Bhr185 1133.1138
Cerebral Cortex May 2012;22:1133--1138doi:10.1093/cercor/bhr185Advance Access publication July 28, 2011
Modulation of Inhibition of Return by the Dopamine D2 Receptor Agonist BromocriptineDepends on Individual DAT1 Genotype
Ariel Rokem1, Ayelet N. Landau2, William Prinzmetal2, Deanna L. Wallace1,2, Michael A. Silver1,3 and Mark D'Esposito1,2
1Helen Wills Neuroscience Institute, 2Department of Psychology and 3School of Optometry, University of California, Berkeley,Berkeley, CA 94720, USA.
Address correspondence to Ariel Rokem, 360 Minor Hall #2020, University of California, Berkeley, Berkeley, CA 94720-2020, USA. Email:
[email protected].
Involuntary visual spatial attention is captured when a salient cue
what extent these 2 phenomena depend on the same neural
appears in the visual field. If a target appears soon after the cue,
response times to targets at the cue location are faster relative to
Here, we examined the role of the neurotransmitter
other locations. However, after longer cue--target intervals,
dopamine (DA) in both involuntary attention and IOR. DA is
responses to targets at the cue location are slower, due to
involved in a variety of cognitive functions, and 3 lines of
inhibition of return (IOR). IOR depends on striatal dopamine (DA)
evidence suggest that it also modulates IOR. First, patients with
levels: It varies with different alleles of the DA transporter gene
Parkinson's disease (PD), a disease characterized by reduced
DAT1 and is reduced in patients with Parkinson's disease, a disease
dopaminergic transmission in the striatum, have reduced IOR
characterized by reduced striatal dopaminergic transmission. We
magnitude, relative to healthy controls (Filoteo et al. 1997;
examined the role of DA in involuntary attention and IOR by
Yamaguchi and Kobayashi 1998; Possin et al. 2009). Second,
administering the DA D2 receptor-specific agonist bromocriptine to
even in healthy individuals, genetic differences in striatal DA
healthy human subjects. There was no effect of either DAT1
transmission predict differences in IOR. In particular, the gene
genotype or bromocriptine on involuntary attention, but participants
DAT1 codes for a DA transporter which facilitates reuptake of
with DAT1 alleles predicting higher striatal DA had a larger IOR.
DA in the striatum (Sesack et al. 1998), and this gene has
Furthermore, bromocriptine increased the magnitude of IOR in
different alleles that are associated with different levels of DA
participants with low striatal DA but abolished the IOR in subjects
clearance from synapses (Mill et al. 2002). Subjects with
with high striatal DA. This inverted U-shaped pattern resembles
a DAT1 allele that predicts higher levels of striatal DA have
previously described relationships between DA levels and perfor-
a larger IOR for short cue--target intervals (less than 750 ms),
mance on cognitive tasks and suggests an involvement of striatal
relative to subjects with a DAT1 allele that predicts lower levels
DA in IOR that does not include a role in involuntary attention.
of striatal DA (Colzato et al. 2010). Finally, DA D2 receptors(DRD2) are enriched in the human striatum (Camps et al. 1989;
Keywords: DAT1, dopamine, inhibition of return, striatum, visual attention
Meador-Woodruff et al. 1996), and long-term cocaine use,which leads to reductions in DRD2 (Volkow et al. 1999),abolishes the IOR (Colzato and Hommel 2009).
Taken together, these results suggest that increased striatal
DA transmission is associated with larger IOR. In order to
When a salient event occurs in the visual field, involuntary
delineate a causal role of striatal DA transmission in the IOR,
visual spatial attention is captured at that location (Yantis and
pharmacological methods can be used. A previous study has
Jonides 1990). As a consequence, performance on discrimina-
shown that the temporal extent of the IOR is increased in a dose-
tion tasks is facilitated and response times (RTs) are faster
dependent manner by the administration of d-amphetamine
when a target appears in the cued location, relative to other
(Fillmore et al. 2005), a drug that increases extracellular DA
locations (Prinzmetal et al. 2005). This effect of cueing, due to
levels. However, the actions of d-amphetamine are not specific to
capture of involuntary attention, develops quickly but is
a particular type of DA receptor. Moreover, d-amphetamine also
transient (Posner and Cohen 1984). On the other hand, with
increases levels of extracellular noradrenaline in the central
longer delays between cue and target, involuntary attention
nervous system (Heal et al. 2009).
dissipates before target presentation, and the opposite effect is
In the present study, we administered the DRD2-specific
observed: RTs are faster for targets presented at noncued
agonist bromocriptine to a group of young healthy participants
locations, relative to the cue location (Posner and Cohen
and tested their performance in a cued visual discrimination
1984). This phenomenon is known as the inhibition of return
task. Bromocriptine is used to treat PD (Radad et al. 2005), and
(IOR; for a review, see Klein (2000)).
