Detection of pyrrolizidine alkaloids in german licensed herbal medicinal teas

Contents lists available at
journal homepage:
Detection of pyrrolizidine alkaloids in German licensed herbalmedicinal teas
M. Schulz J. Meins S. Diemert P. ZR. Goebel D. SM. Schubert-Zsilavecz M. A
a Drug Commission of German Pharmacists (AMK), Jaegerstrasse 49/50, 10117 Berlin, Germanyb Central Laboratory of German Pharmacists, Carl-Mannich-Strasse 20, 65760 Eschborn, Germanyc Department of Food Chemistry and Toxicology, Technische Universität Kaiserslautern, Erwin-Schrödinger-Strasse, 67663 Kaiserslautern, Germanyd Department of Pharmaceutical Chemistry, Goethe-University Frankfurt, Max-von-Laue Strasse 9, 60438 Frankfurt am Main, Germany
Article history:
Background: Because of the hepatotoxic, mutagenic, and cancerogenic effects of pyrrolizidine alkaloids (PAs)
Received 21 October 2014
the German Federal Institute for Risk Assessment (BfR) recommends not to exceed a daily PA intake of
Revised 26 February 2015
0.007 μg/kg body weight (0.42 μg/60 kg adult). In a recent study conducted by the BfR, up to 5647 μg PA/kg
Accepted 23 March 2015
dried herbal material were detected in tea products marketed as food.
Purpose: The present study aimed at elucidating whether medicinal teas licensed or registered as medicinal
products contain PAs as well.
Pyrrolizidine alkaloid
Study design: One hundred sixty-nine different commercially available medicinal teas, i.e. 19 nettle (Urtica
Medicinal herbal tea
dioica L.), 12 fennel (Foeniculum vulgare Mill.), 14 chamomile (Matricaria recutita L.), 11 melissa (Melissa
officinalis L.) and 4 peppermint (Mentha piperita L.) teas as well as 109 tea mixtures were analyzed for the
presence of 23 commercially available PAs.
Method: LC/MS was used for the determination of the PAs
Results: In general, the total PA contents ranging 0–5668 μg/kg. Thirty percent of the tested single-ingredienttea products and 56.9% of the tested medicinal tea mixtures were found to contain PA concentrations abovethe limit of quantification (LOQ) of 10 μg/kg. In 11 medicinal teas PA contents >300 μg/kg dry herb weredetermined thus exceeding the recommended limit for PA intake by BfR. In addition three products of theinvestigated tea mixtures revealed extremely high PA contents of 4227, 5137, and 5668 μg/kg. Generally,single-ingredient tea products contained much less or even no detectable amounts of PAs when compared tothe tea mixtures. PAs in the range between 13 and 1080 μg/kg were also detected in five analyzed aqueousherbal infusions of the medicinal tea mixture products with the highest PA content. Two out of the fiveinvestigated herbal infusions exceeded the recommended BfR limit for PA intake.
Conclusion: This study demonstrates clearly that also medicinal teas licensed as medicinal products maypartly contain high amounts of PAs exceeding current recommendations. For that reason manufacturers areadvised to carry out more rigorous quality control tests devoted to the detection of PAs. This is very importantto minimize PAs in medicinal teas accounting for possible additional exposure of the consumer to PAs fromother food sources (e.g. honey).
2015 Elsevier GmbH. All rights reserved.
Up to date, more than 600 different PAs have been de-scribed
Pyrrolizidine alkaloids (PAs) constitute a group of heterocyclic
Several PAs have been found to cause hepatotoxic, mutagenic and
compounds naturally occurring in a wide variety of plants, mostly
cancerogenic effects – accounting for the toxicological relevance of
Asteraceae, Boraginaceae and Fabaceae They are esters
of hydroxylated methylpyrrolizidines (referred to as necine bases)
and aliphatic mono- or dicarbonic acids (referred to as necine acids)
tain structural requirements have toxic effects. These are all alka-loids derived from 1-hydroxymethyl-1,2-dehydropyrrolizidine withthe primary hydroxymethyl group being esterified with one branched
∗ Corresponding author. Tel.: 49 6196 937 955; fax: +49 6196 937 861.
E-mail address: (M. Abdel-Tawab).
0944-7113/ 2015 Elsevier GmbH. All rights reserved.
