Association analysis of cyp2c9*3 and phenytoin-induced severe cutaneous adverse reactions (scars) in thai epilepsy children
Journal of Human Genetics (2015) 60, 413–417
& 2015 The Japan Society of Human Genetics All rights reserved 1434-5161/15
Association analysis of CYP2C9*3 andphenytoin-induced severe cutaneous adversereactions (SCARs) in Thai epilepsy children
Supharat Suvichapanich1,5, Jiraphun Jittikoon2,5, Nuanjun Wichukchinda3, Wasu Kamchaisatian4,Anannit Visudtibhan4, Suwat Benjapopitak4, Somjai Nakornchai1, Wiparat Manuyakorn4 andSurakameth Mahasirimongkol3
CYP2C9 is the key enzyme in aromatic antiepileptic drugs (AEDs) metabolism. CYP2C9*3 is a loss of function polymorphism.
This study was designed to investigate genetic association between CYP2C9*3 and aromatic AED-induced severe cutaneousadverse reactions (SCARs) in Thai children. The 37 aromatic AED-induced SCARs patients (20 phenobarbital and 17 phenytoin)and 35 tolerances (19 phenobarbital and 16 phenytoin) were enrolled. CYP2C9*3 was genotyped by allele-specific PCRs. Theassociation between CYP2C9*3 with phenytoin-induced SCARs and phenobarbital-induced SCARs were analyzed in comparisonwith tolerances and healthy samples. Significant association between phenytoin-induced SCARs and CYP2C9*3 was discovered(odds ratio = 14.52; 95% confidence interval (CI) = 1.18–∞, P-value = 0.044). CYP2C9*3 was not associated withphenobarbital-induced SCARs. This study is the first report of CYP2C9*3 association to phenytoin-induced SCARs in Thaiepileptic children. The CYP2C9*3 is a reasonable predictive genetic marker to anticipate SCARs from phenytoin.
Journal of Human Genetics (2015) 60, 413–417; do; published online 21 May 2015
carbamazepine treatment. The patients who carry HLA-B*15:02
Steven–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN)
should not use this medicine unless benefits are outweigh. As aromatic
and drug rash with eosinophilia and systemic symptoms (DRESS) are
AEDs share similar aromatic structure, cross-reactivity among
considered Severe Cutaneous Adverse Reactions (SCARs). SJS and
aromatic AEDs were regularly reported.6 Therefore, USFDA states
TEN are characterized by destruction of the epidermis and mucosal
that phenytoin should be avoided as an alternative for carbamazepine
epithelium. These conditions often presented with internal organs
in patients who are positive for HLA-B*15:02 because of the increased
involvement. Mortality rates were 1–5% for SJS and 20–30% for
risk of SJS/TEN in patients of Asian ancestry.7 Recent study of
TEN.1–3 Moreover, DRESS presents as a group of symptoms including
HLA-B*15:02 provided evidence of association between phenytoin and
extensive mucocutaneous rash, fever, lymphadenopathy, hepatitis,
SJS in Thai epilepsy patients (odds ratio (OR) = 18.5, 95%CI 1.82–188.40)
eosinophilic infiltration and multiple organ damages. Onset usually
although it is less strong than the similar evidence of carbamazepine-
begins after 2 weeks and may occur at any time within 3 months.
induced SCARs in Han Chinese (OR = 2504, 95% CI = 126–49 522).8,9
Incidence of DRESS is 1 case in 1000 to 10 000 drug exposures,
In addition, Thai drug label indicates not to use carbamazepine in
whereas incidence of SJS and TENs is 1.2 to 6 cases per millionperson-years approximately.4,5
patients who carry HLA-B*15:02 because there are strong evidences in
Pharmacogenetic study is becoming a key component for SCARs
developing SJS/TEN in such patients. In case of phenytoin, limited
prevention. Moreover, aromatic antiepileptic drugs (AEDs), such
evidences suggest that phenytoin may be a risk factor for
as carbamazepine, phenobarbital and phenytoin, are the most
SJS/TEN, however, consideration to avoid using this drug is given in
common causes of SCARs.5 In 2007, the US Food Drug Administra-
HLA-B*15:02 positive patients. These Thai label warnings are as same
tion (USFDA) recommended to perform genetic screening on
as contents in the US package insert.
