Ncdc.ge
The Georgian Influenza
Pandemic Preparedness
Acronyms ARI Acute
Biosafety Level 2
Biosafety Level 3
Community Network of Reference Laboratories for Human Influenza in Europe
Economic Association Agreement
Electronic Integrated Disease Surveillance System
Food and Agriculture Organization
Health Care Workers
Health and Social Programs Agency
International Classification of Diseases
Municipal Public Health Center
Ministry of Internal Affairs
Ministry of Agriculture
Ministry of Labour Health and Social Affairs
National Centre of Disease Control and Public Health
National Influenza Centre
National Institute for Medical Research
World Organization for Animal Health
Polymerase Chain Reaction
National Institute for Public Health and the Environment
Reverse Transcription Polymerase Chain Reaction
Severe Acute Respiratory Infection
Severe Acute Respiratory Syndrome
Viral Transport Media
World Health Organization Collaborating Centre
World Health Organization Global Influenza Surveillance Network
Purpose of the influenza pandemic preparedness plan . 1
Chapter 1. Background . 1
Clause 1. Influenza and pandemic influenza . 1
Clause 2. Pandemic phases . 1
Clause 3. Estimated impact of influenza pandemic in Georgia . 3
1. Impacts on population health . 3
2. Basic assumptions for drafting preparedness plans . 4
Clause 4.Economic and social consequences . 5
2. Schools and other closed communities . 5
3. Impact on other services . 5
4. Impact on travel . 6
Clause 5. Legal considerations . 6
Clause 6. Ethical considerations . 6
1. Values and principles affecting decision-making . 6
2. Fundamental ethical issues . 7
Chapter 2. Recommended actions before, during and after a pandemic . 1
Chapter 3. Planning and coordination . 3
Clause 9. Government Leadership . 3
Clause 10. The Government Steering Commission on Emergency Situations . 7
Clause 11. Emergency Situation Management Department of the Ministry of Internal Affairs . 8
Clause 12. Regional Governor's Administration . 8
Clause 13. Local Municipal Self-Government . 8
Clause 14. Ministry of Agriculture and its Agencies . 8
Clause 15. The role of the MoLHSA and its agencies . 9
Chapter 4. Animal Health . 11
Chapter 5. Situation monitoring and assessment. 11
Clause 17. Surveillance . 11
1.Phases 1-3 . 11
2. Pre-pandemic Surveillance strategy (phase 4) . 12
3. Phases 5-6 . 12
4. Post-peak period . 13
5. Post Pandemic period . 13
Clause 18. Laboratory diagnostics . 13
Chapter 6. Reducing the spread of disease . 14
Clause 19. Individual and Community Contaitmnet measures . 14
1. General Strategies . 14
2. The pandemic mitigation measures include the following: . 15
3. Management of few human infections and their contacts during pre pandemic . 16
4. Influenza control and prevention in the community during sustained human-to-human transmission . 16
5. Prepardness for implemenation of containment measures . 17
Clause 20. Planning for Vaccination Against a Pandemic Influenza Virus . 18
Clause 21. Antiviral Drug Distribution and Use . 19
Clause 22. Clinical management of Pandemic (H1N1) 2009 influenza cases . 25
Clause 23. Infection Control . 25
4. Recommendations for infection control for home healthcare services . 26
5. Management of influenza patients . 26
6. Infection control measures in the home . 27
7. Recommendations for infection control in schools and workplaces . 27
Clause 24. Management of Mass Fatalities during an Influenza Pandemic . 27
Chapter 7. Continuity of Health Care Provision . 31
Clause 25. Estimated impact on the healthcare system . 31
Clause 26. Proposed operational response . 32
Clause 27. Pre-surge . 33
Clause 28. Managing increased Demand and Capacity (surge) . 34
Clause 29. Recovery . 35
Clause 30. Facility based plans . 35
Clause 31. Planning for provision of care in hospitals . 35
Clause 32. Planning for provision of care in non-hospital settings . 37
Chapter 8. Communication . 40
Clause 33. Goal of the National Communication Strategy on Pandemic Influenza . 40
Clause 34. Major Objectives: . 40
Clause 35. Key Responsibilities of the Communication strategy . 41
1. Capacity strengthening . 41
2. Social Research . 41
3. Message and material development . 42
4. Research . 42
5.Plan and assess current knowledge . 42
6. Expand community mobilization . 42
7. Personal Hygiene Campaign . 42
Chapter 9. Business Continuity . 44
Clause 36. Communications . 44
Clause 37. Energy . 44
Clause 38. Finance . 45
Clause 39. Food . 45
Clause 40. Public transport . 45
Clause 41. Water . 45
Clause 42. Emergency services . 45
Clause 43. Planning by local authorities . 45
Clause 44. Public order . 46
Chapter 10. Implementation of the national plan . 47
Clause 46. Funding Mechanisms for Healthcare. 47
Clause 47. Creation of Pandemic Stockpiles . 47
. Clause 48. Financing of Primary Health Care services . 48
. Clause 49. Financing of Inpatient Services . 48
Clause 50. Financing of Epidemiological Surveillance and Safety measures . 49
Clause 51. Financing of Other Services . 49
Clause 52. Building of National Capacity (Training) . 49
Why influenza pandemics occur . 50
Animal reservoirs . 51
Distinguishing pandemic from seasonal influenza . 51
The post-peak period . 64
The post-pandemic period . 65
Whole virus vaccines are being replaced by less reactogenic split virus and subunit vaccines. 83
Annex 1. Influenza Virus . 50
Annex 2. Legal Documents Regulating Influenza Pandemic and Related Issues . 52 Annex 3: National Actions during the pandemic phases and responsible agencies . 53 Annex 4. Influenza Surveillance National Guidelines . 67 Annex 5. Laboratory diagnostics. 68 Annex 6. Interim Planning guideline for the use of non-pharmaceutical interventions to mitigate influenza pandemic. 81 Annex 7. Recommendations on Vaccination against Seasonal Influenza . 82 Annex 8. Clinical management of Pandemic (H1N1) 2009 Virus Infection (Guideline) . 87 Annex 9. Novel Influenza A (H1N1, H5N1) Infection Control Guidelines for Health Care Facilities . 87 Annex 10. Hospital Pandemic Preparedness Plan . 88 Annex 11. Business Pandemic Influenza Planning Checklist . 89 Annex 12. Contingency Plan for Avian Influenza _Ministry of Agriculture of Georgia . 92
Purpose of the influenza pandemic preparedness plan
The overall aims of pandemic planning in Georgia are to reduce mortality (deaths) and morbidity (sickness) related to influenza pandemic and to minimize the resulting disruption to society. Key objective of this preparedness plan is to provide guidance to all levels of the healthcare services for pandemic preparedness. This Plan has been developed under the supervision of the National Working Group on Influenza Pandemic Preparedness based on recommendations by the World Health Organization for national pandemic plans. It concentrates on the health response but also provides some advice on the planning which must take place across all sectors of society.1 These are divided into five categories: planning and coordination, situation monitoring and assessment, reducing the spread of disease, continuity of health care provision, communications and all have been addressed in this plan. This plan has been developed taking account of pandemic plans developed by other countries2, and the original influenza pandemic preparedness plan of Georgia.3 Incorporated in this plan are a number of specific technical guidelines addressing issues essential during the current phase of pandemic alert. These guidelines may have to be adapted when new knowledge accumulates. The purpose of this document is to spell out critical information about pandemic influenza, to explain what the Government and the health services are doing to prepare for a possible pandemic and most importantly, to advise all stakeholders what they need to do if there is a pandemic. Contingency planning for an event which may occur in the future can be difficult to justify, particularly in the face of more immediate problems and priorities. However, the World Health Organization (WHO) has identified two vital reasons to invest in pandemic preparedness:
1. Preparation will lessen the direct medical and economic effects of a pandemic by making sure
that adequate measures are put in place before the pandemic occurs.
2. Improvements in infrastructure to prepare for the next influenza pandemic which will
provide benefits now and will also mitigate the effect of other epidemics or infectious disease threats.
It is desirable that the present preparedness plan is widely used for training to improve preparedness, and in the preparation and implementation of adapted local plans. This plan also provides the base for strengthening the resources needed in different organizations. Assessment of the economic impact of an influenza pandemic will continue during the implementation of the preparedness plan.
1 Pandemic Influenza Preparedness and Response – A WHO Guidance Document, April 2009 2 We mainly used the plans of USA, the UK, Finland, Ireland, Canada 3 A Preparedness Plan to Fight Influenza (2006)
Chapter 1. Background
Clause 1. Influenza and pandemic influenza
1. Seasonal influenza Influenza is an acute viral infection. In most years, influenza occurs predominantly during a six to eight week period during the winter. For most people, this ‘seasonal' influenza is an unpleasant but self-limiting and not life-endangering illness, but in some people it may be more severe, or complicated by secondary bacterial infections such as bronchitis and pneumonia. The very young, the elderly and people with underlying diseases such as heart or chest disease are particularly at risk of serious illness from influenza. Without interventions such as annual influenza immunization, the elderly and those of all ages in disease-based risk groups suffer significant morbidity and mortality even in a non-pandemic year. Further information on influenza viruses and the illness they cause is at Annex 1. 2. Pandemic influenza In past pandemics, the scale and severity of illness, and hence the consequences, have varied considerably but in general they have been of much greater magnitude than even the most severe ‘epidemic' winters. There have also been material differences in the age groups most affected (for example, working age adults rather than the elderly), the time of year of outbreaks and the speed of spread, all of which influence the overall impact. Despite their variability and unpredictability, much can be learned from previous pandemics Annex 1.
Clause 2. Pandemic phases
The MoLHSA follows the World Health Organization's (WHO) guidance for national pandemic planning, which defines pandemic activities in six phases. (See Table 1). These phases reflect the progression of an influenza pandemic from the first appearance of a new flu virus to wide international spread
Table 1 Summary of WHO Phases of Pandemic Influenza
No viruses circulating among animals have been reported to cause infections in
humans. An animal influenza virus circulating among domesticated or wild animals is
known to have caused infection in humans, and is therefore considered a potential pandemic threat. An animal or human-animal influenza reassortant4 virus has caused sporadic cases
or small clusters of disease in people, but has not resulted in human-to-human transmission sufficient to sustain community-level outbreaks. Verified human-to-human transmission of an animal or human-animal influenza
reassortant virus able to cause "community-level outbreaks."
4 Reassortment – influenza virus antigenic modification, caused by exchange of genetic information between human and animal influenza viruses.
Human-to-human spread of the virus into at least two countries in one WHO region. While most countries will not be affected at this stage, the declaration of
Phase 5 is a strong signal that a pandemic is imminent and that the time to finalize the organization, communication, and implementation of the planned mitigation measures is short. The pandemic phase, is characterized by community level outbreaks in at least one other country in a different WHO region in addition to the criteria defined in
Phase 5. Designation of this phase will indicate that a global pandemic is under way. Pandemic disease levels in most countries with adequate surveillance will have dropped below peak observed levels. The post-peak period signifies that pandemic
Post-peak period
activity appears to be decreasing; however, it is uncertain if additional waves will occur and countries will need to be prepared for a second wave. Levels of pandemic influenza activity in most countries with adequate surveillance
Possible New Wave
rising again Influenza disease activity will have returned to levels normally seen for seasonal influenza. It is expected that the pandemic virus will behave
Post-pandemic period
as a seasonal influenza A virus. At this stage, it is important to maintain surveillance and update pandemic preparedness and response plans accordingly. An intensive phase of recovery and evaluation may be required.
Source: WHO, 2009. It is important to stress that the phases were not developed as an epidemiological prediction, but to provide guidance to countries on the implementation of activities. While later phases may loosely correlate with increasing levels of pandemic risk, this risk in the first three phases is simply unknown. It is therefore possible to have situations which pose an increased pandemic risk, but do not result in a pandemic. The designation of alert phases, including decisions on when to move from one phase to another, is made by the Director-General of the World Health Organization. The transition between phases may be rapid and some phases may be skipped. Each alert phase coincides with a series of recommended activities to be undertaken by the WHO, the international community, Governments and industry. Changes from one phase to another are triggered by several factors, which include the behavior of the disease and the characteristics of circulating viruses. Alternatively, although global influenza surveillance and monitoring systems are much improved, it is also possible that the first outbreaks of a pandemic will not be detected or recognized. For example, if symptoms are mild and not very specific, an influenza virus with pandemic potential may attain relatively widespread circulation before being detected; thus, the global phase may jump from Phase 3 to Phases 5 or 6. If the rapid containment operations are successful, Phase 4 may revert back to Phase 3. When making a change to the global phase, WHO will carefully consider whether the criteria for a new phase have been met. This decision will be based upon all credible information from global surveillance and from other organizations. Local actions would depend on whether cases had been identified in Georgia and the extent of spread. For Georgia purposes, four additional alert levels have therefore been included within WHO Phases 4, 5 and 6; (Table 2)
Table 2Additional alert levels within WHO phases for Georgia
Cases registered only outside Georgia
Virus isolated in Georgia
Outbreak(s) in the Georgia
Widespread activity across Georgia
Clause 3. Estimated impact of influenza pandemic in Georgia
1. Impacts on population health
a) Influenza pandemics have occurred three times in the 20th century: 1918, 1957, and 1968. Experts predict that another influenza pandemic is highly likely, if not inevitable. The impact of an influenza pandemic can be devastating. For example, it has been estimated that over 20 million people died during the pandemic of 1918. Pre-pandemic planning, therefore, is essential if influenza pandemic-related morbidity, mortality, and social disruption are to be minimized. Unfortunately, no one can predict when the next pandemic will occur, nor can they accurately forecast who will become ill and suffer adverse health outcomes such as death and hospitalization. b) Since 2003 two types of novel influenza virus is circulating worldwide, which has potential for pandemic. Influenza type A virus H5N1 subtype was detected since 2003 and until 2009 totally 424 people have been infected, 261 of them had died (61.5%). From the beginning of 2009 human cases with new subtype of Influenza type A (H1N1) have been identified. The course novel influenza currently resembles seasonal influenza in terms of lethality. However, there is a threat that virus may mutate, leading to an outbreak of more severe illness in future. It is very difficult to predict how the pandemic will evolve, i.e. it is impossible to predict the timing, intensity, or impacts on the healthcare system and other societal functions of the next pandemic. However, in order to draft a national pandemic preparedness plan, assumptions must be made on the kind of impacts the next pandemic may have on healthcare and the rest of society. Thus, the following sections are only assumptions made in order to estimate required resources, not forecasts. Only when the pandemic virus has caused relatively extensive epidemics are factors determining its impacts revealed, consisting of at least the following: particular properties of the pandemic virus genotype and structure, human-to-human transmissibility, pathogenicity and drug susceptibility, possible partial immunity in certain population groups and available vaccines, antiviral drugs and other measures that may be employed in an effort to reduce numbers of new infections. c) Adaptation of any novel influenza virus to humans may take a long time. That is why it is possible that before an actual pandemic wave, the pandemic virus may cause infection chains of varying lengths in humans, some of which may also reach Georgia. Attempts should be made to interrupt them through active isolation, treatment and preventative measures. This way, the start of a pandemic might be delayed, but it cannot be prevented. The first pandemic wave, causing great morbidity, may be followed by one or more subsequent waves after months or a year. Their morbidity in previous pandemics has generally – albeit not necessarily – been lower than during the initial wave.
2. Basic assumptions for drafting preparedness plans
2.1 Observations made during pandemics of the past century may be utilized when modeling potential impacts of the next pandemic. The fundamental problem is that the degrees of severity of those three pandemics were very different, particularly in terms of case mortality. 2.2 In the following sections, pandemic preparedness planning is based on the following assumptions in calculations concerning Georgia:
a) Susceptibility to the pandemic influenza subtype will be universal. b) The clinical disease attack rate will be 25% in the overall population. c) Of those who become ill with influenza, 50% of patients will seek outpatient medical care d) The number of hospitalizations and deaths will depend on the virulence of the pandemic virus. Estimates differ about 10-fold between more and less severe scenarios. Because, the virulence of the influenza virus that causes the next pandemic can not be predicated, two scenarios are presented based on extrapolation of past pandemic experience (Table 3).
Table 3 Pandemic Influenza Scenarios - Moderate and Severe
MODERATE (1958/68-LIKE) SEVERE (1918-LIKE)
25% of population
Outpatient medical care
50% of flu patients
1-10% of flu patients
15% of hospitalized patients
Mechanical ventilation
5% of hospitalized patients
f) Risk groups for severe and fatal infections cannot be predicted with certainty. During annual fall and winter influenza season, infants and the elderly, persons with chronic illnesses, and pregnant women are usually at higher risk of complications from influenza infections, in contrast, in the 1918 pandemic, most deaths occurred among young, previously healthy adults. g) Incubation period for seasonal influenza averages 2 days. It is assumed that 2-3 days will be for a novel pandemic strain. Infected persons may shed virus and transmit infection for one-half to one day before the onset of illness. Viral shedding from the infected person and the risk for transmission will be greatest during the first 2 days of illness and will continue for 4-7 days. h) On average about 2 secondary infections will occur as a result of transmission from someone who is ill. i) In an affected territory, pandemic will last about 6 to 8 weeks. At least two pandemic disease waves are likely. j) The seasonality of a pandemic cannot be predicted with certainty. During 20th century pandemics the largest waves occurred in the fall and winter.
Clause 4.Economic and social consequences
1. Absence from work A pandemic would severely impact on the labour resources available to the national economy. It must be assumed that the impacts would be the greatest in labour-intensive service sectors. Some of the alternatives make the assumption that morbidity would be greater in the service sectors than in industry. Sickness absences and increased mortality would also have a long-term adverse effect on the national product. Work patterns have changed so much since previous pandemics that it is unwise to extrapolate from historical data on sickness absence. Absence from work will depend on the age-specific attack rate, although even if working age people are relatively spared, additional absenteeism may result from staff needing to take time off to care for family members, or difficulties with transport. It is suggested that business continuity plans are based on a cumulative total of 25% of workers taking some time off – possibly 5-8 working days – over a period of 3 to 4 months. Modeling suggests absenteeism due to the pandemic will rise to a peak of 5-7%, the higher number including those who would need to look after those who are ill. These equate to about three times the normal average absenteeism in a private sector company and double that in the public sector. Even in the reasonable worst case of a 50% attack rate these figures only rise to 10-15%. However the absenteeism rate would not be uniform and some employers may be particularly badly affected. In the absence of vaccination, those in occupations with particularly high exposure such as health care workers will have higher absenteeism. The skill mix required in some occupations, including health care, may limit the extent to which other staff can be redeployed.
2. Schools and other closed communities
Influenza will spread rapidly in schools. In 1957, for example, up to 50% of schoolchildren developed influenza, but even those schools which were most severely disrupted had returned to normal 4 weeks after the appearance of the first case. In residential schools, attack rates reached 90%, often affecting the whole school within a fortnight. This will impact on working parents. However, closing schools has a significant impact on business continuity and maintenance of essential services, particularly health care, due to parent workers needing to stay at home for childcare. Similar spread is likely in other closed communities such as residential care facilities and prisons.
3. Impact on other services
In the absence of early or effective interventions, there will be an effect on all other services, through staff sickness, any travel restrictions imposed and through the knock on effects of other disrupted businesses and services. This includes all non-health ‘first responder' services (police, fire etc), the military, other essential services (e.g utilities, fuel supply, food production and distribution, transport), prisons, education and businesses. Services such as death registration and funeral directors will have an increased work load. In addition to maintaining continuity of their work, badly affected businesses will need to consider, for example, the security of premises, including manufacturing plants. Further advice on business continuity is available in the Chapter 9.