in healthy young participants, it can increase performance on
The physiological mechanisms underlying the IOR are only
tasks requiring spatial working memory (reviewed in Mehta
partially understood. Brain imaging studies suggest that IOR
and Riedel (2006)). However, these findings are mixed, with
involves frontal and posterior parietal cortical regions (Lepsien
some studies failing to replicate increased spatial working
and Pollmann 2002; Mayer et al. 2004). However, other results
memory performance following bromocriptine administration
implicate subcortical structures in IOR (Sapir et al. 1999;
or replicating them only at lower doses. One possible
Fecteau and Munoz 2005). Although physiological (Fecteau and
explanation of these discrepancies is the inverted U-shaped
Munoz 2005), neuropsychological (Sapir et al. 1999), and
effect of DA transmission on cognitive functions that has been
behavioral (Ro and Rafal 1999) studies suggest that involuntary
observed in several different contexts (Cools and Robbins
attention and IOR can occur independently, it is unclear to
2004; Seamans and Yang 2004; Cools and D'Esposito 2011),
Ó The Author 2011. Published by Oxford University Press. All rights reserved.
For permissions, please e-mail:
[email protected]
including following bromocriptine administration (Kimberg
levels peak ca. 100 min after oral administration and remain
et al. 1997; Cools et al. 2007, 2009). Based on the inverted U-
significantly elevated for several hours; Price et al. (1978)). Subjects
shaped effects of DA, we predicted that participants would
first conducted a brief block of training (20 trials) to acquaint themwith the task, followed by 4 blocks of 80 trials each. They were
respond to pharmacological activation of DRD2 in a non-
instructed to fixate on a central point, and eye movements were
monotonic fashion, depending on their genetic background.
monitored using a camera placed in front of their eyes. Auditory
Specifically, we predicted that participants with low baseline
feedback was provided at the end of a trial if fixation was not
levels of striatal DA would show an increase in the magnitude
maintained, and trials containing eye movements were excluded from
of the IOR following bromocriptine administration, whereas
further analysis. The proportion of trials in which eye movements
bromocriptine would decrease IOR magnitude in participants
occurred was low (ca. 0.3% of all trials) and did not differ between drugand placebo sessions (F
with high baseline levels of striatal DA.
1,15 = 0.21, P = 0.65).
Materials and Methods
Genetic TestingUsing the Oragene DNA Self-Collection Kit (DNA Genotek Inc., Ottawa,Ontario, Canada), we collected saliva samples from each subject, and
the variable number of tandem repeats (VNTR) for the DAT1 gene was
Twenty-one healthy adults (11 females; age: 19.9 ± 1.7) participated in
determined by polymerase chain reaction, using primers designed
the study. The experimental procedures were approved by the
specifically for the 40-bp VNTR polymorphism in the 3
Committee for the Protection of Human Subjects at the University of
region (Creative Genomics, Port Jefferson Station, NY). Of the 19
California, Berkeley, and all experiments were conducted with the
subjects that completed the study, 10 were homozygous for the 10-
written consent of each subject. Two subjects experienced adverse
repeat allele of this gene (10R), 8 were heterozygous (one copy each of
effects of the drug and did not complete the task.
the 10-repeat and 9-repeat alleles), and 1 was homozygous for the 9-repeat (9R) allele. Following Colzato et al. (2010), subjects carrying at
least one copy of the 9R allele were grouped together and referred to
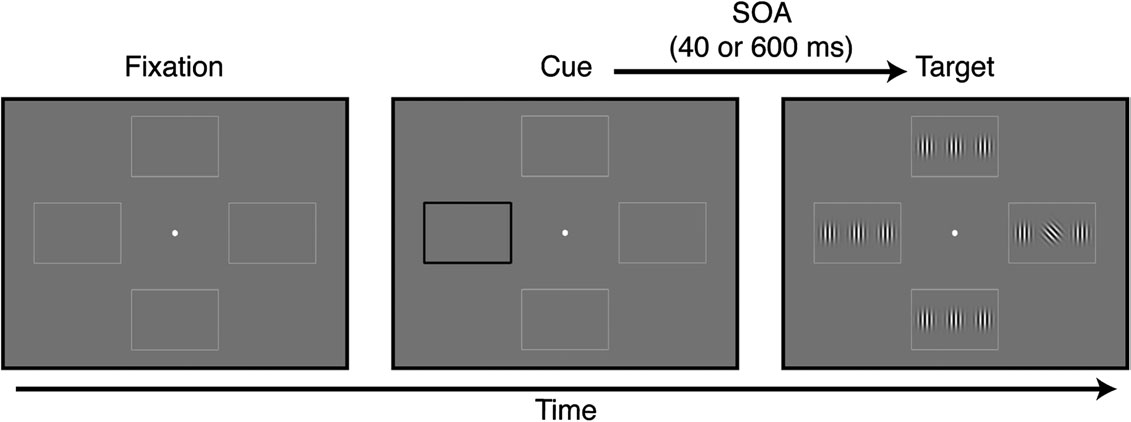
Each trial began with a 200 ms cue: one of the peripheral rectangular
collectively as 9R.