HLA-B*15:02, which has been strongly associated with serious skin
The recommendation to screen HLA-B*15:02 in phenytoin-treated
reactions, in ancestry across broad areas of Asians prior to starting
patient before starting treatment has been recently proposed in
1Department of Pharmacology, Faculty of Pharmacy, Mahidol University, Bangkok, Thailand; 2Department of Biochemistry, Faculty of Pharmacy, Mahidol University, Bangkok,Thailand; 3Medical Genetic Centre, Medical Life Science Institute, Department of Medical Science, Ministry of Public Health, Nontaburi, Thailand and 4Department of Pediatrics,Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand5These authors contributed equally to this work.
Correspondence: Dr W Manuyakorn, Department of Pediatric, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok 10400, Thailand.
E-mail: or Dr S Mahasirimongkol, Department of Medical Sciences, Medical Genetic Centre, Medical Life Sciences Institute, Ministry of Public Health, Nonthaburi 11000, Thailand.
E-mail: Received 17 November 2014; revised 4 April 2015; accepted 7 April 2015; published online 21 May 2015
CYP2C9*3 and phenytoin-induced SCARs
S Suvichapanich et al
Clinical Pharmacogenetics Implementation Consortium (CPIC)
sequence manipulation suite.20 Afterward, the investigation of target DNA
Guidelines for CYP2C9 and HLA-B Genotype and Phenytoin
specificity was performed by blasting novel primers with human genome
sequence on nucleotide blast database.21 In order to validate designed primers,
Genetic polymorphisms also contribute to variability of cytochrome
the known CYP2C9*3 carriers and the CYP2C9*3 noncarriers were used as
P450 (CYP) activities. Interestingly, CYP enzymes, which are the
positive and negative controls.
keystones in metabolism of AEDs, are recently suggested as a
Allele-specific PCR and genotype interpretation
determinant factor for SCARs induced by phenytoin in addition toHLA-B*15:02
AS-PCR method and size determination by gel electrophoresis were selected for
.10 Both CYP2C9 and CYP2C19 are substantial enzymes
genotyping assay. PCR amplification was performed by using T100 Thermal
in phenobarbital and phenytoin metabolism.11,12 These certain
Cycler (Biorad, Hercules, CA, USA). The compositions of PCR reaction were
enzymes transform phenobarbital and
phenytoin to inactive
20 ng of DNA templates and 1 × of KAPA2G Fast multiple PCR Kit (Kapa
Biosystems, Wilmington, MA, USA), 0.2 μM of each primer. KAPA2G contains
respectively.13,14 Thus, loss of function of these enzymes owing to
Hot start DNA polymerase (1 U per 25 μl reaction), KAPA2G Buffer A (1.5 × at
genetic polymorphisms may result in delay clearance and excess toxic
1 × ), dNTPs (0.2 mM each dNTP at 1X), MgCl2 (3.0 mM at 1X) and stabilizers.
metabolites accumulation.10 The association of CYP2C9*3 variant
The condition for each primer was optimized by gradient temperature in
was formerly reported in Korean that done on phenytoin-treated
accordance with primer profiles. PCR conditions were listed as following: an
neurological patients.15 Later, CYP2C9*3 was also determined from
initial denaturation 95 °C for 3 min, 30 cycles of amplification [95 °C for 15 S,68 °C for 30 S, 72 °C for 30 S], a final extension 72 °C for 1 min. Size of PCR
Taiwanese genome-wide association study, which was followed
product was determined in gel electrophoresis. 5 μl of each PCR products were
by direct sequencing of the associated loci in phenytoin-treated epilepsy
analyzed in 2% agarose gel with 0.5X TBE buffer followed by ethidium bromide
patient.10 Though CYP2C9*3 association study was done in epilepsy
staining. The genotype data were interpreted from the length of PCR product
Korean and Taiwanese patients, it has never been investigated in Thais.
which was compared with 100 bps ladder (Invitrogen, Waltham, CA, USA). The
The variability of hepatic CYP2Cs expression and their catalytic
presence of PCR band was observed under UV light with gel documentation
activity may lead to the skin reaction development in children
(Unidoc, UK). DNA samples which were detected both CYP2C9*3 allele and
population. The CYP2C9 activity of children reaches adult level
non CYP2C9*3 allele were interpreted as CYP2C9*3 heterozygous genotype. If
between 5 months to 2 years old.7,16 Therefore, loss of function
DNA samples were identified only CYP2C9*3 allele or non CYP2C9*3 allele in
CYP2C9 might cause more serious problem to this special population.
both reaction, they were considered as homozygous genotype of that specificallele (shown in
The goal for this research was to examine the association of CYP2C9*3and phenytoin-induced SCARs in Thai epilepsy children.
