Basharesearch.com

3rd World Conference on
Applied Sciences, Engineering & Technology
27-29 September 2014, Kathmandu, Nepal
Environmental Risks of Some Non-steroidal Anti-inflammatory
Drugs (NSAIDs) in Surface Water in Ho Chi Minh City
O VU HOANG ANH , BUI QUANG MINH , PHAM HONG NHAT
1Institute of Environmental Technology, Vietnam Academy of Science and Technology, Vietnam
2Vietnam Institute for Tropical Technology and Environmental Protection, Ho Chi Minh City, Vietnam
E-mail: [email protected]
Abstract: An environmental risk assessment of some common NSAIDs including ketoprofen, ibuprofen,
diclofenac sodium and mefenamic acid for surface water in Ho Chi Minh City was performed by determination
of their concentrations in water environment (MEC). Risk levels were obtained from the comparison between
the MEC value and predicted no-effect concentration (PNEC). Analysis results of the surface water samples
collected in Ho Chi Minh City during September, October and November 2013 showed that the environmental
risk levels of ketoprofen, ibuprofen and diclofenac sodium were not high except for ibuprofen at Le Van Sy
Bridge in the first two surveys. Mefenamic acid has consistently been identified as high risk at almost all
sampling sites. The total risk level of the 4 compounds in surface water in Ho Chi Minh City has been found at
high risk levels.According to the hazard index (HI) values calculated from chronic daily intake (CDI) and
reference dose (RfD), however, it is clear that the studied compounds at the existing concentrations cannot pose
negative impacts on human health even if the surface water is used for domestic water supply purpose. In the
long run, however, if the use of these compounds increase and no proper management measures are applied,
their residues in surface water may become a serious problem.
Keywords: Environmental Risk Assessment, Non-Steroidal Anti-Inflammatory Drugs (Nsaids), Surface Water
1. Introduction:
Pharmaceuticals and Personal Care Products (PPCPs)
residues present in the environment are now common
and recognized as a problem. Thousands of ton of
pharmacologically active substances are used every
year over the world to fight diseases or to face the
stresses of modern life. Studies conducted in
Australia,
Germany, Greece, Italy, Spain, Switzerland, the Netherlands and the United States had found the presence of more than 80 compounds including pharmaceuticals and its metabolites in aquatic environment [1]. Notably, Non-Steroidal Anti-
Inflammatory Drugs (NSAIDs) from various sources
has been increasingly identified in the environment.
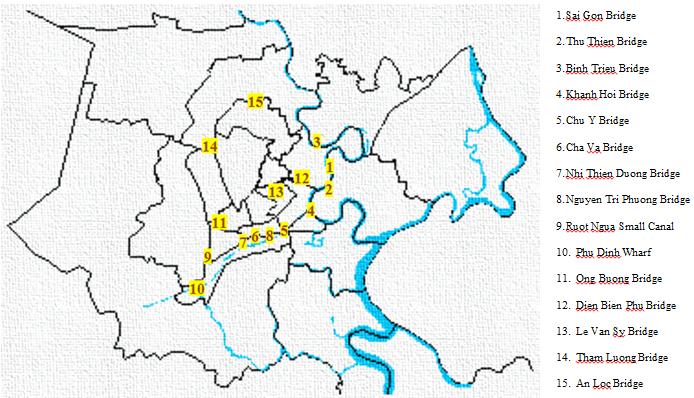
Figure 1. Sampling sites
These NSAIDs account for many cases of ulcers and
Samples were collected at the depth of 0.25 to 1.0
intestinal perforation in chronic users for pain and
meter from the surface of water and stored in 2.5-liter
inflammation. Therefore, PPCPs in general and
amber glass bottles at 4 0C until sample treatment [4].
NSAIDs residues in particular in the environment,
especially in water environment, should gain more
2.2. Sample preparation and analysis [5]
attention from us all. In fact, NSAIDs residues have
2.2.1. Chemicals and instrument:
been intensively studied around the world whereas
Acetic acid (Merck), acetonitrile (Labscan), n-hexane
they are still quite new to Vietnam. Subsequently, an
(Labscan), methanol (Merck), ethyl acetate (Merck),
environmental riskassessment of most commonly
ultrapure water (Millipore), ketoprofen, ibuprofen,
Anti-inflammatory
diclofenac sodium and mefenamic acid standard
(NSAIDs) in surface water in Ho Chi Minh City is
(IDQC, purity >99.9 %). Stock solutions of 1,000
necessary to provide a scientific foundation for future
mg/L were prepared in methanol and stored at 4 0C.
in-depth studies in thisdensely populated area.
