Eprf.org
Journal of the American College of Cardiology
Vol. 58, No. 19, 2011
2011 by the American College of Cardiology Foundation
ISSN 0735-1097/$36.00
Published by Elsevier Inc.
Heart Rhythm Disorders
Cardiovascular Outcomes in theAFFIRM Trial (Atrial FibrillationFollow-Up Investigation of Rhythm Management)
An Assessment of Individual Antiarrhythmic Drug TherapiesCompared With Rate Control With Propensity Score-Matched Analyses
Sanjeev Saksena, MD,* April Slee, MS,* Albert L. Waldo, MD,* Nick Freemantle, PHD,*Mathew Reynolds, MD, MS,* Yves Rosenberg, MD,† Snehal Rathod, MS,* Shannon Grant MS,*Elizabeth Thomas, MS,* D. George Wyse, MD, PHD*
Warren, New Jersey; and Bethesda, Maryland
The impact of individual antiarrhythmic drugs (AADs) on mortality and hospital stay in atrial fibrillation (AF) was
Cardiovascular (CV) outcomes in AF patients receiving pharmacologic rhythm control therapy have not been
compared with rate control therapy on the basis of AAD selection.
We compared CV outcomes in the AFFIRM (Atrial Fibrillation Follow-Up Investigation of Rhythm Management) trial in
subgroups defined by the initial AAD selected with propensity score matched subgroups from the rate arm (Rate).
Seven hundred twenty-nine amiodarone patients, 606 sotalol patients, and 268 Class 1C patients were matched. The
composite outcome of mortality or cardiovascular hospital stays (CVH) showed better outcomes with Rate compared
with amiodarone (hazard ratio [HR]: 1.18, 95% confidence interval [CI]: 1.03 to 1.36, p ⫽ 0.02), sotalol (HR: 1.32,95% CI: 1.13 to 1.54, p ⬍ 0.001), and Class 1C (HR: 1.22, 95% CI: 0.97 to 1.56, p ⫽ 0.10). There was a nonsignifi-cant increase in mortality with amiodarone (HR: 1.20, 95% CI: 0.94 to 1.53, p ⫽ 0.15) with the risk of non-CV deathbeing significantly higher with amiodarone versus Rate (HR: 1.11, 95% CI: 1.01 to 1.24, p ⫽ 0.04). First CVH eventrates at 3 years were 47% for amiodarone, 50% for sotalol, and 44% for Class 1C versus 40%, 40%, and 36%, re-
spectively, for Rate (amiodarone HR: 1.20, 95% CI: 1.03 to 1.40, p ⫽ 0.02, sotalol HR: 1.364, 95% CI: 1.16 to 1.611,p ⬍ 0.001, Class 1C HR: 1.24, 95% CI: 0.96 to 1.60, p ⫽ 0.09). Time to CVH with intensive care unit stay or deathwas shorter with amiodarone (HR: 1.22, 95% CI: 1.02 to 1.46, p ⫽ 0.03).
In AFFIRM, composite mortality and CVH outcomes differed for Rate and AADs due to differences in CVH; CVH
event rates during follow-up were high for all cohorts, but they were higher for all groups on AADs. Death, inten-
sive care unit hospital stay, and non-CV death were more frequent with amiodarone. (Atrial Fibrillation Follow-Up
Investigation of Rhythm Management;
(J Am Coll Cardiol 2011;58:1975–85) 2011 by the
American College of Cardiology Foundation
Atrial fibrillation (AF) is the most prevalent tachyarrhyth-mia and is associated with increased mortality, stroke, and
recurrent hospital stays Health care resource con-
From the *Electrophysiology Research Foundation, Warren, New Jersey; and the
Merck, Pfizer, Eli Lilly, Novo Nordisk, and Medtronic. Dr. Reynolds has received a
†National Heart, Lung and Blood Institute, Bethesda, Maryland. Dr. Saksena is or
research grant and is a consultant/advisory board member for Sanofi-Aventis. Dr. Wyse
has been a consultant, investigator, and research grant recipient for the National Heart
is a consultant to Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, Sanofi-Aventis,
Lung and Blood Institute, Medtronic Inc., St. Jude Medical Inc., Sanofi-Aventis,
Biotronik, Boston Scientific/Guidant, National Heart, Lung and Blood Institute, Duke
Sorin Group, and Aryx Pharmaceuticals; and has been a Speakers' bureau member for
Clinical Research Institute, European Commission, Merck, Medtronic, and Bayer, and
Sanofi-Aventis. Dr. Waldo is a consultant to Sanofi-Aventis, Ortho-McNeil-Janssen,
Speakers' Bureau member for Sanofi-Aventis. All other authors have reported that they
Biotronik, St. Jude Medical, Daiichi, Sankyo Pharmaceuticals, Medtronic Inc.,
have no relationships relevant to the contents of this paper to disclose. For list of
Astellas Pharma, Biosense Webster Inc., Bristol-Myers Squibb, Portola, Boehringer
investigators and affiliated institutions, please see the Online Appendix.
Ingelheim, CardioInsight Technologies, Merck, AtriCure Inc., and Sanofi-Aventis;
Manuscript received April 19, 2011; revised manuscript received July 18, 2011,
and is a speaker for Sanofi-Aventis. Dr. Freemantle is a consultant for Sanofi-Aventis,
accepted July 26, 2011.
Saksena et al.
JACC Vol. 58, No. 19, 2011
CV Outcomes of AADs in the AFFIRM Trial
November 1, 2011:1975– 85
sumption due to AF, primarily
(CVH). Individual components (all-cause mortality and
due to hospital stay, is among the
CVH) were also examined, as were subsets of both CVH
highest for cardiovascular (CV)
and all-cause mortality The AAD subgroups were
AAD ⴝ antiarrhythmic drug
diagnoses, but the patterns of
compared with propensity score matched rate subgroups
AF ⴝ atrial fibrillation
these hospital stays and their re-
(Rate) and included: 1) initial amiodarone therapy (amio-
CI ⴝ confidence interval
lationship to individual therapeutic
darone cohort); 2) initial sotalol (sotalol cohort); and 3)
CV ⴝ cardiovascular
choices in AF have not been evalu-
initial Class 1C drug (flecainide or propafenone, Class 1C
CVH ⴝ cardiovascular
ated The AFFIRM (Atrial Fi-
brillation Follow-Up Investigation
Propensity score matched subgroups were selected
HR ⴝ hazard ratio
of Rhythm Management) trial was
from the rate control strategy arm (Rate) for each AAD
ICU ⴝ intensive care unit
conducted to examine 2 treatment
cohort. The score was derived with 62 baseline patient
Rate ⴝ rate control
strategies for AF, namely rate con-
characteristics from the AFFIRM database deemed a
trol or rhythm control All-
priori to potentially affect AAD selection. Two additional
cause mortality, the primary out-
characteristics that were determined to be important to
come measure, showed a trend
achieve balanced cohorts (left ventricular ejection frac-
toward excess mortality in the rhythm control arm. The antiar-
tion, and history of coronary artery disease) were added in
rhythmic drugs (AADs) used in the rhythm arm have been cited
as a potential cause of the excess mortality Despite concerns
with regard to their safety, most of the AADs used in theAFFIRM trial remain widely used in clinical practice.