Statistical analysisThe protocol was designed with 80% power to detect a significant difference
MATERIALS AND METHODS
(P-value = 0.05, two sided). Statistical analysis was performed by SPSS software,
version 16.0 (SPSS Inc., Chicago, IL, USA). Demographic data (continuous
DNA samples were obtained from Faculty of Medicine, Ramathibodi Hospital.17
variable) was analyzed and presented as mean ± s.d., median or frequency. The
A total 72 DNA samples from epileptic patients were examined in this study.
normality of continuous data were tested by Kolmogorov–Smirnov Test. In
These samples were divided into four groups. Seventeen subjects were in
order to compare the difference among continuous variables, student t-test
phenytoin-induced SCARs group. All of cases were 0–18 years old with SCARs
(normal distribution) or Mann–Whitney U-test (if the data are not normal
diagnosed, composing of SJS, TEN and DRESS, from phenytoin after take drugs
distribution) was used for analyses. The results of study were presented as
within 12 weeks. Sixteen subjects who tolerated phenytoin SCARs after taking
frequencies, P-value, OR and 95% CI. In order to calculate OR, Haldane's
phenytoin at least 12 weeks were allocated to phenytoin-tolerant group. Another
modification, adds 0.5 to all cells, is applied for all variables when there was an
two groups included phenobarbital-tolerant group (19 subjects) and
absence in genotype frequency.
phenobarbital-induced SCARs (20 subjects). Major characteristic of SJS and
χ2-test was used for categorical variables, such as gender. Fisher's exact test was
TEN were skin detachment and mucosal erosion: skin separation o10% of body
performed to find association between genotypes frequencies and the incident of
surface area in SJS; and 430% of body surface area in TEN.18 The diagnostic
SCARs. The Fisher's exact significant level, 95% CI and ORs, were computed in
criteria for DRESS were scored from these symptoms, fever 438.5 °C, lymph
R console 3.1.1 statistical software for window (http://cran.r-project.org/) using
nodes enlargement, eosinophilia, atypical lymphocyte, skin rash 450% of body
‘Exact 2X2' package.22 P-valueo0.05 is considered as statistical significance. The
surface area and organ involvement (liver, kidney, lung, pancreas and so on).19
exact test for Hardy–Weinberg disequilibrium was performed to exclude the
The protocol was approved by the Human Rights And Ethic Committee of
genotype error that might distort the proportion of heterozygotes and homo-
Faculty of Medicine, Ramithibodi Hospital, Mahidol University. Written
zygotes with significant departure from Hardy–Weinberg equilibrium.
informed consents were obtained from each participant and their parents.
The associations of CYP2C9 in AEDs-induced SCARs patients were
compared with drug-tolerant patients and Thai healthy adult population from
Primer design and validation
published data.23
Two pairs of primers were designed for allele-specific PCR (AS-PCR), onepair for CYP2C9*3 allele detection and the other pair for non CYP2C9*3
allele detection. All designed primers were provided as follow: forward
Patient characteristics
primer 1 (5′-TGC ACG AGG TCC AGA GAT ACA-3′), reverse primer 1
Among 72 patients, there were 17 patients of phenytoin-induced
(5′-TAC AAA CCT TTA TAG CCC CAA AC-3′); forward 2 (5′-TGA
SCARs, 16 of phenytoin tolerances, 20 subjects of phenobarbital-
ACG TGT GAT TGG CAG AAA C-3′); and reverse 2 (5′-CTG GTG GGG AGA
induced SCARs and 19 of phenobarbital tolerances. The median ages
AGG TCA AG-3′). PCR products from two reactions were difference in length.
and dosages were summarized in .