4. Impact on travel
Travel will be impacted through:
a) any explicit advice or restrictions on travel and public gatherings as a policy option b) people opting not to travel (e.g. because of cancellation of work/school etc, fear of acquiring infection through travel or fear of leaving home) v) availability of fuel and transport workers
Clause 5. Legal considerations
1. Duties and responsibilities of the government structures, legal and physical persons during pre-pandemic and pandemic phases are defined by the Georgian legislation. 2. Under the Georgian Law on Public Health (June 27, 2007), the State is obliged to plan, direct and supervise the effort of combating infectious diseases within the country. Responsible agencies order restricting the freedom of the individual and make decisions on the matter. Under the same law, the State may order a compulsory health examination. 3. Georgian law on Public Health also addresses issues related to "quarantine," "isolation," and "detention" of cases, which should be implemented in line with the public health goals. In emergency situations implementation of quarantine and isolation activities is the responsibility of the Department of Emergency Situation Management under the Ministry of Internal Affairs. Making of decisions on the quarantine and isolation is made by the Public health services in accordance with the principles of European convention on security of Human Rights. The Government ensures provision of healthcare services to the people placed in isolation or under quarantine. In emergency situations (related to public health as well) government response measure are regulated by the following laws: Georgia Law on the Emergency Situations (#972, October 17, 1997) and Law of Georgia on Protection of Population and Territory from Emergency Situations of Natural and Technogenic Nature (#4922, June 8, 2007). Activities to avoid emergencies and response measured are, are laid down by the Presidential decree # 415 "Planning of National Response to Natural and Technogenic Emergency Situations" from 26 August, 2008. Hence current plan is based on above mentioned plan. 4. Legal framework and list of the legal documents are given in the Annex 3.
Clause 6. Ethical considerations
1. Values and principles affecting decision-making
During a pandemic, decision-makers and those responsible for healthcare may be forced to make difficult decisions affecting the health and freedom of action of their citizens. These decisions must be grounded on jointly defined values. The MoLHSA proposes the following values as bases of decision-making. Balanced reconciliation of these values is important, albeit not easy. a) Individual liberty It may be necessary to restrict individual liberty at the threat of or during a serious epidemic. Possible restrictions must not be exaggerated in relation to the impending danger, and they must be equally applied to all whose liberties it is deemed necessary to restrict in order to protect the community.
b) Equity (egalitarian approach) Equal right of everyone to preventive healthcare and treatment is declared by Georgian laws. Nevertheless, even under normal conditions various treatments and target groups must be placed in order of urgency and priority. In a pandemic situation the need for prioritization is emphasized. It is also possible that quick decisions are required if a means of protection (e.g. vaccine) is not available in sufficient quantity for everyone. c) Utility maximization (utilitarian approach) Utility maximization aims at the maximum possible good for as many as possible. 4) Efficiency Efficient and appropriate use of resources is extremely important in a situation where resources are limited. 5) Transparency Values and principles as the basis of decision-making, and its implementation, must be easily accessible to all concerned. 6) Reasonability of the decisions The decisions must be clearly reasoned and based on best available information on the threat, as well as on previously accepted values and principles. 7) Reciprocity The principle of reciprocity presupposes that society will support and protect especially those who bear a considerably greater than average burden in order to safeguard the common good.
2. Fundamental ethical issues
a) Duty of healthcare personnel to provide treatment Treatment of influenza patients may cause a risk of infection for the healthcare personnel giving treatment. The Georgian Law on Health Care obliges to administer immediate assistance to the seriously ill in all situations. If the number of those infected during the pandemic is very large, application of maximal protective measures sure to prevent infection will not be possible at every patient contact. Nevertheless, treatment of patients cannot be abandoned. Even if mortality of infected people of working age might be very small, conflicting ideas about transmissibility of the infection, severity of the disease and adequate preventive measures may arise. Those responsible for healthcare must ensure safe working conditions for staff by training and by ensuring that units have adequate supplies of structures and equipment needed in protection. Ethical guidelines for healthcare personnel should define in more detail how great a personal risk may be expected of healthcare professionals. They should also define the practical meaning of their duty of not harming patients and colleagues by spreading the disease, as well as when they may be obliged to use all available precautionary measures. b) Just distribution of limited resources
In distribution of healthcare personnel work, treatment units, vaccines and antiviral medication, the aim should be equality on one hand, and the maximum possible health benefit on the other hand. Taking both into consideration, it must further be decided what kind of health benefits are sought. As well as maximizing equality and health benefits, the principle of reciprocity must be taken into consideration when distributing limited resources. While the staff is expected to have an especially large input in taking care of infected patients, and even subject themselves to dangerous infection, they must be given priority in distributing all means of protection. After the objective of alleviating the impacts of a pandemic has been chosen, the means most likely to reach the objective must be assessed. If resources are limited, a decision must be made on the order of targeting the preventive measures on different population groups. Georgia will ensure supply of vaccine for the whole population. Should delivery of the vaccines be partially delayed, the decision must be made as to who should receive the first batches, and who should wait for the remaining deliveries. Considering 25% of attack rate of the pandemic, approximately 1,150,000 will require the treatment, and it is obvious that there will be no sufficient drug supplies for all, and their use must be prioritized. In such case, it can not be used as post-exposure short term prophylaxis or long-term preventive drug treatment, in addition to treatment of the infected. The principles of using vaccines, antiviral drugs and other preventive measures are discussed in detail elsewhere (Chapter 6). The crucial point is that effective efforts are made to halt infection chains created in pandemic phase through measures to isolate and protect those exposed, and through targeted prophylaxis. Once the pandemic is under way, the emphasis is on caring for the infected and protecting exposed healthcare personnel. In healthcare and elsewhere in society, efforts to prevent the spread of infection are made through means other than those based on the use of vaccines and antiviral drugs. The extreme burdening of healthcare brought about by the pandemic will unavoidably reduce availability of healthcare services for treatment of other than influenza patients. Regional and primary healthcare preparedness planning must take into consideration in relation to each problem and disease group, how serious the impacts will be on rescheduling non-emergency preventive services and those for chronic diseases. The planning should identify alternative methods of treatment, through which the difficulties caused by reduction of these services might be minimized. c) Restriction of individual liberty in order to prevent an epidemic The Georgian Law on Public Health defines the responsibilities and duties of various parties in surveillance and prevention of infectious diseases, as well as the situations in which an individual's inviolability, freedom of movement or property may be compromised in order to prevent infection. The municipal agency (usually the Center of Public Health) responsible for prevention of communicable diseases may order an infected person or one suspected of being infected to absent him from work or an educational institution. It has the option of ordering a person to be isolated in a hospital, if the risk of the disease spreading is apparent, and if there is no other way of preventing the spread of the disease. At the place of isolation, a person suffering from an infectious disease involving danger to the general public may be administered treatment necessary for prevention of the spread of the disease, even regardless of his wishes.
Under the Law on Public Health, a general program of vaccination is voluntary to the individual. However, the Government may separately decide on mandatory inoculations to prevent the spread of such an infectious disease as may have considerable adverse effects on the health of the population or a part of it. It is unlikely that mandatory inoculations would be necessary in a pandemic situation. d) Accelerated adoption of new vaccines and drugs Vaccines used in a pandemic may have to be widely used before the safety studies normally required of influenza vaccines have been completed. Accelerated adoption of a new antiviral drug or drug variant is also a possibility during a pandemic. Influenza vaccines and antiviral drugs are usually well tolerated. Pandemic vaccines are unlikely to be significantly different from similar seasonal influenza vaccines, or possible new antiviral drugs from those already in established use. Therefore, application of these preventive methods to reduce health risks caused by a pandemic can be deemed to be ethically acceptable.
Chapter 2. Recommended actions before, during and after a pandemic
1. This section provides specific actions to be taken by national authorities. The new WHO pandemic phases and a summary of recommended actions for each phase are presented in Table 4. Recommendations are grouped by pandemic phases and the five components of preparedness and response which are the following:
a) planning and coordination b) situation monitoring and assessment c) reducing the spread of disease d) continuity of health care provision e) communications
2. The goal of planning and coordination efforts is to provide leadership and coordination across sectors. One important aspect is to integrate pandemic preparedness into national emergency preparedness frameworks. Annex 3 present five components of the preparedness and response by responsible agencies grouped by pandemic phases. 3. The goal of situation monitoring and assessment is to collect, interpret, and disseminate information on the risk of a pandemic before it occurs and, once under way, to monitor pandemic activity and characteristics. To assess if the risk of a pandemic is increasing, it will be important to monitor the infectious agent, its capacity to cause disease in humans, and the patterns of disease spread in communities. It is important to collect data on influenza viruses, the genetic changes taking place and consequent changes in biological characteristics, and to rapidly investigate and evaluate outbreaks. Once a pandemic influenza virus begins to circulate, it will be vital to assess the effectiveness of the response measures. 4. Reducing the spread of disease will depend significantly upon increasing the "social distance" between people. Measures such as individual/household level measures, societal-level measures and international travel measures, and the use of antivirals, other pharmaceuticals, and vaccines will be important. Individual/household level measures include risk communication, individual hygiene and personal protection, and home care of the ill and quarantine of contacts. Societal-level measures are applied to societies or communities rather than individuals or families. These measures require a behavioral change in the population, multiple sector involvement, and mobilization of resources, strong communication, and media support. International travel measures aim to delay the entry of pandemic disease into not-yet-affected countries and will have an impact on international traffic and trade. Countries should balance reducing the risks to public health and avoiding unnecessary interference with international traffic and trade.
The use of pharmaceutical interventions to prevent or treat influenza encompasses a range of approaches. Additionally, the successful prevention and treatment of secondary or pre-existing conditions will be a key factor in many settings for reducing the overall burden of illness and death. 5. During a pandemic, health systems will need to provide health care services while attending to the influx of patients with influenza illness. Planning for surge capacity in health care facilities will help determine the extent to which the existing health system can expand to manage the additional patient load. Health care facilities will need to maintain adequate triage and infection control measures to protect health care workers, patients, and visitors. 6. The goal of communications before and during a pandemic is to provide and exchange relevant information with the public, partners, and stakeholders to allow them to make well informed decisions and take appropriate actions to protect health and safety. Effective communication about the risks related to pandemic influenza is critical at every stage of preparedness and response and is a fundamental part of effective risk management. Communications should be based on the five principles outlined in WHO's Outbreak Communications Guide:25 planning; trust; transparency; announcing early; and listening. Given the complex risks and perceptions associated with an influenza pandemic, communication strategies that simply disseminate outbreak information and recommendations will be insufficient. The scope and complexity of the task demands frequent, transparent, and proactive communication and information exchange with the public, partners, and other stakeholders about decision making, health recommendations, and related information. In addition to the suggested actions which follow below, countries are encouraged to develop core risk communication capacities such as those described in the WHO Outbreak Communication Planning Guide. By developing a solid foundation for pandemic influenza communications, Member States would also strengthen communication response systems for any public health emergency that may arise. 7. Finally the WHO does not encourage application of the following restrictions if not in particular circumstances:
a) Pandemic-related international border closures for people and/or cargo. b) General disinfection of the environment during a pandemic. c) The use of masks in the community by well persons. d) The restriction of travel within national borders during a pandemic, with the exception of a globally led rapid response and containment operation, or in rare instances where clear geographical and other barriers exist.
Chapter 3. Planning and coordination
Planning and coordination of all actions by pandemic phases are the main responsibility of the
Georgian Government. Specific roles and responsibilities of various governmental bodies in
preparedness and response are defined in
Annex 3.
Clause 9. Government Leadership
1. The leading Government bodies responsible for management of emergency citations related to influenza pandemic include:
a) The Government Steering Commission on Emergency Situations b) Emergency Situation Management Department, Ministry of Internal Affairs (MIA) c) Ministry of Health, Labor, and Social Affairs (MoLHSA)
2. The following Government bodies will be directly or indirectly involved in the response to an influenza pandemic:
a) Ministry of Defense b) Ministry of Education and Science c) Ministry of Agriculture d) Ministry of Environment Protection e) Ministry of Finance, Department of income f) Ministry of Economy g) The Governors and their administration h) Municipal Self-Governments
3. All the aforementioned bodies do play important role in providing effective and coordinated response to a pandemic threat. However, it is recognized that the MoLHSA is the lead government body providing technical guidance in preparing Georgia for a response to a pandemic influenza. 4. At the local level, governmental structures responsible for crisis management include:
a) MIA Emergency Situation Management Department branches in Apkhazia and Adjara b) Governor's administration at the regional level c) Local municipal self-governments.
The table below present State and other Government bodies specific functions with regard to pandemic preparedness, response and mitigation of consequences in the country.
Approves national influenza pandemic preparedness and response plans
Ensures financing of executive authorities engaged in organization and implementation of pandemic response measures; Coordinates implementation of the above measures.
Coordinates and directs the work of ministries and other executive
Cabinet of Ministers authorities regarding their preparedness to respond to an influenza
pandemic in the country and mitigate its consequences
Makes inter-governmental agreements related to protection of the population against an influenza pandemic
Resolves other issues within its responsibilities defined by the Georgian legislation
Provides operational management and coordination of measures, implemented by executive and local authorities; institutions, organizations regardless of their ownership form and population regarding prevention of avian and pandemic influenza spread on the territory of the country, pandemic response and mitigation of the pandemic consequences.
Discusses proposals on pandemic preventive measures and implementation of activities
Develops proposals on national response measures and local authorities preparedness plans and programs implementation
Develops proposals on unified national strategies on pandemic
Steering Commission avoidance, mitigation of its consequences and response actions
Develops proposals on material technical resource allocation, rational
distribution and usage needed for pandemic preparedness and response
Ministry of Internal
interagency operation Organizes proposal developed on central, regional and local authorities
obligations and responsibilities during pandemics
Makes decisions on country obligations to international countries and partners based on international agreements and memoranda
Provides practical assistance to regional and local emergency anti-epidemic headquarters in implementation of response measures.
Develops and submits proposals to the Cabinet of Ministers of Ukraine regarding establishment of quarantine and the other restrictive measures on avian and pandemic influenza affected areas
Conducts hearings of government officers' reports regarding implementation of preventive, treatment and response measures, their results and follow up organizational and practical decisions made
Engages health care workers, heads and employees of enterprises, institutions and organizations of all ownership forms into implementation of preventive and response measures
Reviews and analyses materials on pandemic response and mitigation of the pandemic consequences
Develops and ensures ongoing revision of the relevant sections of the Pandemic Plan.
Coordinates operations planning efforts for the health sector. Develops respective guidelines and procedures and oversees implementation of the plan.
Coordinates communication with WHO, vaccine and antiviral drug manufacturers and suppliers. Secures supplies of an effective vaccine, antiviral drugs, antibiotics, and other pharmaceutical products and essential supplies (e.g. masks, gloves, and other supplies).
Provides information and guidance to HESPA, municipal CPH, state agencies and other organizations involved in planning of a response at the national, regional and local levels.
Ministry of Labor Health and Social
Develops strategies, priority groups and recommendations for use of
vaccine and use of antiviral agents
Coordinates antiviral and other drug delivery
Monitors effectiveness of response activities and modifies strategies as necessary
Coordinates provision of consistent, accurate advice to health and public health professionals.
Monitors vaccine, antiviral drug adverse effects
Negotiates for additional resources if necessary for the national response of health services
Initiates meetings of the Governments Steering Commissions on Emergency Situations and Avian Influenza based on epidemiological situation.
Organizes and ensures stable functioning of the state institutions.
Emergency Situation Ensures order in the society. Renders security services at regimen
institutions including health facilities engaged in pandemic response, at
Department of the pandemic vaccine and antiviral storage facilities, etc.
Ministry of Internal
Coordinates regional and local authorities activities in case of
emergency situations during pandemics, provides them with guidance,
technical assistance and formal documents.
In cooperation with other government bodies prepares and implements trainings in preparedness, prevention and response with public and local authorities participation
To mitigate/ eliminate Pandemic consequences ensures material and human resources mobilization, provides logistical support to emergency health services, social support activities to affected and infected population.
Facilitates provision of information to the population on the course of a pandemic and effectiveness of implemented measures.
Ensures stable functioning of the State border and in the frame of early global response measure establishes screening (registration) at the border.
Prepares and ensures revision of the relevant sections of the pandemic preparedness plan
Constantly monitors epizootic situation of diseases with high potential of animal-human transmission including highly pathogenic avian and swine influenza;
Develops guidelines for veterinary surveillance and control of highly pathogenic avian influenza.
Organizes and caries out training of veterinary medicine specialists
National Service of regarding avian influenza response.
Veterinary and Plant Defines list of animal and poultry farms and their employees, which
Protection of the
may become a subject of special surveillance
Provides education and cooperates with the employees of the
veterinary and poultry sector, Implements prevention and disease
control activities in domestic animals and poultry.
Organizes regular laboratory tests for samples obtained from poultry, synanthropic and wild birds. Regulates importation of animals and animal products and controls their safety
Provides information to the Government Commissions and MoLHSA regarding highly pathogenic avian influenza epizootic situation and assists Ministry of Health in implementation of pandemic response activities
Prepares and implements protocols regarding closure of classes at the schools in case of pandemic and severe or disproportional morbidity
Ministry of Education among children.
Ensures distribution of education and communication materials among primary secondary and high school students
Ministry of Refuges Supports provision of preparedness and response measures among IDP
and Accommodation (especially IDP collective centers)
Regulates animal, poultry and their products import, controls their security
Ministry of Finance, Ensures activities as defined by the Law during pandemic emergency
Customs Department situation and ensures safety transportation through the state border of
influenza virus strains, clinical and biological materials for diagnostic studies, equipment, reagents and diagnostic materials for influenza virus testing and identification in Georgia.
Develops projections of pandemic possible economic consequences and
Ministry of Economic their mitigation strategies.
Develops recommendations of the business continuity for the private sector.
Provides logistical, human resources and equipment to support
Ministry of Defense response to the pandemic
Provides information on the pandemic influenza situation and measures to address its health, sanitary and social-economic
Public TV and Radio consequences.
Organizes and conducts presentations of medical experts, consultants, and other authorities.
Coordinates implementation of measures by local emergency situation
management headquarters within its administrative-territorial units.
Prepares local preparedness plans and ensures functioning of the
emergency response headquarters established at the local self-government administration's office
May provide financial and technical assistance.
May participate in joint research activities.
Clause 10. The Government Steering Commission on Emergency Situations
1. The Government Steering Commission on Emergency Situations is a central coordinating body, which ensures functioning of all agencies and supporting organization involved in entire system of emergency situations avoidance, elimination and mitigation of its consequences. The Commission performs its activities through close linkages with the Georgian Government, Autonomous Republic authorities and the local self-governments. The Commission is chaired by the Prime Minister and is represented by the high executive Government representatives, Security Council member deputy Ministers. Participation of other members is defined by the Commission chair.
Clause 11. Emergency Situation Management Department of the Ministry of Internal Affairs
1. The Government Steering Commission on Emergency Situations executes its objectives through the Emergency Situation Management Department of the Ministry of Internal Affairs. The Emergency Situation Management Department is tasked with the objective to organize and coordinate activities targeted at avoidance, elimination and mitigation of consequences of the emergency situations countrywide. 2. Hence the MIA Emergency Situation Management Department following the Government Commission guidance ensures coordination of various agencies activities with aim to mitigate and eliminate pandemic consequences.
Clause 12. Regional Governor's Administration
The President's Representative – the Regional Governor – coordinates operation of the Ministries regional bodies, supervises local self-governments' performance in the frame of the legislation, requests for assistance from the national level when the local resources are not sufficient. In case of emergency situations caused by the Pandemic and following the Government Steering Commissions' decision the regional Governor's administration coordinates operation of the headquarters established at the local level.
