub-
ogy). For spatial normalization, the summed images were first coregis-
jects were using oral contraceptives), and current use of drugs affecting
tered to the individual MR (1.5T MRT Scanner; Philips Gyroscan NT;
the CNS. All 20 subjects are a small subgroup of a large neurogenetic
Philips Medical Systems), and the MR-registered sequence was then nor-
study group of women (
n ⫽ 200) not including molecular imaging tech-
malized to the ICBM-452 template using PMOD
niques The cohort is well characterized in respect
(Brain Normalization II routine), and a 12 parameter rigid-body trans-
to demographics, and by intention highly homogeneous in age, gender,
formation. Decay-corrected time activity curves (TACs) were then cal-
and education. Men were not included to reduce heterogeneity and to
culated for a set of volume of interest templates, including cerebellum,
improve the statistical power for main effects. The 20 PET subjects were
and left and right ventral caudate nucleus and ventral putamen. For one
randomly distributed into two treatment groups (usual or reversed order
participant with contraindications against MR tomography, PET images
of baseline/MPH PET scans). To optimize statistical power for the two
were registered to a normalized [ 18F]FDOPA-template.
main hypotheses, the groups were asymmetrically distributed, with 14
[18F]FDOPA kinetics. Most brain [ 18F]FDOPA studies are quantified
receiving first the unmedicated control scan, followed by the MPH con-
by linear graphical analysis relative to the arterial [ 18F]FDOPA input, or
dition, and six being scanned in reversed order.
a reference tissue surrogate. With PET recordings of 45– 60 min, graph-
Neuropsychology. To investigate the prefrontal cognitive capacities of
ical analysis yields an index of [ 18F]FDOPA utilization that assumes
the participants in the two scanning conditions, we administered the
irreversible trapping, thus ignoring the rapid formation of deaminated
Trail Making Test (TMT-A⫹B) of executive functioning
[ 18F]FDOPA metabolites in living striatum, and their diffusion from
Stroop test d2-concentration test of attention
brain Because this metabolic process entails
and degraded stimulus continuous performance task
useful information about the turnover of the neurotransmitter pool, we
(dsCPT) for measuring attention, in which subjects need to react on
elected to use the reversible IOM for kinetic
either a degraded or contoured target stimulus
analysis of the regional TACs. The IOM is based on principles similar to
Following upon dsCPT was analyzed according
those of the reversible tracer model of as also used by
to the signal detection theory (SDT) The test
both approaches yield outcome parameters for
parameters for hits, missed, false alarms, and correct rejections were
the net blood– brain clearance of [ 18F]FDOPA, a fractional rate constant
entered into analysis using BayesSDT software package for MATLAB
for the diffusion from brain of deaminated [ 18F]FDOPA metabolites,
to obtain the following primary outcome variables: discrim-
and also a distribution volume, which reflects dopamine storage capacity.
inability (sensitivity index, d⬘) and decision-bias (). This bias indicates
The approach of necessarily entails PET acquisition
either a more liberal (negative values) or a more conservative (positive
times of 4 h, whereas the presents IOM applies for recordings of only 2 h
Schabram et al. • [18F]FDOPA PET Study
J. Neurosci., October 29, 2014 • 34(44):14769 –14776
• 14771
Table 1. Cognitive performance scores
Stroop interference
Decision bias 
d2 concentration performance
*
p ⬍ 0.05.
Table 2. Effect of MPH challenge on 关
18F兴
FDOPA PET kinetic parameters
CTR, no drug condition
MPH, methylphenidate condition
CTR, No drug condition; CN, caudate nucleus; PUT, putamen; MPH, methylphenidate condition.
*
p ⬍ 0.05; **
p ⬍ 0.01.
because of a more accurate subtraction of brain radioactivity arising from
ences (percentage of the change) were calculated as ([no drug ⫺ MPH]/no
the peripheral metabolite OMFD. The IOM entails a three-step approach
drug ⫻ 100). ⌬ parameters were calculated for
K,
k , and the cognitive
in which a constrained one tissue compartment model is first used to
measures (MPH condition subtracted by drug-free condition) indicating
calculate the TAC for plasma-derived [ 18F]OMFD in cerebellum, which
the percentage of change. To examine the order of treatment effect, an
contains negligible DOPA-decarboxylase activity. Here the permeability
independent
t test was conducted for ⌬-
K and ⌬-
k . To justify a deeper
ratio (q) for the two substances arising from blood (OMFD/FDOPA) is a
investigation for treatment order effect, a prescreening threshold of
p ⬍
fixed parameter set to a magni-
0.2 was applied for the decision to include parameters in the repeated-
tude of 1.5, which is the mean of the limited number of explicit measure-
measures ANOVA (i.e.,
K and treatment order). Furthermore, Spearman
ments Assuming, like all [ 18F]FDOPA
correlations between baseline PET parameters and baseline neuropsy-
models, homogeneous distribution of [ 18F]OMFD throughout brain,
chology scores, ⌬neuropsychology scores, ⌬-
K and ⌬-
k
the curve calculated in cerebellum is then subtracted from the entire 4D
lated. To correct for multiple testing, a Bonferroni correction at ␣ ⫽ 0.05,
PET recording, to isolate the brain contents of [ 18F]FDOPA, [ 18F]FDOPA,
calculated by the Dubey/Armitage-Pamar ␣ boundary
and its deaminated metabolites, which freely diffuse from brain. The first
was used. This correction includes the correlation among the
20 min are excluded from the IOM analysis because of the need for an
equilibrium for [ 18F]FDOPA in brain Finally,the multilinear form of the IOM is applied to the "cleaned" brain TACs to
calculate the steady-state parameters alluded to above: (1) the net blood–
All 20 subjects successfully completed the two PET scans. In two
brain clearance of [ 18F]FDOPA (
K, ml hg ⫺1 min ⫺1), which is an index
subjects, neuropsychological testing (d2 test, TMT-A⫹B) was
of dopamine synthesis capacity; (2) the washout rate for [ 18F]FDOPA
lost because of technical problems. Mean IQ was 110.43 ⫾ 12.13
together with its deaminated metabolites (
k
; min ⫺1), which is compa-
(SD) The mean (SD) specific activity of fluorine-18 was
rable with the biochemical assays of dopamine turnover; and (3) the
9.4 ⫾ 1.9 MBq/mol (range 5.7–13.6 MBq/mol), indicating a
steady-state distribution volume of [ 18F]FDOPA together with its decar-
total injected mass of ⬃4 pmol; there were no significant differences
boxylated metabolites (
V ; ml/g), which is an index of dopamine storage
in specific activity between the two PET scans (T ⫽ 1.327,
p ⫽ 0.231).