Relating outcomes to clinical and treatment factors. The
The impact of individual AADs on mortality and hospital
severity of CVH was characterized by acuity of hospital stay
stay outcomes in the AFFIRM population in relation to rate
on the basis of concomitant intensive care unit (ICU) stay,
control has not been available. In part, this was related to
CV procedures, CV interventions, or emergency room
the intent of the AFFIRM investigators to test the treat-
visits. Outcomes in AAD subgroups were related to patient
ment strategy hypothesis rather than individual drug ther-
characteristics, underlying disease state, clinical events, and
apies. In this report, we examined the impact on outcomes
treatment strategy.
of the selection of amiodarone, sotalol, or a Class 1C
Study Outcomes and Definitions
antiarrhythmic agent (flecainide or propafenone) as the firstAAD, compared with a rate strategy in the AFFIRM study.
The principal outcome for this analysis was a composite
The AADs were selected for this analysis on the basis of
outcome: the first of death from any cause or a CVH. A
current widespread clinical usage. To address the nonran-
CVH was defined as a hospital admission for CV reasons
dom nature of drug selection in the rhythm arm, we
(per investigator) or for non-CV reasons but with a CV
employed propensity score matching derived from 64 base-
event occurring during the same follow-up interval. Exact
line patient characteristics deemed to affect antiarrhythmic
dates were available for death but not for hospital admission
selection. Propensity score matching has not been employed
or discharge. The midpoint of the previous follow-up visit
to assess individual drug outcomes in the AFFIRM trial
and the follow-up visit when the hospital stay was reported
We compared mortality and hospital stay outcomes in
were used to estimate event time for CVH. Investigators
patient subgroups defined by each type of AAD selected as
recorded total number of hospital days and total number of
first therapy with propensity score matched subgroups from
ICU days. Visits occurred at 2 months after randomization
the rate control arm.
and every 4 months thereafter. Patients who did notexperience CVH or death were censored at the last
follow-up visit. For death alone, follow-up informationfrom a vital status sweep (telephone contact with all subjects
Patient Selection in the AFFIRM Trial
and national death index scan) at the end of the study was
The AFFIRM trial recruited consenting patients who had AF
used to determine censoring date.
that was likely to be recurrent, warranted therapy, and had risk
Statistical Methods and Analytical Techniques
factor(s) for stroke. Patients were candidates for at least 2 drugswithin each strategy and for anticoagulation
Propensity score and establishment of matched cohorts.
The goal of development of propensity score matched
Primary Objective of Analysis
cohorts was to account for possible confounding variables
Reassessment of clinical outcomes by initial AAD therapy.
that might be related to drug selection, because the patients
The primary objective was to reassess clinical outcomes in
were not assigned randomly to specific initial drug therapy
the AF population enrolled in the AFFIRM study by initial
in the AFFIRM trial.
AAD therapy with a composite principal outcome and its
Selection of covariates. Propensity score was calculated
individual components. The principal outcome was a com-
separately for each AAD subgroup (amiodarone, sotalol, or
posite of mortality or first cardiovascular hospital stay
Class 1C). Four patients received more than 1 AAD and
JACC Vol. 58, No. 19, 2011
Saksena et al.
November 1, 2011:1975– 85
CV Outcomes of AADs in the AFFIRM Trial
Covariates Used in Propensity Score Model
Covariates Used in Propensity Score Model
Primary cardiac diagnosis
Coronary artery disease
Year of randomization
Current CCS angina class
History of myocardial infarction
Number of AAD failures
History of pulmonary disease
Failed amiodarone
History of intracranial hemorrhage
Failed disopyramide
History of congestive heart failure, congestive heart failure on enrollment
Failed flecainide
History of cardiomyopathy
Failed moricizine
History of valvular heart disease
Failed procainamide
History of congenital heart disease
Failed propafenone
History of angina
History of diabetes
History of hepatic or renal disease
History of symptomatic brady/atrioventricular block
Previous other CV procedure
History of resuscitated cardiac arrest
Previous percutaneous coronary interventions
History of stroke/transient ischemic attack
Previous coronary artery bypass grafting
History of peripheral vascular disease
Previous thrombolytic therapy
History of systemic embolism
LV ejection fraction
History of hemorrhage or coagulopathy
History of thyroid disease/specific drugs—thyroid replacement
History of carotid disease
Symptoms constellations are
2. Diaphoresis, fatigue, panic, dizziness, syncope
4. Dyspnea, edema, orthopnea, paroxysmal nocturnal dyspnea
5. Fast heart rate, palpitations
AF symptoms frequency
Duration of qualifying AF episode(s)
Hospitalized for qualifying episode
Cardioverted for qualifying episode(s)
Current ventricular/max HR during AF ⬎100 beats/min
Other cardiac neurologic interaction
List of covariates used in propensity score model. Please note that multiple imputation was used for body mass index (BMI) and systolic blood
pressure (SBP).
AAD ⫽ antiarrhythmic drug; AF ⫽ atrial fibrillation; BMI ⫽ body mass index; CCS ⫽ Canadian Cardiovascular Society; CV ⫽ cardiovascular; FADS
⫽ first antiarrhythmic drug substudy; HR ⫽ heart rate; LV ⫽ left ventricular; max ⫽ maximum; NYHA ⫽ New York Heart Association functional class;SBP ⫽ Systolic blood pressure.
were excluded. The propensity score model used data from
Model building. Proc GLIMMIX in SAS (version 9.2, SAS
AFFIRM patients randomized to rhythm control. Identical
Institute, Cary, North Carolina) was used for building the
baseline explanatory variables were included in each model
propensity-matched cohorts. Each model considered all ex-
and were prospectively determined by consensus before data
planatory variables in Site was included as a fixed effect
analysis This model included explanatory vari-
for this step. The functional form of response was assessed for
ables that might be considered by clinicians when selecting
continuous variables to determine whether transformation was
an AAD, including demographic data, clinical characteris-
necessary Then, the model was run twice, with site as a
tics of patients, treating physicians (cardiologists or other),
fixed and then as a G-sided (generalized) random effect. These
centers, and study design factors. Patients in the first AAD
models were compared for evidence of extra binomial variabil-
substudy had their first AAD randomly assigned, so partic-
ity at the investigator site level. Risk score was calculated for
ipation in first AAD sub-study was included as a variable
each patient in the rate subgroup, and the VMATCH algo-
A stepwise model reduction procedure was used to
rithm (Zentrum fur Bioinformatik, Hamburg, Germany) was
produce a parsimonious model for each propensity score
used to construct the cohorts Matching was 1:1 between
equation. After initial cohort construction, imbalances in 2
each AAD cohort and the rate cohort.
additional variables, coronary artery disease and left ventric-
Descriptive reporting. Once the propensity score matched
ular ejection fraction, were identified; these items were
cohorts were established, baseline demographic and clinical
added to the model in a second step.
characteristics were tabulated to be consistent with the main
Saksena et al.