Clause 13. Local Municipal Self-Government
Local emergency headquarters are functional at the local level to adequately respond pandemic. In the emergency (including pandemic related) situations the head of the local self-government calls for the formation of the emergency headquarters and its composition is guided by the emergency response plans functions. The headquarters is headed by the special agency high official, which coordinates local means and resources, continuously communicates with the representative of the MIA Emergency Situation Management Department and prepares written report upon reception of the new information. Until getting assistance from the national level all decisions are made by the head of the headquarters.
Clause 14. Ministry of Agriculture and its Agencies
MoA and its agencies, esp. the National Service of Food Safety, Veterinary and Plant Protection are responsible for the highly pathogenic avian influenza (or anima—human transmittable influenza virus) control activities, which mainly are implemented during the first three phases of the Pandemic. The detailed functions, responsibilities and response actions are described in the "National Preparedness and Response Plan for Avian Influenza in Birds" (2007). The MoA and its agencies closely collaborate with the health institutions at the national, regional and local levels.
Clause 15. The role of the MoLHSA and its agencies
1. The MoLHSA has overall responsibility for planning, initiation, direction and co-ordination of health response. 2. Prior to the declaration of the emergency situation in the country, the responsibility for the basic operative management of a pandemic situation lies with the the MoLHSA and its agencies in cooperation with the MoA. Operative management involves the direction of the service system operations, securing of resources and their appropriate management, obtaining special powers and expert services as may be required by the situation, as well as ensuring adequate cooperation with the authorities. The expert services required for operative management are obtained by the MoLHSA from Pandemic Influenza Expert Group and other expert bodies (e.g., NCDC, NIDC). 3. The specific roles of MoLHSA agencies in response to influenza pandemic are specified in the Table below:
Table 4. Summary of MoLHSA agencies specific roles in response to influenza pandemic
Initiates meetings of the involved parties, making operative decisions
Develops / implements emergency response individual plans to provide timely and adeqautre medical assitance to the affeceted population
Stockpiles and distributes of medical supplies necessary for affected population
Emergency Situation medical assistance, coordination of health facilities performance
Coordination and
Plans, organizes health personnel training activities
Ensures tinely transportation between health faciliteis
Coodination of receit and distribution of humanitarian aid int eh frame of the legislaiton
Coordinates pandemic response activities with MCPH including pandemic vaccination
Conducts clinical and epidemiological influenza surveillance and monitors pandemic health impacts
Guide containment measures as necessary to prevent the spread of pandemic disease in territories and communities
Monitors vaccination program implementation and investigates vaccine adverse effects
Organizes broad community health campaign for different strata and social groups of population to prevent, and control the disease and decrease risk of infection.
Provides guidance on initiating community-based containment measures including social distancing, "snow days", and community-wide quarantine
Conducts and supports viral and epidemiological influenza surveillance and monitoring
Contributes to international surveillance of influenza
National Central
Collaborates with WHO, world and neighboring countries' influenza centers
Influenza Laboratory over potential vaccine candidate strains
Collaborates with the reference laboratories in testing the antiviral susceptibility of isolates
Evaluates factors that influence transmission of influenza viruses
Facilitates import and issues a permission for use of a pandemic vaccine
Medical Care Regulatory Facilitates import and issues a permission for use of a antiviral drugs
Monitors and investigates antiviral drug adverse effects
Provides clinical guidance to health professionals and others involved in provision of medical care to patients affected by pandemic influenza
Pandemic Influenza
Provides public health advice to health professionals and others involved in
pandemic influenza preparedness and response
Provides advice on pandemic preparedness planning and preparedness plan implementation to health care managers
Purchasing hospital services for the patients affected by pandemic virus
Inclusion of necessary services in the basic package of current PHC programs
Pandemic vaccine purchase
Coordination of health care facilities response at the local level
Overseeing implementation pandemic vaccination
Planning distribution of antiviral medications at the local level
Coordination of public health communication at the local level
Development of institutional pandemic preparedness plans Activation of institutional pandemic preparedness plans
Hospitals and polyclinics Mobilization of resources for implementation of the pandemic preparedness
Chapter 4. Animal Health
For the Pandemic phases 1-3 epizootic surveillance, infection control alerts, disease containment measures are described in the avian influenza preparedness and response national plans (Annex 4. Influenza Surveillance National Guidelines presented as a separate document).
Chapter 5. Situation monitoring and assessment
Clause 17. Surveillance
a) Routine clinical surveillance The routine clinical surveillance implies reporting of ILI and SARI group cases by all ambulatory and hospitals.
b) Sentinel Surveillance schemes Selected facilities in the regions collect clinical and epidemiological information on ILI and SARI cases and laboratory samples. This allows viral surveillance and antiviral drug monitoring. c) Sentinel clinical surveillance – aggregate clinical and epidemiological data are collected from patients with ILI from selected sentinel outpatient facilities across the country.
d) An enhanced nationwide notifiable disease surveillance system 1. An enhanced nationwide notifiable disease surveillance system or unusual or unexpected occurrences of acute respiratory infections which allows for the detection, verification, and investigation of novel influenza cases in a timely manner and for the adoption of the necessary control measures. This is combined with collection of lab specimens to determine the viral causes of the aforementioned unusual or unexpected occurrences. 2. For this purposes the following cases or events identified by providers require immediate notification of the local MCPH without any delay, by any existing means of communication (telephone, fax, email, or in person).
a) Cluster of ILI/SARI
b) Suspected case of influenza caused by novel viral type (e.g. H1N1, H5N1).
3. In addition, the following events should trigger public health department notification and inquiry prior to laboratory confirmation:
a) An excess number of ILI/SARI cases in a health care facility or community
b) Any rumors of clusters of SARI or of atypical respiratory infections, including disease related to animal exposure c) Possible other triggers for outbreak investigation may include clusters of animal deaths or excessive absenteeism from schools, institutions, and workplaces.
Surveillance procedures are described in detail in the national surveillance guidelines.
2. Pre-pandemic Surveillance strategy (phase 4)
During the phase 4 the country continues implementation of above mentioned four surveillance schemes (routine clinical surveillance, sentinel surveillance, sentinel clinical surveillance on primary care level and enhanced nationwide notifiable disease surveillance system). During the pre pandemic period, when sustained human-to-human transmission takes place, the primary goal of rapid detection is to quickly identify and contain cases of novel influenza. To limit the need to evaluate an overwhelming number of patients, the screening criteria should be specific, relying on a combination of clinical and epidemiologic features. In addition, it is critically important to monitor and report on international influenza activity, as assessed from official information sources (e.g. the WHO, national ministries of health) and international influenza surveillance reports (e.g. US Centers for Disease Prevention and Control in Atlanta [CDC], European Influenza Surveillance Scheme and theWHO's FluNet). International monitoring of influenza and emerging respiratory infections includes a summary and risk assessment of human infections with the novel (H1N1, H5N1) influenza viruses.
1. The basis for the expanded clinical surveillance is prepared by sentinel and sentinel clinical surveillance system, namely ILI and SARI aggregate data reporting. Using this system the following minimum data elements are reported by all outpatient clinics and hospitals to the local MCPH weekly or daily (reporting frequency will be defined based on situation):
a) Total number of outpatient visits by age
c) Total hospitalizations by age and time
d) Total hospitalized SARI cases by age and
b) Total number of visits of ILI cases by
e) Total hospitalized deaths by age and time
f) Total hospitalized SARI deaths by age
2. Tracking of influenza-associated deaths is done by the vital statistics offices through formal mortality tracking for ICD-10 (J09-J18) codes for pneumonia and influenza. Weekly/daily reporting is done during pandemic activity in the country for all hospital death cases in the
aforementioned ICD 10 categories. Data reports for pandemic influenza should be generated on a weekly/daily basis during pandemic activity in the country. 3. Surveillance procedures are described in detail in the national surveillance guidelines.
4. Post-peak period
1. During the post-peak period, pandemic disease levels will drop below peak observed levels. The post-peak period signifies that pandemic activity appears to be decreasing; however, it is uncertain if additional waves will occur and the country will need to be prepared for a second wave. Hence, all surveillance activities that are proposed for the pandemic phase must be continued.
5. Post Pandemic period
In the post-pandemic period, influenza disease activity will return to levels normally seen for seasonal influenza. At this stage, it is important to maintain surveillance as proposed for phases 1-3 and update pandemic preparedness and response plans accordingly.
Clause 18. Laboratory diagnostics
1. Laboratory diagnostics functional plan according to the pandemic phases is given in the Annex 4. Influenza Surveillance National Guidelines presented as a separate document. 2. Country has good laboratory base for diagnostics of various agents including pandemic viruses. National Influenza Laboratory (NCDC) provides Influenza virus typing by RT-PCR. Below are giver recommendations to strengthen laboratory capacity: a) Buy two extra PCR working stations for the same equipment (app. 60,000 to 70,000$ each) or a different type of real time PCR machine allowing performance of 96-reactions per run in a 96 multiwell plate (app.budget 50,000$) b) Provide training:
1) Primers design and sequencing for 2 NIC laboratory staffs (app. budget 6500 Euros) 2) Viral isolation by cell and egg culture for 2 NIC laboratory staffs (app. budget 6500 Euros 3) Ensure one NIC staff has a valid IATA training certificate for shipment of infectious substances.
c) Participation of the NIC laboratory in the WHO External Quality Assessment Programmes
Chapter 6. Reducing the spread of disease
Clause 19. Individual and Community Contaitmnet measures
1. General Strategies
1) To minimize economic and social costs, it will be important to judiciously match interventions to the pandemic severity level. Without mitigating interventions, even a less severe pandemic would likely result in dramatic increases in the number of hospitalizations and deaths. In addition, an unmitigated severe pandemic would likely overwhelm nation's critical healthcare services and impose significant stress on nation's critical infrastructure.
2) Table below lists factors that may influence decisions on where and when to impose community-based containment measures. Consider measures affecting exposed or at-risk
Consider measures that affect whole communities
when: There is moderate to extensive disease
There is limited disease transmission in the area
transmission in the area
Most cases can be traced to contact with an earlier
Many cases can not be traced to contact with an
case or exposure to a known transmission setting
earlier case or known exposure
(e.g., a school or a workplace)
Cases are increasing among contacts of influenza
The intervention is likely to slow the spread of
infection or decrease the overall magnitude of an
There is significant delay between the onset of
symptoms and the isolation of cases because of the large number of ill persons
3) The analysis and decision should also take into consideration the following parameters: 3.1 Epidemiological information:
a) Number of cases b) Number of hospitalized cases c) Mortality
3.2 Health care resources:
a) Hospital/facility bed capacity b) Staff resources, patient/staff ratio c) Number of all or absent staff members d) Availability of ventilators and other respiratory equipment (in hospitals and in reserve) e) Availability of therapeutic medications (in hospitals and in reserve)
3.3 Public health resources:
a) Public health specialist to cases ratio
3.4 Community compliance:
a) Degree of compliance with voluntary isolation b) Degree of movement out of the community c) Degree of compliance with community-containment measures
2. The pandemic mitigation measures include the following:
a) Individual level
1. Isolation and treatment (as appropriate) with influenza antiviral medications of all
persons with confirmed or probable pandemic influenza. Isolation may occur in the home or healthcare setting, depending on the severity of an individual's illness and /or the current capacity of the healthcare infrastructure.
2. Voluntary home quarantine of members of households with confirmed or probable
influenza case(s) and consideration of combining this intervention with the prophylactic use of antiviral medications, providing sufficient quantities of effective medications exist and that a feasible means of distributing them is in place.
b) Community level
3. Dismissal of students from school (including public and private schools as well as
universities) coupled with protecting children and teenagers through social distancing in the community to achieve reductions of out-of-school social contacts and community mixing.
4. Use of social distancing measures to reduce contact between adults in the
community and workplace, including, for example, cancellation of large public gatherings and/or alteration of workplace environments and schedules to decrease social density and preserve a healthy workplace to the greatest extent possible without disrupting essential services.
c) Border closures or severe travel restrictions are not recommended as a measure to prevent import of influenza into the country due to minimal effectiveness and massive cost and indirect resource expenditures. d) Entry screening is not recommended. The effectiveness of this intervention is minimal because many infected people may be pre-symptomatic or asymptomatic. At the same time direct and indirect losses of material and human resources will be disproportionately high. These resources should be used to implement much more effective interventions.
e) The recommendations to limit one's travel (except essential travel) during the pandemic period will not be effective, either, if unless most of the population will adhere to this recommendation. f) How to plan non-pharmaceutical interventions to mitigate influenza pandemic is described in the Annex 6.
3. Management of few human infections and their contacts during pre pandemic
Infection control strategies understood and implemented by both the public and the health care community are critical to the reduction in transmission of infectious disease entities, including novel respiratory viruses. Since the availability of pharmaceutical interventions is unlikely to be available during the early phase of the pandemic, emphasis on social distancing and personal hygienic practices needs to be continually reinforced pre-pandemic, allowing such practices to become a "norm" and widely accepted and adopted as routine practice. Careful attention to hand washing and respiratory etiquette has been suggested by mathematical models as a core management strategy for the control of respiratory pathogens.
4. Influenza control and prevention in the community during sustained human-to-human transmission
1) From the alert level 2, containment activities will focus on public health and individual measures that attempt to slow and limit viral transmission. 2) Containment measures applied to individuals (e.g., isolation and quarantine) may have limited impact in preventing the transmission of pandemic influenza, due to the short incubation period of the illness, the ability of persons with asymptomatic infection to transmit virus, and the possibility that early symptoms among persons infected with a novel influenza strain may be non-specific. 3) Nevertheless, during the pandemic Phase 4 with a less efficiently transmitted virus, these measures may have some effectiveness, slowing disease spread and allowing time for targeted use of medical interventions. Later, when disease transmission is occurring in communities around the country, individual quarantine is much less likely to have an impact and likely would not be feasible to implement. 4) Emphasizing what individuals can do to reduce their risk of infection (e.g., hand hygiene and cough etiquette) may be more effective disease control tools. Consideration to community-based containment measures (e.g., closing schools or restricting public gatherings) may be warranted if there is widespread public acceptance for such strategies.
5. Prepardness for implemenation of containment measures
Community preparedness for implementation of pandemic influenza containment measures Both individual and community-based containment measures raise legal, logistic, and social challenges that should be addressed during the pre pandemic period (Phase 4). 5.1 Planning for disease control and containment It is expected that local officials will face logistic, economic, ethical, legal, social, and psychological challenges in implementing disease control and containment measures during a Pandemic Period. Although individual quarantine as a control measure is likely only to be used during the Phase 4 and very early during the Pandemic Period - for example, among communities where initial cases are introduced in Georgia – all MCPH and local authorities should anticipate and prepare for the challenges of effectively implementing this measure by working with community partners to review the steps involved in establishing and maintaining quarantine facilities and procedures. Key activities include:
a) Identifying potential isolation and quarantine facilities b) Establishing procedures for medical evaluation and isolation of quarantined persons who exhibit signs of influenza-like illness (ILI) c) Developing tools and mechanisms to prevent stigmatization and provide mental health services to persons in isolation or quarantine, as well as to family members of affected persons and other community members d) Establishing procedures for delivering medical care, food, and services to persons in isolation or quarantine. These efforts should take into account the special needs of children and persons with disabilities. e) Developing protocols for monitoring and enforcing quarantine measures to ensurelegal authorities and procedures exist for various levels of movement restrictions f) Establishing procedures for issues related to employment compensation and job security.
5.2 Legal preparedness Issues related to isolation of cases and quarantine are addressed in the Georgian Law on Public Health, Chapter 4, Paragraph 11-12. 5.3 Planning for influenza clinics and hotlines 1) An influenza pandemic is likely to put great stress on the healthcare delivery system, in particular emergency departments. To prevent overwhelming demand from compromising the function of emergency departments, healthcare providers, organizations, and public health authorities should consider optimal methods for delivering assessment and care to individuals with probable influenza. This may include designating certain offices or clinics for screening, triage, and care of individuals with influenza-like illness. While the large majority of outpatient care during a pandemic will be provided by patients' usual medical care practitioner, health authorities may decide to establish special facilities (influenza clinics) to
provide rapid medical assessment of potentially infected persons, as part of efforts to control and contain small, well defined disease clusters, or in geographical areas that are medically underserved. Ill persons will be encouraged to call special influenza hotlines that provide advice on whether to stay home or to seek medical care. 2) Local MCPH supporting hotlines as triage and information systems must be aware of the healthcare resources available in the community. These "community triage" efforts may help prevent hospitals from being overwhelmed with patients who do not require hospital-level care. Moreover, community triage efforts may also reduce the number of uninfected persons who mingle with infected persons at clinics and hospitals. Preparedness planning for establishing influenza hotlines includes:
1. Establishing telephone hotline numbers that people can call 2. Identifying sites, staff members, and volunteers 3. Developing protocols for hotline staff members that include training components and
triage decision trees or algorithms
4. Establishing communication systems with influenza clinics, if they are established.
5.4 Increasing public understanding of disease containment measures 1) Community preparedness for implementation of both individual and community control measures can be enhanced during the pre pandemic period by improving public understanding of the dangers of pandemic influenza and the benefits of communitywide disease control practices, including personal hygiene and social-distancing measures that can prevent illness and death. Strategies for disease control will be facilitated by clear communication of the rationale for - and duration of - containment measures. 2) Local public health campaigns should explain how individual action (e.g., strict compliance with respiratory hygiene, staying home when ill) and community efforts can help reduce disease transmission. Education campaigns can describe the criteria, justification, role, methodology, and duration of quarantine and the social, medical, and psychological ways in which persons will be supported during the quarantine period. They can also explain that quarantine - which temporarily restricts personal movement - is a collective action implemented for the common good. Key messages prepared for use during the pre pandemic period can be adapted for use during an actual pandemic (see Chapter 8).
Clause 20. Planning for Vaccination Against a Pandemic Influenza Virus
1. Worldwide vaccine production capacity is limited and is primarily in industrialized countries, where most seasonal influenza vaccine is produced. Thus, the level of production is clearly insufficient to supply vaccines to all countries. Only a limited number of vaccine doses would be available, particularly in the early stages of the pandemic, and most of them would likely be supplied to industrialized countries. Many countries, probably including Georgia, will be forced to confront the next pandemic with few or no available vaccines. 2. As vaccine is likely to be in short supply and demand will be high in Georgia and worldwide, vaccine must be administered as it becomes available to predetermined priority groups. The reasons for the priorities must be defensible. The public will need information about vaccine not being generally available.
3. The priority groups for immunization will be based on a number of factors, including the need to maintain the elements of community infrastructure in order to carry out the pandemic plan; to limit mortality among high-risk groups; to minimize social disruption and economic losses; to reduce morbidity in the general population. 4. The priority groups will be defined prior to importing the vaccines in the country. It will be subject to review, depending on the epidemiology and clinical features of the new pandemic virus and depending on availability of vaccine. It is likely that advice will be given by WHO about priority groups for immunization, as soon as epidemiological data from the emerging pandemic is obtained. 5. Authorization for pandemic vaccine use in Georgia
During the influenza pandemic, as in case of other emergencies, the need may arise to import unregistered medical preparations including vaccines. Namely, in emergency situations, the MoLHSA will be using the Law on Drugs and Pharmaceutical Activities dated 10.08.2009, specifically the clause 1113 on "exclusions permitting introduction of the pharmaceuticals product on the Georgian market troughs avoiding established regimes" subsection (t) in cases of state emergency situations (natural disasters, population mass affect, epidemic, rare disease) for humanitarian purposes in agreement with the Minister. 6. Vaccine procurement and distribution The MoLHSA cooperates with the WHO regarding pandemic influenza preparedness. The WHO secretariat is planning to establish and maintain a stockpile of pandemic vaccines and associated equipment, including syringes, needles and applicators, consistent with expert guidance. Alongside with using WHO channels, direct contracting with vaccine manufacturers regarding procurement of pandemic vaccine (when it is available) is being considered by the MoLHSA as well. When it is available in Georgia, each MCPH will receive vaccines in proportion to the size of its population in defined priority groups.