Working solutions were prepared by diluting the
stock standard solutions in methanol. Solid phase
2. Materials and methods:
extraction system (Agilent) with vacuum pump and
2.1. Sampling:
Poly-Sery PSD (Poly styrene divinylbenzene) SPE
Based on the monitoring points of surface water at
tubes (250 mg, 6 mL) of DNW Technologies GmBh.
Ho Chi Minh City [2], tidal levels [3] and weather
LaChrom Hitachi HPLC system with Inspire C18
conditions, samples were taken at 15 locations
(250 mm x 4.6 mm i.d., 5 m) column protected by
spotted in Figure 1 within 3 surveys (September 23th
an Inertsil ODS-3 (10 mm x 4.0 mm i.d., 5 m) guard
2013, October 21th 2013 and November 19th 2013).
WCSET 2014164 BASHA RESEARCH CENTRE. All rights reserved.
DO VU HOANG ANH, BUI QUANG MINH, PHAM HONG NHAT
2.2.2. Sample preparation:
500 mL samples were filtered through a 0.45 m
MEC= measured environmental concentration
cellulose acetate membrane filter to remove
PEC= predicted environmental concentration
suspended matters. The SPE cartridges were
PNEC= predicted no effect concentration
conditioned with 6 mL ethyl acetate, 6 mL methanol
- RQ = 0.01 - 0.1: low risk
and 6 mL ultrapure water. 500 mL samples were then
- RQ = 0.1- 1.0: medium risk
transferred to the SPE cartridges. The loaded
- RQ > 1.0: high risk
cartridges were rinsed with 3mL of methanol:water
To anequitoxic mixture (a mixture where each
(5:95, v/v) solution and 3 mL n-hexane. After the
chemical contributes the same to toxicity): a sum risk
enrichment step, the cartridges were vacuum dried.
quotient (RQmix) is total of risk quotients of the
The elution was performed with three 3-mL aliquots
individual pharmaceuticals (RQi)
of ethyl acetate. Extraction was done under vacuum
at the flow rate of 3 mL/min. The effluent was dried
under the stream of nitrogen. The residues were
dissolved in 0.5mL methanol and injected into the
PNEC values of ketoprofen, ibuprofen, diclofenac
2.2.3. Analysis:
sodium and mefenamic acid are 15.6 g/L, 5.0 g/L,
After solid phaseextraction, samples were injected on
13.5 g/L and 0.428 g/L respectively [7, 8, 9, 10,
HPLC-DAD system under a defined condition (Table
Table 1.HPLC-DAD conditions.
2.3.2. Hazard Index (HI) [13]:
Parameters
Health risk can be assessed by Hazard Index (HI) as follows:
Injection volume
Mobile phase (isocratic)
CDI = chronic daily intake for the ith toxicant in
mg/kg-day, and RfD = chronic reference dose for the ith toxicant in
Oven temperature
NOAEL= no-observed-adverse-effect level
Ufinter = interspecies uncertainty factor
Ufintra = intraspecies uncertainty factor, and
Ufother = additional uncertainty factors
Method detection limit (MDL), method
quantitation limit (MQL) and recovery (H) are
3. Results and discussions
presented in Table 2.
Based on calculated risk quotients (RQ) and total risk
Table 2.MDL, MQL and recovery (H).
quotient (RQmix) of the four studied compounds, the
Compounds
following findings are made:
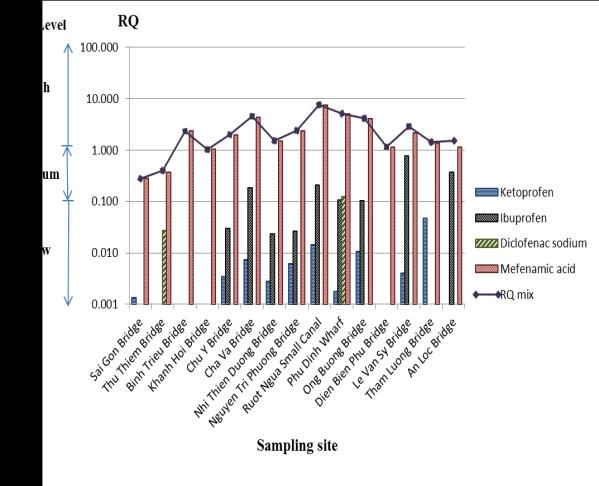
In the first survey, ketoprofen and diclofenac sodium
were found at a low risk level for the studied surface water. Ibuprofen was also at a low risk level except
for at Le Van Sy Bridge sampling site where it was
of a high level. Especially, mefenamic acid wasat
high risk levels in almost all sampling locations
except for only Thu Thiem Bridge where it was at a medium risk level. Total risk quotient of ketoprofen,
ibuprofen, diclofenac sodium and mefenamic acid
2.3. Determination of risk quotient (RQ) and
(RQmix) proved that 14 out of 15 sites were in a high
hazard index (HI)
environmental risk level range whereas the Thu
2.3.1. Risk quotient (RQ) [6]:
Thiem Bridge site was of a medium level.