frames (Fig. 1) became black and thicker and, after a variable stimulusonset asynchrony (SOA), a target display appeared for 240 ms. The targetdisplay contained 12 Gabor patches (100% contrast, spatial frequency: 3
cycles/degree of visual angle; space constant: 1 degree of visual angle), 3
Correct responses were well above 90% in all conditions, and there was
within each frame. The target (always the central of the 3 Gabor
no main effect of the drug on the percentage of correct responses (F1,15
patches) was tilted ±45° away from vertical, and all other patches were
= 0.97, P = 0.34). RTs from incorrect trials and trials with RTs faster than
vertically oriented (Rokem et al. 2010). The tilted grating appeared in
100 ms or slower than 1500 ms were excluded from the analysis, as
one of the 4 locations with equal probability (25% of the trials),
were trials with RTs more than 3 standard deviations (SDs) away from
independent of the cue location, and subjects were told that the cue did
each participant's mean performance in a given condition (combination
not contain any information about the subsequent target location.
of drug/SOA/target location for that subject).
Subjects reported the direction of tilt of the target by pressing one of 2buttons as quickly and accurately as they could. Auditory feedback onperformance was provided at the end of each trial. In different blocks,
the SOA between cue and target appearance was either 40 or 600 ms.
In order to measure behavioral effects of the allocation of
SOA blocks were interleaved, and the order was counterbalanced
involuntary attention and the IOR, we measured RT in a visual
between subjects, such that all combinations of order of SOA and orderof drug administration were approximately equally represented.
discrimination task. In each trial, a cue appeared with equalprobability in one of 4 locations in the visual field (Fig. 1). Thelocation of the cue was not predictive of subsequent target
location, which was also randomly selected on each trial. The
A crossover design was employed: each subject received placebo priorto one experimental session and 1.25 mg bromocriptine prior to the
effects of cueing were assessed by comparing RTs from trials in
other. Drug administration was double blind. Testing was conducted
which the target appeared in the cue location (25% of trials)
approximately 3--4 h after bromocriptine administration (drug plasma
with RTs from trials in which targets appeared in other
Figure 1. Visual cueing task. At the beginning of each trial, one of the 4 peripheral rectangular frames became black and thicker. Following a SOA of either 40 or 600 ms, thetarget appeared in one of the 4 locations (25% probability at each location). The target was a Gabor patch oriented ±45° relative to vertical. Subjects indicated target orientationas quickly and accurately as they could by pressing one of 2 buttons.
Rokem et al.
locations (75% of trials). Separate sets of blocks with differentcue-to-target SOAs were used to assess involuntary attentionand IOR. In half of the blocks, a 40 ms SOA was used, consistentwith the SOA required for allocation of involuntary attention(Posner and Cohen 1984). In the other half of the blocks, a 600ms SOA was used, consistent with the time course of the IOR(Posner and Cohen 1984). To test the effects of the DRD2agonist bromocriptine, we employed a placebo-controlled,double blind crossover design in which each subject partici-pated in 2 sessions: one with a placebo and the other aftertaking a pill containing bromocriptine.
In order to assess the effects of cueing, SOA and bromocrip-
tine, we conducted a mixed-model analysis of variance(ANOVA) on mean RTs, with target location (cued vs. other),SOA (short vs. long), and drug (bromocriptine vs. placebo) as
within-subject factors. In addition, DAT1 genotype (10R, or lowstriatal DA, vs. 9R, or high striatal DA) was also entered asa between-subjects factor in the ANOVA to test the effects ofindividual differences in baseline striatal DA levels. Finally, toaccount for effects of learning between the 2 sessions, order ofdrug administration (bromocriptine first vs. placebo first) was
also entered as a between-subjects factor.
We did not find a main effect of bromocriptine on RT (F1,15 =
0.01, P = 0.9), suggesting that the drug did not have an overall
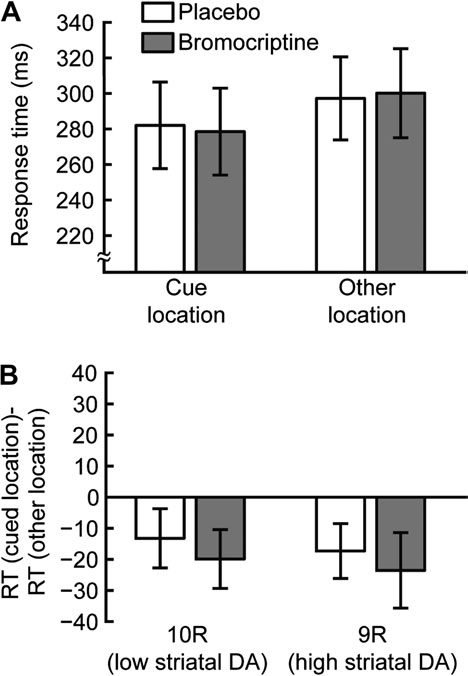
Figure 2. Short SOA blocks. (A) Average RTs for 40 ms SOA blocks. RTs are shown
effect on motor response or arousal. In addition, there was no main
for trials in which the target appeared in the cue location (25% of trials, left) and for
effect of DAT1 genotype on RT (F
trials in which the target appeared in one of the other locations (75% of trials, right).
