Foodtech-portal.eu
Surface-plasmon-resonance-based biosensors for food diagnostics
SPR based biosensors
Key words
surface plasmon resonance, SPR, biosensor, additive, residue, contaminant,pesticide, herbicide, veterinary drug, antibiotic, bacteria, pathogen, toxin, allergen,analysis
Completed by
How does it work?
analytical tool
detection and (semi)quantification of low levels of biological and chemical
substances in foods (e.g. veterinary drugs, pathogenic bacteria/toxins, vitamins,
pesticides, allergens) based on the principle of specific biological recognition.
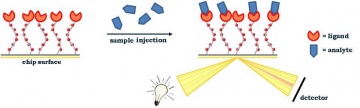
Surface-plasmon-resonance (SPR)-based biosensor detection and quantification arebased on label-free monitoring of the interaction between a target analyte insolution and a biomolecular recognition element immobilized on the sensorsurface. In most cases, a specific antibody towards this target is used asrecognition element. Assays rely in principle on the measurement of massconcentration changes (as a refractive index change) at the sensor surface, whichare detected by use of the optical phenomenon of SPR. (1-3)
Briefly, SPR occurs in thin conducting films (mostly gold) at an interface betweenmedia of different refractive index. Under specific conditions of wavelength andangle of incidence, incident light excites plasmons (= electron charge densitywaves) in the gold film, resulting in absorption of energy (part of the energy of thelight is transferred to the metal film). SPR is seen as a dip in the intensity of thereflected light. A change in the refractive index of the medium near the gold filmalters the light characteristics (e.g., angle, wavelength) at which this dip inintensity occurs. Different types of SPR sensors are distinguished depending onwhich light characteristic is measured as SPR response (mostly SPR angle shift) inorder to detect changes in mass concentration at the surface (as changes inrefractive index). (1-3)Practically, in an SPR biosensor, one interaction partner (mostly the biorecognitionelement) is immobilized on the sensor surface using an appropriate couplingmethod. Different immobilization chemistries and specific tailored surfaces havebeen described. Next, a sample containing the target analyte is brought intocontact with the surface to allow interaction (often by a microfluidic system). Thesensor surface is then regenerated with an appropriate washing solution for thenext sample to be tested. Binding and dissociation events are followed on asensorgram (= SPR response, as a measure for bound mass, vs. time). Bycomparing the SPR response of an unknown sample with those of knownconcentrations of analyte, quantification of the unknown samples is achieved. Forlow-molecular weight analytes (mass below ca. 5 kDa), the sensitivity/limit ofdetection can be enhanced by using sandwich (in which a second binder to theanalyte is injected, sometimes even linked to nanoparticles), inhibition (withimmobilized target molecule) or competitive assays, since binding of smallcompounds generates only small SPR responses (1-3).
Besides detection and quantification of target substances, SPR biosensors alsoallow the specific study of affinity, kinetics (association and dissociation rates),thermodynamics (based on binding data at different temperatures) andstoichiometry of interactions between biomolecules without the need for labelling(1,2).
The application field of SPR biosensors is not restricted to food analysis, but alsoincludes medical diagnostics (e.g. cancer markers, antibodies, drugs, hormonesetc.), environmental monitoring (e.g. pesticides, heavy metals, dioxins, PCBs etc.)and interaction analysis in the life sciences and drug discovery (1-3).
Several parameters directly impact the sensitivity , specificity and detection limit
of the SPR-based biosensors (3), including:• Choice of (specific) bio-recognition element (antibody or other);• Choice of immobilization strategy (retaining biological activity and minimizingnonspecific binding to the surface);• Choice of detection format (direct, sandwich, inhibition or competition assay);• Choice of sample preparation/pretreatment.
What can it be used for?
Basically, all kinds of food products are possible candidates to be analyzed by SPRbiosensor technology, provided that an appropriate sample preparation isexecuted. This sample preparation can be restricted to dilution in a running buffer(for some liquid samples) or entail homogenization, extraction and/orcentrifugation/filtration (for complex liquid and solid samples) (2).