Clause 21. Antiviral Drug Distribution and Use
1. Antiviral agents are considered effective for an influenza pandemic. They are particularly useful in the early stages of a pandemic when there is a shortage of vaccines. Two groups of antiviral agents for influenza are currently available, including M2 ion-channel inhibitors (amantadine and rimantadine) and neuraminidase inhibitors (oseltamivir and zanamivir). Neuraminidase inhibitors are preferred because some influenza viruses show high frequencies of resistance to M2 ion-channel inhibitors. All four drugs are registered by the Pharmacological agency of the MoLHSA and imported into the country by a number of suppliers. Appropriate use of these agents during an influenza pandemic may reduce morbidity and mortality and diminish the overwhelming demands that will be placed on the healthcare system. Antivirals might also be used during the Pandemic Alert Period in limited attempts to contain small disease clusters and potentially slow the spread of novel influenza viruses. 2. Stockpiling of neuraminidase inhibitors is under way in many industrialized countries as part of national influenza pandemic preparedness. However, the stockpiles of antiviral agents
available in developing countries are small and limited. WHO has global and regional stockpiles of antiviral agents, which are limited and are specifically used for early response and containment. The stockpile of antiviral agents is insufficient for a global pandemic. 3. A huge and uncoordinated demand for antivirals early in a pandemic could rapidly deplete national and local supplies. Preparedness planning for optimal use of antiviral stocks is therefore essential. 4. Use of antiviral medications in management of cases of novel influenza during pandemic alert period For treatment of cases of novel influenza: A patient with a suspected case of influenza A (H1N1) or another novel strain of influenza should be treated in accordance with the clinical algorithm provided in the clinical guidelines. The recommendations include the use of oseltamivir (Tamiflu) administered as early as possible and ideally within 48 hours after onset of symptoms. Neuraminidase inhibitors are preferred because the majority of influenza A (H1N1) viruses currently affecting humans are susceptible to the aforementioned drugs. 5. Recommendations on pandemic antiviral drug use 5.1 During a pandemic the following assumptions specific for antiviral drugs should be considered:
a) Treatment with a neuraminidase inhibitor (e.g., Tamiflu) will be effective in decreasing risk of pneumonia, will decrease hospitalization by about half, and will also decrease mortality. b) Antiviral resistance to amantadine and rimantadine may limit their use during a pandemic. c) The primary source of antiviral drugs for a pandemic response will be the supply of antiviral drugs that have been stockpiled. d) Treating earlier after the onset of disease is most effective in decreasing the risk of complications and shortening illness duration. Generally, treatment should be given within the first 48 hours. e) Assumptions for the amount of antiviral drug needed for defined priority groups are based on the population in those groups and assumptions that 25% of persons in the priority groups will be affected. f) Unlike vaccines, where each tier would be protected in turn as more vaccine is produced, for antiviral drugs, the number of priority groups that can be covered would be known at the start of the pandemic based on the amount of drug that is stockpiled. Additional supply that would become available during the pandemic could provide some flexibility.
Table 5: Antiviral drug priority group recommendations
Consistent with medical practice and ethics to treat
1. Patients admitted to
those with serious illness
and who are most likely to die
Healthcare workers are required for quality medical
care. There is little surge
2. Health care workers
capacity among health
and emergency medical
sector personnel to meet
service providers with
increased demand. To
direct patient contact and
ensure psychological
their family members
support and commitment to
work, family members of
healthcare workers should be also covered.
3. Highest risk outpatients
– immuno-compromised
persons, chronic CVD,
Groups at greatest risk of
chronic RD, malignant
hospitalizations and death.
neoplasms, DM, renal
failure, and pregnant
4. Pandemic health
responders (public
health), public safety
These groups are critical for
(police, fire, MoIA rescue
an effective public health
personnel, corrections)
response to a pandemic
and government decision
5. Increased risk
outpatients – young
children 6-23 months old,
These groups are at high
persons > 60 years old,
risk for hospitalization and
and persons with
underlying medical
5.2) During an actual pandemic, these recommendations could be modified based on the characteristics of the causative virus (e.g., drug susceptibilities, initial geographic distribution,
fatality rate, age-specific morbidity and mortality rates) and the effectiveness of implemented strategies. 6. Ensuring antiviral drugs supplies 6.1) Currently, WHO holds stockpiles of oseltamivir. The document "WHO interim protocol: rapid operations to contain the initial emergence of pandemic influenza (updated October 2007)" outlines a strategy for using antivirals and other measures (for example, quarantine, isolation and social distancing) in any country in order to stop (if possible) or slow the spread of an influenza virus with pandemic potential, if that virus is detected and reported rapidly enough. The WHO interim protocol provides practical guidance on use of the antivirals and on their release from the rapid response stockpile. The interim protocol includes standard operating procedures that provide processes and procedures for: (1) countries to request antivirals; (2) WHO to evaluate countries' requests and notify the manufacturer of the decision to deploy antivirals; (3) WHO and the manufacturer to discharge their responsibilities for deploying oseltamivir; (4) recipient countries to fulfill their responsibilities for receiving, storing, distributing and dispensing the antivirals, and for monitoring and reporting on their use. Once a decision to launch a rapid containment operation has been made by national authorities in collaboration with WHO, the manufacturer is responsible for transporting the antivirals to the nearest international airport in the country in which the operation is being conducted, respecting a time limit of 24 hours from receipt of a request from WHO. A direct handover to the WHO Country Office will take place at the airport. National authorities should be ready to authorize any package type and composition, waiver liability, and assume responsibility for customs release and compliance with importation requirements. 6.2) The MoLHSA is also considering a possibility of creating a national strategic reserve of antiviral drugs and other life-saving medicines. As for May 2009, MoLHSA has purchased 107,000 doses of oseltamivir (Tamiflue), which equals to approximately 50% of projected minimum of needed amount for abovementioned target groups. Appropriateness of further purchasing of antiviral drugs and time of purchasing will be defined by the MoLHSA. 6.3) The establishment of national, local, or institutional stockpiles should take into account the expiration dates of the purchased material. All drugs are marked with an expiration date, based on review of stability data, at the time of manufacture. However, when purchased, the drugs might have been stored for some time in warehouses so that the time to expiration might be shorter than the time from initial manufacture to expiration date. Moreover, one shipment might consist of several batches with different expiration dates. Antivirals maintained in the national stockpile may be tested for potency and dating can be extended by the Pharmacological Committee. 6.4) The decision to deploy national supplies of antivirals will be made by the MoLHSA officials. Antivirals will be delivered to regional storage facilities under the emergency committee under Regional Governor Office, which will include the following members:
a) HESPA regional branch representative
b) NCDC regional representative c) MoLHSA Emergency Situation Coordination and Regime Department regional
7. Regional level planning 7.1) Emergency committee under Regional Governor office should do the following:
a) Determine antiviral need (according to predefined criteria) b) Employ formal mechanism for obtaining antiviral drugs from the national
c) Distribute medicines for targeted use d) Collect data on drug u se e) Conduct training f) Disseminate public health information.
7.2) These planning efforts require coordination and collaboration with healthcare providers who will administer antivirals during a pandemic. Local MCPH will determine priority groups in their jurisdictions. Planning steps for distribution of antivirals to priority groups might include:
a) Estimating the size and needs of priority groups in region and rayons using
provisional recommendations
b) Developing a communication plan to explain the rationale for establishing these
8. Monitoring of antivirals use To ensure optimal use of antiviral drugs during an influenza pandemic, local MCPH and healthcare partners should collect data on:
a) Occurrence of adverse events following administration of antiviral drugs b) Serious adverse events associated with the use of antiviral drugs should be
reported to the MoLHSA and the Pharmacological Committee and will be investigated using established procedures.
c) Effectiveness of treatment and prophylaxis d) Development of drug resistance (when laboratory capacity is available).
9. Antiviral effectiveness and antiviral drug resistance Studies to evaluate the effectiveness of antiviral drug use during a pandemic may be conducted by the MoLHSA and the National Center of Infectious Diseases, the NCDC in collaboration with local MCPH and other healthcare and academic partners. 10. Training Enhance training and education efforts related to use of antiviral drugs during a pandemic. Exercises that involve healthcare providers who will administer antivirals to individual patients are essential to ensure that distribution systems are in place and that roles and responsibilities are well understood.
11. Public health information Local MCPH should develop and implement plans to educate the public, the medical community, and other stakeholders about:
a) Role of antivirals in responding to pandemic influenza b) Need to prioritize use of limited antiviral supplies for treatment and
prophylaxis and rationale for the priority groups identified
c) Importance of appropriate use (i.e., using the drugs as prescribed and for the
full number of days recommended) to minimize the development of drug resistance.
12. Estimated number of antibiotics and supplies Selection of antibiotics is based on national guidelines for pneumonia treatment and clinical management Estimations in the table are based on following assumptions:
Target population
Attack rate (% of population)
Complication with pneumonia (% of infected)
Hospitalized cases (severe pneumonia)(% of infected)
not severe pneumonia
Ventilators (% of hospitalized)
Table 6: Estimated requirement of antibiotics and supplies during pandemic
Estimated number of
Estimated #
Unit Dosage
patients requiring
Total quantity
of patients
antibiotics
not severe (100%)
not severe (100%)
Doxicycline
> 12 years. (20%)
II and III generation cephalosporines (cefuroxime,
cefotaxime)
Fluoroqinolones (Levofloxacine)
severe (on ventilation 80%) 645
Infusions (NaCl, Ringer, Glucose)
Glucose (D5, D10)
Disposable syringes and needles
Peripheral venous catheters (G24, G22, G20, G18)
Calculations are based on adult dosage only, however number of days (duration of treatment) is minimal, which is correction for lower dosages for children. Oral drugs are only in tablets - can be substituted by suspension considering the dosage
Clause 22. Clinical management of Pandemic (H1N1) 2009 influenza cases
Recommendations on clinical management of pandemic influenza cases are described in the guidelines (Annex 7.)
Clause 23. Infection Control
1. When a pandemic begins, a vaccine may not yet be widely available, and the supply of antiviral drugs may be limited. The ability to limit transmission in healthcare settings will, therefore, rely heavily on the appropriate and thorough application of infection control measures. The infection control guidance provided in this chapter is based on our knowledge of routes of influenza transmission, the pathogenesis of influenza, and the effects of influenza control measures used during past pandemics and pre pandemic periods. Given some uncertainty about the characteristics of a new pandemic strain, all aspects of preparedness planning for pandemic influenza must allow for flexibility and real-time decision-making that take new information into account as the situation unfolds. The specific characteristics of a new pandemic virus - virulence, transmissibility, initial geographic distribution, clinical manifestation, risk to different age groups and subpopulations, and drug susceptibility - will remain unknown until the pandemic gets underway. If the new virus is unusual in any of these respects, the MoLHSA will provide updated infection control guidance. 2. Recommendations for infection control in healthcare settings Basic infection control principles for preventing the spread of pandemic influenza in healthcare settings entail the following:
a) Standard infection control precautions b) Respiratory hygiene/cough etiquette c) Early recognition, isolation, and reporting of possible AI cases d) Recommendations for ambulatory-care settings e) Pre-hospital care and transport outside health-care facilities f) Isolation precautions for suspected or confirmed AI-infected patients g) Specimen collection/transport/handling within health-care facilities h) Patient transport within health-care facilities i) Duration of infection control precautions j) Family member/visitor recommendations k) Waste disposal l) Dishes and eating utensils m) Dishes and eating utensils n) Environmental cleaning and disinfection o) Patient-care equipment p) Patient discharge q) Occupational health recommendations
1). Recommendations for health-care facility administrators 2) Recommendations for all HCWs 3) Recommendations for HCWs who have provided care for AI-infected patients
r) Other occupational health issues s) Administrative control strategies for health-care facilities
t) Prioritizing the use of PPE when supplies are limited u) Environmental control strategies for health-care facilities v) Care of the deceased
3. These principles are described in details in the national guidelines on Infection Control Guideline for Health Care Facilities Annex 9.
4. Recommendations for infection control for home healthcare services
Most patients with pandemic influenza will be able to remain at home during the course of their illness and can be cared for by other family members or others who live in the household. Anyone residing in a household with an influenza patient during the incubation period and illness is at risk for developing influenza. A key objective in this setting is to limit transmission of pandemic influenza within and outside the home. When care is provided by a household member, basic infection control precautions should be emphasized (e.g., segregating the ill patient, hand hygiene). Infection within the household may be minimized if a primary caregiver is designated, ideally someone who does not have an underlying condition that places them at increased risk of severe influenza disease. Although no studies have assessed the use of masks at home to decrease the spread of infection, use of surgical or procedure masks by the patient and/or caregiver during interactions may be of benefit.
5. Management of influenza patients
a) Physically separate the patient with influenza from non-ill persons living in the home as much as possible. b) Patients should not leave the home during the period when they are most likely to be infectious to others (i.e., 5 days after onset of symptoms). When movement outside the home is necessary (e.g., for medical care), the patient should follow cough etiquette (i.e., cover the mouth and nose when coughing and sneezing) and wear procedure or surgical masks if available.
Management of other persons in the home
a) Persons who have not been exposed to pandemic influenza and who are not essential for patient care or support should not enter the home while persons are actively ill with pandemic influenza. b) If unexposed persons must enter the home, they should avoid close contact with the patient. c) Persons living in the home with the pandemic influenza patient should limit contact with the patient to the extent possible; consider designating one person as the primary care provider. d) Household members should monitor closely for the development of influenza symptoms and contact a telephone hotline or medical care provider if symptoms occur.
6. Infection control measures in the home
a) All persons in the household should carefully follow recommendations for hand
hygiene (i.e., handwashing with soap and water or use of an alcohol-based hand rub) after contact with an influenza patient or the environment in which care is provided.
b) Although no studies have assessed the use of masks at home to decrease the spread of
infection, use of surgical or procedure masks by the patient and/or caregiver during interactions may be of benefit. The wearing of gloves and gowns is not recommended for household members providing care in the home.
c) Soiled dishes and eating utensils should be washed either in a dishwasher or by hand
with warm water and soap. Separation of eating utensils for use by a patient with influenza is not necessary.
d) Laundry can be washed in a standard washing machine with warm or cold water and
detergent. It is not necessary to separate soiled linen and laundry used by a patient with influenza from other household laundry. Care should be used when handling soiled laundry (i.e., avoid "hugging" the laundry) to avoid contamination. Hand hygiene should be performed after handling soiled laundry.
e) Tissues used by the ill patient should be placed in a bag and disposed with other
household waste. Consider placing a bag for this purpose at the bedside.
f) Normal cleaning of environmental surfaces in the home should be followed.
7. Recommendations for infection control in schools and workplaces
a) In schools and workplaces, infection control for pandemic influenza should focus on: B) Keeping sick students, faculty, and workers away while they are infectious. c) Promoting respiratory hygiene/cough etiquette and hand hygiene as for any respiratory infection. The benefit of wearing masks in these settings has not been established.
d) School administrators and employers should ensure that materials for respiratory hygiene/cough etiquette (i.e., tissues and receptacles for their disposal) and hand hygiene are available. Educational messages and infection control guidance for pandemic influenza are available for distribution.
Clause 24. Management of Mass Fatalities during an Influenza Pandemic
1. During a pandemic, local authorities will have to be prepared to manage additional deaths due to influenza, over and above the number of fatalities from all causes currently expected during the pre pandemic period. Within any locality, the total number of fatalities (including influenza and all other causes) occurring during a 6- to 8-week pandemic wave is estimated to be similar to that which typically occurs over 6 months in the pre pandemic period. This guideline aims to assist local authorities in preparing to cope with large-scale fatalities due to an influenza pandemic. A number of issues have been identified, which should be reviewed jointly by local authorities, health workers, coroners/medical examiners, specialized communal enterprises and ritual services administrations of local authorities, religious groups, etc. The following laws should be consulted when planning the above measures: a) The Law of Georgia on Public Health; b) The Law of Georgia on Healthcare;
c) The Law of Georgia on Patients' Rights; d) The Law of Georgia on Protection of Population and Territory from Emergency
Situations of Natural and Technogenic Nature;
e) The Law of Georgia on Emergency Situation. 2. In order to identify planning needs for the management of mass fatalities during a pandemic, it is important to examine each step in the management of a corpse under normal circumstances and then to identify what the limiting factors will be when the number of corpses increase over a short period of time.
Table 7. Corpse Management Process
PLANNING / POSSIBLE SOLUTIONS
Inform population re. how to access an authorized person. Train sufficient number of staff that can certify death and issue certificates.
Availability of people Plan and test an on call system 24/7 specifically for
Person authorized to
able to do this task.
perform this task:
Allow keeping corps for no longer than 1 day in
health workers and
If death occurs in the cases where the situation does not require autopsy
personnel of civilian
home then one of
or coroner's investigation.
registry offices
these people will
need to be contacted. Establish a system for issuing death certificates
right at the place of death by a health worker that certifies it. Engage the Red Cross Society, Social Services, religious organizations and communities to facilitate delivery of death certificates.
Supply of human and Envision a rotating 6 month inventory of body physical (body bags)
bags, given their shelf life
Plan training or expanding the role of current staff
Person(s) trained to
to include this task
perform this task
enterprises and ritual
Envision engagement of additional staff of the
administrations of
MoIA and military units
local authorities, and ambulances
Provide this service in the home in conjunction
with pronouncement and transportation to
morgue or place of burial
In hospital: trained
In hospital: plan training of additional staff
staff and stretcher
working within the facility
Availability of human
to the morgue Outside hospital:
Look for alternate suppliers of equipment that
physical resources
informed person(s),
could be used as stretchers in an emergency
stretcher and vehicle
suitable for this
Outside hospital: inform population or provide
specific instructions through a phone service re.
where to take corpses if the family must transport
A suitable facility
Identify and plan for possible temporary morgue
Capacity of such
can be maintained at
Optimize (reduce) morgue storage time
4 to 8 degrees Celsius
Availability of human and
Person qualified to
Ensure that physicians and families are aware that
physical resources
perform autopsy and
an autopsy is not required for confirmation of
suitable facility with
May be required in
as cause of death
some Circumstances
Determine resources of specialized communal enterprises and ritual services administrations of local authorities, and other similar institutions
regardless of their form of ownership and
Appropriate location
departmental subordination to create a rotating 6
(s), caskets, bags
month inventory.
funeral director
location for service
Entrust heads of specialized communal enterprises
and ritual services administrations of local
authorities to determine surge capacity and possibly the need for additional sites (e.g., use of churches etc. for visitation)
Identify alternate vehicles that could be used for
Availability of human
Suitable vehicle and
this purpose (MoIA, military units, etc.)
vault or burial Driver
physical resources
Consider use of volunteer drivers
Availability of grave
Identify sources of supplementary workers (MoIA,
diggers and cemetery military units, volunteers).