1,15 = 1.41, P = 0.25), suggesting
(B) The cueing effect is defined for each subject as the mean RT of trials in which the
that overall task performance was not determined by baseline
target appeared in the cue location minus the mean RT of trials in which the target
striatal DA levels. However, there was a significant interaction of
appeared in one of the other locations. Cueing effects are shown separately for 10R
the effects of drug and order of drug administration (F1,15 = 23.23,
(left) and 9R subjects (right) and for placebo (white) and bromocriptine (gray)
P < 0.01). Specifically, if participants were administered placebo in
sessions. The negative values indicate a reduction in RT for trials in which the target
the first session and bromocriptine in the second session, they
appeared in the cue location, reflecting capture of involuntary attention. Thisinvoluntary attention effect was not affected by either bromocriptine administration or
were faster in the bromocriptine session. If they were adminis-
DAT1 genotype. Error bars are standard error of the mean within group/condition.
tered bromocriptine and then placebo, they were faster in theplacebo session. This suggests that participants' performance
computed within-subject cueing effects in order to eliminate
improved through their experience with the task and that they
variability due to overall RT differences between subjects. The
were generally faster in the second testing session, regardless of
average cueing effect, defined as the difference in RT between
whether bromocriptine or placebo was administered in this
trials in which the target was in the cue location and trials in
session. We controlled for this order effect by counterbalancing
which the target appeared in one of the other locations, was
the order of drug and placebo sessions between subjects.
significantly less than zero (–15 ms, within-subject 2-tailed t-
Collapsing across both SOA conditions and drug and placebo
test: t18 = 2.38, P < 0.05) and was negative for 14 of the 19
sessions, we found no main effect of target location (cued vs.
subjects in the placebo sessions. In the bromocriptine sessions,
other, F1,15 = 2.95, P = 0.1). However, there was a main effect of
subjects were also faster to respond to targets presented at the
SOA (F1,15 = 6.60, P < 0.05): subjects were faster in 600 ms
cued location than other locations (cue location: 278 ms, SD
compared with 40 ms SOA blocks. This reflects the fact that
104, other locations: 300 ms, SD 106, Fig. 2A), again resulting in
subjects have more time to prepare their response in long SOA
a significant cueing effect due to involuntary attention (–22 ms,
trials. In addition, there was a significant interaction of target
within-subject 2-tailed t-test: t18 = 2.92, P < 0.01). There was no
location and SOA (F1,15 = 14.37, P < 0.01), indicating that, as
significant difference between the cueing effect observed in
predicted, the cue had opposite effects in the 2 SOA
the placebo sessions and the cueing effect in the bromocrip-
conditions. Specifically, RTs were faster for trials in which the
tine sessions (within-subject 2-tailed t-test: t18 = 0.88, P = 0.39).
cue and target location were the same for the short SOA
Additionally, involuntary attention cueing effects were not
condition (involuntary attention), but they were slower for
significantly different for 9R and 10R subjects in either placebo
these trials in the long SOA condition (IOR). Therefore, we will
(9R: –17 ms, 10R: –13 ms, t17 = 0.3, P = 0.76) or bromocriptine
separately examine the effects observed in each SOA condition.
(9R: –24 ms, 10R: –20 ms, t17 = 0.57, P = 0.58, Fig. 2B) sessions. Weconclude that differences in DA transmission in the striatum,
Short SOA: Involuntary Attention Is Unaffected by Striatal
resulting either from individual genetic differences or from
bromocriptine administration, do not affect involuntary attention.
In 40 ms SOA trials, capture of involuntary attention occurredat the cue location, and RTs (placebo sessions, combining bothgenotype groups) were faster when the target appeared in this
Long SOA: IOR Depends on Bromocriptine Administration
cued location versus other locations (cue: 282 ms, SD 103;
and Baseline Levels of Striatal DA
other: 297 ms SD 99, Fig. 2A). As there is substantial between-
In 600 ms SOA blocks (placebo sessions, combining both
subject variance in mean RT in these measurements, we
genotype groups), RTs were slower when the target appeared
Cerebral Cortex May 2012, V 22 N 5 1135
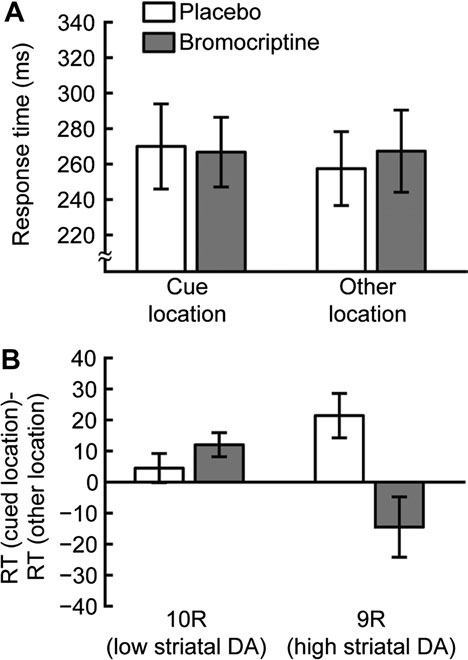
(placebo: 21 ms, bromocriptine: –15 ms, within-subject 2-tailedt-test: t8 = 2.88, P < 0.05, Fig. 3B).