capacity comparable with the effective distribution volume (EDV;ml 䡠 g ⫺1) defined by
The mean (SD) dose of MPH was 33.0 ⫾ 6.4 mg (range 20–50 mg).
Statistical analyses. Wilcoxon's rank order tests for paired samples
There was no group differences in the stage of menstrual cycle at the
were conducted to investigate effects of MPH on cognition and PET
scanning day, nor any main effect of cycle or oral contraceptive use
parameters (
K,
k
, and
V ). The baseline versus MPH condition differ-
on any [18F]FDOPA PET parameter at baseline.
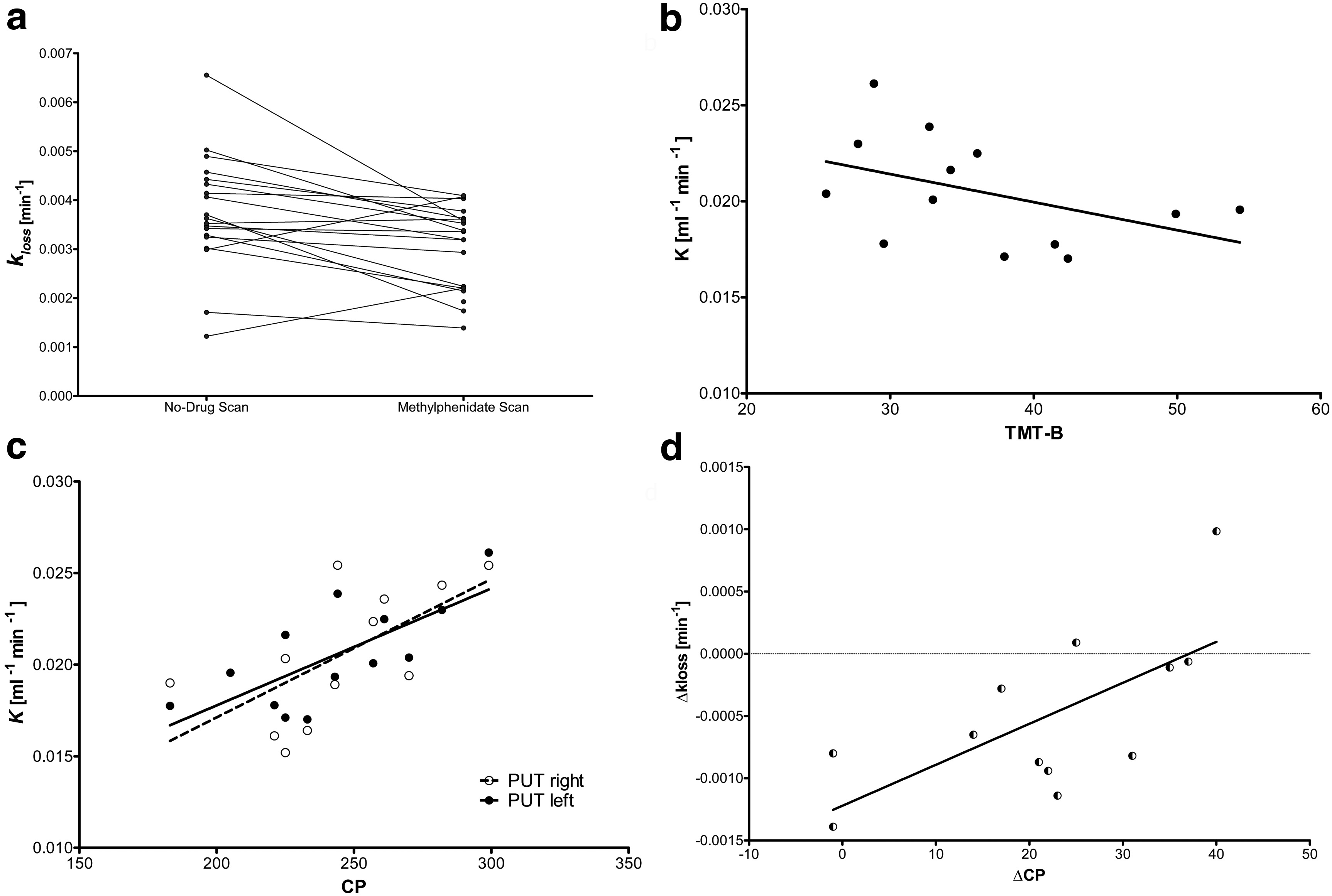
14772 • J. Neurosci., October 29, 2014 • 34(44):14769 –14776
Schabram et al. • [18F]FDOPA PET Study
Figure 1.