Examples are water, milk, orange/apple juice, urine, honey, barley, wheat, corn,adipose tissue, muscle tissue, mussel, prawn, shrimp, etc (4,5).
SPR biosensor methods have been described for detection and/or quantification ofa wide variety of biological and chemical substances in complex food products.
Targeted analytes include:• Pathogens: bacteria (Escherichia coli O157:H7, Salmonella spp., Listeriamonocytogenes, Campylobacter jejuni, a.o.), protozoa, fungi (1,3,5)• Toxins: staphylococcal enterotoxins, botulinum neurotoxins, domoic acid,mycotoxins (1,3,5)• Veterinary drug residues: growth-promoting hormones (e.g. β-agonists),antibiotics (e.g. penicillin, streptomycin, chloramphenicol) (2-6)• Pesticide and herbicide residues: atrazine, simazine (3,4)• Vitamins: Vit B2 (riboflavin), Vit B5 (pantothenic acid), Vit B8 (biotin), Vit B9 (folicacid), Vit B12 (cobalamine) (2,3)• Allergens: from peanut (Ara h1), egg white, sesame seed, hazelnut (3,7)
Solutions for short
The ability to identify and quantify low levels of biological and chemical
compounds that are relevant for food quality and food safety is of greatimportance to the food processing industry as well as to regulatory agencies. Thereis a need for sensitive and accurate techniques to rapidly detect the presence ofcontaminants. SPR is one such technique.
The main advantages of SPR-based detection over alternative analyticaltechniques (microbial assays, ELISA, PCR, chromatography a.o.) include ease ofuse, reduced assay time, sensitivity (although not for all cases), minimal samplepreparation and versatility. SPR biosensors are capable of performing real-timedetections, making on-line monitoring of food processing possible (rather thanend-product testing) (1-6).
What can it NOT be used for?
The analysis by SPR biosensors is restricted to liquid samples (i.e. either a solutionwhich is pumpable through a microfluidic system or a liquid in which a dipstick-likeprobe can be immersed). Consequently, solid foods should at least behomogenized and filtered/centrifuged. Sometimes, an extraction is required. "Insitu" analysis is not possible.
An SPR biosensor technique for a particular target analyte can only be designed ifa specific bio-recognition element is available for that target (e.g. antibody oraptamer).
The size of the target also matters, since large targets (e.g. live bacteria) haveextremely low rates of diffusion. To overcome this limitation, various treatmentmethods can be applied to create smaller fragments (e.g. heat-killing, cell lysisusing ethanol or detergents) (1). For some analytes, current SPR biosensors do notreach the required limits of detection and further optimization is needed (1).
Most current commercial SPR equipment is rather bulky in size and expensive,consequently not very suited for "field use".
Risks or hazards
Generally, there are no specific risks for the operators of this analytical technique.
The risk may however be in the conclusions one draws from experimental results(see legal aspects).
Quite a number of SPR sensor platforms from various manufacturers are availablecommercially. Besides, an even more diverse spectrum of tailor-made laboratoryinstruments have been described, including fiber optic SPR sensors (e.g. dipsticktype). Systems often differ in SPR optical configuration, liquid handling system(flow cell, cuvette, microfluidic chip), level of automation and miniaturization, levelof sensitivity and high-throughput capacity. In the context of food analysis, BiacoreQ is worth mentioning, since is concerns a fully-automated, wizard-driveninstrument, which is dedicated to safety and quality analysis (qualitative orquantitative) within the food industry. To make the analysis process as simple andconsistent as possible, an extensive range of Qflex Kits have been created for usewith the Biacore Q system (1-3).
The development of low-cost, compact, portable alternatives for field use (e.g.
fiber optic SPR biosensors) is currently subject of lab-scale research (7-8).
Procedures for a wide variety of analytes have been described in scientificliterature. For specific analytes, ready-to-use analysis kits can even be purchased(e.g. QFlex Kits of Biacore) (2,9). For others, method implementation andoptimization may be needed.