JACC Vol. 58, No. 19, 2011
CV Outcomes of AADs in the AFFIRM Trial
November 1, 2011:1975– 85
AFFIRM publication Tests for differences across
patients were receiving the initially selected drug at first
matched cohorts were conducted (Fisher exact or chi-square
CVH. There was no increased mortality risk for sotalol and
for categorical variables, analysis of variance or Wilcoxon for
Class 1C cohorts, but an increase in risk was observed for
amiodarone (HR: 1.20, 95% CI: 0.94 to 1.53, p ⫽ 0.15),compared with Rate, which was not statistically significant.
Time to first CVH was shorter for all AADs, compared
The principal outcome analyzed was a comparison of event
with Rate. First CVH event rates at 3 years were 47% for
time with the log-rank test on an intention-to-treat basis,
amiodarone, 50% for sotalol, and 44% for Class 1C com-
similar to the primary AFFIRM analysis. Unadjusted
pared with 40%, 40%, and 36%, respectively, for the
Kaplan-Meier survival curves were examined for each
matched Rate cohorts. The CV mortality did not differ
propensity-score matched cohort pair. Proportional hazards
between Rate and any of the AAD cohorts (p ⬎ 0.15 for all
models were used to obtain hazard ratios (HRs) and 95%
comparisons). There was an increased risk of noncardiovas-
confidence intervals (CIs) and to determine the effect in
cular mortality with amiodarone (HR: 1.11, 95% CI: 1.01 to
clinically important subgroups.
1.24, p ⫽ 0.04) but not with sotalol or Class 1C drugs,
Sensitivity analyses. To determine the impact of treatment
compared with Rate. However, deaths attributable to cancer
strategy-related hospital stays, (e.g., cardioversions) and
or pulmonary causes were comparable across each cohort.
further define acuity of CVH, we repeated the analysis with
A composite of death or ICU hospital stays showed
a composite of death and first hospital stay requiring ICU
moderately increased risk with amiodarone (HR: 1.22, 95%
stay. To evaluate the propensity score methodology, a Cox
CI: 1.02 to 1.46, p ⫽ 0.03) but not with sotalol or Class 1C
proportional hazards model with a frailty term for site was
agents (HR: 1.06, 95% CI: 0.87 to 1.30, p ⫽ 0.56, and HR:
1.07, 95% CI: 0.78 to 1.46, p ⫽ 0.67, respectively),compared with Rate There was no difference intime to ICU hospital stays for sotalol and Class 1C,
compared with Rate, but a nonsignificant increased risk was
Patient population. Seven hundred twenty-nine AF pa-
noted for amiodarone (HR: 1.18, 95% CI: 0.95 to 1.47, p ⫽
tients initially received amiodarone therapy, 606 received
0.14) All-cause hospital stays were increased in
initial sotalol therapy, and 268 received either initial flecain-
amiodarone compared with Rate (HR: 1.19, 95% CI: 1.05
ide or propafenone. The clinical characteristics of these 3
to 1.35, p ⫽ 0.008) and in sotalol compared with Rate (HR:
AAD cohorts on the basis of initial drug therapy selection
1.22, 95% CI: 1.06 to 1.41, p ⫽ 0.005). There was no
are shown in The AAD cohorts were generally
increased risk of all-cause hospital stay with Class 1C
well-matched. Patients were usually elderly, predominantly
compared with Rate.
male, and had recurrent AF associated with cardiac disease.
Concomitant beta-blocker therapy did not alter outcomes
The amiodarone cohort had a slight excess of men, com-
for either sotalol or Class 1C cohorts for either mortality or
pared with its matched Rate cohort (67.4% vs. 61.3%,
CVH risk (CVH for sotalol HR: 1.09, 95% CI: 0.89 to
respectively). More patients in the sotalol cohort had a
1.34, for death HR: 1.15, 95% CI: 0.81 to 1.63; CVH for
history of angina, compared with Rate (11.1% vs.
Class 1C HR: 0.75, 95% CI: 0.60 to 1.03), for death HR:
6.9%).There were no other significant differences. The C
0.65, 95% CI: 0.40 to 1.07). Amiodarone-Rate cohort
statistic for the 3 propensity models were 0.814 for amio-
patients who were concomitantly taking beta-blockers had
darone, 0.837 for sotalol, and 0.837 for Class 1C subgroups.
an increased mortality risk (CVH risk for amiodarone HR:
Outcomes analysis. HRs and 95% CIs for the overall com-
1.06, 95% CI: 0.90 to 1.25, for death HR: 1.53, 95% CI:
parison (rhythm compared with rate) in the AFFIRM trial and
1.16 to 2.02). There was no evidence of a treatment–
individual AAD subgroups with the matched rate cohort are
digoxin interaction for the principal outcome. Time-
shown for the composite principal outcome of mortality and
dependent digoxin use was significantly associated with
first CVH in All AAD cohorts had inferior
CVH in the amiodarone-Rate cohorts (HR: 1.43, 95% CI:
principal outcomes, compared with Rate (HR for amioda-
1.21 to 1.68) and in the Class 1C-Rate cohorts (HR: 1.36,
rone: 1.18, 95% CI: 1.03 to 1.36, p ⫽ 0.02; HR for sotalol:
95% CI: 1.04 to 1.77) but not in the sotalol-Rate cohorts
1.32, 95% CI: 1.13 to 1.54, p ⬍ 0.001; and HR for Class
(HR: 1.15, 95% CI: 0.96 to 1.37). After adjusting for
1C: 1.22, 95% CI: 0.97 to 1.56, p ⫽ 0.10). In the smaller
time-dependent digoxin use, AADs still increased the risk
Class 1C cohort, this difference did not reach statistical
of CVH (HR for amiodarone: 1.34, 95% CI: 1.13 to 1.57,
significance. shows the individual components of
HR for sotalol: 1.40, 95% CI: 1.17 to 1.67, compared with
the composite endpoint. Risk of CVH was increased for all
matched rate patients; HR for Class 1C: 1.34, 95% CI: 1.03
3 AAD cohorts (amiodarone HR: 1.20, 95% CI: 1.03 to
to 1.75, compared with the respective AAD rate-matched
1.40, p ⫽ 0.05; sotalol HR: 1.36, 95% CI: 1.16 to 1.61, p ⬍
patients). The increased risk of CVH or death was consis-
0.001; and Class 1C HR: 1.24, 95% CI: 0.96 to 1.64, p ⫽
tent across clinically important subgroups including coro-
0.09, compared with Rate). Ninety-one percent of amioda-
nary disease, female sex, and age for amiodarone and sotalol
rone patients, 88% of sotalol patients, and 78% of Class 1C
patients, presence of thyroid disease only in amiodarone
Baseline Patient Characteristics for Entire Rate Cohort in AFFIRM
Baseline Patient Characteristics for Entire Rate Cohort in AFFIRM
Ethnic minority group
Predominant cardiac diagnosis
Coronary artery disease (MI, angina, and so on)
Dilated cardiomyopathy
Valvular heart disease
No apparent heart disease
History of congestive heart failure
Duration of qualifying AF ⱖ2 days
First episode of AF (vs. recurrent episode)*
Any pre-randomization failure of an antiarrhythmic drug
Size of left atrium normal†
Baseline CCS class
Class II or greater
Baseline NYHA functional class
Values are mean ⫾ SD or n (%). Baseline patient characteristics for entire Rate cohort in AFFIRM (Atrial Fibrillation Follow-Up Investigation of Rhythm Management) trial (Overall Rate) and the 3 paired propensity (PS) matched cohorts for individual antiarrhythmic drugs
and matched rate control groups. The size of the left atrium was unknown in 185 of 3,311 cases, and left ventricular function (where normal was defined as left ventricular ejection fraction [LVEF] ⫽ 0.50) was unknown in 279 of 3,311. Electrocardiogram information was
not used in PS models. *This information was not collected on the initial version of the data form and therefore was imputed for 143 patients. †Electrocardiograms were obtained in 3,311 of 4,060.