M. Schulz et al. / Phytomedicine 22 (2015) 648–656
tissue, fibrosis, nodular regeneration, cirrhosis and subsequent liverfailure may occur Symptoms include colickyabdominal pain, vomiting and diarrhea, ascites (within days), en-largement and induration of the liver (within weeks) and in somecases hematemesis Mortality following PA ingestionoccurs due to liver failure or complications arising from cirrhosis likerupture of esophageal varices Cases of suspected PA poisoning have been reported from both de-veloping and industrialized countries An overviewidence, that PAs not only cause hepatotoxicity but also possessmutagenic and carcinogenic properties. To the best of our knowl-edge no data are available with regard to the long-term follow-up of humans exposed to PAs. The frequent occurrence of liver tu-mors in certain regions of Central and South Africa, however, is as-cribed, at least in part, to the consumption of PA containing herbs
Despite the pronounced toxicity of PAs, only little regulatory
guidance concerning limits of intake of PAs for medicinal prod-ucts, food including food supplements exists. Several EU-memberstates, however, have adopted national regulations on the consump-tion of PAs. In Germany, for example, a graduated plan set up in1992 limits the maximum daily intake of PAs for medicines for in-ternal use to 1 μg for a maximum of 6 weeks/year and 0.1 μgfor medicines with no limited duration of treatment. Evaluatingthe non-cancer effects of PAs, the British "Committee on Toxicityof Chemicals in Food, Consumer Products and Environment" (COT)came to the conclusion that doses of PAs below 0.007 μg/kg bodyweight/day, would unlikely be of concern. Accordingly the GermanFederal Institute for Risk Assessment (BfR) identified that for 1,2-unsaturated PAs a daily intake of 0.007 μg/kg (0.42 μg/60 kg adult)should not be exceeded. Also the EMA/HMPC permits a daily intakeof 0.007 μg PA/kg body weight in its finalized public statement onthe use of herbal medicinal products containing toxic, unsaturatedPAs released in November 2014 With regard tothe mutagenic effects of PAs, the Dutch National Institute for Pub-lic Health and the Environment stated in 2005, that a "VirtuallySafe Dose" (VSD) for PAs would be 0.00043 μg/kg body weight/day
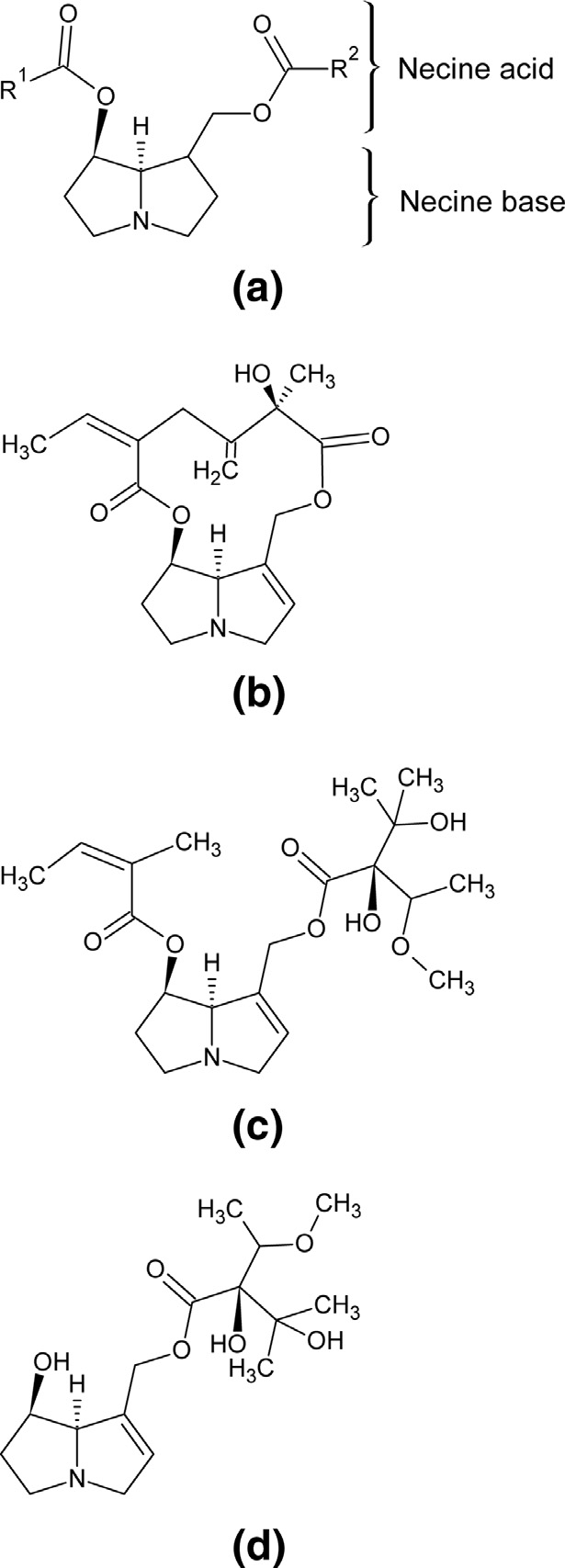
Fig. 1. Chemical structures of PAs showing (a) structural requirements for toxicity
Recently, the BfR conducted a study to assess the content of PAs
and exemplary structures of (b) seneciphylline as example of PA with cyclic diester,
in 184 tea products marketed as food as well as in 37 medicinal
(c) lasiocarpine as example of a diesterified PA and (d) europine as example of a
teas from pharmacies Even though none of the plants,
monoesterified PA.