To summarize the results, although an involuntary attention
cueing effect was observed with a short SOA, we did not findany effects of either bromocriptine administration or DAT1genotype on involuntary attention. In addition, we replicatedprevious results (Colzato et al. 2010) showing that for an SOAof 600 ms, IOR is larger for 9R (higher striatal DA) subjects thanfor 10R (lower striatal DA) subjects. Moreover, bromocriptineadministration had differential effects on IOR, depending onDAT1 genotype: bromocriptine increased IOR in 10R subjectsand abolished it in 9R subjects.
The neurotransmitter DA is involved in a variety of cognitivefunctions through its activity in multiple brain areas, includingthe prefrontal cortex (PFC) and striatum (Cools and Robbins2004; Cools and D'Esposito 2009). In this study, we focused onthe role of DA transmission in the striatum in modulating visual
discrimination performance following a nonpredictive cue.
This type of cue leads to capture of involuntary attention for
Figure 3. Long SOA blocks. (A) Average RTs for 600 ms SOA blocks. RTs are shown
short cue-to-target SOAs and causes IOR for longer SOAs
for the cue location (left) and other locations (right) for sessions in which placebo
(Posner and Cohen 1984).
(white) or bromocriptine (gray) was administered. (B) Cueing effects. Data are plottedseparately for 10R (left) and 9R subjects (right) for sessions in which placebo (white)
We manipulated striatal DA transmission by administering
or bromocriptine (gray) was administered. Positive values indicate IOR. In placebo
the DRD2 receptor--specific agonist bromocriptine to healthy
sessions, 9R subjects had greater IOR than 10R subjects. Bromocriptine increased
participants. DRD2 levels are much higher in striatum than in
IOR in 10R participants and abolished IOR in 9R participants. Error bars are standard
other parts of the human brain, including PFC, where DRD1 is
error of the mean within group/condition.
more abundant (Camps et al. 1989; Meador-Woodruff et al.
in the cue location compared with one of the other locations
1996). A non-monotonic effect of DA transmission levels on
(cue: 267 ms, SD 101; other: 257 ms, SD 89, Fig. 3A). This
cognitive functions has been identified in many different
resulted in a significant mean positive cueing effect of 13 ms
contexts (Cools and Robbins 2004; Seamans and Yang 2004),
(within-subject 2-tailed t-test: t18 = 2.76, P < 0.05), indicating IOR
and the effects of bromocriptine on a variety of measures can
(Posner and Cohen 1984). This positive cueing effect was found
be described by an inverted U-shaped function of DA levels
in 13 of 19 subjects in the placebo sessions. Differences in striatal
(Cools and D'Esposito 2011). Thus, bromocriptine benefits
DA transmission, as indicated by subjects' DAT1 genotype, have
performance on cognitive tasks in subjects with low memory
previously been found to predict differences in IOR for SOAs less
spans (who have lower baseline striatal DA; Cools et al. (2008)),
than 750 ms (Colzato et al. 2010). We also found a significant
while subjects with high memory spans are impaired on these
difference of DAT1 genotype on the magnitude of the IOR in the
tasks following bromocriptine administration (Kimberg et al.
placebo sessions, with 9R participants (higher striatal DA) having
1997). Similarly, bromocriptine reduces the behavioral costs of
a greater IOR (9R: 21 ms, 10R: 5 ms, between-subject one-tailed
task switching and their neural correlates in the striatum in
t-test: t17 = 2.02, P < 0.05).
high-impulsive but not in low-impulsive subjects (Cools et al.
In the bromocriptine session, the RT in 600 ms SOA blocks
2007), and impulsivity is a personality trait that is linked to low-
for all subjects was not significantly different for cue versus
binding availability of striatal DA D2/D3 receptors (Dalley et al.
other target locations (cue: 266 ms, SD 84; other: 267 ms, SD
2007). In addition, an inverted U-shaped curve accounts for the
98; cueing effect: 1 ms, within-subject 2-tailed t-test: t18 = 0.1,
differential effects of bromocriptine on reversal learning as
P = 0.93, Fig. 3A). However, when the cueing effects were
a function of baseline striatal DA synthesis capacity, as
separately analyzed for the 2 DAT1 genotypes, significant
measured using positron emission tomography (Cools et al.
differences were found, indicating that bromocriptine had
2009). These findings suggest that cognitive functions are most
different effects on IOR, depending on DAT1 genotype (drug
efficiently performed at intermediate levels of striatal DA
by target location by DAT1 interaction: F1,15 = 4.85, P < 0.05;
activation and that higher or lower levels of DA transmission at
drug by target location by SOA by DAT1 interaction: F1,15 =
striatal synapses may lead to suboptimal performance.