CHF ⫽ congestive heart failure; MI ⫽ myocardial infarction; other abbreviations as in
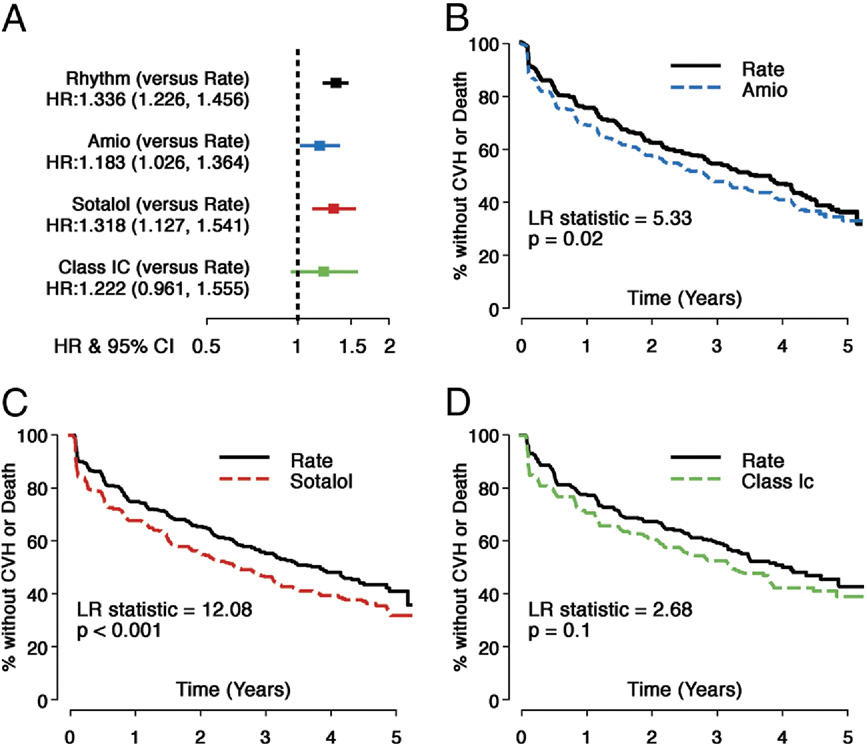
Saksena et al.
JACC Vol. 58, No. 19, 2011
CV Outcomes of AADs in the AFFIRM Trial
November 1, 2011:1975– 85
Comparison of Composite Principal Outcome: Individual AADs Versus Rate
Hazard ratios (HRs) and Kaplan-Meier survival analyses comparing individual antiarrhythmic drugs (AADs) with matched rate control strategy arm (Rate) cohorts for the
composite principal outcome (time to first cardiovascular hospital stay [CVH] or death). Individual panels are shown as follows: (A) HRs and 95% confidence intervals
(CIs) (HR: rhythm drug/Rate); (B) propensity score matched Rate and amiodarone (Amio) subgroups; (C) propensity score matched Rate and sotalol subgroups; and
(D) propensity score matched Rate and Class 1C subgroups. All AADs and matched Rate cohorts show substantial event rates for the principal outcome during follow-
up, but all AADs studied had a higher risk of events during follow-up. LR ⫽ log rank.
patients but in none of the subgroups examined for the
sex was associated with increased risk in sotalol and Class
Class 1C patients. These results are detailed in the next
1C cohorts, compared with matched Rate cohorts, but this
was not observed in the amiodarone-Rate cohort compari-
CVH categorized by intensity, duration, and associated
son. A history of heart failure, coronary disease, and diabetes
procedures are tabulated in There were substantially
at enrollment were associated with increased risk for CVH
more hospital stays of ⬍3-day duration associated with
in all AAD cohorts. Pulmonary disease at baseline was
cardioversion in the amiodarone and sotalol cohorts than
associated with increased risk of CVH with amiodarone,
matched rate cohorts. Cardioversion occurred at similar
and age ⬎75 years was associated with increased risk of CVH
rates in the matched Class 1C and Rate cohorts (7.2%).
with sotalol. There was evidence of significant AAD–
Cardiovascular hospital stays with a length of stay of ⬍3
comorbidity interactions only in the amiodarone cohort; age
days with a cardioversion procedure alone (without another
⬎75 years and thyroid disease were associated with increased
CV procedure, emergency room visits, or ICU stay [i.e.,
risk for amiodarone patients but not for their matched Rate
events that might reflect adherence to AF rhythm control
counterparts. A significant increased risk for CVH was main-
treatment strategy only]) constituted 6.1%, 6.1%, and 4.0%
tained for amiodarone and sotalol, compared with Rate,
of first CVH for amiodarone, sotalol, and Class 1C,
despite adjustments for age, sex, or any of these comorbidities.
respectively. The corresponding rates in the matched Rate
Time-dependent changes in clinical status that increased
cohorts were 1.9%, 1.6%, and 0.9%, respectively. Stroke,
risk of CVH are shown in In the amiodarone
embolism, and major bleeds accounted for only a minority
patient cohort, relapse from sinus rhythm to AF and
of first CVH in both AAD and rate cohorts
increase in New York Heart Association (NYHA) func-
Warfarin use at first CVH or death was slightly but not
tional class by 1 or more were associated with a 1.9- and
significantly higher in the rate cohorts.