which had been used for the tea-products endogenously producesPAs, the BfR found up to 3430 μg PAs/kg dried herbal material. Nodistinguishment was made by the BfR between medicinal teas and tea
group is present in position C-7 Exemplary structures for
products marketed as food in reporting the results. The BfR concluded
diesterified and monoesterified PA are shown in , c and d, re-
that consumers drinking tea regularly and with a tendency to stick
to a certain (supposedly contaminated) brand product might be at
PAs are readily absorbed from the intestine and partly hydrolyzed
increased risk. As a worst case scenario, the BfR estimated that adults
by esterases. The resulting cleavage products of necine bases and
might consume as much as 0.144 μg PA/kg body weight per day, hence
necine acids are relatively non-toxic and believed to be renally ex-
greatly exceeding the aforementioned limits. In another study of the
creted. The majority of PAs, however, is metabolized by liver mono-
BfR on 274 tea samples total PA concentrations up to 5647 μg/kg
As all previous studies focused on herbal tea products sold as food
and nucleic acids. Due to the relatively high reactivity of these
two independent, not-for-profit organizations, the Drug Commission
metabolites, damage is mainly confined to the liver but may also
of German Pharmacists (AMK) and the Central Laboratory of German
affect extrahepatic blood vessels and the lung, leading to pulmonary
Pharmacists (ZL) carried out the present study on herbal tea products
hypertension. Other organs less frequently affected by PA toxicity in-
licensed or registered as medicinal products to elucidate whether
clude the kidneys, the gastro-intestinal tract, the pancreas and bone
licensed medicinal teas contain PAs as well. In contrast to herbal
teas marketed as food for which good manufacturing practice isn't
In man, ingestion of a toxic dose of PAs corresponding to
always guaranteed registered and licensed medicinal herbal teas are
0.015 mg/kg of body weight per day causes acute veno-occlusive
subject to intense quality control measures. For that reason they are
disease For a 70 kg adult, that would
generally considered to be safe. Similar to the study conducted by the
correspond to 1 mg total PAs per day. As a consequence of ve-
BfR, none of the investigated plants is known to produce PAs on its
nous occlusion and restricted blood flow, necrosis of the surrounding
M. Schulz et al. / Phytomedicine 22 (2015) 648–656
Material and methods
Chemicals and solvents
Based on that background, 2.5 g of each herbal product were
ground and sieved through a sieve of 500 μm mesh, then through
All chemical reagents were purchased from Merck, Roth
a sieve of 300 μm mesh. Afterward 2 × 1 g of the ground herb were
or Sigma–Aldrich and were of analytical grade. All solvents
extracted with 10 ml 0.05 M sulfuric acid for 15 min on a vertical
used were of HPLC–MS grade purity. The standard substances
shaker at 200 rpm, followed by sonication for 15 min in an ultrasonic
for the tested PAs echimidine, erucifoline, erucifoline-N-oxide,
bath. Afterward the samples were centrifuged at 4618 g at -4 °C.
europine, europine-N-oxide, heliotrine, heliotrine-N-oxide, inter-
The supernatant was removed and the residue was extracted again
medine, jacobine, jacobine-N-oxide, lasiocarpine, lasiocarpine-N-
with 10 ml 0.05 M sulfuric acid as mentioned before. Both super-
oxide, lycopsamine, monocrotaline, monocrotaline-N-oxide, retror-
natants were combined and adjusted to pH 6–7 with 6.3% ammoniac
sine, retrorsine-N-oxide, senecionine, senecionine-N-oxide, seneci-
phylline, seneciphylline-N-oxide, senkirkine, and trichodesmine
After filtration through a Rotilabo R� -Fibre glass syringe filter (Roth,
were acquired from PhytoLab, Germany. Propranolol hydrochloride
Germany), 10 ml of the neutralised combined extract was subjected
(100.5% purity), used as control standard was purchased from Fagron,
to SPE using DSC-C18 SPE cartridges (500 mg, Supelco, Germany)
Germany. Blank plant material was donated by BfR, Germany, which
preconditioned with 5 ml methanol followed by 5 ml water. Then
is gratefully acknowledged.
the cartridges were washed two times with 5 ml water, and driedunder vacuum for 5–10 min. Elution was performed with 2 × 5 ml
methanol. The eluate fraction was dried under a gentle stream of ni-trogen at 50 °C and redissolved by shaking with 1 ml methanol/water
For this study, 169 different commercially available medicinal
(5/95, v/v). Finally, 100 μl of control standard solution (1 mg/ml pro-
teas licensed or registered in Germany including 60 single-ingredient
pranolol hydrochloride in methanol:water, 5:95, v/v) were added
herbal tea products as well as 109 tea mixture products were pur-
and the samples centrifuged for 15 min at 4000 rpm (3345 g)
chased from the wholesaler Alliance Healthcare Deutschland AG,
Frankfurt am Main, Germany. The investigated assortment comprised
The standard stock solutions for calibration were prepared by dis-
19 single-ingredient medicinal tea products containing dried net-
solving 15 mg of each PA reference standard in 25 ml acetonitrile
tle leaves (Urtica dioica subsp. dioica), 12 single-ingredient prod-
yielding a concentration of 0.6 mg/ml. The standard working solution
ucts containing dried fennel fruits (Foeniculum vulgare subsp. vulgare
(PA-Mix) representing a mix of all PA reference standards at a con-
(Mill.) var. vulgare (Mueller) Thellung), 14 single-ingredient products
centration of 1 μg/ml each was prepared by pipetting the respective
containing dried chamomile flowers (Matricaria recutita subsp. re-
volume of each PA stock solution into a volumetric flask and filling up
cutita (L.) Rauschert), 11 single-ingredient products containing dried
to mark with acetonitrile.
Melissa leaves (Melissa officinalis subsp. altissima (Sm.) Arcang.), and 4
In order to compensate for possible matrix effects, calibration so-
single-ingredient products containing dried peppermint leaves (Men-
lutions were prepared by spiking reconstituted blank herbal extracts
tha × piperita var. officinalis Sole.) in addition to the investigated tea
(free of PAs) with the respective volume of PA-Mix covering a con-
mixtures. All plant names have been checked with the plant list on
centration range of 10–300 μg/kg and 100 μl of the internal stan-
produced by the Royal Botanic Gardens,
dard solution. For that purpose blank plant material mix composed of
the Missouri Botanical Garden and other collaborators worldwide. De-
equal amounts of peppermint, chamomile, caraway and fennel was
pending on the indication, tea mixtures contained one of the above
processed exactly as described before for the medicinal teas.
mentioned herbs selected for the single-ingredient products in addi-tion to for example valerian, hop cones, lavender flowers in case of
sleeping teas or in addition to for example caraway, anise, corianderin case of gastrointestinal teas.