Envision communal graves upon getting consent of
Space at cemetery
Extreme cold and
local sanitary control offices
3. Autopsies Many deaths in a pandemic would not require autopsies since autopsies are not indicated for the confirmation of influenza as the cause of death. However, for the purpose of public health surveillance (e.g., confirmation of the first cases at the start of the pandemic), respiratory tract specimens or lung tissue for culture or direct antigen testing could be collected post-mortem. 4. Death Registration Death registration is a territorial responsibility; it should be carried out according to the country laws and regulations. A medical death certificate and a death certificates issued by a civilian registry office are required for burial. In the pandemic situation, with the increased number of deaths, medical death certificates can be issued at the place of death or in morgues. If the person's death does not meet any of the criteria for needing to be reported to a coroner, then the person could be moved to a holding area (a morgue) soon after being pronounced dead or left at home for burial. A simplified procedure for issuing death certificates by civilian registry offices needs to be developed.
5,Infection Control Special infection control measures are not required for the handling of persons who died from influenza, as the body is not "contagious" after death. Visitations could be a concern in terms of influenza transmission amongst attendees. 6. Transportation No special vehicle or driver license is needed for transportation of a corpse. Therefore, there are no restrictions on families transporting bodies of family members if they have a death certificate. 7. Supply Management Special ritual services regardless of their form of ownership and departmental subordination are recommended not to order excessive amounts of supplies such as embalming fluids, body bags, etc., but that they have enough on hand in a rotating inventory to handle the first wave of the pandemic (that is enough for 6 months of normal operation). 8. Special Populations A number of religious and ethnic groups have specific directives about how bodies are managed after death, and such needs must be considered as a part of pandemic planning.
Chapter 7. Continuity of Health Care Provision
Clause 25. Estimated impact on the healthcare system
1. An influenza pandemic will place a huge burden on the country healthcare system. Estimates based on extrapolation of the 1957 and 1968 pandemics suggest that there could be about 16,000 hospitalizations and 800,000 outpatient visits in Georgia. Estimates based on extrapolation from the more severe 1918 pandemic suggest that substantially more hospitalizations and deaths could occur. Pre-pandemic planning by healthcare facilities is therefore essential to provide quality, uninterrupted care to ill persons and to prevent further spread of infection. Despite planning and preparedness, however, in a severe pandemic it is possible that shortages, for example of mechanical ventilators, will occur and medical care standards may need to be adjusted to most effectively provide care and save as many lives as possible. Flusurge 2.0 software5 developed by the US Centers for Disease Control and Prevention was used to estimate the impact of an influenza pandemic on hospital surge capacity in Georgia. 2. The following assumptions were used: Total number of staffed non-ICU beds in the country
Total number of staffed ICU beds
Total number of ventilators available
Attack rate (% of population that will become ill due to pandemic influenza)
Proportion of influenza patients who will require hospitalization (%)
Average length of non-ICU hospital stay for influenza-related illness (days)
Average length of ICU stay for influenza-related illness (days)
Average length of ventilator usage for influenza-related illness (days)
Average proportion of admitted influenza patients who will need ICU care (%)
Average proportion of admitted influenza patients who will need ventilators (%)
Average proportion of influenza deaths assumed to be hospitalized (%)
Daily percentage increase of cases arriving compared to previous day (%)
3. The results are presented in the table below. They are based on the most likely scenario.
PANDEMIC INFLUENZA IMPACT
Hospital Weekly admissions
929 1,548 2,321 2,941 2,941 2,321 1,548 929
admission Peak admissions/day
Hospital # of flu patients in hospital
683 1,138 1,706 2,162 2,238 1,967 1,509 990
5 http://www.cdc.gov/fl u/fl usurge.htm
capacity % of hospital capacity needed 23% 38% 57% 72% 75% 66% 50% 33%
# of influenza patients in ICU 139 295 454 599 648 631 501 346
capacity % of ICU capacity needed
153% 234% 310% 335% 326% 259% 179%
# of flu patients on
70 148 227 300 324 315 251 173
capacity % usage of ventilator capacity 42% 90% 138% 182% 197% 192% 152% 105%
# of deaths from influenza
189 315 473 599 599 473 315
# of influenza deaths in
132 221 331 419 419 331 221
Total hospital admissions– 15,477 (most likely); Total deaths– 3,151 (most likely); Estimates for the most severe (1918-type) scenario may need to be increased 5-10-fold 4. Notes:
1) Number of influenza patients in hospital, in ICU, and number of influenza patients on
ventilators are based on maximum daily number in a relevant week
2) Hospital capacity used, ICU capacity used, and % usage of ventilator are calculated as a
percentage of total capacity available
3) Under this scenario, it is assumed that 50% of total hospital beds, 50% of total ICU
beds, and 50% ventilators will be available for influenza patients
4) The maximum number of influenza patients in the hospital each week is lower than
the number of weekly admissions because we assume a 5-day stay in general wards
5. Based on these projections, Georgia is likely to experience a severe shortage of intensive care beds beginning on the 2nd week of a pandemic and severe shortage of ventilators beginning on the 3rd week of a pandemic.
Clause 26. Proposed operational response
From an operational perspective, a pandemic consists of three stages:
1. Pre - surge – when Georgia alert level 1 is declared. It will continue into Georgia
alert level 2. there is sustained human-to-human transmission.
2. Surge – when local triggers indicate the potential for a sudden escalation in patient
numbers, e.g., an influenza death, an outbreak in a school or other institution or
increased staff absence, this would take place at Georgia alert levels 3 and 4, and partly
post peak period .
3. Recovery – when it is clear that local influenza activity is declining, which
corresponds with late post peak and post pandemic period.
The term "Surge" is used in healthcare to describe "The ability of the health service to expand beyond normal capacity to meet an increased demand for clinical care".
Clause 27. Pre-surge
1. General preparation for a pandemic and activation of business continuity plans would take place at WHO Phase 5. However, the declaration of Georgia alert level 1 would trigger the pre-surge preparation for the MoLHSA. This would involve making sure that operational plans are tested; staff education, training and skilling up strategies are implemented; and stores and supplies are topped up. Surveillance would be enhanced and the algorithms on returning travellers developed by the NCDC would be widely distributed. Examples of the types of activity that might take place at this stage are listed in Table 3 below.
Table 8. Examples of activities in the pre-surge stage, Georgia alert level 1
a) Ensure that business continuity plans are in place and tested
b) Normal local admission and referral criteria apply
c) Consider identifying patients with chronic problems for review
d) Initiate training/upskilling programmes for staff with specific pandemic support and
cross-cover roles
e) Update staff infection control guidance f) Outpatient referrals as normal g) Reinforce messages on self-care and how to protect and look after yourself
a) Ensure that business continuity plans are in place and tested
b) Normal local admission criteria apply
c) Initiate training/skilling up for staff with specific pandemic support and cross-cover
d) Update staff infection control guidance e) Some hospitals may wish to use this period to fast-track urgent elective procedures
and investigations
f) Links between district general hospitals and paediatric intensive care units should be
reinforced and any extra training needs addressed
2. On the declaration of Georgia alert level 2 the above described processes would continue but, in addition, surveillance should be intensified to allow the start of the surge to be identified locally, since the start of any local pandemic activity may differ by two to three weeks across the Country (Table 9). This stage would also see the activation of the arrangements and procedures for the assessment and management of people with influenza-like illness, as described in Clinical Guidelines.
Table 9. Examples of activities in the pre-surge stage, Georgia alert level 2
a) Increase surveillance for influenza-like illness and ensure that reporting mechanisms
and reporting of influenza-like illness are agreed and in place
b) Reinforce infection control advice and procedures in consultation with NCDC,
c) Be prepared to switch to surge mode of operating at short notice. Normal local
admission and referral criteria would apply
d) Reinforce messages on self-care and how to protect and look after yourself
a) Increase surveillance for influenza-like illness and ensure that facilities and
procedures for the triage, isolation, assessment and treatment of affected patients and
their contacts are in place
b) Reinforce infection control advice and procedures in consultation with NCDC, MCPHc) Be prepared to switch to surge mode of operating at short notice. Normal local
admission criteria still apply
It should be recognised that the end of surge activities may represent the initiation of surge activities in other sectors e.g. social care.
Clause 28. Managing increased Demand and Capacity (surge)
1. The activation of this stage will be determined as the country reports sustained community level outbreaks (alert level 3). It is likely that there will be two steps to the surge response:
a) Initially, efforts will concentrate on expanding capacity: canceling all elective
procedures, dealing with emergencies only, early discharge and redeployment of staff. This is likely to offer increased capacity for only a few days.
b) The second stage of the surge response will involve the introduction of prioritization
criteria and restrictions on treatment options – to be introduced when deemed necessary in the local setting. This should be introduced for as short a period as practicable, due to the nature of the restrictions.
2. It is important to remember that it is likely that over 40% of the total patient volume will occur over a two-week period at the peak of the pandemic. Examples of activities in the surge stage are given in Table 5. Table 10: Examples of activities in the surge stage 2.1
a) Business continuity plans activated
b) All but essential elective procedures cancelled
c) Emergency admissions only introduce phased responses to any increasing demand
a) Business continuity plans activated
b) All but essential elective procedures cancelled
c) Phased admissions and treatments policy introduced and implemented as necessary
Clause 29. Recovery
1. On the basis of previous pandemics, it is likely that the initial local surge will last for three to four weeks before there is evidence of patient numbers tailing off. The decision about the relaxation of prioritisation criteria will have to be made locally, according to resource availability and dictated partly by staff availability. It is likely that the priorities will be to restore pre-surge standards of clinical care in the emergency setting, followed by the gradual resumption of urgent and then non-urgent elective procedures (Table 6). During this recovery period, the emphasis will be on getting services back to normal, learning from the experiences of the first wave, refining the response and preparing for a potential second wave. However, this needs to be set against the situation where they may be many tired and bereaved people and large backlogs of annual leave. Table 11: Examples of activities in the recovery stage 1.1
a) Gradual relaxation of restrictions on admissions
b) Phased reintroduction of general consultations
c) Gradual reintroduction of non-emergency outpatient referrals and investigations
a) Gradual relaxation of restrictions on admissions and treatment policies
b) Reintroduction of pre-pandemic standards of clinical care for emergencies
c) Phased reintroduction of elective treatments and investigations
2. Command and trigger The trigger to activate surge plans should be authorized by the MoLHSA. If epidemiological data indicate the beginning of local surge, the decision will be made about the area to which the activation trigger should apply and local health care facilities will move to this mode.
Clause 30. Facility based plans
1. The essential activity during pre pandemic period is planning, i.e. preparation for the pandemic. It is strongly recommended for every hospital and polyclinic to develop an institutional pandemic preparedness plan. These plans should be guided by the principles and approaches outlined below
Clause 31. Planning for provision of care in hospitals
1. Hospital pandemic preparedness plan (PPP) is a pre-established plan of action in case of pandemic that causes a consistent unbalance in the request for services of acute care compared to the actual capacities of the hospital. The purpose of developing PPP is to improve resilience
and capacity of the hospital to face a pandemic, namely, to define all procedures and actions to be pursued by all concerned staff in a crisis in order to provide the best attainable quality in delivering medical care to all patients in the hospital. 2. An influenza pandemic will place a huge burden on the country healthcare system. Pre-pandemic planning by healthcare facilities is therefore essential to provide quality, uninterrupted care to ill persons and to prevent further spread of infection. All hospitals of the country must be prepared for the rapid pace of pandemic influenza. Hospitals should be equipped and ready to care for a limited number of patients infected with a pandemic influenza virus, or other novel strains of influenza, as part of normal operations; and a large number of patients in the event of escalating transmission of pandemic influenza. 3. Main Recommendations
a) The key to readiness in critical situation is to have simple plans!
b) The plan must be widely shared and understood by all concerned actors (staff, patients,
volunteers, institutions, etc.)
c) It must clearly define roles, behaviors and protocols for all those involved, and enable
everyone to automatically act in accordance with a functional and functioning mechanism that can ensure the optimization of time, space and resources; the latter are usually inadequate and crucial in all critical situations and represent either the weak point or the clue to success.
d) The protocols in PPP should not substantially differ from the daily practice and vice
versa. By doing so, the planned actions will be more effective in one or the other situation.
e) It is of fundamental importance to pay particular attention to the following general
recommendations.
f) In the first place, preparation for a crisis preparedness plan for a hospital is a
fundamental exercise that will also provide a huge benefit to the general organization and management of emergency medical services in daily practice.
g) Planning should be done by a multidisciplinary group. PPP is the responsibility of all
staff. The management team or the head doctor should identify the person/s with capacity and willingness to develop the plan, but all staff must be involved in the drafting of the plan to the maximum extent.
h) Either in preparedness or in response, a hospital should mainly rely on internal
resources, especially with regard to the human resources, as long as this remains feasible. They know what, when, where and how to undertake all actions required, and
most important of all, hospital staff will understand why things have to be done in a certain way, if they have taken active part in the planning process.
i) Cooperation and coordination with national and regional epidemiological and
infectious diseases centers is fundamental.
j) Drills and tests of the PPP are a crucial factor. Getting ready for the worst is a matter of
practice. The more the hospital tests its capacity to respond to crises, the more it acquires knowledge and experience in organization and management of inestimable value.
4. Hospital response plans for pandemic influenza should:
a) Outline administrative measures for detecting the introduction of pandemic influenza,
preventing its spread, and managing its impact on the facility and the staff
b) Incorporate planning suggestions from NCDCPH c) Identify criteria and methods for measuring compliance with response measures (e.g.,
infection control practices, epid surveillance, patient placement, healthcare worker illness surveillance).
d) Review and update inventories of supplies that will be in high demand during an
influenza pandemic.
e) Review procedures for the receipt, storage, and distribution of assets received from
national stockpiles.
5. Guiding principles for hospital pandemic influenza planning are given in (Annex 10). In addition the guidelines cover the following topics.
a) Triage protocol b) Surge Management c) Service prioritization during the surge stage d) Bed expansion outside hospital e) Admission to, utilization of and discharge from services f) Patient placement, segregation and cohorting
Clause 32. Planning for provision of care in non-hospital settings
1. Primary care facilities 1.1 Planning and effective delivery of care in outpatient settings is critical. Appropriate management of outpatient influenza cases will reduce progression to severe disease and thereby reduce demand for inpatient care. A system of effective outpatient management will have several components. Telephone hotlines should be established to provide advice on whether to stay home or to seek care. Most persons who seek care can be managed appropriately by outpatient providers. 1.2 Pandemic-specific pre-hospital patient assessment and treatment protocols should recognize that hospital capacity will be extremely limited, emphasizing treatment at home and
ensuring that only patients with life-threatening conditions are actually conveyed to emergency departments. Local response plans should also consider the extent to which the field assessment and treatment skills of ambulance staff could be utilized to support the wider delivery of home care. 2. Mental health Mental health establishments will face specific challenges. Many, particularly those providing secure care, are relatively closed environments with the attendant risk of rapid spread of influenza amongst patients and staff. The welfare of patients being cared for in the community is largely dependent on staff availability for domiciliary visits and the supply of psychopharmacological agents necessary to maintain health. Contingency plans should include infection control measures to minimize the spread of influenza in residential establishments, based on the assumption that it will not be possible to move those with significantly disturbed behavior to other settings, and should contain explicit agreements for utilizing available staff according to greatest need.
3. Pharmacy 3.1 The contribution that pharmacies can make in a pandemic scenario will depend on the setting in which they routinely provide services and the qualifications, expertise and area of practice of their pharmacists. Pharmacies are often located in the heart of communities. They can make an important contribution in support of self-care, dispensing/repeat dispensing of routine medicines, supplying regular medicines to vulnerable people and maintaining medicine supplies under contracts with various facilities.
3.2 Formal consultation will precede any proposed changes to legislation. Where there are shortages of some medicines, pharmacists are well placed to advise on the use of alternative medicines that have a similar effect. Pharmacies will play an important part in educating the community, providing positive health messages and advising patients and members of the public on medicine supply issues. As the pandemic escalates, some of the routine functions and services provided by pharmacies may have to be reduced, or stopped for short or longer periods, as demands increase elsewhere. Clinical pharmacists may be able to support doctors and other healthcare professionals in all settings, including primary care, hospitals and the community.
4. Dentistry Current infection control advice suggests that health professionals should avoid aerosol-generating procedures on symptomatic patients as far as possible during a pandemic and wear respirators and suitable protective equipment where that is not possible. Many dental procedures have the potential to generate aerosols and risk assessments will therefore be necessary. Local plans should ensure that emergency care remains available throughout a pandemic, but dental practitioners may find normal demand reduced because of limits on the procedures they are able to carry out on those with respiratory symptoms and patients themselves deferring treatment or facing travel difficulties. Local planning should explore opportunities to use the assessment and treatment skills of dental practitioners or other health professionals to support the wider delivery of healthcare in a pandemic.
5. Prison health 5.1 Prison Health Service reports directly to the Governmental Standing Commission to inform planning for managing the impact upon the prison system. The Prison Health Service has to prepare contingency plan for Pandemic Influenza, which will set out the requirements and parameters to meet the contingencies of pandemic influenza.
5.2 Prison senior medical staff has to be involved at all stages of planning. The prison authorities must establish links with the MoLHSA and local MCPH to finalize arrangements for antiviral access and infection control management, and to adapt local community policies to a prison setting. This is necessary to establish the appropriate lines of command and control between the governor and the local MCPH communicable disease control expert. This agreed division of authority and responsibility must be specified in the contingency plan.
6. Alternative care sites If an influenza pandemic causes severe illness in large numbers of people, hospital capacity might be overwhelmed. In that case, communities will need to provide care in alternative sites (e.g., shuttered hospitals, schools, convention centers). The selection of alternative care sites for pandemic influenza should specifically address the following infection control and patient care needs:
a) Bed capacity and spatial separation of patients b) Facilities and supplies for hand hygiene c) Lavatory and shower capacity for large numbers of patients d) Food services (refrigeration, food handling, and preparation) e) Medical services f) Staffing for patient care and support services g) PPE supplies h) Cleaning/disinfection supplies i) Environmental services (linen, laundry, waste) j) Safety and security.
Chapter 8. Communication
Clause 33. Goal of the National Communication Strategy on Pandemic Influenza
The National Communication Strategy on Pandemic Influenza will, with other components of the National AI Plan, reinforce the capacity to protect Georgian society from the emergence of an highly pathogenic avian influenza (or other new influenza virus) pandemic, and if it occurs, contribute to the containment of and recovery from the epidemic.
Clause 34. Major Objectives:
1. The National Communication Strategy on Pandemic Influenza:
a) Ensure a comprehensive, evidence-based communication approach to prepare the
people of Georgia for protection from an Influenza pandemic.
b) Work to limit the spread of Pandemic Influenza through all 6 pandemic phases
from animal-to-animal, from animals to humans and humans to humans;
c) Promote hygiene behavior change to limit the spread from animals to humans; d) Provide detailed communication guidelines to all institutional sectors involved in
Influenza pandemic prevention and containment.
e) Strengthen the capacity of government and other institutional preparedness and
response should a pandemic occur.
f) Develop a comprehensive, evidence-based communication approach that informs
people how and motivates them to protect themselves and their families from influenza.
2. The outcome of these objectives will be to provide accurate information, raise awareness, promote priority prevention behaviors and engage collaborative dialogue among major institutions responsible for pandemic influenza prevention and containment. 3. During this time when it might appear there is no need for concern because no recent evidence has been found regarding infection in poultry and animals, it is very tempting to turn to other issues. On the contrary, this is the opportunity to update and further refine comprehensive communication interventions. During the pandemic alert period, national, regional and local health communications professionals should focus on preparedness planning, capacity building and building flexible, sustainable communications networks to keep the public and other target groups updated about risks if the threat of a pandemic evolves. Communication efforts prior to an outbreak seek generally to educate, inform, advocate, prepare and promote animal-to-animal prevention activities. When an outbreak or pandemic emerges, communication needs must quickly shift to prevention of animal-to-human and human-to-human transmission and disease containment. During such a time, the public urgently demands information on how to protect
their families. Health personnel must be informed, prepared and ready to act. Building capacity lays the groundwork for effective pandemic communication.