1.7-fold increase in CVH risk, respectively. For sotalol,
Potential risk factors for CVH. Baseline historical char-
relapse from sinus rhythm to AF, increase ⱖ1 in NYHA
acteristics that increased risk of CVH with AAD, compared
functional class, increase in angina class by 1 or more, and
with matched Rate cohorts, are shown in Female
ventricular rate increase ⱖ15 beats/min were all associated
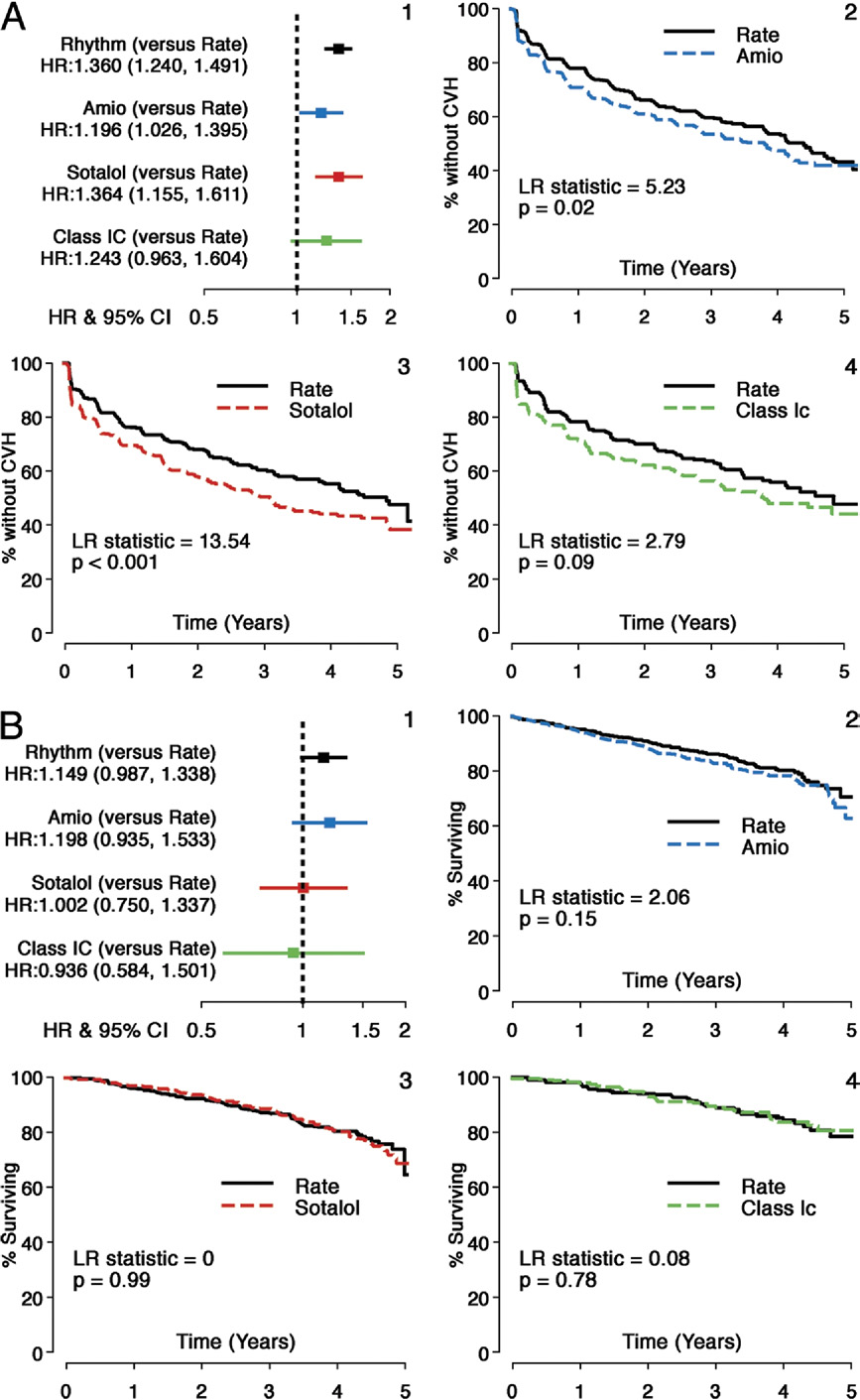
JACC Vol. 58, No. 19, 2011
Saksena et al.
November 1, 2011:1975– 85
CV Outcomes of AADs in the AFFIRM Trial
Components of Principal Outcome—First CVH and Mortality: Individual AADs Versus Rate
(A) First CVH: individual AADs versus Rate. The HRs and Kaplan-Meier survival analyses comparing individual AADs with matched Rate cohorts for a component of princi-
pal outcome: time to first CVH. Individual panels are shown as follows: 1) HRs and 95% CIs (HR: rhythm drug/Rate); 2) propensity score matched Rate and Amio sub-
groups; 3) propensity score matched Rate and sotalol subgroups; 4) propensity score matched Rate and Class 1C subgroups. All AADs and matched Rate cohorts show
substantial event rates during follow-up, but all AADs studied had a significantly higher risk of a first CVH during follow-up. (B) Mortality: individual AADs versus Rate. The
HRs and Kaplan-Meier survival analyses comparing individual AADs with matched Rate cohorts for a component of principal outcome: time to death. Individual panels
are shown as follows: 1) HRs and 95% CIs (HR: rhythm drug/Rate); 2) propensity score matched Rate and Amio subgroups; 3) propensity score matched Rate and sota-
lol subgroups; and 4) propensity score matched Rate and Class 1C subgroups. Sotalol and Class 1C groups and matched rate cohorts show comparable event rates for
risk of death during follow-up, but there is a nonsignificant increase in mortality with Amio compared with its matched Rate cohort. Abbreviations as in
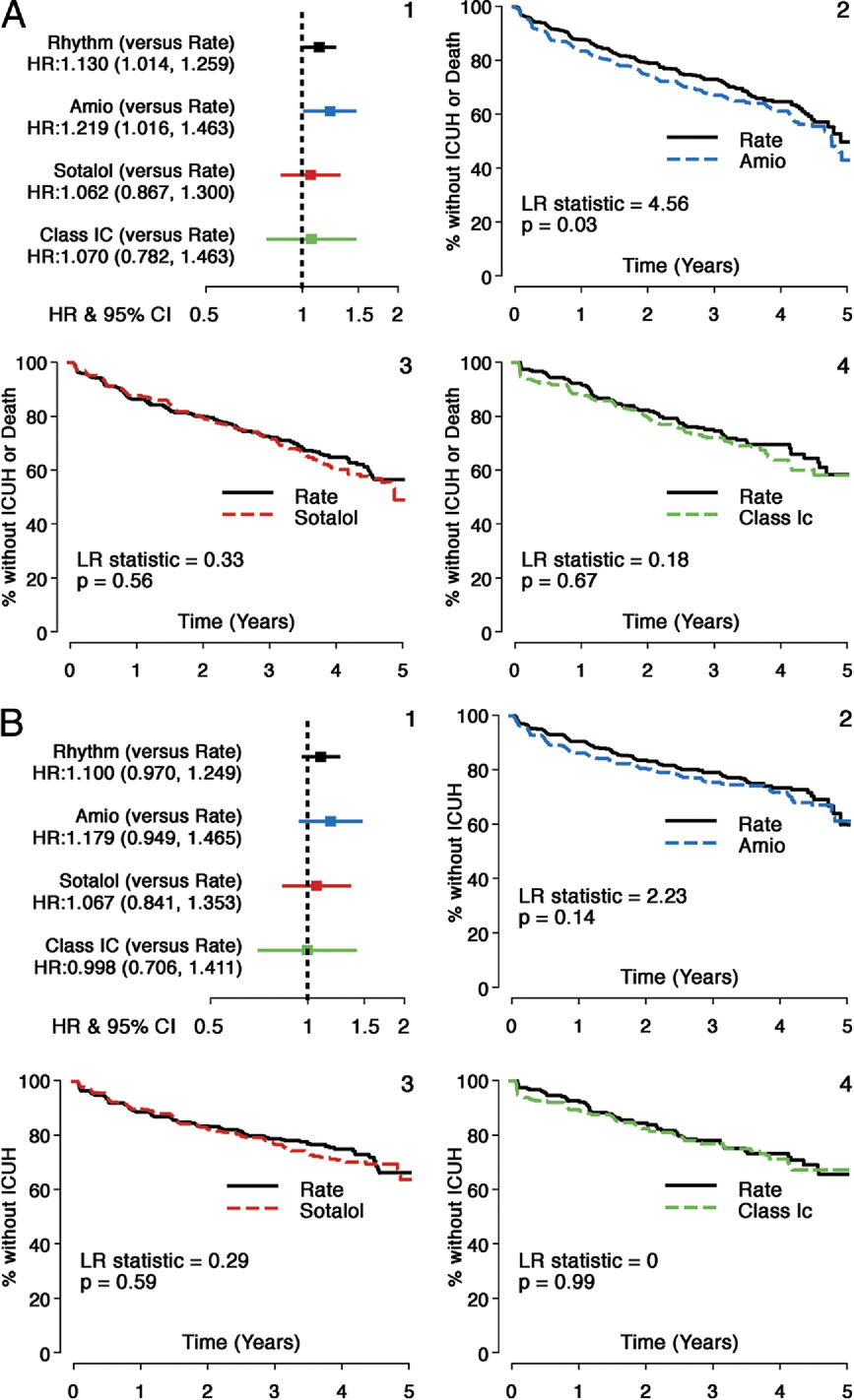
Saksena et al.
JACC Vol. 58, No. 19, 2011
CV Outcomes of AADs in the AFFIRM Trial
November 1, 2011:1975– 85
Comparison of Secondary Composite Outcome—ICUH or Death: Individual AADs Versus Rate
(A) Secondary composite outcome (intensive care unit hospital stays [ICUH] or death): individual AADs versus Rate. The HRs and Kaplan-Meier survival analyses
comparing individual AADs with matched rate cohorts for secondary composite outcome: time to first ICUH or death. Individual panels are shown as follows: 1)
HRs and 95% CIs (HR: rhythm drug/Rate); 2) propensity score matched Rate and Amio subgroups; 3) propensity score matched Rate and sotalol subgroups; 4)
propensity score matched Rate and Class 1C subgroups. Composite outcome shows that time to ICUH or death was shorter with Amio but not with sotalol or
Class 1C versus Rate during follow-up. (B) Comparison of ICUH: individual AADs versus Rate. The HRs and Kaplan-Meier survival analyses comparing individual
AADs with matched rate cohorts for secondary outcome: time to first ICUH. Individual panels are shown as follows: 1) HRs and 95% CIs (HR: rhythm drug/Rate);
2) propensity score matched Rate and Amio subgroups; 3) propensity score matched Rate and sotalol subgroups; 4) propensity score matched Rate and Class
1C subgroups. Time to ICUH was comparable for sotalol and Class 1C groups, compared with matched Rate cohorts, but a nonsignificant increased risk was
seen with Amio compared with Rate during follow-up.
JACC Vol. 58, No. 19, 2011
Saksena et al.
November 1, 2011:1975– 85
CV Outcomes of AADs in the AFFIRM Trial
Patient Cohorts for
Individual Antiarrhythmic Drugs in the AFFIRM Trial