a, Reductions in [ 18F]FDOPA k
in bilateral putamen after MPH challenge ( p ⫽ 0.007, n ⫽ 19, complete sample). b, Correlation between time at Trail Making Test B and no drug
[ 18F]FDOPA K in left putamen ( p ⫽ 0.029, n ⫽ 14, conventional order group). c, Correlation between no drug [ 18F]FDOPA K and concentration performance parameter (CP) in right and left putamen
(PUT) (right: p ⫽ 0.039, n ⫽ 12; left p ⫽ 0.007, n ⫽ 13, conventional order group). d, Correlation between change in [ 18F]FDOPA k and change in concentration performance under MPH ( p ⫽
0.040, n ⫽ 12, conventional order group).
Table 3. ANOVA for 关18F兴FDOPA K in PUT and CN
The results of the neuropsychological tests are displayed in MPH increased concentration performance parameter (CP) in
the d2 task (control condition: 245.95 ⫾ 30.86, MPH: 260.75 ⫾
关18F兴FDOPA K
28.06 (mean ⫾ SD); ⫺6.0%, Z ⫽ ⫺2.87, p ⫽ 0.004). However,
there was no effect of MPH on TMT or dsCPT parameters.
关18F兴FDOPA K
PET parameters baseline
The mean (SD) magnitudes of [ 18F]FDOPA IOM parameters
kloss, K, and Vd in ventral caudate nucleus and in ventral putamen
PUT, Putamen; CN, caudate nucleus; TO, treatment order.
are reported in The baseline kloss was 0.0036 ⫾ 0.0014
*p ⬍ 0.05.
min ⫺1 in the bilateral caudate nucleus and 0.0038 ⫾ 0.0012min ⫺1 in the bilateral putamen. There were no significant sidedifferences. Baseline K was 0.0200 ⫾ 0.005 ml 䡠 g⫺1 䡠 min⫺1 in
Order of treatment effect
To test whether the order of the drug treatment had an effect on
ml 䡠 g⫺1 䡠 min⫺1 in the bilateral putamen. Mean (SD) baseline
the PET parameters, we first performed an independent-sample t
Vd was 6.96 ⫾ 3.28 ml 䡠 g⫺1 in the bilateral caudate nucleus and
test; this showed that ⌬K in bilateral caudate nucleus and bilateral
7.14 ⫾ 3.23 ml 䡠 g⫺1 in the bilateral putamen. In one partici-
putamen differed between groups (caudate: T ⫽ 1.90, p ⫽ 0.075
pant, two ROIs (caudate right and putamen right) were excluded
n ⫽ 19; putamen: t ⫽ 2.90, p⫽0.010, n ⫽ 19). The ⌬-kloss param-
from consideration because of poor fitting outcome.
eter showed no general effect of treatment order, except in leftputamen (T ⫽ 2.589, p ⫽ 0.019, n ⫽ 20). Because of these results,
Effect of MPH on PET parameters
we performed repeated-measures ANOVAs, including K as a
In the MPH challenge condition, mean (⫾SD) kloss was 0.003 ⫾
within-subjects variable and "order of treatment" as a between-
0.0009 min ⫺1 in bilateral caudate nucleus and 0.003 ⫾ 0.0008
subject variable. The tests revealed no main effect of K but a main
min ⫺1 in bilateral putamen. Thus, MPH reduced kloss by 22% in
effect of order of treatment and an interaction effect in bilateral
right caudate nucleus (Z ⫽ ⫺1.97, p ⫽ 0.048, n ⫽ 19), 22% (Z ⫽
putamen (F ⫽ 8.426, p ⫽ 0.01*; K*order of treatment; see also
⫺2.95, p ⫽ 0.003, n ⫽ 20) in left putamen, and 15% (Z ⫽ ⫺2.09,
. Bilateral caudate nucleus showed a trend in order of treat-
p ⫽ 0.036, n ⫽ 19) in right putamen ). Mean (SD)
ment effect. The post hoc analyses revealed that, in those subjects with
K was 0.0197 ⫾ 0.003 ml 䡠 g⫺1 䡠 min⫺1 for bilateral caudate
reversed order of drug application (MPH scan first), baseline K esti-
nucleus and 0.0220 ⫾ 0.003 ml 䡠 g⫺1 䡠 min⫺1 for bilateral puta-
mates in bilateral putamen were 24% higher than for the conven-
men; there were no significant effects of MPH on K (n ⫽ 20).
tional order group (Z ⫽ ⫺2.368, p ⫽ 0.018, n ⫽ 19), with a trend in
Schabram et al. • [18F]FDOPA PET Study
J. Neurosci., October 29, 2014 • 34(44):14769 –14776 • 14773
Table 4. Treatment order effect: post hoc analyses within group differences in bilateral 关18F兴FDOPA K
关18F兴FDOPA K
CTR, No drug condition; CN, caudate nucleus; PUT, putamen; MPH, methylphenidate condition.