A forward 1 and a reverse 1 primer were designed to detect a non CYP2C9*3
The clinical characteristics of SCARs patients were presented in
allele, and they amplified 263 base pairs of DNA. Conversely, using a forward 2
. Majority of phenytoin cases (88%) exhibited DRESS
primer and a reverse 2 primer to detect CYP2C9*3 allele produced 114 basepairs of DNA.
symptoms, whereas 12% of cases were diagnosed as SJS–TEN. Ninety
Analysis of primers was based on electronic database to prevent the
percentages of phenobarbital cases (18 subjects) were diagnosed as
formation of primer–dimers, hairpin and inappropriate melting temperature.
DRESS but SJS–TEN were diagnosed in two subjects (10%). Liver
First, the general properties of primers were examined by going to site on the
enzymes from SCARs patients were slightly higher than normal range
Journal of Human Genetics
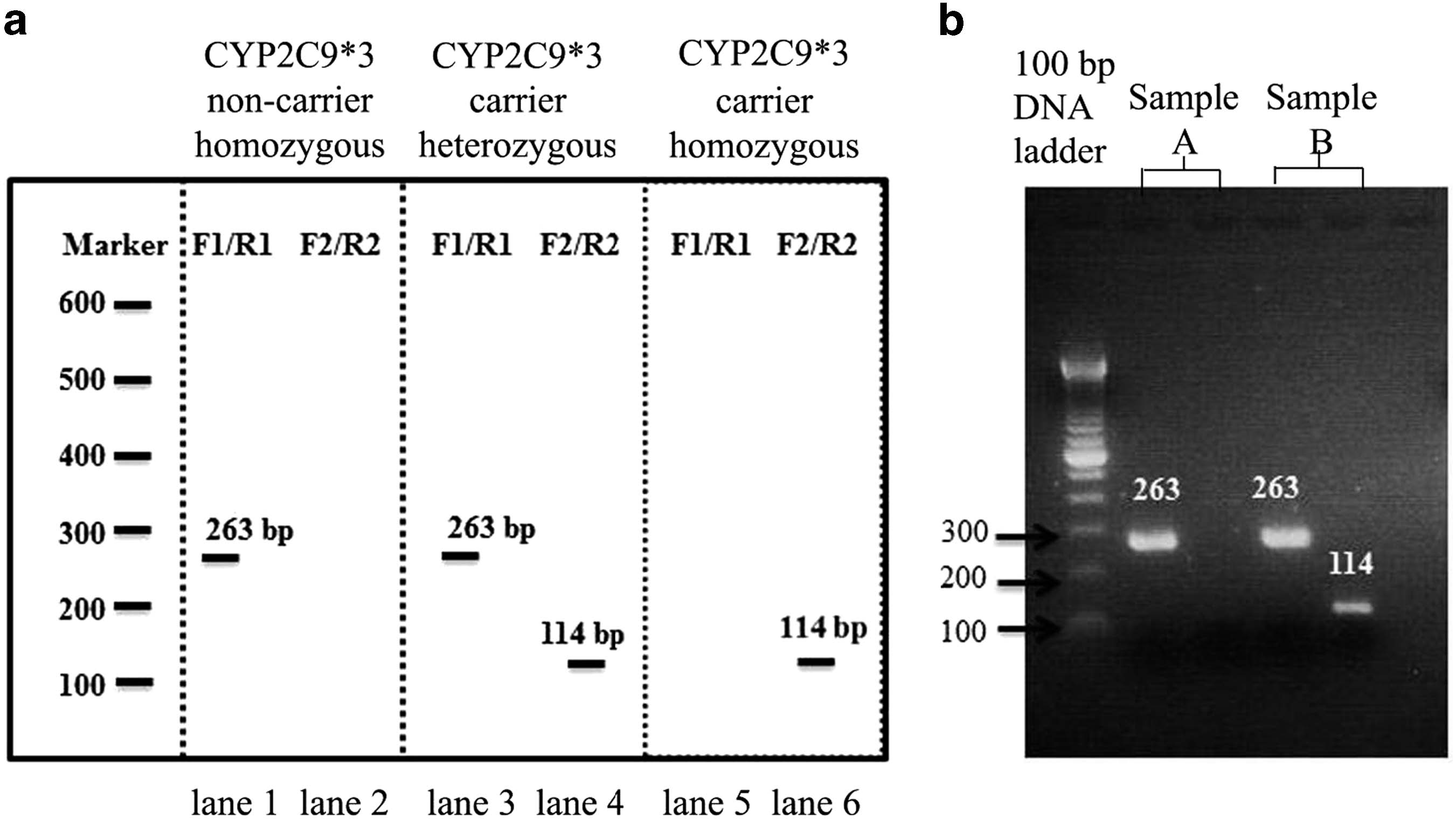
CYP2C9*3 and phenytoin-induced SCARsS Suvichapanich et al
Figure 1 Experimental design for CYP2C9*3 genotyping. (a) Model of expected PCR products from designed primer. The presence of PCR product fromforward 1(F1) and reverse 1 (R1) primers (263 bps) demonstrated non CYP2C9*3 allele. The detection of DNA (114 bps) from forward 2 (F2) and reverse 2(R2) primers indicated CYP2C9*3 allele. The left panel pattern determined CYP2C9*3 non carrier. The pattern of CYP2C9*3 carrier were shown in middlepanel (heterozygous) and right panel. (b) Examples of PCR band from the experiment. Sample A was interpreted as CYP2C9*3 non carrier, and sample Bwas CYP2C9*3 carrier (heterozygous).
Table 1 Demographic data of phenytoin-treated, phenobarbital-treated patients
Tolerance (n = 16)
(A) Phenytoin-treated subjects
Age (years), median
Dose (mg kg per day) median (IQR)
Tolerance (n = 19)
(B) Phenobarbital-treated subjects
Age (years), median
Dose (mg kg per day) median (IQR)
Abbreviation: IQR, interquartile range.
aK.S. test (Po0.05): Man–Whitney U-test.