Environmental risks of NSAIDs for surface water
were determined via risk quotient (RQ), which was calculated by the equation:
Proceedings of the 3rd World Conference on Applied Sciences, Engineering and Technology
27-29 September 2014, Kathmandu, Nepal, ISBN 13: 978-81-930222-0-7, pp 724-727
Environmental Risks of Some Non-steroidal Anti-inflammatory Drugs (NSAIDs)
in Surface Water in Ho Chi Minh City
Figure 2.RQs and RQmix at 15 sampling sites in the
Figure 4.RQs and RQmix at 15 sampling sites in the
first survey
third survey
Data from the second survey show the same trend as
In summary, calculations from ketoprofen, ibuprofen,
of the first survey. Ketoprofen and diclofenac sodium
were also under a high risk range. Ibuprofen at Le
concentrations in surface water in Ho Chi Minh City
Van Sy Bridge was at a medium risk level whereas it
in the three surveys indicates that the environmental
was found at a high risk during the first survey.
risk levels of the first three compounds were not high
Mefenamic acid was identified at high risk levels for
except for ibuprofen at Le Van Sy Bridge in the first
almost all sampling sites except for Saigon Bridge
and second surveys. Mefenamic acid consistently
and Thu Thiem Bridge. Total risk of the four
was of high risk levels at almost all sampling sites.
compounds at Saigon Bridge and Thu Thiem Bridge
Furthermore, total risk levels of the four studied
was of a medium risk level while at the remaining 13
compounds in surface water in Ho Chi Minh City,
sites of a high level. RQs during this survey slightly
which were greatly affected by mefenamic acid risk
decreased as compared to those at the first survey.
levels, were at high levels in most cases. The question is if the surface water is suitable for human drinking and other daily domestic activities. To partly answer this question, hazard index (HI) has been assessed for the four studied compounds. HI values calculated from CDI and RfD for each individual as well as for all the four studied compounds were both smaller than unity. This means that at present no adverse health effect can be caused by the four studied NSAIDs. Therefore,the surface water can still be used as a safe source for drinking and other daily domestic activities. In the future, however, the presence of these compounds in the study surface water needs to be monitored.
The four studied NSAIDs including ketoprofen,
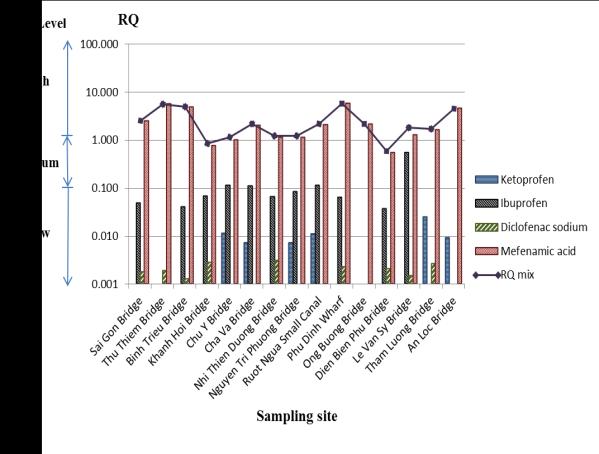
Figure 3.RQs and RQ
ibuprofen, diclofenac sodium and mefenamic acid are
mix at 15 sampling sites in the
second survey
currently present in surface water in Ho Chi Minh
In the third survey, the risk levels of ketoprofen,
City at high environmental risk levels. Although the
ibuprofen, and diclofenac sodium still could not be
surface water is still safe for drinking and other daily
rated as high. Similarly to the earlier surveys, all
domestic activities, the studied compounds might
mefenamic acid risk levels were at a high range
have potential impacts on the aquatic ecosystem in
except for at Khanh Hoi Bridge and Dien Bien Phu
the future if their use consistently increases.
Bridge. Although the total risk quotients of the four
compounds (RQmix) were lower than those in the
The authors acknowledge support from the laboratory
second survey, they were still at high levels except
of the Institute of Environmental Technology, within
for at Khanh Hoi Bridge and Dien Bien Phu Bridge.
the Vietnam Academy of Science and Technology.
Special thanks are to Mr. Nguyen DuyLinh for taking part in the development of analysis method, Ms. Tran Minh Huong for revising the English and Ms. Tran Phuong Lien for editing the text.