*p ⬍ 0.05.
Table 5. Correlations between neuropsychological parameters and 关18F兴FDOPA K in bilateral CN and PUT
Decision bias 
Discriminability d⬘
CN, Caudate nucleus; PUT, putamen; NS, not significant.
*p ⬍ 0.05; **p ⬍ 0.01.
bilateral caudate nucleus (Z ⫽ ⫺1.842, p⫽0.072, n ⫽ 19, Wilcoxon
MPH treatment, such that the observed changes of volume of
test). Insofar as this may indicate a carryover effect 2 weeks after
distribution (Vd) are mostly driven by kloss. Baseline parameters
MPH on [18F]FDOPA kinetics, we analyzed post hoc differences for
for attention performance (CP [d2 test] and TMT-B) correlated
both groups The results indicate that the increase was K
with baseline [ 18F]FDOPA K in putamen; similar results for
only significant in the reversed-order group.
other cognitive parameters are found in previous studies We also detected significant correla-
Relationships between cognitive performance and baseline K
tions between [ 18F]FDOPA ⌬-kloss and change of cognitive
Because of the order of treatment effect, correlations between K
performance parameters evoked by MPH: in particular, the indi-
and neuropsychological measures were conducted only within
vidual change of the decision bias (⌬) and the extent of kloss
the conventional order group (n ⫽ 14). We found that baseline
reduction correlated positively. Furthermore, ⌬CP correlated
magnitude of K in left putamen correlated negatively with base-
strongly with ⌬kloss, indicating more pronounced attentional im-
line TMT-B: the lower K, the better the performance )
provement in subjects with the greatest pharmacodynamic effect
(TMT-B is measured in seconds, such that briefer duration of the
of MPH. Interestingly, our randomized study design revealed a
test indicated better working memory). Furthermore, there was a
strong PET scan-ordering effect, such that there was an apparent
negative correlation between CP (d2 test) scores and K in left and
increase in drug-free [ 18F]FDOPA K (the dopamine synthesis
right putamen ).
capacity) persisting at 2 weeks after a single, moderate MPH dose.
It is well known that MPH inhibits the plasma membrane
Correlations with change in kloss and change in
catecholamine transporters, causing an increase of intrasynaptic
dopamine and noradrenaline concentrations.
There was a strong correlation between ⌬-kloss in bilateral puta-
described DAT occupancy of up to 74% in human
men and the improvement in CP under MPH (r ⫽ 0.599, p ⫽
subjects treated with MPH. Microdialysis studies have shown
0.040, n ⫽ 12, Spearman; ). Furthermore, ⌬-kloss in right
doubling of the interstitial dopamine concentration in rat stria-
putamen correlated negatively with the change in decision bias
tum after oral MPH administration
(⌬-) within the conventional order group (r ⫽ ⫺0.630, p ⫽
0.028, n ⫽ 12, Spearman).
2/3-receptor ligand PET studies have
shown availability decreases in healthy subjects ranging from 6%
to 27% with MPH challenge
Using [ 18F]FDOPA PET in conjunction with the IOM approach
The construct validity of the
for estimation of the dopamine turnover, the present study was
competition paradigm, however, has some caveats because re-
designed to quantify direct effects of acute MPH on nigrostriatal
sults depend on which D2/3 ligand is used
dopamine metabolism in healthy females and to link these phar-
and an imperfect relation between
macodynamic effects to individual cognitive performance
interstitial dopamine changes and the time course of alterations
changes. We found the expected decrease of k
in receptor availability seen by PET exists
loss in the MPH
condition. Most likely, k
This discrepancy may be related to receptor
loss is decreased because of particular
action of MPH as a reuptake inhibitor, which decreases the reen-
internalization and affinity states induced by dopamine agonists.
try of released dopamine into the intracellular substrate pool for
Studies showed internalization effects for amphetamine, dopa-
monoamine oxidase. Thus, under MPH, the oxidative deamina-
mine, and dopamine agonists
tion of released dopamine to the diffusible metabolite DOPAC by
Given these vagaries, we proposed that the [18F]FDOPA IOM
monoamine oxidase is largely disabled
should provide a more interpretable assay of the pharmacody-
namic effects of a psychostimulant challenge. Indeed, the per-
feedback reduces the formation of diffusible dopamine metabo-
centage change of kloss exceeded that of the D2/3-receptor
lites. Nonetheless, the magnitude of K was unaffected by acute
availability seen in many MPH challenge studies.