in both drug-induced SCARs groups because the majority of cases in
subjects. All CYP2C9*3 carriers were heterozygous and they belong to
this study belong to DRESS, in which hepatitis is a common
phenytoin-induced SCARs group ). The CYP2C9*3 carrier
characteristic. These SCARs symptoms from both agents prolonged
rate was 29.4% in phenytoin-induced SCARs patients. CYP2C9*3
hospitalization for at least 1 week.
significantly associated with phenytoin-induced SCARs (OR = 14.52;95% CI = 1.1754—infinity, P-value exact = 0.044; ). Genotype
frequency of CYP2C9*3 in phenobarbital-induced SCARs versus
The examined alleles did not differ from Hardy–Weinberg equilibrium
tolerant group were 5 and 15.8%. However, phenobarbital-induced
in both SCARs group and control group (P-value40.05). The data of
SCARs group was not associated with CYP2C9*3. In addition, the
healthy samples from published study were included in this analysis.23
association between phenytoin-induced SCARs and CYP2C9*3 allele
The attribution of these samples was summarized below. There were
were confirmed when compared with published genotyping data of
326 Thai healthy samples. The age range was between 26 and 62 years
CYP2C9*3 in healthy adult Thais23 (OR = 4.43; 95% CI = 1.39–13.97,
old (mean 39 ± 15 years old).
P-value exact = 0.016) (.
Five cases from 33 phenytoin-treated individuals were CYP2C9*3
Another analysis is determination of the additive effects from
carriers (heterozygous). None of this allele was identified in tolerant
CYP2C9*3 and HLA-B*15:02, two reported alleles associated with
Journal of Human Genetics
CYP2C9*3 and phenytoin-induced SCARs
S Suvichapanich et al
Table 2 Clinical characteristic of SCARs patients
chromosome 10, juxtaposed by CYP2C19. CYP2C9*3 is nonsynon-ymous exchange of isoleucine to leucine at position 359.24 Both
Clinical characteristics
homozygous and heterozygous CYP2C9*3 reduce metabolic clearanceof phenytoin.24 From current analysis, significant associations were
Onset of symptom (days), mean (s.d.)
