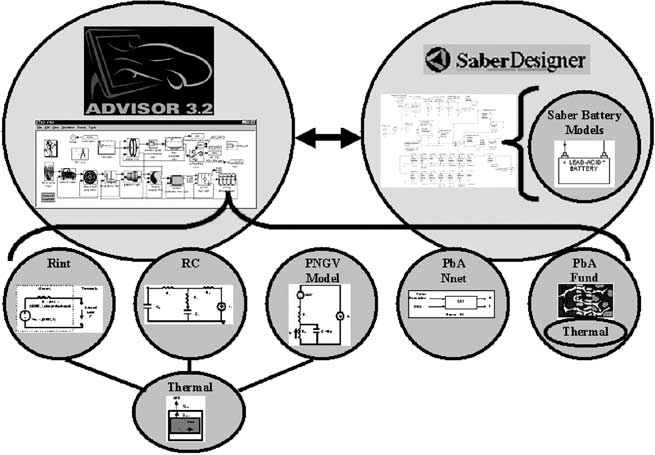
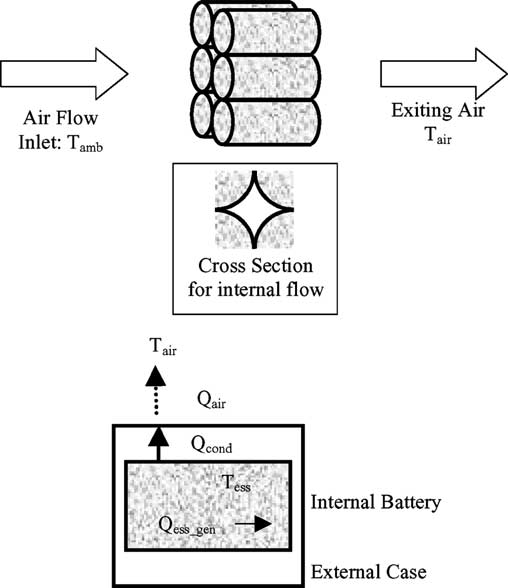
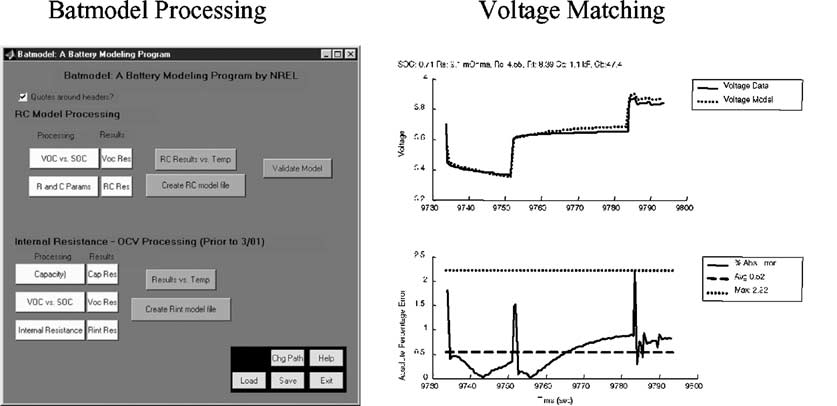
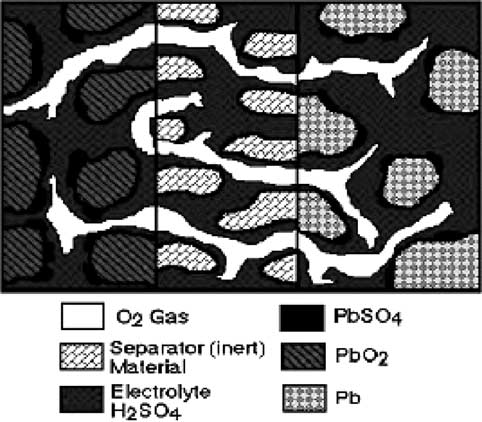
Classification of brain tumor type and grade using mri texture and shape in a machine learning scheme
Magnetic Resonance in Medicine 62:1609 –1618 (2009) Classification of Brain Tumor Type and Grade Using MRITexture and Shape in a Machine Learning Scheme Evangelia I. Zacharaki,1,2* Sumei Wang,1 Sanjeev Chawla,1 Dong Soo Yoo,1,3Ronald Wolf,1 Elias R. Melhem,1 and Christos Davatzikos1 The objective of this study is to investigate the use of pattern