4.85, P < 0.05). Specifically, the 10R subjects (lower striatal
In order to examine individual differences in drug effects as
DA) showed a numerically higher IOR in bromocriptine
a function of baseline striatal DA levels, we determined the
compared with placebo sessions, although this was not
genotype of DAT1, a DA transporter that is enriched in the
statistically significant (placebo: 5 ms, bromocriptine: 12 ms,
striatum (Sesack et al. 1998), in every subject. Replicating
within-subject 2-tailed t-test: t9 = 1.25, P = 0.24, Fig. 3B). On the
previous results (Colzato et al. 2010), we found that carriers of
other hand, participants with the 9R allele of DAT1 (higher
the 9R allele (higher striatal DA) have a larger IOR in 600 ms
striatal DA) exhibited larger IOR than 10R subjects under
SOA blocks. In addition, we found a differential effect of
placebo (see above) as well as a significant decrease in the
bromocriptine, resulting in reduced IOR in 9R subjects and
magnitude of IOR following bromocriptine administration
increased IOR in 10R subjects. These results are consistent
Rokem et al.
with an inverted U-shaped function of the effects of striatal DA
involuntary attention and the IOR rely on different neural
transmission on IOR magnitude.
Brain imaging studies suggest that IOR involves regions of the
frontal and posterior parietal cerebral cortex (Lepsien andPollmann 2002; Mayer et al. 2004), but the pattern of deficits in
a human patient with a focal midbrain lesion (Sapir et al. 1999), as
National Institutes of Health (R01-DA20600 to M.D. and NEI
well as electrophysiological evidence from non-human primates
CORE grant EY003176).
(Dorris et al. 2002; Fecteau and Munoz 2005), suggest that theIOR is also mediated by the superior colliculus (SC), a brainstem
structure involved in oculomotor control and in allocation of
Jon Kelvey and Alexandra Carstensen helped collect the data. Conflict
visual spatial attention (Cavanaugh and Wurtz 2004). Furthermore,
of Interest: None declared.
functional imaging studies have shown that bromocriptinemodulates striatal and prefrontal cortical activity (Cools et al.
2007) as well as the functional connectivity between these
Camps M, Corte´s R, Gueye B, Probst A, Palacios JM. 1989. Dopamine
regions (Wallace et al. 2011). Therefore, despite the systemic
receptors in human brain: autoradiographic distribution of D2 sites.
administration of bromocriptine in our study, the known
anatomical distribution of DRD2, the observed effects of DAT1
Cavanaugh J, Wurtz RH. 2004. Subcortical modulation of attention
genotype, and previous functional magnetic resonance imaging
counters change blindness. J Neurosci. 24:11236--11243.
results are all consistent with a striatal site of action of this drug.
Colzato LS, Hommel B. 2009. Recreational use of cocaine eliminates
The evidence presented above suggests that both the SC and
inhibition of return. Neuropsychology. 23:125--129.
the striatum are components of the neural circuit mediating the
Colzato LS, Pratt J, Hommel B. 2010. Dopaminergic control of
IOR. Striatal signals are known to influence activity in the SC
attentional flexibility: inhibition of return is associated with the
(Hikosaka et al. 2000). A substantial proportion of neurons in the
dopamine transporter gene (DAT1). Front Hum Neurosci. 4:53.
Cools R, D'Esposito M. 2009. Dopaminergic modulation of flexible
caudate nucleus exhibit spatially specific responses to visual
cognitive control in humans. In: Bjo¨rklund A, Dunnett SB, Iversen
stimuli, eye movements, and allocation of attention (Hikosaka
LL, Iversen SD, editors. Dopamine handbook. Oxford: Oxford
et al. 1989). These caudate neurons affect SC activity through 2
University Press.
parallel pathways: one that excites SC neurons by disinhibition
Cools R, D'Esposito M. 2011. Inverted-U-shaped dopamine actions on
via the substantia nigra pars reticulata and the other inhibitory to
human working memory and cognitive control. Biol Psychiatry.
the SC, via the external globus pallidus. The balance between
these 2 pathways is regulated by the relative levels of activation
Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M. 2009.
of DRD1 (excitatory pathway) and DRD2 (inhibitory pathway) in
Striatal dopamine predicts outcome-specific reversal learning andits sensitivity to dopaminergic drug administration. J Neurosci.
the caudate by projections from the substantia nigra pars
compacta (Gerfen and Surmeier 2011). Importantly, nigrostriatal
Cools R, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M. 2008. Working
projections are affected in PD (Davie 2008). Previous studies in
memory capacity predicts dopamine synthesis capacity in the
patients with PD have shown that for short cue- target intervals,
human striatum. J Neurosci. 28:1208--1212.
no impairment is observed in the allocation of visual spatial
Cools R, Robbins TW. 2004. Chemistry of the adaptive mind. Philos
attention (Rafal et al. 1984). However, longer intervals reveal
Transact A Math Phys Eng Sci. 362:2871--2888.
reduced magnitude of IOR in patients with PD (Filoteo et al.