# fatal first CVH
CVH ⬍3 days ⫹ CV
CVH ⬍3 days, CV, no ER/ICU
ICU days first CVH
Warfarin use at first CVH (% of CVH)
Bleeds/stroke/embolic events (% of CVH)
Warfarin use at above event (% of event)
Values are n or n (%).
AFFIRM ⫽ Atrial Fibrillation Follow-Up Investigation of Rhythm Management; CV ⫽ cardiovascular event; CVH ⫽ cardiovascular hospital stay(s); ER ⫽ emergency room visit; ICU ⫽ intensive care unit stay.
with increased risk for CVH. For Class 1C, ventricular rate
To evaluate these agents individually, we employed propen-
increase ⱖ15 beats/min was associated with increased risk.
sity score matching to permit comparative analysis with the
Higher absolute ventricular rate (in steps of 15 beats/min)
rate control patients In this report, it produced highly
was associated with increased risk for sotalol and Class 1C
comparable Rate and AAD cohorts for demographic data,
patients. Overall, a higher NYHA functional class was
disease status and severity, prior interventions, and therapy
associated with increased risk for all cohorts and higher
angina class for amiodarone and Class 1C patients.
Major Findings of Study
Clinical outcomes, especially CVH, are affected by initial
Analyses of overall and secondary outcomes for the AF
AAD selection. The present analysis demonstrates inferior
population in the AFFIRM study have suggested no overar-
performance in the principal clinical outcome for the indi-
ching benefit of a particular strategy There was,
vidual AADs studied versus rate control for the AFFIRM
however, a nonsignificant increase in mortality in the rhythm
population. This difference in composite outcome was
arm with an excess in pulmonary and cancer deaths
largely due to excess and earlier CVH for each AAD.
This finding raised the specter of AAD therapy-related mor-
Sotalol and Class 1C cohorts were comparable to Rate for
tality risk. The impact of individual AAD selection on both
all-cause mortality. The HR comparing amiodarone with
mortality and hospital stay, compared with Rate, has not been
Rate was very similar to the overall AFFIRM study result
available due to the investigator-determined process for AAD
for mortality risk with rhythm control, but in this small
selection, which makes unbiased comparisons challenging.
matched cohort the power to see a significant difference was
However, such an analysis is still relevant and potentially
low (⬍30%). Initial amiodarone therapy was associated with
informative, because most of these agents are currently in
significantly increased risk of non-CV death and mortality
widespread clinical use and still employed in clinical trials
plus ICU hospital stay. The sotalol and Class 1C cohorts
were similar to Rate with respect to these outcomes,
Relationship Between Baseline Characteristics and Risk of CVH
Relationship Between Baseline Characteristics and Risk of CVH
Amiodarone-Rate Cohort
Sotalol-Rate Cohort
Class 1C-Rate Cohort
Baseline variable
1.63 (1.4–1.91)*
1.55 (1.29–1.86)*
1.5 (1.08–2.08)†
1.08 (0.92–1.27)
1.23 (1.04–1.46)†
1.37 (1.06–1.78)†
Coronary artery disease
1.83 (1.57–2.14)*
1.4 (1.18–1.65)*
1.37 (1.01–1.85)†
Pulmonary disease
1.3 (1.08–1.58)‡
1.07 (0.82–1.4)
1.23 (0.86–1.74)
1.62 (1.36–1.92)*
1.29 (1.07–1.57)‡
1.56 (1.13–2.15)‡
1.44 (1.16–1.79)‡
1.12 (0.88–1.43)
1.26 (0.92–1.73)
1.14 (0.97–1.35)
1.25 (1.05–1.5)†
1.1 (0.81–1.49)
Interactions with treatment
Rate control ⫻ age ⬎75 yrs
0.93 (0.72–1.21)
Amiodarone ⫻ age ⬎75 yrs
1.35 (1.08–1.69)
Rate ⫻ thyroid disease
1.10 (0.80–1.51)
Amiodarone ⫻ thyroid disease
1.92 (1.43–2.59)
Values are hazard ratio (95% confidence interval). *p ⬍ 0.001; †p ⬍ 0.05; ‡p ⬍ 0.01.
