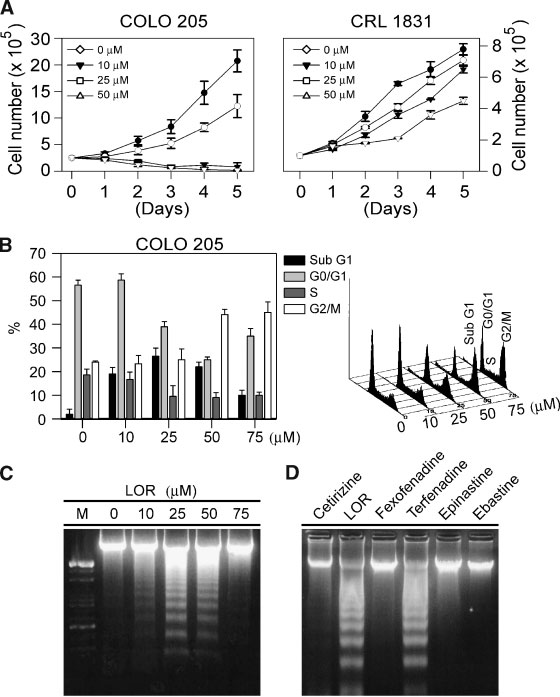
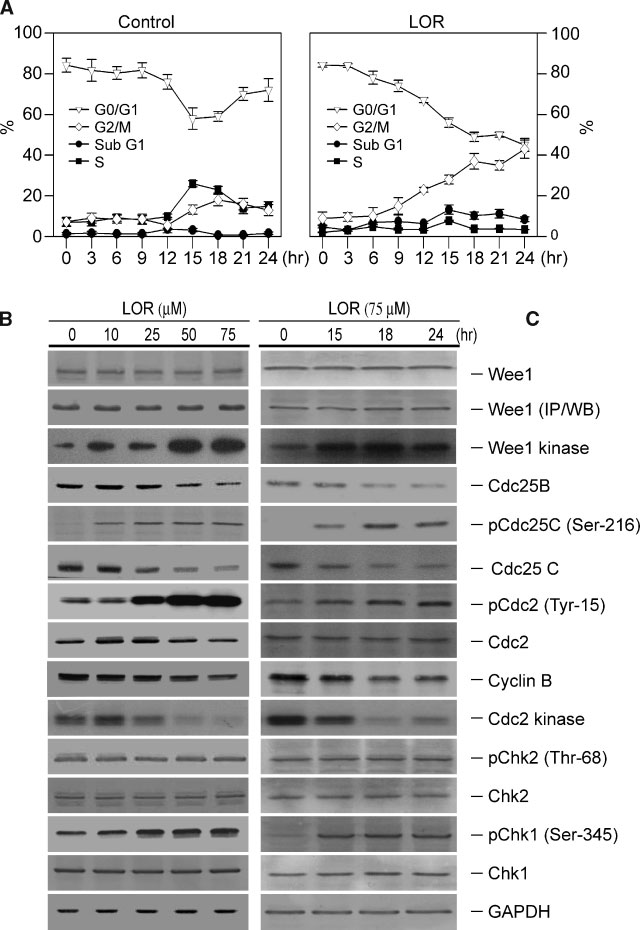
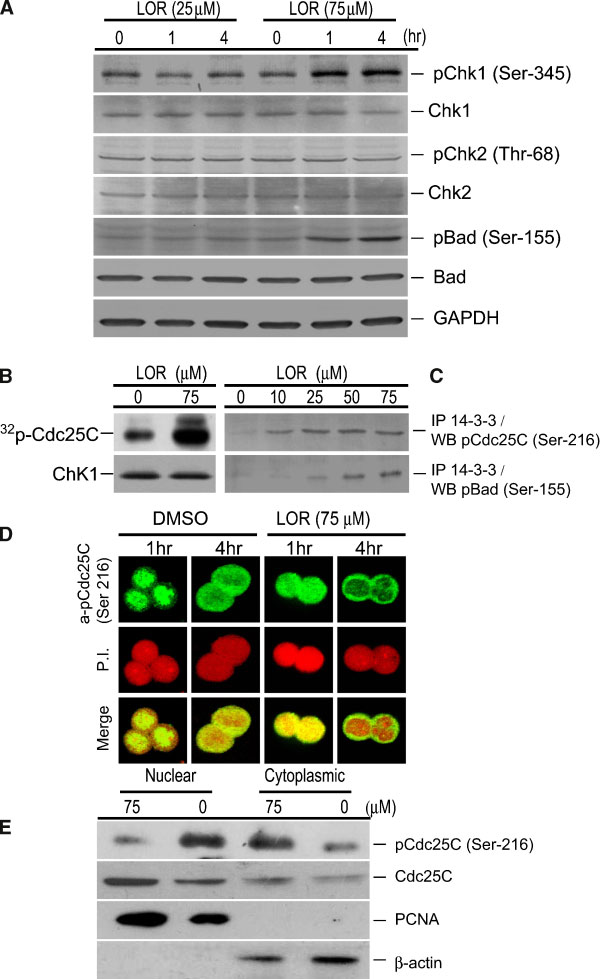
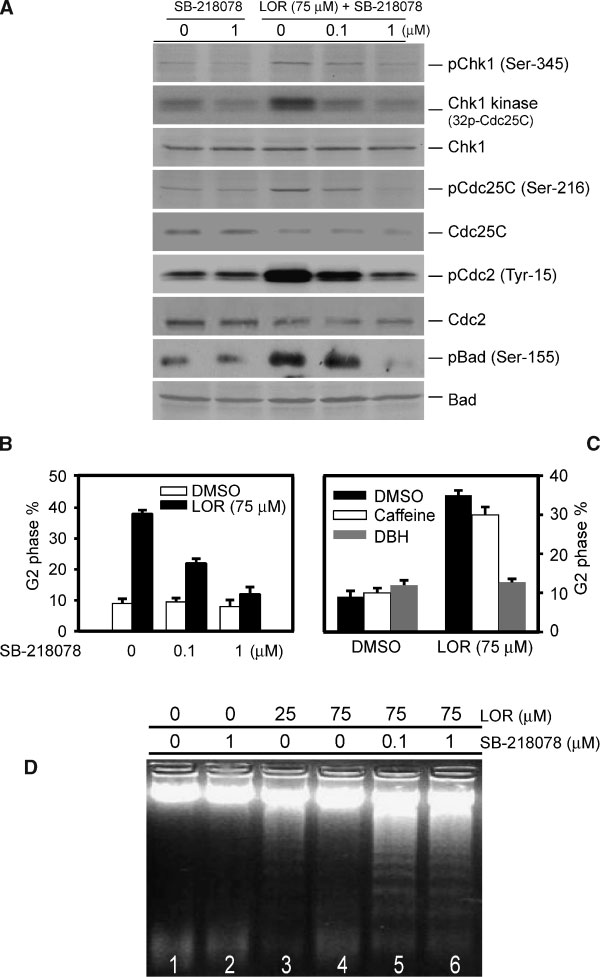
Jahresbericht des bundespatentgerichts für das jahr 2005
herausgegeben von der Stelle für Presse und Öffentlichkeitsarbeit des Bundespatentgerichts Hausanschrift: Cincinnatistraße 64 Internet: www.bpatg.de E-Mail: [email protected] Schwerpunkt auch dieses Jahresberichts ist die Dokumentation der Rechtsprechung des Bundespatentgerichts. In den Bereichen Patentrecht, Gebrauchsmusterrecht, Geschmacksmusterrecht und Markenrecht werden insbesondere die Entscheidungen