Cools R, Sheridan M, Jacobs E, D'Esposito M. 2007. Impulsive
1997; Yamaguchi and Kobayashi 1998; Possin et al. 2009).
personality predicts dopamine-dependent changes in frontostriatal
We propose that for subjects with low baseline striatal DA
activity during component processes of working memory. J Neuro-sci. 27:5506--5514.
levels, increasing DRD2 transmission with bromocriptine
Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, La¨a¨ne K,
enhances caudate-mediated inhibition of the SC, thereby
Pen˜a Y, Murphy ER, Shah Y, Probst K, et al. 2007. Nucleus
increasing IOR magnitude. However, DRD2 are also located
accumbens D2/3 receptors predict trait impulsivity and cocaine
presynaptically in nigrostriatal projections, where they func-
reinforcement. Science. 315:1267--1270.
tion as autoreceptors and negatively regulate DA release in
Davie CA. 2008. A review of Parkinson's disease. Br Med Bull.
striatum (Gonon and Buda 1985). One possibility is that for
subjects with high baseline striatal DA levels, further increase
Dorris MC, Klein RM, Everling S, Munoz DP. 2002. Contribution of the
of DRD2 signaling by bromocriptine could reduce striatal DA
primate superior colliculus to inhibition of return. J Cogn Neurosci.
14:1256--1263.
release via nigrostriatal autoreceptors. Indeed, behavioral and
Fecteau JH, Munoz DP. 2005. Correlates of capture of attention and
microdialysis studies in rats suggest that under conditions of
inhibition of return across stages of visual processing. J Cogn
low extracellular DA in the striatum, bromocriptine acts mainly
on postsynaptic DRD2, while in the presence of high striatal
Fillmore MT, Rush CR, Abroms BD. 2005. d-Amphetamine-induced
extracellular DA, bromocriptine has primarily presynaptic
enhancement of inhibitory mechanisms involved in visual search.
DRD2 effects (Maruya et al. 2003). Such a differential activation
Exp Clin Psychopharmacol. 13:200--208.
of pre- and postsynaptic DRD2 by bromocriptine, depending on
Filoteo JV, Delis DC, Salmon DP, Demadura T, Roman MJ, Shults CW.
baseline striatal DA levels, could account for the inverted U-
1997. An examination of the nature of attentional deficits in patients
shaped effects of striatal DA on IOR that we have observed.
with Parkinson's disease: evidence from a spatial orienting task. J IntNeuropsychol Soc. 3:337--347.
Finally, we found that involuntary attention cueing effects
Gerfen CR, Surmeier DJ. 2011. Modulation of striatal projection systems
were not affected by either bromocriptine administration or
by dopamine. Annu Rev Neurosci. 34:441--466.
DAT1 genotype, suggesting that involuntary attention is not
Gonon FG, Buda MJ. 1985. Regulation of dopamine release by impulse
likely to be substantially influenced by striatal DA transmission.
flow and by autoreceptors as studied by in vivo voltammetry in the
These results provide further evidence that the allocation of
rat striatum. Neuroscience. 14:765--774.
Cerebral Cortex May 2012, V 22 N 5 1137
Heal D, Cheetham S, Smith S. 2009. The neuropharmacology of ADHD
Prinzmetal W, McCool C, Park S. 2005. Attention: reaction time and
drugs in vivo: insights on efficacy and safety. Neuropharmacology.
accuracy reveal different mechanisms. J Exp Psychol Gen.
Hikosaka O, Sakamoto M, Usui S. 1989. Functional properties of monkey
Radad K, Gille G, Rausch WD. 2005. Short review on dopamine agonists:
caudate neurons. II. Visual and auditory responses. J Neurophysiol.
insight into clinical and research studies relevant to Parkinson's
disease. Pharmacol Rep. 57:701--712.
Hikosaka O, Takikawa Y, Kawagoe R. 2000. Role of the basal ganglia in the
Rafal RD, Posner MI, Walker JA, Friedrich FJ. 1984. Cognition and the
control of purposive saccadic eye movements. Physiol Rev. 80:953--978.
Kimberg DY, D'Esposito M, Farah MJ. 1997. Effects of bromocriptine on
basal ganglia. Separating mental and motor components of
human subjects depend on working memory capacity. Neuroreport.
performance in Parkinson's disease. Brain. 107(Pt 4):1083--1094.
Ro T, Rafal RD. 1999. Components of reflexive visual orienting to
Klein RM. 2000. Inhibition of return. Trends Cogn Sci. 4:138--147.
moving objects. Percept Psychophys. 61:826--836.