14774 • J. Neurosci., October 29, 2014 • 34(44):14769 –14776
Schabram et al. • [18F]FDOPA PET Study
The parameter kloss reflects the composite of partitioning of
The IOM, which accommodates the reversibility of [18F]FDOPA
[ 18F]FDOPA between cytosolic and vesicular compartments, re-
trapping, is necessarily a simplification of the biological complex-
lease and reuptake, subsequent exposure to monoamine oxidase,
ity of dopamine metabolism. As noted above, our main endpoint
and diffusion of deaminated metabolites from brain
kloss is explicable as an index of dopamine turnover, traced by the
Despite this complexity, we have shown that the kloss mag-
elimination of the [ 18F]FDOPA pool formed in striatum, such
nitude depicts the steady-state fractional rate constant for dopa-
that a reduction of kloss reflects a decline in turnover. Our previ-
mine turnover As such, our observations of a
ous results confirm the (patho)physiological relevance of dis-
positive association between decrease of kloss and MPH-related
turbed kloss in psychiatric and neurological disorders
modulations of cognitive performance link the pharmacody-
In contrast, the [ 18F]FDOPA parameter K represents
namic effect of MPH on dopamine metabolism; the more
the capacity to use (exogenous) [ 18F]FDOPA in brain, which is
pronounced the decrease in kloss, the less improvement in concen-
hardly subject to regulation upon acute MPH challenge. We pre-
tration performance was observed. Analogously, a previous
viously reported changes in [ 18F]FDOPA K upon treatment for
[ 18F]FDOPA IOM study showed that individual changes in kloss
3 d with a D2/3-anatgonist whereas
following haloperidol challenge likewise correlate with cognitive
short-term antipsychotic treatment had no effect on correspond-
changes In the present study, MPH did
ing m-tyrosine PET findings
not evoke cognitive improvement in every case; we suppose that
Our design deliberately entailed a test of order effect on
many in our cohort were high achievers with normal IQ, and
[ 18F]FDOPA kinetics, which revealed kloss to be robust to this
already in a state of optimal dopamine balance, which could not
factor, whereas K was increased by previous MPH exposure. Our
be improved by MPH challenge. Using the SDT analyses, we
subjects did not otherwise report any previous use of psycho-
detected another important association consistent with procog-
stimulants, so we feel confident in our observation of a main
nitive and attention effects of MPH in ADHD patients; partici-
effect of MPH challenge on kloss. In clinical [18F]FDOPA studies
pants who manifested the strongest impact of MPH in reducing
of ADHD patients, drug treatment history is very likely to be
kloss also showed the strongest shift to a more conservative deci-
relevant, given that K was strongly affected by previous one-time
sion bias. The decision bias provides a measure of how liberal or
MPH use. Nevertheless, the unequal group sizes call for caution
conservative the decisions were, regardless of overall perfor-
in the interpretation of this finding. The group with the reversed
mance. These findings might be relevant for several psychiatric
treatment order (n ⫽ 6) was smaller than the group with standard
disorders that include cognitive disabilities
scanning order (n ⫽ 14). This was, by design, to provide suffi-
cient power for testing the main hypotheses concerning cor-
The widespread clinical use of psychostimulants in ADHD has
relations between PET and cognitive changes. Also notably,
raised concerns with respect to long-term treatment effects. Be-
the test–retest variability is 10% for conventional reference tis-
havioral sensitization to amphetamine is well known in rodent
sue [ 18F]FDOPA PET the corresponding
studies and previous MPH treatment
covariance remains to be established for the IOM method. Given
evokes persistent changes in interstitial dopamine
that a possibly higher variance in the outcome parameters of the
and NMDA receptors in rat brain
IOM might exist, which would impair the test–retest reliability,
A human PET study revealed potentiation of reductions in
from a statistical point of view, this would not relativize the fact
amphetamine-evoked D2/3-receptor availability some months af-
that, based on the present observations, the probability to reject a
ter the previous dose of amphetamine, indicating persistent sen-
possible true null hypothesis is ⬍5% (risk of false positive as
sitization Based on these results, our study
depicted by ␣ ⱕ 0.05). Lower test–retest reliability, however, de-
design included a subgroup with a reversed order of the drug-free
pends on higher necessary effects to contrast against the higher
and pharmacological challenge [ 18F]FDOPA PET scans. We did
level of noise in the data.
see an order effect on the magnitude of K; MPH treatment 2weeks before the scan significantly (⬃18%) increased striatal do-
pamine synthesis capacity. This presynaptic change might have
Supplemental material for this article is available at
arisen through feedback regulation of dopamine autoreceptors,
which could likewise be a factor in the amphetamine sensitization
Detailed description of the reversible Inlet/Out-
reported by Verification of the presynaptic
let Model. Giving a more complete overview and a better understanding
potentiation would require prospective [ 18F]FDOPA studies
of the kinetic model which is used in this manuscript. This material hasnot been peer reviewed.
with repeated psychostimulant challenge. found that ADHD patients had increased [ 18F]FDOPA uptake inmidbrain compared with healthy subjects, despite being drug free
for several weeks. Present findings suggest that this, too, may have
Bartlett SE, Enquist J, Hopf FW, Lee JH, Gladher F, Kharazia V, Waldhoer M,
reflected a carryover effect rather than an ADHD trait per se. Our
Mailliard WS, Armstrong R, Bonci A, Whistler JL (2005) Dopamine re-
main endpoint in the present study, k
sponsiveness is regulated by targeted sorting of D2 receptors. Proc Natl
loss, obviously is a more
Acad Sci U S A 102:11521–11526.
volatile parameter, being related to present state of dopamine
Ba¨umler G (1985) Farbe-Wort-Interferenztest (FWIT) nach J. R. Stroop
turnover. Consistent with this conception, we saw no evidence
(Handanweisung). Go¨ttingen, Germany: Verlag fur Psychologie Hogrefe.
for a carryover in the magnitude of kloss. If further studies repli-
Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE,
cate our finding of persistently increased dopamine synthesis ca-
Schmeichel B, Hamilton C, Spencer RC (2006) Methylphenidate pref-
pacity following MPH, it will become an issue whether this effect
erentially increases catecholamine neurotransmission within the prefron-
contributes to the beneficial clinical effects in ADHD, which are
tal cortex at low doses that enhance cognitive function. Biol Psychiatry60:1111–1120.
usually attributed to the acute increase of dopamine levels.
Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C
Some limitations of the present study should be noted. In
(2006) Modeling sensitization to stimulants in humans: an [ 11C]raclo-
general, the complex nature of [ 18F]FDOPA metabolism in brain
pride/positron emission tomography study in healthy men. Arch Gen
hampers the interpretation of our findings.
Psychiatry 63:1386 –1395.
Schabram et al. • [18F]FDOPA PET Study
J. Neurosci., October 29, 2014 • 34(44):14769 –14776 • 14775
Brickenkamp R (2002) Test d2 Aufmerksamkeits und Konzentrationstest,
In: Befunderhebungin der psychiatrie: Lebensqualita¨t, negativsymptom-
Ed 9. Go¨ttingen, Germany: Hogrefe.
atik und andere aktuelle entwicklungen (Mo¨ller HJ, Engel RR, Hoff P,
Calipari ES, Ferris MJ, Salahpour A, Caron MG, Jones SR (2013) Methyl-
eds), pp 331–338. Vienna: Springer.
phenidate amplifies the potency and reinforcing effects of amphetamines
Kumakura Y, Cumming P, Vernaleken I, Buchholz HG, Siessmeier T, Heinz
by increasing dopamine transporter expression. Nat Commun 4:2720.
A, Kienast T, Bartenstein P, Gru¨nder G (2007) Elevated [ 18F]fluorodo-
pamine turnover in brain of patients with schizophrenia: an [ 18F]fluo-
Calipari ES, Ferris MJ, Melchior JR, Bermejo K, Salahpour A, Roberts DC,
rodopa/positron emission tomography study. J Neurosci 27:8080 – 8087.
Jones SR (2014) Methylphenidate and cocaine self-administration pro-
duce distinct dopamine terminal alterations. Addict Biol 19:145–155.
Kumakura Y, Gjedde A, Caprioli D, Kienast T, Beck A, Plotkin M, Schlagen-
hauf F, Vernaleken I, Gru¨nder G, Bartenstein P, Heinz A, Cumming P
Cumming P, Gjedde A (1998) Compartmental analysis of dopa decarbox-
(2013) Increased turnover of dopamine in caudate nucleus of detoxified
ylation in living brain from dynamic positron emission tomograms. Syn-
alcoholic patients. PLoS One 8:e73903.
apse 29:37– 61.
Laruelle M, Iyer RN, al-Tikriti MS, Zea-Ponce Y, Malison R, Zoghbi SS,
Cumming P, Boyes BE, Martin WR, Adam M, Grierson J, Ruth T, McGeer EG
Baldwin RM, Kung HF, Charney DS, Hoffer PB, Innis RB, Bradberry CW
(1987) The metabolism of [18F]6-fluoro-L-3,4-dihydroxyphenylalanine
(1997) Microdialysis and SPECT measurements of amphetamine-
in the hooded rat. J Neurochem 48:601– 608.
induced dopamine release in nonhuman primates. Synapse 25:1–14.
Cumming P, Le´ger GC, Kuwabara H, Gjedde A (1993) Pharmacokinetics of
plasma 6-[18F]fluoro-L-3,4-dihydroxyphenylalanine ([18F]Fdopa) in
Lee MD (2008) BayesSDT: software for Bayesian inference with signal de-
humans. J Cereb Blood Flow Metab 13:668 – 675.
tection theory. Behav Res Methods 40:450 – 456.
Cumming P, Munk OL, Doudet D (2001) Loss of metabolites from monkey
Ludolph AG, Kassubek J, Schmeck K, Glaser C, Wunderlich A, Buck AK,
striatum during PET with FDOPA. Synapse 41:212–218.
Reske SN, Fegert JM, Mottaghy FM (2008) Dopaminergic dysfunction
in attention deficit hyperactivity disorder (ADHD), differences between
Deep P, Gjedde A, Cumming P (1997) On the accuracy of an [ 18F]FDOPA
pharmacologically treated and never treated young adults: a 3,4-
compartmental model: evidence for vesicular storage of [ 18F]fluorodop-
dihdroxy-6-[18F]fluorophenyl-l-alanine PET study. Neuroimage 41:
amine in vivo. J Neurosci Methods 76:157–165.
Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW (2011) The roles
Mamo D, Remington G, Nobrega J, Hussey D, Chirakal R, Wilson AA, Baker
of dopamine and noradrenaline in the pathophysiology and treatment of
G, Houle S, Kapur S (2004) Effect of acute antipsychotic administration
attention-deficit/hyperactivity disorder. Biol Psychiatry 69:e145– e157.
on dopamine synthesis in rodents and human subjects using 6-[ 18F]-L-
m-tyrosine. Synapse 52:153–162.