identified between phenytoin-induced SCARs and CYP2C9*3 carriers,in particular heterozygous because the homozygous for CYP2C9*3
Clinical manifestation, numbers of cases
(*3/*3) was rare in Thais and not present in this study.23 The
Cutaneous symptoms
CYP2C9*3 carriers has 14.5 times higher OR for development of SCARs
Respiratory symptoms
from phenytoin over those CYP2C9*3 noncarriers and significant
Diagnosis, numbers of cases (%)
difference of genotype distribution when compared with Thai healthy
adults. These associations demonstrated the same trend as data from
Taiwanese.10 Taiwanese data also suggested that CYP2C9*3 carriers hadhigher phenytoin plasma level than that of noncarriers. Moreover, their
Initial laboratory parameters, median (range)
meta-analysis study showed significant association between CYP2C9*3
Absolute eosinophil (cell/ml),
and SCARs from phenytoin in Japan, Malaysia and Taiwan.10 Our
supplied evidence in Thais can confirm the association of CYP2C9*3
and phenytoin-induced SCARs in Southeast Asia region.
The exact mechanism explaining why reduced function of CYP2C9
Length of hospitalization (days)
contributed to phenytoin-induced SCARs was not known at the time
Abbreveiations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; DRESS, drug
of this study. To the best of our knowledge, intermediate toxic
rash with eosinophilia and systemic symptoms; GGT, gamma-glutamyl transferase; PB,
metabolites might be accumulated more owing to slower clearance in
phenobarbital; PHT, phenytoin.
combination with nonlinear pharmacokinetic properties of phenytoin.
These metabolites bind to cellular macromolecules resulted in haptenformation and stimulated immunological reactions.6,25
Table 3 Association testing among phenytoin-induced SCARs
HLA-B*15:02 was suggested as predictive marker for SCARS
patients, tolerant patients and healthy adult Thais
from phenytoin albeit with inconsistencies when compared with
Odds ratio (95%CI)
Negative result of HLA-B*15:02 was demonstrated in this study.17
Comparison between SCARs and tolerances
The screening for HLA-B*15:02 or CYP2C9*3 in our study exhibited
14.52b (1.18–∞)
the superior results compared with that for HLA-B*15:02 alone but of
those are inferior compared with CYP2C9*3 alone.
0.28 (0.01–2.9)
This study carried some limitations. First, sample sizes were small
because SCARs were not common adverse reaction. Previous Koreanstudy provided 10 cases of phenytoin-induced cutaneous adverse
Odds ratio (95% CI)
reactions, 3 out of 10 were heterozygous of CYP2C9*3.15 Second,
Comparison between SCARs and healthy
CYP2C9*3 has lower clearance activity of enzyme, but we did not
4.43 (1.39–13.97)
measure phenytoin plasma level of SCARs patients in this study. These
DNA samples came from retrospective study, which almost all patients
0.56 (0.03–3.47)
had recovered from those reactions, and rechallenging of phenytoin in
SCARs are prohibited owing to patient safety. Therefore, it is not
Abbreviations: CI, con
feasible to measure phenytoin concentration in their plasma. In
fidence interval; PB, phenobarbital; PHT, phenytoin; SCARs, severe
cutaneous adverse reactions.
addition, the case and control populations were not perfectly matched
aAll positive CYP2C9*3 are heterozygous.
bOdd ratio with Haldane's modification.
due to difficulty in enrolled control group resulted in significant
cPo0.05 (Fisher's exact test).
difference in demographic data except gender. In pediatric practice in
Data from Busakornyuangrat et al.22
Thailand, phenobarbital was frequently prescribed among the first-linedrug for epilepsy in children owing to its lowest cost.
phenytoin-induced SCARs group. Some patients carries either
Further study may need to clarify these limitations. However, given
CYP2C9*3 or HLA-B*15:02 or both of them. As association between
the association in other Asian populations, this association analysis is
HLA-B*15:02 and phenytoin-induced SCARs was absence in this
providing important information for the association of these pharma-
study, combined information from HLA-B*15:02 and CYP2C9*3
cogenetic markers in Thai population. The precise determination of
genotypes demonstrated significant association with phenytoin-
these allele effect sizes will require a larger sample sizes in a well-
induced SCARs (OR = 10.5; 95% CI = 1.22–247.93, P#-value = 0.039)
matched cases–control study or prospective study; it is unlikely that
but less association than CYP2C9*3 alone.