Liquid chromatography was performed on an Agilent 1200 series
The choice of samples was meant to reflect the huge variety of
HPLC system equipped with a gradient pump with vacuum degasser,
medicinal teas containing the aforementioned herbal ingredients.
an autosampler and a column oven. A Thermo Hypersil Goldۚ C18 col-
Further, it was intended to incorporate at least one sample from each
umn (150 × 2.1 mm; 1.9 μm; Thermo scientific, Germany), and an
manufacturer of medicinal teas. Being licensed medicinal products
upstream VICI-Inline Filter, ID 0.75 μm with frit (VICI, Switzerland)
the manufacturer is responsible for ensuring the traceability and re-
were used for chromatography. Mobile phase A was prepared by dis-
producible quality of every processed herbal material. All tested tea
solving 315 mg ammonium formate and 1 ml formic acid (98–100%)
products were assigned voucher numbers and representative voucher
in 999 ml water. Mobile phase B was prepared by dissolving 315 mg
specimen have been deposited in the Central Laboratory of German
ammonium formate, 5 ml water, 1 ml formic acid (98–100%) in 994 ml
Pharmacists, Eschborn, Germany.
methanol. After injection of 10 μl, separation was achieved using agradient program starting with 95% mobile phase A and 5% mobile
phase B for 0.5 min, changing to 65% mobile phase A within 11.6 min.
This gradient was held constant for 4.4 min and was then changed to
The analysis of PAs was performed according to the analytical
20% mobile phase A within 0.5 min, which was kept constant again
method applied by the BfR in its study on PA content in tea products
for 3 min. Afterward the gradient was changed to 0% mobile phase
marketed as food with minor modifications. In brief, it is based on
A within 0.2 min and was kept 9.8 min at this level. Finally, mobile
acidic extraction, clean-up and enrichment of PAs by means of solid
phase A was increased to 95% within 0.1 min and kept constant for
phase extraction (SPE) followed by liquid chromatographic separa-
9.9 min till the end of the run. The total run time was 40 min at a
tion and mass spectrometric detection (LC–MS/MS) Using
flow rate of 0.3 ml/min. The column oven was set to 40 °C and the
diluted aqueous acid as extraction solvent allows simultaneous pro-
autosampler was cooled to 20 °C.
filing of both the free bases and the N-oxides from the herbal product
MS analysis was performed in the positive multiple reaction
in contrast to other extraction procedures like Soxhlet extraction de-
monitoring (MRM) mode on an Agilent Triple Quadrupole LC/MS
scribed for the determination of PAs Moreover
6410 series (Agilent Technologies, Germany) equipped with an
the combination of SPE with LC–MS/MS represents a powerful and
Electro Spray (ESI) Ionization source. Dwell time was chosen to
sensitive procedure for generating a complete PA profile of the tested
M. Schulz et al. / Phytomedicine 22 (2015) 648–656
following equation:
Mass transfers and retention times for the individual PAs.
Content PA = β × DF = y − b × 1/a × Vextract/msample
Retention time [min]
× 1/Vapplied × Vsample
where β = concentration in ng/ml, DF = conversion factor from ng/ml
to μg/kg, y = peak area of the respective PA, b = axis intercept of the
calibration curve, b = slope of the calibration curve, Vextract = volume
of the extraction solvent, msample = mass of the weighted sample,
Vapplied = volume of the extract applied to SPE, Vsample = end volume
of the sample [ml]. The total PA content represents the sum of the
individual PA contents determined.
The control standard propranolol hydrochloride was added in or-
der to control the chromatographic system.
The analytical method applied in this study had been extensively
validated by BfR. The validity of the analytical method had been fur-
ther established in the frame of an international collaborative trial
carried out by BfR based on the ISO/IUPAC/AOAC protocol involving
24 different laboratories testing six tea samples purchased from re-
tail markets in Berlin containing PAs and one spiked recovery sample.
The relative repeatability standard deviation (RSDr) indicating the re-
peatability of the first and second measurement of a sample in one lab-
oratory ranged between 5.7% (retrorsine-N-oxide) and 9.8% (seneci-
phylline) and the relative reproducibility standard deviation (RSDR)
comparing analytes and test samples tested by different laborato-
ries ranged between 18.4% (lasiocarpine-N-oxide) and 27.3% (seneci-
phylline). In addition the Horwitz Ratio (HorRat value) describing the
ratio between the reproducibility standard deviation and the pre-
dicted reproducibility standard deviation (PRSD), which is calculatedfrom the Horwitz equation, was determined.
whereas PRSD = 2(1-log MR/2) and MR is the normalized mean value.
This performance parameter is used as measure to evaluate the
acceptability of analytical methods with respect to among-laboratory
precision (reproducibility) Values ࣘ0.5 point out,
that method reproducibility may be in question due to lack of study
independence, values >2.0 are a sign for a problematic method re-
producibility, and values ࣘ1.5 reflect a method reproducibility as
normally would be expected. The HorRat values determined in the
frame of the collaborative study ranged between 0.8 for lasiocarpine-
N-oxide and 1.2 for seneciphylline indicating sufficient precision and
a good inter-laboratory comparability of the applied method for the
detection of PAs. The recovery rate was determined by spiking a PA-
free herbal mixture with a PA-mix of known concentration. The ob-
tained recoveries ranged between 76% for lasiocarpine and 125% for
senkirkine. According to the AOAC-International Guidelines for Stan-
dard Method Performance Requirements (2012) they are considered
to be sufficient for reliable analysis.