Egerton A, Demjaha A, McGuire P, Mehta MA, Howes OD (2010) The
Markowitz JS, Straughn AB, Patrick KS, DeVane CL, Pestreich L, Lee J, Wang
test–retest reliability of 18F-DOPA PET in assessing striatal and extras-
Y, Muniz R (2003) Pharmacokinetics of methylphenidate after oral ad-
triatal presynaptic dopaminergic function. Neuroimage 50:524 –531.
ministration of two modified-release formulations in healthy adults. Clin
Pharmacokinet 42:393– 401.
Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM (1998) DOPA
Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T,
decarboxylase activity in attention deficit hyperactivity disorder adults: a
Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D,
[fluorine- 18]fluorodopa positron emission tomographic study J Neurosci
Iacoboni M, Schormann T, Amunts K, Palomero-Gallagher N, Geyer S,
Parsons L, et al. (2001) A probabilistic atlas and reference system for the
Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Cohen RM
human brain: International Consortium for Brain Mapping (ICBM). Phi-
(1999) High midbrain [ 18F]DOPA accumulation in children with atten-
los Trans R Soc Lond B Biol Sci 356:1293–1322.
tion deficit hyperactivity disorder. Am J Psychiatry 156:1209 –1215.
Morris ED, Yoder KK (2007) Positron emission tomography displacement
sensitivity: predicting binding potential change for positron emission to-
Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller
mography tracers based on their kinetic characteristics. J Cereb Blood
D, Molina PE, Dewey SL (2000) Comparison between intraperitoneal
Flow Metab 27:606 – 617.
and oral methylphenidate administration: a microdialysis and locomotor
Nielsen JA, Chapin DS, Moore KE (1983) Differential effects of d-amphetamine,
activity study. J Pharmacol Exp Ther 295:51–57.
beta-phenylethylamine, cocaine and methylphenidate on the rate of do-
Gillings NM, Bender D, Falborg L, Marthi K, Munk OL, Cumming P (2001)
pamine synthesis in terminals of nigrostriatal and mesolimbic neurons
Kinetics of the metabolism of four PET radioligands in living minipigs.
and on the efflux of dopamine metabolites into cerebroventricular perfu-
Nucl Med Biol 28:97–104.
sates of rats. Life Sci 33:1899 –1907.
Gjedde A, Wong DF (1987) Positron tomographic quantitation of neurore-
Peterson WW, Birdsall TG, Fox WC (1954) The theory of signal detectabil-
ceptors in human brain in vivo: with special reference to the D2 dopamine
ity. Trans IRE Group on Information Theory PGIT 4:171–212.
receptors in caudate nucleus. Neurosurg Rev 10:9 –18.
Reitan RM (1955) The relation of the trail making test to organic brain
Gjedde A, Reith J, Dyve S, Le´ger G, Guttman M, Diksic M, Evans A, Kuwabara
damage. J Consult Psychol 19:393–394.
H (1991) Dopa decarboxylase activity of the living human brain. Proc
Robinson TE, Becker JB, Presty SK (1982) Long-term facilitation of
Natl Acad Sci U S A 88:2721–2725.
amphetamine-induced rotational behavior and striatal dopamine release
Gru¨nder G, Vernaleken I, Mu¨ller MJ, Davids E, Heydari N, Buchholz HG,
produced by a single exposure to amphetamine: sex differences. Brain Res
Bartenstein P, Munk OL, Stoeter P, Wong DF, Gjedde A, Cumming P
(2003) Subchronic haloperidol downregulates dopamine synthesis ca-
Rosa-Neto P, Lou HC, Cumming P, Pryds O, Karrebaek H, Lunding J, Gjedde
pacity in the brain of schizophrenic patients in vivo. Neuropsychophar-
A (2005) Methylphenidate-evoked changes in striatal dopamine corre-
macology 28:787–794.
late with inattention and impulsivity in adolescents with attention deficit
Houston GC, Hume SP, Hirani E, Goggi JL, Grasby PM (2004) Temporal
hyperactivity disorder. Neuroimage 25:868 – 876.
characterisation of amphetamine-induced dopamine release assessed
Sankoh AJ, Huque MF, Dubey SD (1997) Some comments on frequently
with [ 11C]raclopride in anaesthetised rodents. Synapse 51:206 –212.
used multiple endpoint adjustment methods in clinical trials. Stat Med
16:2529 –2542.
Huang SC, Barrio JR, Yu DC, Chen B, Grafton S, Melega WP, Hoffman JM,
Schabram I, Eggermann T, Siegel SJ, Gru¨nder G, Zerres K, Vernaleken I
Satyamurthy N, Mazziotta JC, Phelps ME (1991) Modelling approach
(2013) Neuropsychological correlates of transcription factor AP-2Beta,
for separating blood time-activity curves in positron emission tomo-
and its interaction with COMT and MAOA in healthy females. Neuropsy-
graphic studies. Phys Med Biol 36:749 –761.
chobiology 68:79 –90.
Jokinen P, Karrasch M, Bru¨ck A, Johansson J, Bergman J, Rinne JO (2013)
Skinbjerg M, Liow JS, Seneca N, Hong J, Lu S, Thorsell A, Heilig M, Pike VW,
Cognitive slowing in Parkinson's disease is related to frontostriatal dopa-
Halldin C, Sibley DR, Innis RB (2010) D2 dopamine receptor internal-
minergic dysfunction. J Neurol Sci 329:23–28.
ization prolongs the decrease of radioligand binding after amphetamine:
Kathmann N, Wagner M, Satzger W, Engel RR (1996) Vigilanzmessung auf
a PET study in a receptor internalization-deficient mouse model. Neuro-
verhaltensebene: Der continuous performance test- mu¨nchen (cpt-m).
image 50:1402–1407.