Clause 35. Key Responsibilities of the Communication strategy
1. Capacity strengthening
a. According to financial resources provision of refresh training courses for health
personnel, veterinarians and poultry farms employees on zoonone diseases, including avian influenza to support.
b. Urgent risk communication training for trainers that should carry out trainings in their
c. Trainings for media representatives and journalists that they have right and reliable
information for population.
d. Provide tools and resources through the MoH website, hotlines and other avenues
to enhance the capacity of regional and local communications staff.
e. Simulation trainings for pandemic regional communication committee to instill their
obligation and assist effective communications between agencies during the pandemic outbreak.
f. Develop web-page on avian influenza by the MoLHSA and MoA, with aim to
strengthen collaboration on preventive issues. Develop a clearinghouse of all communication outcomes such as technical materials, software, data, best practices, TV and radio spots, communication materials (print materials, leaflets, etc.)
2. Social Research
a) Interviewing med staff, vets, community leaders to define dependence and practice. b) Evaluating communication strategy of salmonella 2006, to define inevitability of
inspection and in case of necessity inspect if there will be financial sources.
c) Extra research for selected target population to improve their conduct alternation
d) Major objectives
1) Which target group conduct assist to spread influenza? 2) Which conducts of these groups assist to spread the risk? 3) Which conduct change will shorten the risk? 4) What will serve best to change their behavior?
3. Message and material development
a) For spokesperson, develop pandemic alert and pandemic messages and materials based
on risk communication principles, as outlined in the WHO Outbreak Communication Guidelines.
b) Define specific target audiences, especially those most vulnerable and at highest risk,
and develop materials for these audiences.
c) Refine current message maps and concepts appropriate for each "Phase" of an influenza
pandemic development as approved by the CDC and WHO. These message maps form the basis for all communication interventions.
d) Conduct pretests of all messages and materials before finalizing. Evaluate all materials
after distribution and revise as necessary.
The 2006 KAPB study comprised of (a quantitative survey and a qualitative assessment) was conducted concurrently with the development of the National Strategic Communication Plan of Avian and Pandemic Influenza, 2006-08. As a result no baseline data was developed to evaluate the communication materials developed. There is a need for follow up research to assess the reach, behavioral response and impact of the communication interventions or messages.
5.Plan and assess current knowledge
5.1 Determine what communication actions will be taken and by whom in advance of a pandemic and once a pandemic is confirmed by WHO; Identify communication needs of various audience segments (i.e. what materials, resources, processes, and systems, will be necessary) 5.2 Conduct an assessment of current knowledge of pandemic influenza, which will include:
a) Literature review on pandemic flu, public health risks, public and response to
similar incidents (e.g., SARS);
b) Assess and analyze existing media and public baseline knowledge and attitudes;
5. 3Review current national and international efforts and programs to control the pandemic and work with international partners, such as WHO, to coordinate activities
6. Expand community mobilization
Strengthen community response through community mobilization of local leaders in
rural and hard to reach populations.
7. Personal Hygiene Campaign
a) To further the focus on preparedness planning, the 2009-2011communication strategy
will incorporate behavioral interventions related to the upcoming personal hygiene campaign administered by the Social Projects Implementation Team and funded by the
World Bank. The goal of the campaign is to prevent seasonal influenza and promote food safety to prevent zoonones such as salmonella.
b) Many of the behavioral interventions planned for the hygiene campaign are the same
as those for avian influenza prevention and protection (maintains hygiene behavior efforts and uses a combination of mass media and interpersonal channels). The target audiences are similar - mothers, young children and young adults. Careful attention to hand washing and respiratory etiquette is a core strategy for the control of respiratory pathogens. Pre-pandemic personal hygiene practices need to be continually reinforced to encourage such practices to become the social norm. By focusing on similar behavioral actions, the updated communication strategy ensures coordination, consistency and reinforcement of key messages (wash your hands) between campaigns.
c) Full version of the National Communication Strategy on Avian and Pandemic
Influenza for the Republic of Georgia for 2009-2011 is given separately.
Chapter 9. Business Continuity
1. Planning in all sectors must recognize that no pharmaceutical countermeasures (antiviral medicines or vaccines) are likely to present a ‘silver bullet' solution, particularly during the first wave of a pandemic. 2. The Government has recommended that local government and other sectors – should build on and review their generic business continuity arrangements to reflect the potentially large number of staff who might be absent during a pandemic and identify other key interdependencies. The overall aim is to maintain business as usual for as long and as far as that is possible and particularly around the peak of a Georgia epidemic when staff absences are likely to be at their highest.
Clause 36. Communications
1. At the onset of a pandemic, the telecommunications industry would expect to be able to provide a near-normal service. However, like other sectors, the degree to which services may be affected will depend on a number of factors including the nature of the crisis, the number of workers who contract the virus and the resulting level of absenteeism. Above-normal absenteeism rates during a pandemic are likely to result in a gradual increase in the time taken for telecommunications providers to deal with customer requests.
2. The telecommunications industry would respond to a crisis by seeking to limit the impact on services by prioritizing fault repairs at the expense of routine maintenance and the provisioning of new services. New services provided during such a crisis would generally be restricted to urgent requests.
3. Whilst telecommunications networks have the capacity to support a significant increase in home working, the reconfiguration of networks to enable them to handle significant short-term changes in the location. Organizations planning to increase home working in a pandemic must therefore talk to their telecommunications providers in advance and will also need to ensure that they have the necessary arrangements in place to ensure support, oversight and audit of home workers.
4. There may be some disruption to postal services due to the high level of staff absence at the peak of the pandemic, although a wide range of postal operators should ensure that the market maintains priority delivery services.
Clause 37. Energy
The energy sector is planning to maintain supplies of gas and electricity at near-normal service levels during a pandemic. Though there may be some service disruption during the peak of staff absences. Service supply also may be disrupted by technical or weather related problems.
Clause 38. Finance
Pandemic planning in this sector is coordinated by banking and financial sectors, which share responsibility for maintaining financial stability in Georgia. Planning – involving financial firms and infrastructure regulators – is advanced and has primarily focused on business continuity, also provision of basic services, such as cash circulation, banking and payment systems.
Clause 39. Food
Companies across the food sector will work together through their representative organizations, to maintain supplies as far as possible. However, at the peak of the pandemic, there may be a reduction if some local outlets close due to non-availability of staff.
Clause 40. Public transport
Public transport operators aim to run as near to normal services for as long and as far as that is possible during a pandemic. Government is not planning to impose closure of public transport. The aviation sector may also experience difficulties if airlines have operational problems or stop operating.
Clause 41. Water
All water companies must be confident that they will have sufficient staff to sustain essential operations during a pandemic. They must work with suppliers and contractors to check preparedness arrangements, particularly in critical areas such as chemical supplies for water treatment. Though there may be some reduction in this sector due to non-availability of staff.
Clause 42. Emergency services
The general aim in Business planning will be to maintain emergency provision support at near-normal level and wider response to a pandemic.
Clause 43. Planning by local authorities
1. Local authorities will be key players in the local-level multi-agency response to an influenza pandemic and are planning accordingly in the following main areas:
a) business continuity to sustain key local services
b) arrangements to support central government in communicating public messages
c) implementation of possible social measures that the Government may recommend on an
advisory basis to reduce the risk to individuals of infection
d) supporting the health and social care response
e) preparing for the wider impacts of a pandemic in their area
f) reviewing capacity to handle excess deaths
Clause 44. Public order
1. An influenza pandemic is likely to cause public concern and anxiety, particularly if the virus causes high levels of illness and death and/or the communications strategy has limited success. 2. In the event of any civil disorder, the Government would rely on existing legislation and normal enforcement measures as far as possible, but may consider the need for additional powers should that become necessary. In this regard, it should be recognized that any request for police support is likely to be in the context of reduced police availability through illness and the need to service similar requests for policing support from other sectors. 3. Every above mentioned sector must plan its work during the influenza pandemic accordingly to the business pandemic influenza planning check list presented in the Annex 11.
Chapter 10. Implementation of the national plan
National influenza pandemic preparedness and response plan will be developed as a separate document. In this section are reviewed some important aspects in implementing the national plan, such as financing mechanisms for pandemic activities and priorities for the development of national pandemic preparedness capacities.
Clause 46. Funding Mechanisms for Healthcare
1. Federal and regional funds, as well as funds from donor organizations will finance activities envisaged by the influenza pandemic preparedness and response plan. Some pre-pandemic and pandemic preparedness activities can be financed from assigned funds to those responsible for activities authorities. In case of the need of creating national pandemic stockpiles or in case influenza pandemic will severely affect Georgia's population, additional funds of possibly large amounts will be required, which can be sourced from:
a) President's and government's reserve funds, mobilization, management and spending
of which is regulated by the Georgian law on "Georgia budget systems," and allocation of which for pandemic needs will be assured by the decision of the governmental committee on emergency, the cabinet, and the President.
b) International financial institutions' and donor organizations' funds in the forms of
grants and credits (e.g. possible sources can be World Bank's "Avian influenza control and human pandemic preparedness and response plan in Georgia," WHO antiviral stockpile etc.). Specific recommendations on financing mechanisms for certain national plan activities and directions are also provided in following sub-sections.
Clause 47. Creation of Pandemic Stockpiles
Following the recommendations from the previous sections, the responsibility on creating pandemic stockpiles is shared among federal authorities (MoLHSA and its agencies, Ministry of Agriculture and its agencies), local governance and self-governance bodies, and health care and laboratory network institutions. On central level, Governmental Committee on Emergencies decides on creation of pandemic preparedness and response national stockpiles of vaccines, antiviral drugs, medical equipment, and supplies, which is refilled and updated using the central budget funds (from the funds assigned to relevant ministries, or from the additional funds from government's reserve). Simultaneously, healthcare institutions are advised to create under individual preparedness and response plans minimum recommended stockpiles of intravenous transfusion solutions, antibacterial medications and medical supplies, which will be replenished and renewed from the funds of the local authority budgets (through local governance and self-governance bodies' decisions) and from the own funds of healthcare institutions.
. Clause 48. Financing of Primary Health Care services
During the pre-pandemic phase or the pandemic phase 5, national alert being below level 3, when human to human transmission either do not take place, or there are single cases, primary healthcare facilities do not face outpatient surge and primary health care services for the distinct cases are financed within the existing governmental programs ("Governmental Program on Primary Healthcare," "Governmental Program on Providing Insurance to the Population being under the Poverty Line") provided by the agency for healthcare and social programs. For this relevant changes are applied to the list of services covered by the governmental programs. During the pandemic peak, when outpatient referrals are significantly increased, additional funds will be made available for these programs from presidential and governmental reserve funds if needed. Primary healthcare network provides special vaccines and antiviral drugs for the purposes of prophylaxis and treatment from the national strategic stockpile based on rule established by the Ministry of Labor, Health and Social Affairs.
. Clause 49. Financing of Inpatient Services
1. During influenza pre-pandemic and pandemic phases financing of inpatient services for those pandemic influenza diseased population, which requires such a care, is provided by the government through governmental programs according to the scheme below:
Governmental Program
Groups Covered by the Program
Program for the treatment of infectous diseases
Children under 3 years
Medical Insurance program for the population 950,000 insured persons being under poverty line being under the poverty line Referral program
Part of the population, which is not covered by other governmental programs
2. Within the mentioned programs contacting and financing of the inpatient institutions is carried out by the Agency for Health and Social Programs, for the purpose of which relevant changes are applied to the list of services covered by governmental programs. During the pandemic peak, when significant increase of hospitalizations is anticipated, additional funds will be made available for these programs from presidential and governmental reserve funds if needed. Provision of inpatient institutions with antiviral drugs and when required with antibiotics, medical equipment and supplies from the national strategic stockpile based on rule established by the Ministry of Labor, Health and Social Affairs. Referral of pandemic influenza cases within the country is made to defined hospitals, the list of which is approved by the Ministry of Labor, Health and Social Affairs. When number of pandemic cases exceeds the number of referral hospitals or the capacities of existing referral system, patients are admitted by any hospital in the country, which possesses minimum required gear, medical supplies, equipped bedding and medical staff. Agency for Health and Social Programs defines the disbursement for the hospital cases.
Clause 50. Financing of Epidemiological Surveillance and Safety measures
Financing of epidemiological surveillance activities during all phases of influenza pandemics is provided within existing governmental programs ("The Program for Ensuring Epidemiological Safety").
Clause 51. Financing of Other Services
Out of other pandemic preparedness and response activities important are those, to be implemented by the Ministry of Agriculture and its subordinate agencies for the control of animal pandemic influenza diseases. These activities generally are financed through the routine budgetary assignations made available for relevant services. In case of acute epizootic situation, costs related to quarantine measures and the depopulation of domestic poultry and animals may reach significant figures, and additional funds for these activities are made available from presidential and governmental reserve fund by the decision of Governmental Committee on Emergency Management.
Clause 52. Building of National Capacity (Training)
For every section of this plan, training of health care workers and public health personnel is one of the most critical components to ensure that all activities are implemented as planned. At this pre pandemic stage, in order to ensure an adequate capacity of the country to respond to the pandemic treat, the highest priority should be given to the following three areas:
1. Development of institutional preparedness
Hospital managers/ PHC managers
2. Clinical management of pandemic patients
Providers working at hospitals/ polyclinics
3. Infection control in health care facilities
Providers working at hospitals/ polyclinics
4. Community disease prevention measures
Local MCPH professionals
5. Epid-surveillance
Providers of sentinel sites
Annex 1. Influenza Virus The agent of pandemic influenza is the influenza virus, which is also responsible for causing seasonal influenza, known by most persons as the flu. Seasonal influenza, a common disease characterized by symptoms such as fever, fatigue, body pain, headache, dry cough, and sore throat, affects large numbers of people each year. Influenza viruses are negative-stranded RNA viruses that have been classified taxonomically as orthomyxoviruses; they are divided into two types: "A" and "B" viruses. Influenza type C is not known to cause disease in humans and so is not applicable to this discussion. The remarkable variation of influenza strains—particularly type A—and their ability to cause annual epidemics of respiratory illness of varying intensity and severity, continue to be the focus of intense investigation. Only type A viruses are known to cause pandemics. Type A viruses are further divided into subtypes based on the specific hemagglutinin (H) and neuraminidase (N) proteins on the virus surface. Currently, two subtypes of viruses A are in worldwide circulation in humans: H3N2 and H1N1. The emergence of both of these subtypes in the 20th century led to separate pandemics. For example, the 1918 pandemic resulted from the emergence and spread of the H1N1 virus while the 1968 pandemic was associated with the H3N2 virus. The 1957 pandemic was associated with the emergence and spread of the H2N2 virus; however, this virus subtype stopped circulating in 1968. Influenza pandemics are believed to have occurred for at least 300 years at unpredictable intervals
Why influenza pandemics occur
1. Drift and shift An important feature of influenza viruses that helps to explain much of their epidemiological patterns is the ability and propensity of these viruses to modify (drift) or replace (shift) two key viral proteins, hemagglutinin and neuraminidase, on the viral surface. Because these proteins are the main targets for the immune system, changes in these proteins can have minor to profound effects on the antigenicity of influenza viruses. a) Drift Influenza viruses can change through antigenic drift, which is a process in which mutations to the virus genome produce changes in the viral H or N. Drift is a continuous ongoing process that results in the emergence of new strain variants. The amount of change can be subtle or dramatic, but eventually one of the new variant strains becomes dominant, usually for a few years, until a new variant emerges and replaces it. In essence, drift affects the influenza viruses that are already in worldwide circulation. This process allows influenza viruses to change and re-infect people repeatedly through their lifetime and is the reason the influenza virus strains in vaccine must be updated each year. b) Shift In contrast to drift, pandemic viruses arise through a process known as antigenic shift. In this process, the surface existing viral H and N proteins are not modified, but are replaced by significantly different H and Ns. Since influenza A viruses that bear new (or novel) H or H/N
combinations are perceived by immune systems as new, most people do not have pre-existing antibody protection to these novel viruses. This is one of the reasons that pandemic viruses can have such severe impact on the health of populations.
Animal reservoirs
Novel influenza viruses occasionally emerge among humans as part of the natural ecology and biology of influenza viruses. Wild birds are considered the reservoir for influenza viruses because more influenza A subtypes circulate among wild birds than humans or other animal species. Normally, animal influenza viruses do not infect humans. However, avian influenza viruses can sometimes cross this barrier and directly infect humans. This was demonstrated in 1997, when an outbreak of avian influenza A (H5N1) viruses infected both domestic poultry and humans in Hong Kong, leading to 18 hospitalizations and 6 deaths. Since then, other outbreaks of avian viruses (such as H9N2 in 1999, H7N2 in 2002, H7N7 in 2003, and H5N1 again in 2004) have occurred and been found to directly infect people. Fortunately, these avian viruses lacked the ability to spread easily from person-to-person and therefore did not precipitate larger outbreaks or a pandemic. Pandemic viruses can also arise when some of the genes from animal influenza viruses mix or reassort with some of the genes from human influenza viruses to create a new hybrid influenza virus. This can occur when a single animal (for example, a pig or possibly a person) is simultaneously co-infected by both a human influenza virus and an avian influenza virus. In this situation, genes from the human and avian viruses can reassort and create a virus with the surface proteins derived from the avian virus (hence, creating a new subtype) and the internal proteins derived from the human virus, enhancing the transmissibility of the hybrid virus. The process of reassortment is not theoretical. Reassorted viruses have been frequently identified and are thought to have been responsible for the 1957 and 1968 pandemic viruses.
Distinguishing pandemic from seasonal influenza
Several epidemiological features distinguish pandemic influenza from seasonal influenza. Pandemics of influenza are unusual events and their timing cannot be predicted. For example, only three pandemics occurred in the 20th century (1918, 1957, and 1968). The infrequency and unpredictable timing of these events is explained by the fact that influenza pandemics occur only when a new (or novel) influenza A virus emerges and spreads globally. By definition, most people have never been exposed to these viruses and therefore are susceptible to infection by them. In contrast, seasonal influenza virus strain variants are modified versions of influenza A viruses that are already in widespread circulation. Therefore, there is usually some level of pre-existing immunity to strain variants. Because of the frequent appearance of new variants, virus strains contained in seasonal inter-pandemic trivalent influenza vaccines must be updated annually.
Annex 2. Legal Documents Regulating Influenza Pandemic and Related Issues
Law of Georgia on Public Health (N5069, 27 June 2007) Law of Georgia on Protection of Population and Territory from Emergency Situations
of Natural and Technogenic Nature (N4922, June 8, 2007).