Based on the excellent HorRat values and the above mentioned re-
sults for recovery and precision the method revealed to be applicable
In total, 23 PAs which are commercially available as reference
for the determination of PAs in herbal products.
substances were determined in this study. Each PA was identifiedby comparing the retention time with that of the standard sub-
Preparation of herbal infusions
stances and the detection of two substance-specific fragment ions (see). Due to the large number of samples and analytes all PA con-
Following the manufacturer's instruction one teabag of the five
centrations determined in this study are unique values generated by
most contaminated medicinal tea products was extracted by adding
a single analysis of the respective sample. Based on the validation
150 ml of boiling water and leaving to brew for 15 min. Then 10 ml
results mentioned below a maximum variability of ±27.3% may be
of the tea preparation was subjected to SPE as described under sec-
assumed. The Mass Hunter software (Agilent, Germany) was used for
tion "Sample preparation" and quantified by LC–MS/MS as described
data acquisition and processing.
under section "LC–MS/MS analysis".
A representative chromatogram is shown in Quantification was carried out using the external standard method
Results and discussion
by comparing the peak areas obtained for the individual PA intea samples with those in the calibration solution. The final PA
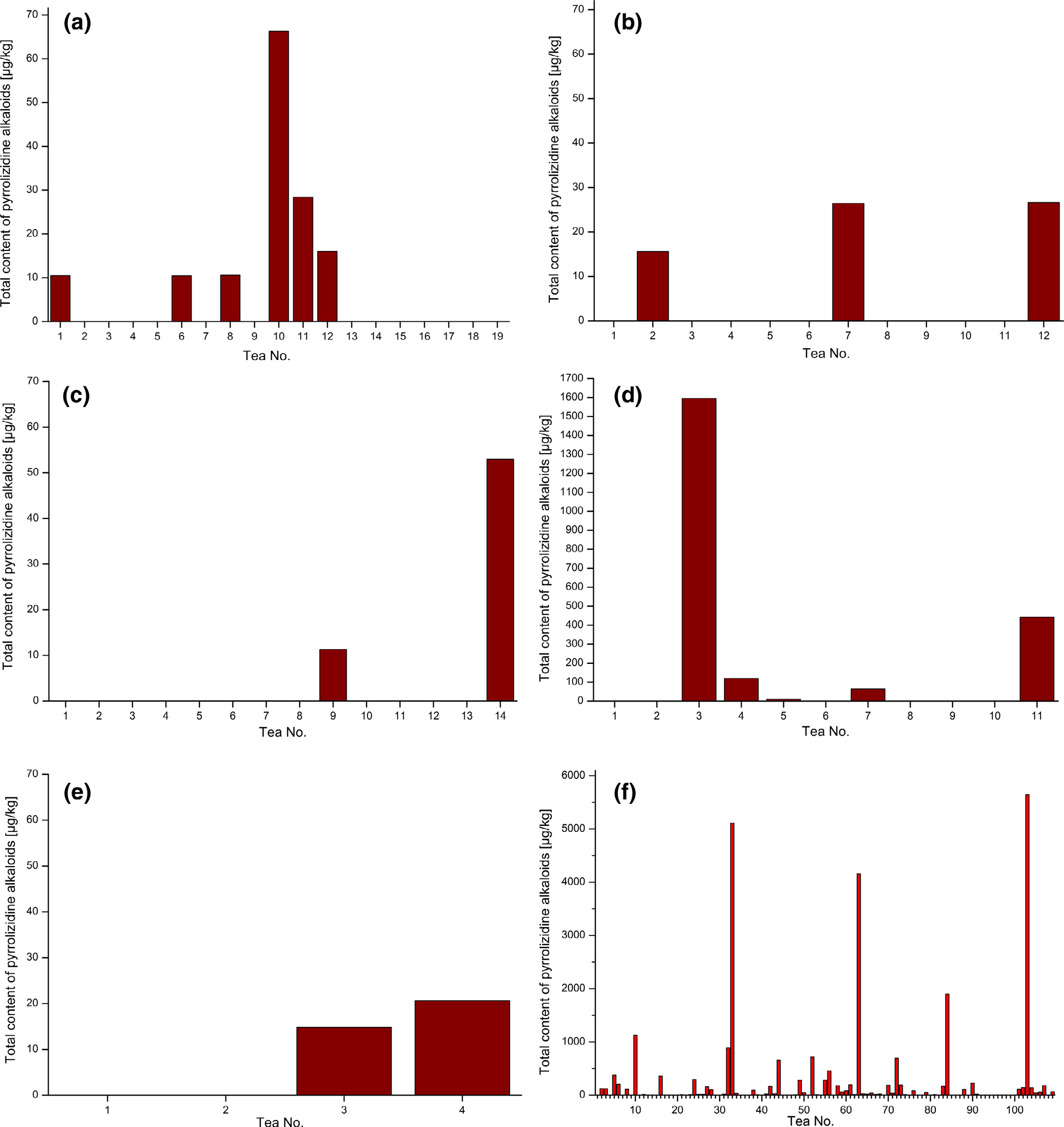
An overview of the PA content determined in nettle, fennel,
content expressed as [μg/kg] was calculated according to the
chamomile, melissa, peppermint and in the tea mixtures is presented

M. Schulz et al. / Phytomedicine 22 (2015) 648–656
Fig. 2. Representative chromatogram of the PAs analyzed. The signals of the individual PAs correspond to 250 μg/kg.