this association is happened by spurious association. As these markersare not explaining all phenytoin-induced SCAR, other enzymes in
phenytoin metabolizing pathway should also be investigated in
Both CYP2C9 and CYP2C19 enzymes are involved with metabolizing
pathway of phenytoin. Previous pharmacogenetic study with
This study is the first report demonstrated the association of
CYP2C19*2, a loss of function allele of CYP2C19 and phenytoin-
CYP2C9*3 to phenytoin-induced severe cutaneous drug reaction in
induced SCARs has failed to prove their association. Therefore,
Thai epileptic young patients. CYP2C9*3 is a reasonable predictive
CYP2C9*3, an allele that encodes decrease activity enzyme, is another
genetic marker of phenytoin-induced SCARs to identify the risk of
candidate target to examine this association. CYP2C9 is located on
phenytoin-induced SCARs in Asian populations.
Journal of Human Genetics
CYP2C9*3 and phenytoin-induced SCARsS Suvichapanich et al
CONFLICT OF INTEREST
9 Chung, W. H., Hung, S. I., Hong, H. S., Hsih, M. S., Yang, L. C., Ho, H. C. et al.
The authors declare no conflict of interest.
Medical genetics: A marker for Stevens-Johnson syndrome. Nature 428, 486 (2004).
10 Chung, W., Chang, W. C., Lee, Y. S., Wu, Y. Y., Yang, C. H., Ho, H. C. et al. Genetic
variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA
312, 525–534 (2014).
This study was financially supported by National Research Council of Thailand
11 Glauser, T. A. Biomarkers for antiepileptic drug response. Biomark. Med. 5,
635–641 (2011).
under the project entitled ‘Pharmacogenomics studies of common adverse
12 Yasumori, T., Chen, L.-s., Li, Q.-h., Ueda, M., Tsuzuki, T., Goldstein, J. A. et al.
reactions in Thais (PGx-COMART)' to Dr Surakameth Mahasirimongkol and
Human CYP2C-mediated stereoselective phenytoin hydroxylation in Japanese: differ-
Department of Medical Sciences. We offer special thanks for Ms Wimala
ence in chiral preference of CYP2C9 and CYP2C19. Biochem. Pharmacol. 57,1297–1303 (1999).
Inunchote in Medical Genetic Unit (Medical Life Science Institute, Department
13 Thorn, C. F., Whirl-Carrillo, M., Leeder, J. S., Klein, T. E. & Altman, R. B. PharmGKB
of Medical Science, Ministry of Public Health, Thailand) to help in handle
summary: phenytoin pathway. Pharmacogenet. Genomics 22, 466–470 (2012).
14 Desta, Z., Zhao, X., Shin, J. G. & Flockhart, D. A. Clinical significance of the cytochrome
P450 2C19 genetic polymorphism. Clin. Pharmacokinet. 41, 913–958 (2002).
15 Lee, A. Y., Kim, M. J., Chey, W. Y., Choi, J. & Kim, B. G. Genetic polymorphism of
cytochrome P450 2C9 in diphenylhydantoin-induced cutaneous adverse drug reactions.
Eur. J. Clin. Pharmacol. 60, 155–159 (2004).
16 Koukouritaki, S. B., Manro, J. R., Marsh, S. A., Stevens, J. C., Rettie, A. E.,
1 Rzany, B., Mockenhaupt, M., Baur, S., Schroder, W., Stocker, U., Mueller, J. et al.
McCarver, D. G. et al. Developmental expression of human hepatic CYP2C9 and
Epidemiology of erythema exsudativum multiforme majus, Stevens-Johnson syndrome,
CYP2C19. J. Pharmacol. Exp. Ther. 308, 965–974 (2004).
and toxic epidermal necrolysis in Germany (1990-1992): structure and results of a
17 Manuyakorn, W., Siripool, K., Kamchaisatian, W., Pakakasama, S., Visudtibhan, A.,
population-based registry. J. Clin. Epidemiol. 49, 769–773 (1996).
Vilaiyuk, S. et al. Phenobarbital-induced severe cutaneous adverse drug reactions are
2 Schneck, J., Fagot, J.-P., Sekula, P., Sassolas, B., Roujeau, J. C. & Mockenhaupt, M.
associated with CYP2C19*2 in Thai children. Pediatr. Allergy. Immunol. 24,
Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal
299–303 (2013).