Law of Georgia on Food Safety and Quality (N2548, 27 December 2005) Law of Georgia on Veterinary (N757, 14 June 1995) Law of Georgia on Agricultural Quarantine (N716, 15 May 1997) Law of Georgia on Emergency Situations (N972, 17 October 1997) Law of Georgia on Georgian Consular Institutions (N605, 22 November 1994) Law of Georgia on Drugs and Pharmaceutical Activities (10 August 2009) Code of Administrative Offences (15 December 1984, as amended) Presidential decree on "Planning of National Response to Natural and Technogenic
Emergency Situations" (N 415, 26 August, 2008)
Financing Agreement (Avian Influenza Control and Human Pandemic Preparedness
and Response Project) between Georgia and International Development Association (14 April 2006)
Resolution of the Government of Georgia No. 14 on establishing the Governmental
Steering Commission on Avian Influenza (18 January 2006)
Order N257 of the Prime Minister on Establishing the Team Implementing Avian
Influenza Control and Human Pandemic Preparedness and Response Project as Foreseen in the Financing Agreement between Georgia and International Development Association (11 October 2006)
Order N2-71 of the Minister of Agriculture on Establishing the Rules for Animal
Quarantine (1 May 2006)
Joint Order N987-N2-184 of the Minister of Finance and the Minister of Agriculture
on establishing rule of state phyto-sanitary border quarantine and state veterinary border quarantine control implementation (31 December 2008)
Order N215/n of Minister of Labor, Health and Social Affairs on approving the list of
infectious diseases and potentially dangerous products and/or cargo subject to sanitary- quarantine control at border and customs zones and obligatory documentation for their export, import and transit (22 September 2003)
Order N336/n of the Minister of Labor, Health and Social Affairs on approving the
rules of organization and implementing disinfection, disinsection, deratization and deactivation in the means of international transportation (18 December 2003)
Order N101/n of the Minister of Labor, Health and Social Affairs on the rule of
processing and submitting the medical statistical information (5 April 2005)
Annex 3: National Actions during the pandemic phases and responsible agencies
Actions taken during pandemic Phases 1-3 are aimed at strengthening pandemic influenza preparedness and response capacities at national and sub-national levels.
Integrate pandemic preparedness and response plans into existing national emergency preparedness Governmental
and response programmes and coordinate its implementation.
Emergency Situations
Assess capacities and identify priorities for pandemic preparedness planning and response at
national and sub-national levels.
Commission on Emergency Situations
Develop, exercise, and periodically revise national and sub-national influenza pandemic
preparedness and response plans in close collaboration with human and animal health sectors and other relevant public and private partners with reference to current WHO guidance.
Establish, as needed, full legal authority and legislation for all proposed interventions.
Governmental Commission on Emergency Situations, Ministry of Justice, Sectoral Ministries
Anticipate and address the resources required to implement proposed interventions at national and
sub-national levels including working with humanitarian, community-based, and non-
governmental organizations.
Emergency Situations
Develop an ethical framework to govern pandemic policy development and implementation.
Provide to public and private sectors the key assumptions, guidance and relevant information to
facilitate their pandemic business continuity planning.
Commission on Emergency Situations
Identify and address trans-border issues, including interoperability of plans across borders.
Georgia Border Police, MIA
Participate, when possible, in regional and international pandemic preparedness planning initiatives Governmental
Commission on Emergency Situations
Develop national surveillance systems to collect up-to-date clinical, virological, and epidemiological MoLHSA;
information on trends in human infection with seasonal influenza viruses, which will also help to
estimate additional needs during a pandemic.
Detect animal and human infections with animal influenza viruses, identify potential animal sources MoA;
of human infection, assess the risk of transmission to humans, and communicate this information to
WHO and relevant partners.
Detect and investigate unusual clusters of influenza-like respiratory illness or deaths and assess for
Characterize and share both animal and human influenza virus isolates and associated information
with relevant international agencies, such as WHO, FAO and OIE, to develop diagnostic reagents,
candidate vaccine viruses, and monitor antiviral resistance.
Strengthen the national laboratories in influenza diagnostic capabilities.
Identify, regularly brief, and train key personnel to be mobilized as part of a multisectoral expert
response team for animal or human influenza outbreaks of pandemic potential.
Preventing human influenza infection from animals
Reduce infection risk in those involved in responding to animal outbreaks (education and training
regarding the potential risk of transmission; correct use of personal protective equipment; making
antivirals available if indicated by the risk assessment).
Recommend measures to reduce human contact with potentially infected animals.
Control potentially contaminated environments such as wet markets and ponds with free grazing
In conjunction with animal health authorities, establish national guidance on food safety, safe
agricultural practices, and public health issues related to influenza infection among animals.
Individual / household level measures
Promote hand and respiratory hygiene.
Develop infection control guidance for household settings.
Develop plans to provide necessary support for ill persons isolated at home and their household
Societal level measures
Establish protocols to suspend classes, especially in the event of a severe pandemic or if there is
disproportionate or severe disease in children.
Emergency Situations;
Promote development of mitigation strategies for public and private sector workplaces (such as
adjusting working patterns and practices).
Promote reduction of unnecessary travel and overcrowding of mass transport systems.
Ministry of Education
Develop a framework to facilitate decision-making for cancellation/restriction of mass gatherings at
Ministry of Economic
the time of the pandemic.
International travel measures
MIA in the frame of
Develop capacities for emergency public health actions at designated points of entry in accordance
with IHR (2005) Annex 1 B.2.
Antivirals and other pharmaceuticals Estimate and prioritize antiviral requirements for treatment and prophylaxis during a pandemic. Develop mechanisms and procedures to select, procure, stockpile, distribute, and deliver antivirals
based on national goals and resources.
Plan for the increased need for antibiotics, antipyretics, hydration, oxygen, and ventilation support
within the context of national clinical management strategies.
Assess effectiveness and safety of antiviral therapy using standardized protocols when possible.
Work to increase seasonal influenza vaccine coverage levels of all high risk people. Establish goals and priorities for the use of pandemic influenza vaccines. Develop a deployment plan to deliver pandemic influenza vaccines to national distribution points within seven days from when the vaccine is available to the national government. Consider the feasibility of using pneumococcal vaccines as part of the routine immunization
program in accordance with WHO guidelines.
Identify priorities and response strategies for public and private health care systems for triage, surge
capacity, and human and material resource management.
Review and update continuity of health care provision strategies at national and sub national levels. MoLHSA
Develop strategies, plans, and training to enable all health care workers, including community level
workers, to respond during animal outbreaks and a pandemic.
Develop case-finding, treatment, and management protocols, and algorithms. Develop national infection control guidance. Estimate and plan for procurement and distribution of personal protective equipment for protection
Develop and implement routine laboratory biosafety and safe specimen-handling and shipping
policies and procedures.
Explore ways to provide drugs and medical care free of charge (or cover by insurance) to encourage
prompt reporting and treatment of human cases caused by an animal influenza virus or virus with pandemic potential.
Develop the capacity for the rapid deployment of diagnostic tests once available. Assess health system capacity to detect and contain outbreaks of human influenza disease in hospital
Update leadership and other relevant sectors regarding global and national pandemic influenza risk
Build effective relations with key journalists and other communications channels to familiarize
them with influenza and pandemic related issues.
Develop effective dialogue and listening mechanisms with the general public.
Develop effective communication strategies and messages to inform, educate, and communicate
with individuals and families so they are better able to take appropriate actions before, during, and
after a pandemic.
Initiate public health education campaigns in coordination with other relevant authorities on
individual-level infection control measures.
Situation Management
Increase public awareness of measures that may be available to reduce the spread of pandemic
Interagency Operation
Create messages and feedback mechanisms targeted towards hard-to-reach, disadvantaged, or
minority groups.
Public TV Broadcast
Test communications procedures through exercises.
Update communications strategies as feedback from the general public and stakeholder
organizations is collected and analysed.
An important goal during WHO pandemic Phase 4 is to contain the new virus within a limited area or delay its spread to gain time to implement interventions, including the use of vaccines.
Responsible structure
Direct and coordinate rapid pandemic containment activities in collaboration with
WHO to limit the spread of human infection.
Activate procedures to access and mobilize additional human and material resources.
Deploy operational and logistics response teams.
Identify needs for international assistance.
Designate special status as needed (such as declaring a state of emergency) to facilitate
rapid containment interventions.
Provide regular updates on the evolving situation to WHO as required under IHR
Governmental Commission on
(2005) and to other partners to facilitate coordination of response.
Emergency Situations;
Encourage cross-border collaboration with surrounding countries through
information sharing and coordination of responses.
Activate pandemic contingency plans for all sectors as deemed critical for the
provision of essential services.
Finalize preparations for a possible pandemic including procurement plans for
essential pharmaceuticals.
Not yet affected Finalize preparations for a possible pandemic by activating internal organizational
arrangements within the command-and-control mechanism and mobilizing staffing surge capacity in critical services.
Enhance surveillance to rapidly detect, investigate, and report new cases and clusters.
Collect specimens for testing and virological characterization using protocols and
procedures developed in collaboration with WHO.
Share specimens and/or strains to develop diagnostic reagents and prototype vaccines
and for antiviral susceptibility.
Collect more detailed epidemiological and clinical data as time and resources permit. To the extent possible, monitor compliance, safety, and effectiveness of mitigation
measures and share findings with the international community and WHO.
Not yet affected Enhance virological and epidemiological surveillance to detect possible cases and
clusters, especially if sharing extensive travel or trade links with affected areas.
Report any suspect cases to national authorities and WHO. When affected
Provide advice to travellers.
Undertake rapid pandemic containment operations in collaboration with WHO and
the international community.
Request and distribute antivirals from the WHO global stockpile and/or other
national or regional stockpiles for treatment of cases and prophylaxis of all persons in the designated areas.
Consider deploying pandemic vaccine if available. Implement individual/household and societal-level disease control measures. Limit all non-essential movement of persons in and out of the designated
containment area(s) and implement screening procedures at transit points.
Not yet affected Reassess the capacity to implement mitigation measures to reduce the spread of
pandemic influenza.
Distribute stockpiles of pharmaceuticals and other materials according to national
Use appropriate individual/household disease control measures for suspect cases and
their contacts.
Provide guidance to health care workers to consider influenza infection in patients
with respiratory illness and to test and report suspect cases.
Implement appropriate infection control measures and issue personal protective
equipment as needed.
Activate contingency plans for responding to the possible overload of health and
laboratory facilities to deal with potential staff shortages.
Activate alternative strategies for case isolation and management as needed. Not yet affected Activate pandemic contingency planning arrangements for the health sector. Advise health care workers to consider the possibility of influenza infection in
patients with respiratory illness, especially those with travel or other contact with persons in the affected country(ies).
Activate communications mechanisms to ensure widest possible dissemination of
Update and disseminate "Talking Points" so that all spokespeople convey consistent
Governmental Commission on
Conduct frequent and pre-announced public briefings through popular media outlets such Emergency Situations;
as the web, television, radio, and press conferences to counter panic and dispel rumours.
When affected Regularly communicate via established mechanisms:
What is known and not known about the virus, the state of the outbreak, use and effectiveness of measures and likely next steps.
The importance of limiting all non-essential movement of persons in and out of the designated containment area(s) and relevant screening procedures at transit points.
The importance of compliance with recommended measures to stop further spread of the disease.
How to obtain medicines, essential services and supplies in the containment area(s).
Gather feedback from the general public, vulnerable populations and at-risk groups
on attitudes towards the recommended measures and barriers affecting their willingness or ability to comply. Incorporate the findings into communication and health education campaigns targeted to the specific groups.
Collaborate with surrounding countries on information sharing.
During Phases 5-6 (pandemic), actions shift from preparedness to response at a global level. The goal of recommended actions during these phases is to reduce the impact of the pandemic on society.
Responsible structure
Maintain trust across all agencies and organizations and with the public through a
commitment to transparency and credible actions.
Designate special status as needed, such as declaring a state of emergency.
Governmental Commission on
Provide leadership and coordination to multisectoral resources to mitigate the societal and
Emergency Situations;
economic impact of a pandemic.
Work for rational, ethical, and transparent access to resources. Assess if external assistance is required to meet humanitarian needs. Not yet affected Finalize preparations for an imminent pandemic. Update, if necessary, national guidance and recommendations taking into account
information from affected countries.
Pandemic disease surveillance
Undertake a comprehensive assessment of the earliest cases of pandemic influenza.
Document the evolving pandemic including geographical spread, trends, and impact.
Document any changes in epidemiological and clinical features of the pandemic virus.
Maintain adequate virological surveillance to detect antigenic and genetic changes, as well
Ministry of Economic
as changes in antiviral susceptibility and pathogenicity.
Modify national case definitions and update clinical and laboratory algorithms for
diagnosis, as necessary.
Monitoring and assessment of the impact of the pandemic Monitor essential health-related resources such as: medical supplies; antivirals, vaccines and
other pharmaceuticals; health care worker availability, hospital occupancy/availability; use of alternative health facilities, laboratory material stocks; and mortuary capacity.
Monitor and assess national impact using criteria such as workplace and school
absenteeism, regions affected, groups most affected, and essential worker availability.
Assess the uptake and impact of implemented mitigation measures. Forecast economic impact of the pandemic, if possible.
Measures regarding crossing the State Border
Take into account WHO guidance and information when issuing international travel
advisories and health alerts.
Individual/household level measures
Advise people with acute respiratory illness to stay at home and to minimize their contact
Governmental Commission on
with household members and others.
Emergency Situations;
Advise household contacts to minimize their level of interaction outside the home and to
isolate themselves at the first sign of any symptoms of influenza.
Provide infection control guidance for household caregivers taking into account the WHO
Georgia Border Police (MIA)
Societal level measures Implement social distancing measures as indicated in national plans, such as class
suspensions and adjusting working patterns.
Encourage reduction in travel and crowding of the mass transport system. Assess and determine if cancellation, restriction, or modification of mass gatherings is
International travel measures Consider implementing exit screening as part of the early global response. Provide advice to travellers. Pharmaceutical measures Distribute antivirals, and other medical supplies in accordance with national plans. Implement vaccine procurement plans. Plan for vaccine distribution and accelerate preparations for mass vaccination campaigns. Modify/adapt antiviral and vaccine strategies based on monitoring and surveillance
Implement medical prophylaxis campaigns for antivirals and/or vaccines according to
priority status and availability in accordance with national plans.
Monitor safety and efficacy of pharmaceutical interventions to the extent possible and
Not yet affected
Be prepared to implement planned interventions to reduce the spread of pandemic disease. Update recommendations on the use of planned interventions based on experience and
information from affected countries.
Implement distribution and deployment plans for pharmaceuticals, and other resources as
Consider implementing entry screening at international borders. WHO recognizes individual country considerations will affect national decisions, but, in general, does not encourage: Pandemic-related international border closures for people and/or cargo. General disinfection of the environment during a pandemic. The use of masks in the community by well persons. The restriction of travel within national borders during a pandemic, with the exception of a
globally led rapid response and containment operation, or in rare instances where clear geographical and other barriers exist
Implement pandemic contingency plans for full mobilization of health systems, facilities,
and workers at national and sub-national levels.
Implement and adjust the triage system as necessary.
Enhance infection control practices in healthcare and laboratory settings and distribute
personal protective equipment in accordance with national plans.
Provide medical and non-medical support for patients and their contacts in households and
alternative facilities if needed.
Provide social and psychological support for health care workers, patients, and
Implement corpse management procedures as necessary. Not yet affected Prepare to switch to pandemic working arrangements. Regularly update the public on what is known and unknown about the pandemic disease,
including transmission patterns, clinical severity, treatment, and prophylaxis options.
Governmental Commission on
Provide regular communications to address societal concerns, such as the disruption to
Emergency Situations;
travel, border closures, schools, or the economy or society in general.
Regularly update the public on sources of emergency medical care, resources for dealing
Public TV Broadcast
with urgent non-pandemic health care needs, and resources for self-care of medical conditions.
The post-peak period
The overall goal of actions during the post-peak period is to address the health and social impact of the pandemic, as well as to prepare for possible future pandemic waves.
The pos-pick period
Responsible structure
Determine the need for additional resources and capacities during possible future
Governmental Commission on
Emergency Situations;
Begin rebuilding of essential services
Address the psychological impacts of the pandemic, especially on the health
Consider offering assistance to countries with ongoing pandemic activity. Review the status of and replenish national, local, and household stockpiles and
Review and revise national plans.
Activate the surveillance activities required to detect subsequent pandemic waves.
Governmental Commission on
Evaluate the resources needed to monitor subsequent waves.
Emergency Situations;
Evaluate the effectiveness of the measures used and update guidelines, protocols, and
algorithms accordingly.
Continue with vaccination programmes in accordance with national plans, priorities,
and vaccine availability.
Ensure that health care personnel have the opportunity for rest and recuperation.
Restock medications and supplies and service and renew essential equipment.
Review and, if necessary, revise pandemic preparedness and response plans in
anticipation of possible future pandemic wave(s).
Revise case definitions, treatment protocols, and algorithms as required.
Regularly update the public and other stakeholders on any changes to the status of the MoLHSA;
Communicate to the public the ongoing need for vigilance and disease-prevention
efforts to prevent any upswing in disease levels.
Continue to update the health sector on new information or other changes that affect
disease status, signs and symptoms, or case definitions, protocols and algorithms.
The post-pandemic period
The goal of activities during the post-pandemic period is to address the long-term health and social impact of the pandemic, as well as to restore normal health and social functions.
The post-pandemic period
Responsible structure
Evaluate the effectiveness of specific responses and interventions and share findings
Governmental Commission on
with the international community.
Emergency Situations;
Review the lessons learned and apply to national emergency preparedness and
response programmes.
Revise national and sub-national pandemic preparedness and response plans. Collect and analyse available data to evaluate the epidemiological, clinical, and
Governmental Commission on
virological characteristics of the pandemic.
Emergency Situations;
Review and revise situation monitoring and assessment tools for the next pandemic
and other public health emergencies.
Resume seasonal influenza surveillance incorporating the pandemic virus subtype as
part of routine surveillance.
Conduct a thorough evaluation of individual, household, and societal interventions
Conduct a thorough evaluation of all the pharmaceutical interventions used,
antiviral effectiveness, safety, and resistance; and
vaccine coverage, effectiveness, and safety.
Review and update relevant guidelines as necessary. Continue with vaccination programmes in accordance with national plans, priorities,
and vaccine availability.
Collect and analyse available data to evaluate the response of the health system to the MoLHSA;
Review the lessons learned and share experiences with the international community.
Amend plans and procedures to include lessons learned. As needed, provide psychosocial services to facilitate individual and community-level
Publicly acknowledge the contributions of all communities and sectors.
Communications Communicate to the public and other stakeholders the lessons learned about the
effectiveness of responses during the pandemic and how the gaps that were discovered will be addressed.
Encourage stakeholders across all sectors, public and private, to revise their pandemic
and emergency plans based upon the lessons learned.
Extend communications planning and activities to cover other epidemic diseases and
use the principles of risk communications to build the capacity to dialogue with the public on all health matters of potential concern to them.
Improve and adjust communications plan in readiness for the next major public
Annex 4. Influenza Surveillance National Guidelines
Presented as a separate document.
Annex 5. Laboratory diagnostics
Perform integrated epidemiological and virological seasonal influenza surveillance (see Chapter 5.1 on epidemiological surveillance) Public health goals for routine surveillance of influenza viruses are to identify and characterize circulating strains and strains with pandemic potential. The National Influenza Centre will therefore:
Participate in both the European Region Influenza Network and the WHO GISN. The
Community Network of Reference Laboratories for Human Influenza in Europe (CNRL) is a network of labs in all EU countries and some other EAA and European Region countries. The CNRL are coordinated by 3 laboratories located in the Health Protection Agency in the United Kingdom (UK), the WHO Collaborating Centre in the UK (NIMR), and the RIVM in the Netherlands.
Coordinate, together with the national surveillance unit, communication between the
WHO and the CNRL, in questions relating to virological and epidemiological surveillance of influenza in Georgia and in the provision of influenza virus isolates to GISN.
Be responsible for the laboratory confirmation of influenza in respiratory specimens
that are collected from SARI, ILI, and/or ARI cases.
Have a national virological focal point that may be a person or persons that are
responsible for the laboratory component of surveillance system. The national virological focal point(s) should be responsible for:
o Receiving, registering and storing specimens from cases of SARI, ILI, and/or
ARI. Following laboratory testing, the focal point should archive and store original clinical specimens at -70C or in liquid nitrogen for at least one year.
o Providing confirmatory testing for SARI, ILI, and/or ARI specimens received,
Seasonal influenza virus typing and subtyping by molecular techniques
(RT-PCR, sequencing). Virus isolation methods should be undertaken on a representative sample of influenza positive specimens.