in b, c, d, e and f, respectively. A great variety of the PA con-
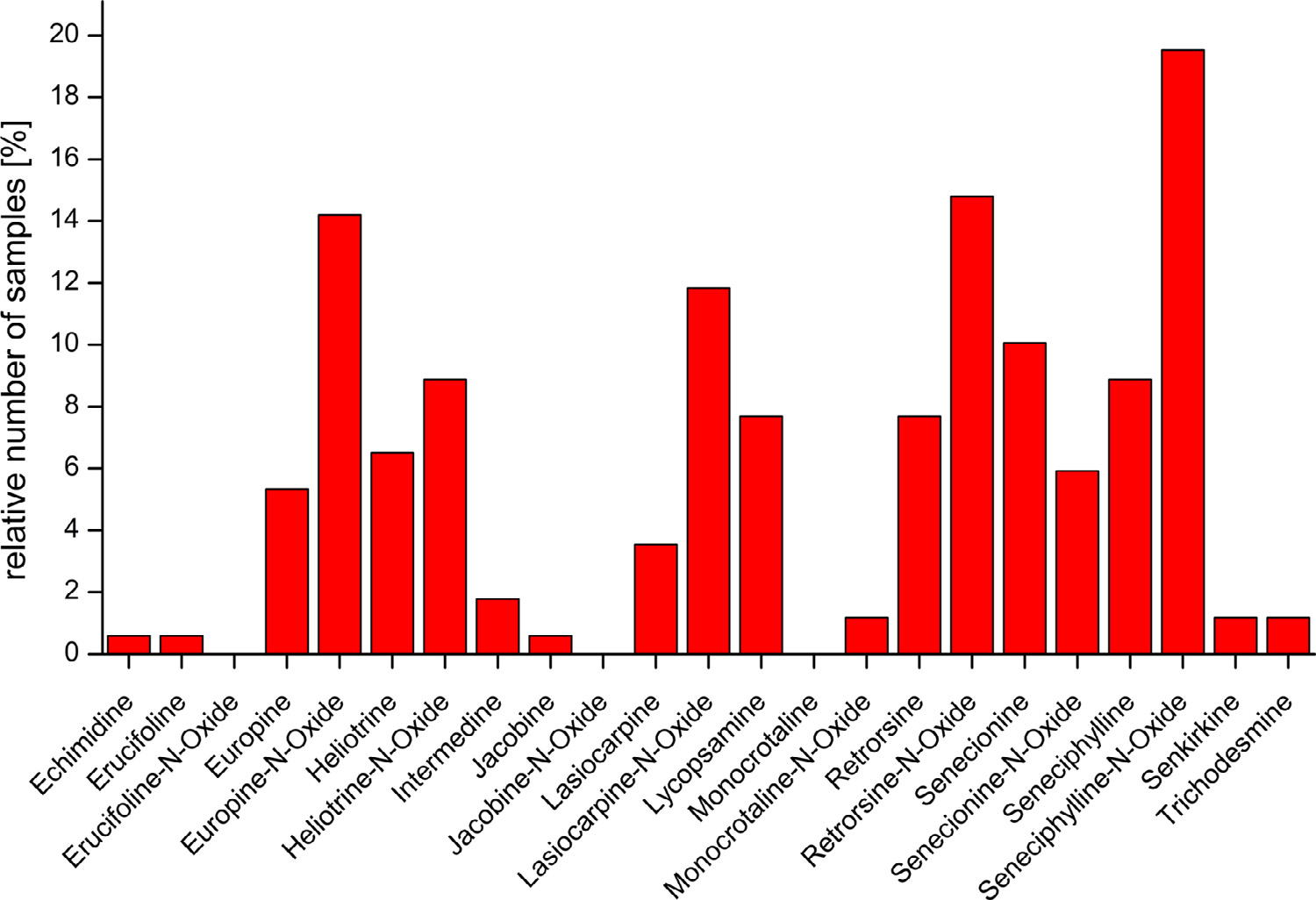
esters like monocrotaline, monocrotaline-N-oxide and trichodesmine
tent within the same species can be noticed. This is mainly attributed
tested in this study could not be detected in any investigated prod-
to the fact, that the samples originate from different manufacturers
ucts with the exception of two fennel products containing 16 μg/kg
and consequently have experienced different cultivation, harvesting,
monocrotaline-N-oxide and 16 μg/kg trichodesmine, respectively.
storage and transport conditions.
In addition lasiocarpine-N-oxide, europine-N-oxide and heliotrine-
Both, the free PA bases and their corresponding N-oxides were de-
N-oxide were most frequently detected.
tectable with the N-oxides present in larger amounts. In general, the
In general the tea mixtures revealed the highest percentage
total PA contents ranged from 0 to 5668 μg/kg, being <300 μg/kg in
of products containing PAs. With regard to the single-ingredient
most products. Nevertheless, 30% of the tested single-ingredient tea
tea products fennel and chamomile revealed the lowest share and
products and 56.9% of the tested medicinal tea mixtures were found
melissa, and peppermint the largest share of samples with PA find-
to contain PA concentrations above the LOQ of 10 μg/kg. In eleven
ings. It becomes also apparent that melissa teas tend to have higher
products PA contents >300 μg/kg were determined and three addi-
PA contents on average compared to the other single-ingredient tea
tional products revealed extremely high PA contents of 4227, 5137
and 5668 μg/kg. Generally, single-ingredient tea products contained
Our study coincides with the BfR study "Pyrrolizidine alkaloids in
much less or even no detectable amounts of PAs when compared to
herbal teas and teas" carried out on 221 nettle, fennel, chamomile and
the tea mixtures. This is an indication that the PAs detected in the tea
peppermint teas which is part of a research project on the "Determi-
mixtures may not necessarily result primarily from the five herbs net-
nation of pyrrolizidine alkaloids in food and feed" As it is
tle, fennel, melissa, chamomile or peppermint, and that they rather
the case in the present study, very high PA concentrations were mea-
result from the other herbal components of the mixture that have not
sured by the BfR only in a few individual herbal tea samples. Also in
been subject to PA analysis in this study but were often present in
the BfR study, melissa teas were affected most by PA contamination.
even larger amounts.