18 Roujeau, J. C. & Stern, R. S. Severe adverse cutaneous reactions to drugs. N. Engl. J.
EuroSCAR Study. J. Am. Acad. Dermatol. 58, 33–40 (2008).
Med. 331, 1272–1285 (1994).
3 Aihara, M. Pharmacogenetics of cutaneous adverse drug reactions. J. Dermatol. 38,
19 Kardaun, S. H., Sidoroff, A., Valeyrie-Allanore, L., Halevy, S., Davidovici, B. B.,
246–254 (2011).
Mockenhaupt, M. et al. Variability in the clinical pattern of cutaneous side-effects of
4 Criado, P. R., Criado, R. F., Avancini, J. M. & Santi, C. G. Drug reaction with
drugs with systemic symptoms: does a DRESS syndrome really exist? Br. J. Dermatol.
156, 609–611 (2007).
Syndrome (DIHS): a review of current concepts.
20 Stothard, P. The sequence manipulation suite: JavaScript programs for analyzing and
435–449 (2012).
formatting protein and DNA sequences. BioTechniques 28: 1102 1104 (2000).
5 Roujeau, J. C., Kelly, J. P., Naldi, L., Rzany, B., Stern, R. S., Anderson, T. et al.
21 Altschul, S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Zhang, Z., Miller, W. et al.
Medication use and the risk of Stevens–Johnson syndrome or toxic epidermal necrolysis.
Gapped BLAST and PSI-BLAST: a new generation of protein database search programs.
New. Engl. J. Med. 333, 1600–1608 (1995).
Nucleic Acids Res. 25, 3389–3402 (1997).
6 Shear, N. H. & Spielberg, S. P. Anticonvulsant hypersensitivity syndrome. In vitro
22 Fay, M., Proschan, M. & Brittain, E. Combining One Sample Confidence Procedures for
assessment of risk. J. Clin. Invest. 82, 1826–1832 (1988).
Inferences in the Two Sample Case. Biometrics 71, 146–156 (2014).
7 Caudle, K. E., Rettie, A. E., Whirl-Carrillo, M., Smith, L. H., Mintzer, S., Lee, M. T.
23 Busakornyuangrat, S., Chuansumrit, A., Angchaisuksiri, P., Sasanakul, W. &
et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for
Kadekasem, P. Frequencies of Polymorphism Associated with Cytochrome P450 2C9
CYP2C9 and HLA-B Genotypes and Phenytoin Dosing. Clin. Pharmacol. Ther. 96,
in Thais. Thai J. Hematol. Transf. Med. 16, 213–220 (2006).
542–548 (2014).
24 Xie, H. G., Kim, R. B., Wood, A. J. & Stein, C. M. Molecular basis of ethnic
8 Locharernkul, C., Loplumlert, J., Limotai, C., Korkij, W., Desudchit, T., Tongkobpetch,
differences in drug disposition and response. Annu. Rev. Pharmacol. Toxicol. 41,
S. et al. Carbamazepine and phenytoin induced Stevens-Johnson syndrome is
815–850 (2001).
25 Leeder, J. S. Mechanisms of idiosyncratic hypersensitivity reactions to antiepileptic
2087–2091 (2008).
drugs. Epilepsia 39(Suppl 7), S8–16 (1998).
Journal of Human Genetics
Source: http://budgetitc.dmsc.moph.go.th/research/pdf/201538.pdf
Centre de Ressources Autisme BULLETIN D'INFORMATIONS DU CENTRE DE RESSOURCES AUTISME PACA Sept - Octt 2012 1/ COLLOQUES, JOURNEES, DEBATS, CONGRES : Journées Nationales d'étude sur l'autisme 28 et 29 septembre 2012, Caen Les prochaines journées nationales 2012 organisées par l'ANCRA, en
Cancer Facts Estimated numbers of new cancer cases for 2016, excluding basal cell and squamous cell skin cancers and in situ carcinomas except urinary bladder. Estimates are not available for Puerto Rico.Note: State estimates are offered as a rough guide and should be interpreted with caution. State estimates may not add to US total due to rounding.