Saksena et al.
JACC Vol. 58, No. 19, 2011
CV Outcomes of AADs in the AFFIRM Trial
November 1, 2011:1975– 85
Relationship Between Time Dependent Changes in Clinical Status and Risk of CVH
Relationship Between Time Dependent Changes in Clinical Status and Risk of CVH
Amiodarone vs. Rate
Class 1C vs. Rate
1.87 (1.40–2.50)
1.76 (1.29–2.41)
1.11 (0.64–1.94)
NYHA functional class
1.82 (1.45–2.29)
1.35 (1.00–1.82)
2.17 (1.30–3.63)
2.28 (1.78–2.93)
1.81 (1.19–2.77)
1.95 (1.00–3.82)
3.51 (2.42–5.09)
3.72 (2.19–6.33)
4.23 (1.49–12.04)
7.44 (3.42–16.20)
15.61 (4.65–52.47)
22.45 (6.01–83.82)
Increase in NYHA functional class
1.72 (1.35–2.20)
1.98 (1.39–2.83)
1.25 (0.67–2.34)
2.20 (1.68–2.89)
1.26 (0.82–1.92)
2.58 (1.28–5.19)
3.57 (2.40–5.30)
1.62 (0.87–3.01)
5.42 (2.09–14.01)
3.73 (1.61–8.64)
2.20 (0.64–7.48)
6.37 (1.19–34.01)
4.08 (1.29–12.88)
1.96 (0.45–8.61)
28.74 (3.10–266.46)
1.25 (0.87–1.80)
2.35 (1.40–3.92)
0.90 (0.36–2.22)
1.13 (1.04–1.24)
1.10 (1.00–1.21)
0.99 (0.84–1.16)
Increase in VR by ⱖ15 beats/min
1.25 (0.96–1.64)
1.58 (1.20–2.07)
1.62 (1.04–2.51)
Values are hazard ratio (HR) (95% confidence interval). HR for ventricular rate (VR) is the increase in risk associated with a 15-beat/min increase in VR.
CHC ⫽ Canadian Heart Association classification for angina pectoris; CVH ⫽ cardiovascular hospital stay; Sota ⫽ sotalol; SR ⫽ sinus rhythm; other abbreviations as in
suggesting that the excess CVH seen with these drugs were
CVH in AF are costly, with average costs
less serious events than those seen with amiodarone.
estimated to exceed $12,000/AF admission in the United
CVH was extremely common with AF therapies in the
States and $3 billion in annual costs Atrial fibrillation
AFFIRM trial. From our data, we can estimate overall
hospital stays are widely assumed to be related to AF
CVH risk for AF populations and its relation to therapy
recurrences, but such an assumption has neither been
selection during the period 1995 to 2001. Cardiovascular
critically verified and quantified, nor has the uniformity of
hospital stay incidence ranged from 36% to 50% at 3 years
this risk been assessed across AF subpopulations or treat-
for rate and rhythm therapies. Cardiovascular hospital
stay rates in the AFFIRM Rate subgroups were similar to
To date, small trials of nonpharmacologic therapies
those seen in the placebo (rate control therapies only) arm
and 1 large pharmacologic therapy trial have provided
of the ATHENA (A placebo-controlled, double-blind,
some information about CVH in AF Anal-
parallel-arm Trial to assess the efficacy of dronedarone
ysis of the AFFIRM database provides important addi-
400 mg BID for the prevention of cardiovascular Hospi-
tional data from a large randomized controlled trial over
talization or death from any cause in patiENts with Atrial
a long follow-up. CVH presaged mortality, but it was
fibrillation/atrial flutter) trial (36.3% at 2.5 years)
unclear how these events related to treatment strategy
Clinical characteristics and initial AAD selection rather
and clinical condition Given the observations with
than treatment strategy influenced CVH risk. Potential
respect to ICU hospital stays, CVH are usually related to
mechanisms proposed for increased CVH include hospi-
serious morbidity, with treatment strategy-related hospi-
tal stays related to change in AAD therapy with associ-
tal stays—such as for a change of drug therapy or for
ated cardioversion or possible higher warfarin discontin-
cardioversion— being a relatively small component. Ex-
uation rates with potential complications Ouranalysis of CVH related solely to cardioversions for the
cess CVH events observed with the AADs evaluated are
rhythm control strategy, although higher than in
associated with age, sex, and comorbidity status. There is
matched Rate cohorts, demonstrated a fairly low inci-
a residual excess CVH risk even after adjustment for
dence in all AAD cohorts. Stoke, embolism, and major
these historical factors, which is related to AAD use.
bleeds also had a low incidence that was comparable in
Additionally, CVH risk can be related to changes in
the matched Rate cohorts. Longer hospital stays, ICU
cardiovascular disease status longitudinally. Time-
stays, and other CV procedures constituted the bulk of
dependent changes that impact risk can include either AF
CVH, suggesting more serious clinical conditions. Dif-
relapses or worsening of major cardiovascular symptoms
ferences in CVH rates persisted across clinically impor-
of the underlying disease. We propose, on the basis of our
tant subgroups, such as elderly persons, women, and
analysis, that both baseline patient characteristics and
coronary disease patients.
time-dependent changes in clinical status contribute to
CVH in AF: insights from the AFFIRM trial. CVH has
CVH risk. Any heart failure or coronary disease was
become a major endpoint for clinical trials. It can impact
associated with increased risk in all 3 matched cohorts
treatment strategy recommendations and regulatory ap-
but was more common in the amiodarone and matched
proval of new therapies but is rarely used in AF trials
Rate cohorts. An increase in heart failure or angina class
JACC Vol. 58, No. 19, 2011
Saksena et al.
November 1, 2011:1975– 85
CV Outcomes of AADs in the AFFIRM Trial
by 1 or more increased risk of CVH. These findings make
5. The AFFIRM Investigators. A comparison of rate control and rhythm
a strong case for baseline disease state variables and
control in patients with atrial fibrillation. N Engl J Med 2002;347:1825–33.
change in clinical status leading to CVH.