In contrast, however, to the present study 91.7% of the 12 investi-
As can be seen in the PAs identified in the samples of
gated nettle teas were contaminated with PAs being thus comparable
the present study included also PAs with cyclic diesters, which are
to peppermint teas with 86.2% of 29 products and chamomile with
thought to be most toxic and carcinogenic Hence,
87.1% of 31 products being affected. Notably, 56.7% of the 30 investi-
seneciphylline, retrorsine, senecionine, and/or their corresponding N-
gated fennel teas in the BfR study were also contaminated with PAs.
oxides represent the cyclic diesters most often detected in the present
For the assessment of possible health risks to consumers it is gen-
study. The highest total amount of these cyclic diesters was detected
erally assumed that PAs migrate completely from the dry material
in a tea mixture and was found to be 3891 μg/kg. The other cyclic di-
into the finished beverage However, with the exception
M. Schulz et al. / Phytomedicine 22 (2015) 648–656
Fig. 3. Total content of PAs in different medicinal teas: (a) nettle, (b) fennel, (c) chamomile, (d) melissa, (e) peppermint, and (f) tea mixtures.
of one study carried out by on the content of
and 0.954 μg per cup of tea in 24 out of 70 tested teas, herbal teas and
symphitine and echimidine in teas prepared from comfrey leaves,
instant teas purchased in Swiss supermarkets and teashops. Never-
no data have been published until a short time ago, which support
theless the knowledge of PA migration from the herbal drug into the
this assumption. Only recently demonstrated
aqueous herbal infusion is still very limited. To address this limitation
on the example of nine PAs that PA concentrations in tea beverages
the present study determined PAs in tea preparations. Herbal infu-
prepared with boiling water were similar to the PA concentrations
sions were prepared from five tea products, which had been found
determined in the herbal material following acidic extraction indi-
to contain the highest PA content (1127–5137 μg/kg), according to
cating complete PA migration into the herbal infusion. In this study
the manufacturer's instruction. The total PA content determined in
the sum of the nine targeted PA concentrations ranged between 0.021
the aqueous herbal infusions ranged between 13 and 1080 μg/kg. For
M. Schulz et al. / Phytomedicine 22 (2015) 648–656
Fig. 4. Overview on the PAs detected in medicinal tea products.
Table 2
Overview of the maximum, and mean amount of total PAs determined in medicinal herbal teas and the relative number
of affected samples.
Relative number of
samples with PA [%]
better comparability, the PA concentrations in the aqueous herbal
The idea that herbal drugs are generally safe and free from side
infusions have been converted from μg/L to μg/kg.
effects has been disproved for a long time as reflected by the with-
In this context, it has to be pointed out, that the procedure for sam-
drawal of many herbal drugs in Germany from the market includ-
ple preparation and the LC–MS/MS method have not been validated
for their applicability to aqueous herbal infusions. As, however, the
dilute aqueous acid extract of the tea products is being neutralized
been associated with an ingestion of PA-containing plants as herbal
to yield the same pH as the aqueous herbal infusion, no great differ-
ences in the matrix composition following the preparation of aque-
ous herbal infusions and dilute aqueous acid extraction of the dry tea
herbal medicines and traditional
Indian medicine including Ayurveda
Investigation of targeted PAs in traditional Chinese Medicines and
vide a good preliminary estimate on PA migration from the dry herbal
selected herbal teas in Ireland by revealed PA con-
tea product into the aqueous infusion. Based on these results it may
tents ranging between 13 μg/kg and 3668 μg/kg in 78% of the inves-
be assumed that the PAs contained in medicinal tea products in fact
tigated Chinese medicines and 10–1733 μg/kg in 50% of tested herbal
migrate into the aqueous herbal infusion being thus ingested after
teas. Whereas the presence of PAs in herbal teas of PA-containing
consumption. As in the case of the medicinal tea products, mostly the
more hydrophilic N-oxides of the PAs were detected in the aqueous
herbal infusions. In general, the total PA content in the aqueous herbal
mainly focused in the past on honey and herbal
infusion was found to be relatively smaller than that of the respec-
teas marketed as food The present study focused on de-
tive dry herbal product. Moreover the N-oxides of the highly toxic
termining the PA content in licensed medicinal teas which meet the
and carcinogenic PAs with cyclic diesters (seneciphylline-N-oxide,
requirements of pharmacopoeias and are frequently believed to be of
senecionine-N-oxide, retrorsine-N-oxide) have been also detected in
higher quality than herbal teas marketed as food. The large number
the aqueous infusions. This finding is of particular relevance, since
of PA-positive medicinal teas identified in the present study includ-
orally ingested N-oxides of PAs have similar hepatotoxic properties
ing some samples with extremely high PA contents is striking, as
as their parent alkaloids, following their reduction to the correspond-
all of the investigated medicinal teas are not known to inherently
contain PAs. Hence, the high PA content in medicinal teas may be
rather attributed to contamination of the medicinal herbs with one
M. Schulz et al. / Phytomedicine 22 (2015) 648–656
or several PA-containing plants during cultivation, harvesting, stor-
and herbal tea products marketed as food in drugstores and super-
age and/or transport This is
markets. The present study focused on herbal tea products licensed
not surprising as many PA-containing plants like Senecio jacobaea
as medicinal teas and revealed that also high quality medicinal teas
(tansy ragwort) are foreign invasive weeds that invade pastures and
generally believed to be safe may partly contain high amounts of PAs
fields or expand on field edges and thus may accidentally contami-
exceeding current safety recommendations, resembling thus tea sam-
nate medicinal herbs. This might also have an impact on the miling
ples marketed as food. This is an important insight for the consumer,
and homogenization process during the sample preparation of the
who might switch to those high quality tea products to minimize
medicinal tea products for analysis. Hence depending on the physical
the risk of PA exposure arising from herbal tea products marketed
properties of the contaminating plant or part of the plants in the tea
as food. Based on that background additional more rigorous quality
samples, the distribution of PAs may vary, which might be associated
control tests should be implemented to detect and minimize possi-
with a great variation in the PA content within different samples of
ble PAs in medicinal teas, so that the exposure of the consumer to
the same batch. In future studies it may be thus useful to optimize the
PAs becomes as low as practically achievable. This is also necessary
homogenization process and sampling step for tea samples in order
since possible additional exposure to PAs from other food sources
to reduce the variability in the analytical results. For example it might
(e.g. honey) may occur. For that reason the permitted daily intake of
be useful to increase the used amount of sample and the extraction
0.007 μg/kg body weight recommended by various scientific organi-
volume, keeping the same sample/extraction volume ratio. Another
zations includes herbal tea products and food as well.