Proceedings of the 3rd World Conference on Applied Sciences, Engineering and Technology
27-29 September 2014, Kathmandu, Nepal, ISBN 13: 978-81-930222-0-7, pp 724-727
DO VU HOANG ANH, BUI QUANG MINH, PHAM HONG NHAT
References:
[1]
Thomas Heberer, "Occurrence, fate, and
B. Ferrari, R. Mons, B. Vollat, B. Fraysse, N.
removal of pharmaceutical residues in the
Paxeus, R.L. Giudice, A, Pollio and J. Garric,
aquatic environment: A review of recent
"Environmental risk assessment of six human
research data", Toxicology Letters,131 1-2,5-
pharmaceuticals:
environmental risk assessment procedures
MONRE, NEA, The Environmental
sufficient for the protection of the aquatic
Monitoring Network of Ho Chi Minh City,
environment",Environmental Toxicology and
2009. Retrieved on January 8, 2014 at:
Chemistry, 23 5,1344-1354,2004.
[10] T.H. Fang, F.H. Nan, T.S. Chan and H.M.
Feng,"The occurrence and distribution of
pharmaceutical compounds in the effluents of
a major sewage treatment plant in Northern
Centre of Oceanography, Tide Table 2013,
waters",Marine Pollution Bulletin, 64 7,
Natural Science and Technology Publisher,
1435–1444, 2012.
T. Kosjeket, E. Heath and A. Krbavcic,
Alonso,"Occurrence and risk assessment of
pharmaceutically
inflammatory drug (NSAIDs) residues in
wastewater treatment plants. A case study:
water samples", Environment International, 31
Environment
International, 33 4,596-601,2007.
D.V.H. Anh, B.Q. Minh, N.D. Linh and P.H.
A. Tauxe-Wuersch, L.F. De Alencastro, D.
Nhat, "Determination of non-steroidal anti-
Grandjean and J. Tarradellas,"Occurrence of
inflammatory drugs (NSAIDs) in surface
several acidic drugs in sewage treatment plants
water at Ho Chi Minh City",Journal of
in Switzerland and risk assessment",Water
Science, Natural Sciences and Technology, 30,
Research, 39 9,1761–1772,2005.
[12] U.S. EPA Region 6,Human Health Risk
B. I.Escher, R. Baumgartner, M. Koller, K.
Assessment
Protocol,
Treyer, J. Lienert and C.S. McArdell,
Characterizing Risk and Hazard, 2005.
[13] Department of Health,Acute reference doses
assessment of pharmaceuticals from hospital
for agricultural and veterinary chemicals,
wastewater", Water Research, 45 1, 75-92,
Australian Goverment, Department of Health,
Office of Chemical Safety, 2013.
Stuer-Lauridsen, M. Birkved, L.P. Hansen,
[14] The European Agency for the Evauation of
H.C. Holten Lutzhoft and B. Halling-
Medicinal Products, Diclofenac, 2003.
Sorensen, "Environmental risk assessment of
[15] Risk Profile Ibuprofen, 2012. Retrieved on
human pharmaceuticals in Denmark after
normal therapeutic use", Chemosphere, 40
O.A. Jones, N. Voulvoulis and J.N. Lester,
"Aquatic environmental assessment of the top
[16] Pfizer, Material Safety Data Sheet Mefenamic
25 English prescription pharmaceuticals",
acid, 2007. Retrieved on January 9, 2014 at:
Water Research, 36 20, 5013–5022,May 2002.
Proceedings of the 3rd World Conference on Applied Sciences, Engineering and Technology
27-29 September 2014, Kathmandu, Nepal, ISBN 13: 978-81-930222-0-7, pp 724-727
Source: http://basharesearch.com/WCSET2014/wcset2014164.pdf
Biblioteca Digital - Dirección de Sistemas de Informática y Comunicación UNIVERSIDAD NACIONAL DE TRUJILLO FACULTAD DE FARMACIA Y BIOQUÍMICA ESCUELA ACADÉMICO PROFESIONAL DE FARMACIA Y BIOQUÍMICA "CONSUMO Y COSTO DEL HALOPERID BIOQUIMICA OL Y ZIPRASIDONA POR VIA ORAL EN EL SERVICIO DE SALUD Y
Osteoporosis: Beyond Bone Mineral Density (Part II)John Neustadt, ND, and Steve Pieczenik, MD, PhD Primary osteoporosis occurs with bone loss as people age, and life-shortening fractures in patients. Early detection and while secondary osteoporosis is caused by other factors such as treatment of risk factors for osteoporosis and osteoporotic frac-medications and medical conditions. Debilitating acute and tures are essential for practicing clinicians.chronic pain in the elderly is often attributed to fractures from