6. Corley SD, Epstein AE, DiMarco JP, et al., AFFIRM Investigators.
Relapse from sinus rhythm to AF was also related to
Relationships between sinus rhythm, treatment, and survival in the
CVH, suggesting failure of rhythm control as a potential
Atrial Fibrillation Follow-Up Investigation of Rhythm Management(AFFIRM) Study. Circulation 2004;109:1509 –13.
mechanism. Finally, specific interactions of antiarrhythmic
7. Rosenbaum PR, Rubin DB. The central role of the propensity score in
agents such as amiodarone with comorbidities such as
observational studies for causal effects. Biometrika 1983;41–55.
thyroid disease suggest additional mechanisms leading to
8. Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite
outcomes in randomized trials— greater precision with greater uncer-
hospital stay. The reasons for CVH are multiple and
tainty. JAMA 2003;289:2554 –9.
multifactorial. Atrial fibrillation patients have varying risk
9. AFFIRM First Antiarrhythmic Drug Substudy Investigators. Main-
for the principal outcome in this analysis on the basis of
tenance of sinus rhythm in patients with atrial fibrillation: an AFFIRM sub
these factors.
study of the first antiarrhythmic drug. J Am Coll Cardiol 2003;42:20 –9.
10. Akaike H. A new look at the statistical model identification. IEEE
Trans on Automatic Control 1974;19:716 –23.
Propensity score matching cannot correct for erroneous
11. Sherman DG, Kim SG, Boop BS, et al., National Heart, Lung, and
Blood Institute AFFIRM Investigators. Occurrence and characteris-
omission or inclusion of variables that might have affected
tics of stroke events in the Atrial Fibrillation Follow-up Investigation
AAD selection, but it is a significant improvement over
of Sinus Rhythm Management (AFFIRM) study. Arch Intern Med
naïve subgroup analyses. Some of the hospital stays might
12. Wyse DG, Slee A, Epstein AE, et al. Alternative endpoints for
be the result of routine patient care for rhythm control
mortality in studies of patients with atrial fibrillation: the AFFIRM
rather than for medical necessity, but these still occur in
study experience. Heart Rhythm 2004;1:531–7.
current clinical practice. The AFFIRM study did not
13. Jenkins LS, Brodsky M, Schron E, et al. Quality of life in atrial
fibrillation: the Atrial Fibrillation Follow-up Investigation of Rhythm
capture detailed reasons for hospital stay or drug doses.
Management (AFFIRM) study. Am Heart J 2005;149:112–20.
Exact dates of hospital stay were not collected, which results
14. Steinberg JS, Sadaniantz A, Kron J, et al. Analysis of cause-specific
in decreased precision in estimates of time to hospital stay
mortality in the Atrial Fibrillation Follow-up Investigation of RhythmManagement (AFFIRM) study. Circulation 2004;109:1973– 80.
but probably not for the comparison of matched cohorts.
15. Hohnloser SH, Crijns HJ, van Eickels M, et al., ATHENA Investi-
gators. Effect of dronedarone on cardiovascular events in atrial fibril-
lation. N Engl J Med 2009;360:668 –78.
16. Wilber DJ, Pappone C, Neuzil P, et al., ThermoCool AF Trial
CV hospitalizations were common in AFFIRM with both
Investigators. Comparison of antiarrhythmic drug therapy and radio-frequency catheter ablation in patients with paroxysmal atrial fibrilla-
treatment strategies but more frequent with amiodarone,
tion: a randomized controlled trial. JAMA 2010;303:333– 40.
sotalol and class 1C agents. The severity of this risk varied
17. Bosco JL, Silliman RA, Thwin SS, et al. A most stubborn bias: no
with the individual AAD, patient characteristics and time
adjustment method fully resolves confounding by indication in obser-vational studies. J Clin Epidemiol 2010;63:64 –74.
dependent changes in clinical status, but was largely unre-
18. Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency
lated to the rhythm treatment algorithm. Death, intensive
ablation vs antiarrhythmic drugs as first-line treatment of symptomatic
care unit hospital stay, and non-CV death were more
atrial fibrillation: a randomized trial. JAMA 2005;293:2634 – 40.
frequent with amiodarone.
19. Stabile G, Bertaglia E, Senatore G, et al. Catheter ablation treatment
in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation for the Cure
Reprint requests and correspondence: Dr. Sanjeev Saksena,
of Atrial Fibrillation Study). Eur Heart J 2006;27:216 –21.
20. Rao BH, Saksena S. Impact of "hybrid therapy" on long-term rhythm
RWJ Medical School, Department of Medicine, 161 Washington
control and arrhythmia related hospitalizations in patients with drug-
Valley Road, Suite 201, Warren, New Jersey 07059. E-mail:
refractory persistent and permanent atrial fibrillation. J Interv Card
21. Naccarelli G, Johnston S, Lin J, Patel P, Schulman K. Cost burden of
cardiovascular hospitalization and mortality in ATHENA-like pa-
tients with atrial fibrillation/atrial flutter in the United States clinicalcardiology 2010;33:270 –9.
1. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB,
22. Khairallah F, Ezzedine R, Ganz LI, London B, Saba S. Epidemiology
Levy D Impact of atrial fibrillation on the risk of death: The
and determinants of outcome of admissions for atrial fibrillation in the
Framingham Heart Study. Circulation 1998;98:946 –52.
United States from 1996 to 2001. Am J Cardiol 2004;94:500 – 4.
2. AF Hospitalizations in the USA, June 2009. Available at:
chart 3-47& 3-48. Accessed September 13, 2011.
Key Words: antiarrhythmic drugs y atrial fibrillation y cardiovascular
3. Miyasaka Y, Barnes ME, Gersh BJ, et al. Changing trends of hospital
hospitalizations y cardiovascular outcomes y clinical trials y outcomes
utilization in patients after their first episode of atrial fibrillation. Am J
Cardiol 2008;102:568 –72.
4. The Planning and Steering Committees of the AFFIRM Study for the
NHLBI AFFIRM Investigators. Atrial fibrillation follow-up investi-gation of rhythm management—the AFFIRM study design. Am J
For list of investigators and affiliated institutions,
Cardiol 1997;79:1198 –202.
please see the online version of this article.
Source: http://www.eprf.org/downloads/AFFIRM_final_published_paper.pdf
Trends in the Abuse of Prescription Drugs by Jane Carlisle Maxwell, Ph.D. The sale of narcotic analgesic pills is increasing, as is diversion and the non-medical use of prescription drugs. These drugs are easy to obtain and they are viewed as "safer" than street drugs. Young adults have the highest rates of lifetime use of these drugs and fewer teenagers in 2005
Preventive journalism A media and coverage of professional's risk situations avian influenza ANDI ANDI LATIN AMERICAN NETWORK UNICEF Preventive journalism A media and coverage of professional's risk situations avian influenza ANDI – BRAZILIAN NEWS AGENCY FOR PREVENTIVE JOURNALISM AND COVERIAGE OF RISK SITUATIONS