possibility might be the reduction of the particle size, leading to amore homogenous distribution of PAs in the tea sample. Yet though
Conflict of interest
this assumption seems reasonable it has to be verified in separatestudies. Nevertheless manufacturers are advised to carry out more
We wish to confirm that there are no known conflicts of inter-
rigorous quality control tests for the presence of PAs in their products
est associated with this publication and there has been no signifi-
in order not to expose consumers to unneeded risks.
cant financial support for this work that could have influenced its
Taking into consideration that in case of a total PA content of
300 μg/kg dry herb a tea bag weighing in average 2 g contains 0.6 μgPAs, the consumption of one cup of tea by an adult is sufficient to
exceed the recommended limit for PA intake of 0.007 μg/kg body
weight/day (corresponding to 0.42 μg/60 kg). The limit of exposure of
0.007 μg/kg/day recommended by BfR is derived from the same point
of departure applying a margin of exposure (MoE) of 10 000 as rec-
ommended by EFSA for the safety assessment of impurities which are
both genotoxic and carcinogenic In the present study, 16
BfR opinion no. 018/2013 of 5 July 2013. Pyrrolizidine alkaloids in herbal teas
medicinal teas revealed PA contents >300 μg/kg dry herb. Moreover,
two out of five tea infusions prepared from the mostly contaminated
medicinal teas were found to contain >300 μg/kg PAs thus exceeding
the recommended maximal intake. These measured concentrations
of PAs correspond to a MOE <117, calculated by dividing the BMDL
value for lasiocarpine (70 μg/kg bw per day) by the exposure value
of one cup of herbal infusion. Being far below 10.000, the determined
MOE value gives cause for great concern. Especially children are at
increased risk because their PA intake relative to their body weight
is higher than that of adults when consuming such high quantities
of PAs. Keeping in mind that the PA content may vary widely even
within the same tea brand, it may be assumed that even higher PA
amounts may be ingested.
Nevertheless it should be pointed out in this context that the tox-
icity of PAs has been mainly demonstrated for purified PAs and their
metabolites. Taking into consideration the complexity of herbal ex-
tracts, it cannot be excluded in principle that synergistic and antago-
nistic actions of the various ingredients in herbal extracts may fortify
EMA/HMPC, 2014. Public statement on the use of herbal medicinal products contain-
or weaken the toxicity of PAs when taken in form of the whole extract.
ing toxic, unsaturated pyrrolizidine alkaloids (PAs). EMA/HMPC/893108/2011.
However reported that toxic DNA adducts were
not only formed after the administration of the isolated PA riddelli-
ing senecionine and seneciphylline exhibited significant cytotoxicity
to HepG2 cells comparable with that of the isolated PAs. Yet no clear
assessment can be made on the comparabilitiy of toxicity of purified
PAs and plant extracts because of the insufficient data available at the
moment. Therefore, until clarity is provided by further studies in the
future, the potential toxicity of tea products containing PAs should be
taken seriously in any case.
Several previous studies highlighted the problem of PA contami-
nation in honey, pollen, traditional Chinese as well as Indian medicine
M. Schulz et al. / Phytomedicine 22 (2015) 648–656
World Health Organization, 1988. Pyrrolizidine alkaloids. Environmental Health
Source: http://s02f60f53ac7845f4.jimcontent.com/download/version/1458469478/module/6298203864/name/Schulz_PA%20in%20med%20herbal%20teas_Phytomedicine%202015.pdf
MINISTERE DE L'EDUCATION NATIONALE REPUBLIQUE DE COTE D'IVOIRE ET DE L'ENSEIGNEMENT TECHNIQUE ********* INSPECTION GENERALE ******** DIRECTION DE LA PEDAGOGIE ET DE LA FORMATION CONTINUE Mot de Madame la Ministre de l'Education Nationale et de l'Enseignement
α-Glucosidase inhibitory and antidiabetic activity of Pisonia alba International Journal of Integrative Biology A journal for biology beyond borders ISSN 0973-8363 α-Glucosidase inhibitory and antidiabetic activities of ethanolic extract of Pisonia alba Span. leaves Sunil Christudas1,*, Latha Gopalakrishnan2, Palanisamy Mohanraj 3,