Lepsien J, Pollmann S. 2002. Covert reorienting and inhibition of return:
Rokem A, Landau AN, Garg D, Prinzmetal W, Silver MA. 2010. Cholinergic
an event-related fMRI study. J Cogn Neurosci. 14:127--144.
enhancement increases the effects of voluntary attention but does not
Maruya H, Watanabe Y, Okita M, Lawlor GF, Utsumi H, Niitsuma T. 2003.
affect involuntary attention. Neuropsychopharmacology. 35:2538--2544.
Inhibitory effects of D2 agonists by striatal injection on excessive
Sapir A, Soroker N, Berger A, Henik A. 1999. Inhibition of return in
release of dopamine and hyperactivity induced by Bay K 8644 in
spatial attention: direct evidence for collicular generation. Nat
rats. Neuroscience. 118:1091--1098.
Mayer AR, Seidenberg M, Dorflinger JM, Rao SM. 2004. An event-related
Seamans JK, Yang CR. 2004. The principal features and mechanisms of
fMRI study of exogenous orienting: supporting evidence for the
dopamine modulation in the prefrontal cortex. Prog Neurobiol. 74:1--58.
cortical basis of inhibition of return? J Cogn Neurosci. 16:1262--1271.
Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. 1998. Dopamine
Meador-Woodruff JH, Damask SP, Wang J, Haroutunian V, Davis KL,
Watson SJ. 1996. Dopamine receptor mRNA expression in human
axon varicosities in the prelimbic division of the rat prefrontal
striatum and neocortex. Neuropsychopharmacology. 15:17--29.
cortex exhibit sparse immunoreactivity for the dopamine trans-
Mehta MA, Riedel WJ. 2006. Dopaminergic enhancement of cognitive
porter. J Neurosci. 18:2697--2708.
function. Curr Pharm Des. 12:2487--2500.
Volkow ND, Fowler JS, Wang GJ. 1999. Imaging studies on the role of
Mill J, Asherson P, Browes C, D'Souza U, Craig I. 2002. Expression of the
dopamine in cocaine reinforcement and addiction in humans.
dopamine transporter gene is regulated by the 3' UTR VNTR:
J Psychopharmacol. 13:337--345.
evidence from brain and lymphocytes using quantitative RT-PCR.
Wallace DL, Vytlacil JJ, Nomura EM, Gibbs SE, D'Esposito M. 2011. The
Am J Med Genet. 114:975--979.
dopamine agonist bromocriptine differentially affects fronto-striatal
Posner MI, Cohen Y. 1984. Components of visual orienting. In: Bouma
functional connectivity during working memory. Front Hum
H, Bouwhais DG, editors. Attention and performance X: control of
Neurosci. 5:32.
language processes. Hillsdale (NJ): Erlbaum. p. 531--556.
Yamaguchi S, Kobayashi S. 1998. Contributions of the dopaminergic
Possin KL, Filoteo JV, Song DD, Salmon DP. 2009. Space-based but not
object-based inhibition of return is impaired in Parkinson's disease.
system to voluntary and automatic orienting of visuospatial
attention. J Neurosci. 18:1869--1878.
Price P, Debono A, Parkes JD, Marsden CD, Rosenthaler J. 1978. Plasma
Yantis S, Jonides J. 1990. Abrupt visual onsets and selective attention:
bromocriptine levels, clinical and growth hormone responses in
voluntary versus automatic allocation. J Exp Psychol Hum Percept
Parkinsonism. Br J Clin Pharmacol. 6:303--309.
Rokem et al.
Source: http://www.landaulab.com/uploads/5/4/9/9/54994549/modulation_of_inhibition_of_retunr_by_dopamine_d2_receptor_agonist_bromocriptine_depends_on_individual_dat1_genotype.pdf
Sept. - Oct. 2007 GSIA BI-MONTHLY NEWS BULLETIN GOA STATE INDUSTRIES ASSOCIATION (An Apex Association for Micro, Small, Medium Enterprises in Goa) ISO 9001:2000 Certified 4 FLOOR, GOA-IDC HOUSE, PATTO PLAZA, PANAJI, GOA 403 001. Office Timings: 9.30 a.m. 6.00 p.m. (Mon.-Fri.) & 9.30 a.m. 1.30 p.m. (Sat.) Ph.: 2438395 Fax: 2438210 E-mail : [email protected] Website: www.gsia.in
Ley del Régimen Penitenciario DECRETO NÚMERO 33-2006 EL CONGRESO DE LA REPÚBLICA DE GUATEMALA Que es deber del Estado de Guatemala, garantizar a los habitantes de la República la vida, la libertad, la justicia, la seguridad, la paz y el desarrollo integral de la persona. Que son fines del Sistema Penitenciario la readaptación social y reeducación de las personas reclusas; así como cumplir con las normas mínimas para la custodia y tratamiento de las mismas.