14776 • J. Neurosci., October 29, 2014 • 34(44):14769 –14776
Schabram et al. • [18F]FDOPA PET Study
Sossi V, Doudet DJ, Holden JE (2001) A reversible tracer analysis approach
are predictive of haloperidol-induced changes in dopamine turnover and
to the study of effective dopamine turnover. J Cereb Blood Flow Metab
cognitive performance: a positron emission tomography study in healthy
subjects. Neuroimage 40:1222–1231.
Udo de Haes JI, Kortekaas R, Van Waarde A, Maguire RP, Pruim J, den Boer
Vernaleken I, Rademacher L, Henkel K, Dietrich C, Prinz S, Winz O, Schmal-
JA (2005) Assessment of methylphenidate-induced changes in binding
johann J, Mohammadkhani Shali S, Mottaghy F, Gru¨nder G (2013) A
of continuously infused [(11)C]-raclopride in healthy human subjects:
longitudal [ 18F]FDOPA PET study on nicotine addicted patients. J Nucl
correlation with subjective effects. Psychopharmacology (Berl) 183:322–
Med 54. (Supplement 2):31.
Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R, Lieber-
Urban KR, Li YC, Gao WJ (2013) Treatment with a clinically-relevant dose
man J, Angrist B, Pappas N, MacGregor R (1994) Imaging endogenous
of methylphenidate alters NMDA receptor composition and synaptic
dopamine competition with [ 11C]raclopride in the human brain. Synapse
plasticity in the juvenile rat prefrontal cortex. Neurobiol Learn Mem
Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Hitzemann R,
van Beilen M, Portman AT, Kiers HA, Maguire RP, Kaasinen V, Koning M,
Pappas N (1998) Dopamine transporter occupancies in the human
Pruim J, Leenders KL (2008) Striatal FDOPA uptake and cognition in
brain induced by therapeutic doses of oral methylphenidate. Am J Psy-
advanced non-demented Parkinson's disease: a clinical and FDOPA PET
chiatry 155:1325–1331.
study. Parkinsonism Relat Disord 14:224 –228.
Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Dewey SL,
Vernaleken I, Kumakura Y, Cumming P, Buchholz HG, Siessmeier T, Stoeter P,
Hitzemann R, Gifford AN, Pappas NR (1999) Blockade of striatal dopa-
Mu¨ller MJ, Bartenstein P, Gru¨nder G (2006) Modulation of [ 18F]fluo-
mine transporters by intravenous methylphenidate is not sufficient to
rodopa (FDOPA) kinetics in the brain of healthy volunteers after acute hal-
induce self-reports of "high." J Pharmacol Exp Ther 288:14 –20.
operidol challenge. Neuroimage 30:1332–1339.
Volkow ND, Wang GJ, Newcorn J, Fowler JS, Telang F, Solanto MV, Logan J,
Vernaleken I, Buchholz HG, Kumakura Y, Siessmeier T, Stoeter P, Barten-
Wong C, Ma Y, Swanson JM, Schulz K, Pradhan K (2007) Brain dopa-
stein P, Cumming P, Gru¨nder G (2007) ‘Prefrontal' cognitive perfor-
mine transporter levels in treatment and drug naive adults with ADHD.
mance of HS positively correlates with cerebral FDOPA influx: an
Neuroimage 34:1182–1190.
exploratory [ 18F]-fluoro-L-DOPA PET investigation. Hum Brain Mapp
Zetterstro¨m T, Sharp T, Collin AK, Ungerstedt U (1988) In vivo measure-
ment of extracellular dopamine and DOPAC in rat striatum after various
Vernaleken I, Kumakura Y, Buchholz HG, Siessmeier T, Hilgers RD, Barten-
dopamine-releasing drugs; implications for the origin of extracellular
stein P, Cumming P, Gru¨nder G (2008) Baseline [ 18F]-FDOPA kinetics
DOPAC. Eur J Pharmacol 148:327–334.
Source: http://dnp.ku.dk/news/openaccesspapers/2014/november/Schabram_I_et_al.__J_Neurosci__2014.34_44__14769-76.pdf
Protocolo para el manejo perioperatorio de la medicación crónica Cristina Roure Nuez, David López Sisamón, Margarita Prats Riera Grupo de trabajo sobre el manejo perioperatorio de la medicación habitual de la Societat Catalana de Farmàcia Clínica: Coordinadores: Cristina Roure (SCIAS-Hospital de Barcelona) David López (Hospital de la Santa Creu i Sant Pau)
Li QN et al / Acta Pharmacol Sin 2003 Jun; 24 (6): 599-604 ©2003, Acta Pharmacologica Sinica Chinese Pharmacological Society Shanghai Institute of Materia Medica Chinese Academy of Sciences Effects of low doses of hydrochloride tetracycline on bone metabolism and uterus in ovariectomized rats1 LI Qing-Nan2,4, HU Bin, HUANG Lian-Fang, CHEN Yan, WENG Ling-Ling3, ZHENG Hu3, CHEN Huai-Qing4