SPR biosensors are capable of performing real-time detections, making on-line
monitoring of food processing possible (rather than end-product testing) (1).
The biosensors may also be coupled offline or online with mass spectrometrydevices for identification of interactants (4,9).
Not applicable. It will be in the consumers' interest to detect f.i. allergens, but it isnot expected that the consumers will have a specific attitude towards thedetection method .
Certification of an analysis (e.g. to reach minimum required performance limits)requires documented evidence for the quality of several assay parameters(specificity, accuracy, repeatability, reproducibility, limit of detection, etc.) andprovides assurance that an independent third party has tested the assay and foundthat the product fulfills all performance claims. Several analyses of the Qflex Kits ofBiacore, for instance, received AOAC approval after rigorous performance trialscarried out in independent laboratories (2,6).
Local legislation should be checked to obtain appropriate certifications if claims aremade based on analytical results.
Environmental aspects Given the limited sample preparation and the principle of detection, SPR
biosensors often require reduced use of (organic) solvents (for extraction and/orelution) compared to alternative analytical techniques (chromatography, microbialassays, ELISA, PCR, etc).
Facilities that might be interesting for you
1. Homola, J. (2006). Surface Plasmon Resonance Based Sensors. Berlin , Germany:Springer.
2. Schasfoort, R.B.M., Tudos, A.J. (2008). Handbook of Surface Plasmon Resonance.
Cambridge, UK: RSC Publishing.
3. Homola, J. (2008). Surface plasmon resonance sensors for detection of chemicaland biological species. Chemical Reviews, 108, 462-493.
4. Petz, M. (2009). Recent applications of surface Plasmon resonance biosensorsfor analyzing residues and contaminants in food. Monatshefte für Chemie, 104,953-964.
5. Ricci, F., Volpe, G., Micheli, L., Palleschi, G. (2007). A review on noveldevelopments and applications of immunosensors in food analysis. AnalyticaChimica Acta, 605, 111-129.
6. Huet, A.-C., Fodey, T., Haughey, S.A., Weigel, S., Elliot, C., Delahaut, P. (2010).
Advances in biosensor-based analysis for antimicrobial residues in foods. Trends inAnalytical Chemistry, 29, 1281-1294.
7. Pollet, J., Delport, F., Janssen, K.P.F., Tran, D.T., Wouters, J., Verbiest, T.,Lammertyn, J. (2011). Fast and accurate peanet allergen detection with nanobeadenhanced optical fiber SPR biosensor. Talanta, 83, 1436-1441.
8. Fernandez, F., Hegnerova, K., Piliarik, M., Sanchez-Baeza, F., Homola, J., Marco,M.-P. (2010). A label-free and portable multichannel surface plasmon resonanceimmunosensor for on site analysis of antibiotics in milk samples. Biosensors andBioelectronics, 26, 1231-1238.
9. Situ, C., Buijs, J., Mooney, M.H., Elliot, C.T. (2010). Advances in surface plasmonresonance biosensor technology towards high-throughput, food-safety analysis.
Trends in Analytical Chemistry, 29, 1305-1315.
Source: http://www.foodtech-portal.eu/index.php?title=Special:PdfPrint&page=SPR+based+biosensors
African Journal of Medical Sciencesfo Citation: Balsam A. Gagran and Nossiba O. Ali. Prevalence of Extended Spectrum Beta- Lactamase Producing Escherichia coli in Hospital Acquired Urinary Tract Patients. African Journal of Medical Sciences, 2016, 1(3) ajmsc.info Prevalence of Extended Spectrum Beta-Lactamase Producing Escherichia coli in Hospital Acquired Urinary Tract Patients
First Quarter 2012 P&T Update Vol. IX, Issue 1 Special Points of P&T members discussed the formulary status of two tetanus toxoid, reduced diphtheriatoxoid and acellular pertussis (Tdap) vaccines: Adacel® and Boostrix®. Both vaccines are • P&T Update-Formulary currently maintained on the hospital formulary. The subcommittee agreed to keep