Subtyping viruses other than seasonal influenza viruses.
o Maintaining the linkage between the Unique ID numbers assigned by the site
sending the specimen and the specimen identification number assigned by the laboratory. This link between the laboratory specimen results and the
epidemiologic data collected at the sites is crucial to the analysis of the surveillance data.
o Identifying and doing preliminary characterization of any novel influenza A
o Wherever possible, conduct antiviral susceptibility testing on influenza viruses. o Consolidating and analyzing national laboratory data. o Providing technical support and guidance to sampling sites on appropriate
specimen collection, packaging, transport and storage.
o Supporting the national surveillance unit during investigations of unusual
outbreaks of influenza, including those due to avian influenza A(H5N1) or other novel influenza viruses.
o Sharing a representative sample of seasonal virus isolates with the WHO
Collaborating Centres
o Having all necessary training and materials for the shipment of specimens
and/or viruses to a WHO CC. This should include receiving the WHO reagents kit for standardized identification of influenza viruses at least once per year.
o Developing national diagnostic standards and assays that are periodically
o Checking participation and results of an external quality assessment for
Communication and exchanges of information will take place between NIC and laboratories dealing with animal samples (Central Laboratory of the Ministry of Agriculture in Tbilisi, and Regional Veterinary Laboratories) that are in charge of the laboratory confirmation in animal surveillance. These laboratories will:
Analyse by RT PCR samples received for the national animal surveillance system and
help collect up-to-date virological information on animal infection (National veterinary laboratory).
Characterize and share animal influenza virus isolates and associated information with
relevant international agencies, such as WHO, FAO and OIE, to develop diagnostic reagents, candidate vaccine viruses, and monitor antiviral resistance. They can also share RNA extracts and associated information for further subtyping with the NIC.
See Annexe 1 for testing algorithm in veterinary laboratories for specimen collected from animals. Ensure notification and report
NIC should be aware, for alert and response purposes and pandemic early warning, of
national procedures for communication with the IHR National Focal Point (NFP).
The national virological focal point will:
o communicate the results of all individual confirmatory tests for SARI cases
back to the sampling site as soon as they are known.
o coordinate with the national surveillance focal point to report data to the
WHO via the current EISS platform.
o provide a weekly report during the influenza season to FluNet . This should
include information on influenza virology and epidemiology, and details of any shipments.
o work closely with the national surveillance focal point to prepare the Weekly
Influenza Report and the Annual Influenza Report. Reports should include epidemiologic and virological data, including any results of antigenic and genetic analyses that have been obtained.
Strengthen the national capacity in influenza diagnostic capabilities to cope with the emergence of novel influenza and a possible pandemic During influenza pandemic, the volume of requests for laboratory testing is expected to increase dramatically. To meet these demands, the laboratory should become proficient in methods that allow efficient testing of large numbers of specimens at a lower biosafety level than BSL 3—which is required for viral culture of novel influenza viruses such as avian influenza A (H5N1), Swine-origin Influenza A (H1N1), other virus subtypes not widely circulating in humans or influenza A viruses that were unsubtypable. To ensure adequate virological surveillance during a pandemic, laboratories should:
Be equipped and trained to use RT-PCR for routine influenza testing For BSL3, be equipped and trained to use RT-PCR for routine influenza testing and to
detect novel influenza viruses by RT-PCR or by viral culture, using proper safety precautions
Maintain reagents and supplies to allow influenza virus testing year-round Develop surge capacity to handle increased testing and reporting during a pandemic
Implement pandemic contingency plans for full mobilization of facilities, and workers at national and sub-national levels. Develop and implement routine laboratory quality policies and procedures In order to get the best possible results, quality assurance measures need to be followed. To ensure good quality and reliable results, laboratories should:
Implement a quality system Train all staff to quality management/quality assurance Train all staff in charge of sampling to perform adequate and good quality samples Participate in an EQA scheme for influenza. It will be the national virological focal
point(s)'s responsibility to check participation and results.
Develop and implement routine laboratory biosafety and safe specimen-handling and shipping policies and procedures. To ensure proper biosafety laboratories should:
Implement biosafety measures Train all staff to bisosafety, use of PPE and safe specimen handling Train all staff responsible of transport of specimen to safe specimen-handling Train all staff in charge of referring samples to national shipping policies
Ensure that at least one person in the laboratory has IATA certified training to perform
shipment of category A and category B substances. This will be under the national virological focal point(s)'s responsibility.
Influenza laboratory specimen processing Laboratory specimens should accompany the epidemiologic data collection. Specimen flow Human samples For seasonal influenza surveillance, specimens are collected in sentinel sites and in different health care facilities in case of ILI, SARI or ARI. These specimens are transported to the NIC laboratory for analysis. The NIC is in charge of shipping newly isolated strains to WHO Collaborating Centres to establish a basis for WHO recommendations on the composition of influenza vaccine, each year. In case of a new A influenza subtype, the system implemented for extremely dangerous pathogens (EDP) would be activated. This means that zonal diagnostic laboratories (ZDL) in place in Kutaisi and Batumi would group the specimens collected from the surrounding health care facilities, store them appropriately if needed and arrange for transport to the NIC in Tbilisi. Laboratory support sites (LSS) are going to be constructed in the coming year. These sites will then be in charge of sampling, packaging the specimens and transporting them to the ZDL for further transport to the NIC laboratory. The human specimen flow is summarized in Figure 1. Animal samples Veterinary LSS are in charge of sampling, packaging and transporting specimens to the ZDL in Kutaisi and Akhaltsikhe for analysis. Specimens can also be sent to the ZDL by the private sector. The national veterinary laboratory in Tbilisi is acting as a ZDL for general analysis of samples coming from the surrounding LSS or private sector and as a national reference laboratory to which specific specimens will be sent to for further typing. In case of an unsubtypable influenza A virus, the specimen will be sent to a reference OIE laboratory for further analysis. The animal specimen flow is also summarized in Figure 1. The national veterinary laboratory and the NIC laboratory will both be integrated in the future Central Reference Laboratory (CRL) that is actually under construction.
Figure 1. Specimen flow for influenza
Specimen Flow for Infuenza
NCDC / CRL (BSL-3)
National Vet Laboratory
NCDC / Surveillance Department /
Influenza Unit - NIC (BSL-2)
Human ZDL (BSL-2)
No diagnostic for
Health Care Facility
Health Care Facility
Central Reference Laboratory
Electronic Integrated Disease Surveillance System
Government of Georgia
Ministry of Labor, Health and Social Affairs
National Center for Disease Control and Public Health
World Organization for Animal Health
Zonal Diagnostic Laboratory
Under construction
Collection Respiratory virus detection depends on the collection of high-quality specimens, their rapid transport to the laboratory and appropriate storage before laboratory testing. Specimens for the direct detection of viral antigens or nucleic acids and virus isolation in cell cultures should be
taken not later than 72 hours after the onset of clinical symptoms. Specimens should preferably be taken before commencement of anti-viral chemotherapy. The time between the onset of illness and specimen collection should be recorded on the data collection form. Although informed consent from the patient is not considered necessary for routine surveillance, a verbal explanation of the reason for specimen collection as well as how the specimen will be used should be given to each patient. All procedures are further outlined in the National laboratory guidelines for influenza.
Type of Specimens to Collect
The following specimens from the upper respiratory tract (URT) may be collected for the detection of influenza and other respiratory viruses:
Nasal swab Throat swab Nasopharyngeal (NP) swab Nasopharyngeal aspirates or washes Nasal wash
Nasopharyngeal swabs, aspirates and washes are the best specimens for virus isolation and PCR. However, these specimens can be technically difficult to obtain and unpleasant for the patient. An acceptable alternative is to collect a nasal and a throat swab and then combine them in a single vial of virus transport medium (VTM). The nasal swab will allow detection of seasonal influenza viruses while the throat swab allows detection of both seasonal influenza viruses and, potentially, avian influenza virus A(H5N1). It is therefore recommended to take the following specimens from each SARI or ILI case selected for testing:
These swabs should be placed into a vial containing VTM.
Before taking any specimens, mark all specimen tubes with the patient unique identifier, the specimen date, time of sampling and the type of specimen in the tube (e.g. nasal swab, throat swab etc.). Packaging
Specimens should be packed in three layers of packaging that complies with P650 packaging requirements for infectious substances in UN 3373 category B, to protect them from damage during transport and to protect the safety of personnel responsible for transport and for receiving/unpacking the specimens. Specimens should be transported to the laboratory together with the data collection form for each specimen and processed as soon as possible. Additionally, an itemized list of contents should be enclosed together with a copy of the data collection form (which also serves as a laboratory request form) between the secondary packaging and the outer packaging. If the
specimen is not being collected as part of the surveillance system then an abbreviated version of the data collection form may be used as a laboratory request form. However the minimum data elements that should be included on a laboratory request form include:
A specimen tracking number that should link the request form to the actual specimens Demographic information (i.e. the patients name, address, date of birth, sex). Date of symptom onset Date of specimen collection Type of specimen Clinical information such as use of antiviral agents Any pertinent exposure history
Specimens that may be delayed should be refrigerated prior to transportation to the laboratory or frozen. Packaging must include ice packs or dry ice, depending on whether the specimens are to be transported refrigerated or frozen. Specimens should not be transported at room temperature. Storage and Transport Successful recovery of viruses from clinical specimens depends on the quality of material received for inoculation onto cells or eggs. Many viruses are susceptible to drying, adverse pH and varying osmotic potential. For this reason samples should be placed in virus transport medium (VTM) immediately after they have been collected and stored at 40C at the sampling site. Specimens for virus isolation should be refrigerated immediately after collection and inoculated into susceptible cell cultures as soon as possible. If specimens cannot be processed within 48–72 hours, they should be kept frozen at or below –70 °C. Ideally all respiratory swabs should be transported to the laboratory in VTM within 24 to 48 hours of collection. However if this is not possible then they should be stored in a -700C freezer or in liquid nitrogen and thawed prior to processing. Each specimen may be divided into aliquots for additional testing, re-testing, or archiving prior to freezing at -70ºC for long-term storage. It is essential that the number of freeze-thaw cycles be minimized as freezing and thawing can ruin the specimen. Do not store specimens in standard household freezer (-20 °C) with a freeze-thaw ("defrost") cycle; it is better to keep a sample on ice even for as long as a week, than to allow the sample to freeze and thaw repeatedly. Blood may be stored at room temperature overnight or incubated at 560C for 30 minutes to allow the blood to clot. The serum should be removed to a new tube by mechanical pipette in a biosafety cabinet and either stored at 4°C for up to one week or immediately put into long-term storage at -20°C. This may be done at the hospital. Specimen processing All specimens suspected of containing an infectious agent should be handled and processed in a laboratory that has a minimum of BSL2. Laboratory procedures that may give rise to infectious aerosols must be conducted in a class II microbiological safety cabinet. For manipulations involving seasonal influenza, disposable
gloves and gown should be worn at all times. For specimens suspected of containing avian influenza, or other pathogens causing severe respiratory disease like SARS, specimen handling and processing should only occur in a BSL2 laboratory when BSL3 practices are strictly adhered to. Otherwise, specimen handling and processing should occur in a BSL3 laboratory. Laboratory Testing Specimens from routine surveillance Test samples from routine surveillance for currently circulating influenza A/H1, A/H3 and B viruses by RT PCR. Any specimen with a positive result for influenza A virus but negative for seasonal influenza H1 and H3 should have the test repeated. If the repeat test also shows A unsubtypable, in the absence of indications of human infections with novel influenza viruses, send to WHO CC. If novel influenza viruses have been detected in humans, test for the novel virus according to the case definition (WHO). Once the virus type and subtype has been determined, virus isolation by cell culture can be performed, providing results in 2-10 days. Upon isolation, identify the virus by RT-PCR. To ensure that the isolation of influenza viruses occurs under appropriate biosafety conditions, virus isolation should be performed according to the following principles:
At BSL2 level the laboratory may safely undertake the isolation of influenza viruses
only from clinical specimens in which the presence of seasonal influenza A(H1N1), influenza A(H3N2) or influenza B has been confirmed by RT-PCR; clinical specimens that are negative for influenza A and B by RT-PCR may be tested for the presence of other common respiratory viruses like RSV and Adenovirus by a rapid test or inoculation onto cells to isolate the virus.
Only in conditions conforming to BSL3, will the laboratory attempt to isolate influenza
viruses from specimens in which:
o influenza A is confirmed but the subtype is not identified o influenza A is confirmed, the subtype is identified but is not human seasonal
A(H1N1) or human seasonal A(H3N2), e.g. swine A(H1N1), avian A(H5N1)
All results should be entered into EIDSS as soon as each result is available. DNAs generated by using subtype-specific primers can be further analysed by molecular genetic techniques such as sequencing. Testing Algorithm for Cases of SARI and ILI According to the above principles, clinical specimens collected from SARI patients should be tested for the presence of influenza A(H1N1), A(H3N2) and influenza B by RT-PCR. Typing and subtyping by RT-PCR may be done in a single step or in two consecutive steps (i.e. determine the presence of influenza A or B before performing subtyping). Virus may be isolated from all specimens positive for seasonal influenza or from a selection thereof: this will depend on the number of specimens taken, number of positive specimens, prevalence of
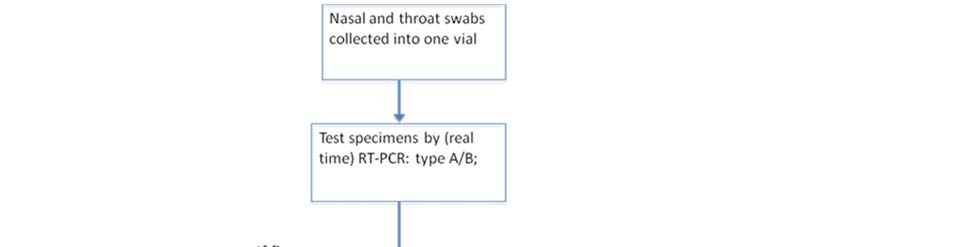
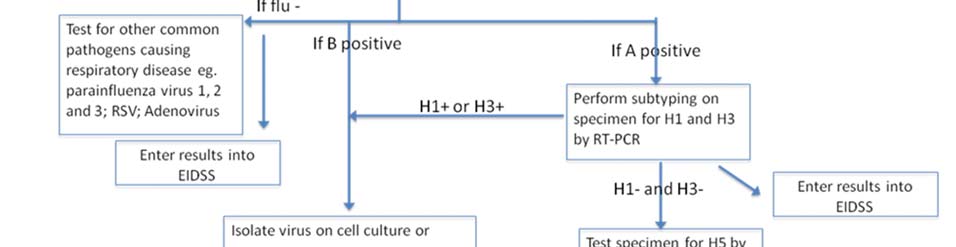

different viruses, unusual events such as the emergence of antiviral resistant virus strains etc. Chicken embryo culture in eggs is the traditional gold standard for virus isolation and should be performed on at least a sample of specimens to provide material for antigenic determination and potential vaccine production.
Figure 2.: Testing Algorithm for Specimens Collected from Cases of SARI and ILI:
Shipment of specimens and viruses to a WHO Collaborating Center NIC should ship clinical specimens and/or viruses to a WHO CC: WHO Collaborating Centre for Reference and Research on Influenza
National Institute for Medical Research
Mill Hill
London NW7 1AA
United Kingdom
Fax: +44 208 906 44 77
Email: [email protected]
http://www.nimr.mrc.ac.uk/wic/ An export permit or similar document stating that the laboratory has permission from the government to ship infectious substances is required. In order not to delay shipments, it is recommended that the permit be valid for a certain period of time rather than permission being required for each shipment. For transport by air, the Technical Instructions for the Safe Transport of Dangerous Goods by Air published by the International Civil Aviation Organization (ICAO) is the legally binding international regulations. The International Air Transport Association (IATA) publishes
Dangerous Goods Regulations (DGR) that incorporates the ICAO provisions and may add further restrictions. All detailed description of how to package and ship specimens and viruses are further outlined in the National laboratory guidelines for influenza. To ensure safe shipment of specimens and viruses to a WHO CC, laboratory, shipment should be performed by a trained person according to current international regulations. Specimens should be shipped frozen. Isolated influenza viruses can be shipped on ice packs providing:
these are fresh isolates that have not been frozen the vials containing the isolates are insulated (e.g. by a thick layer of paper to prevent
freezing to the ice packs)
delivery will be within 48 hours or refrigeration at 4C along the way is ensured
Viruses that have been frozen should be shipped frozen on dry ice to avoid multiple freezing and thawing. Viruses should be accompanied by the data collection form or information described above and by an itemized list of contents enclosed between the secondary packaging and the outer (third layer) packaging Specimens and Viruses that should be Shipped to a WHO CC: 1. Forward representative seasonal influenza viruses for virus strain characterization and
vaccine strain selection: representative seasonal influenza viruses, A(H3N2), A(H1N1) and B, isolated from
cases of SARI and ILI, should be sent at the beginning of the influenza season, during the peak of the epidemic, and towards the end of the season
2. Dispatches immediately by means of the WHO Global Shipping Project to a WHO CC:
any low-reacting viruses as determined using the WHO reagents kit provided through
all specimens containing influenza A for which the influenza subtype was not
all specimens suspected of containing avian influenza or other novel influenza viruses
Feedback from the WHO CC to the NIC will occur as soon as results are available in the case of novel influenza viruses and within one month in the case of seasonal influenza viruses. Testing algorithm for SARI cases that meet the trigger criteria The testing algorithm shown below is for suspected cases of A(H5N1). For all cases of SARI meeting the trigger criteria it is essential to isolate the causal pathogen for risk assessment purposes, antiviral susceptibility testing, vaccine development etc. In the case of influenza A subtype other than A(H1N1) and A(H3N2), virus isolation may only be performed in a laboratory conforming to BSL3. NIC should have procedures in place to ship an aliquot of each virus isolated (i.e. one virus per patient) to a WHO CC. For the diagnosis of influenza from suspected cases of A(H5N1),


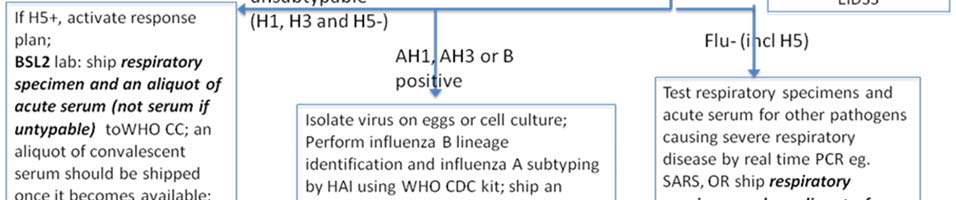

detection of influenza nucleic acid by RT-PCR should also be performed on serum specimens collected during the acute phase of infection.
Figure 3: Testing Algorithm for Specimens Collected from All Cases that Comply With One or More of the Five Trigger Criteria
Enhance virological surveillance During phase 4, the NIC will need to have the capacity to detect the first cases of a novel influenza virus with pandemic potential in Georgia and to characterize the virus in BSL3 laboratory. Laboratories will:
Increase testing. The most intense testing will be necessary during the early stages of a
pandemic, when detecting the introduction of the virus is the primary goal.
Monitor antigenic and genetic changes (BSL3 structure). In phase 4, send all positive specimens and/or strains to WHO CC for the development
of diagnostic reagents, vaccine strains and monitoring of antiviral susceptibility
Ensure additional resources Activate procedures to access and mobilize additional human and material resources
Ensure sufficient resources Activate contingency plans for responding to overload of laboratory facilities to deal with potential staff shortages. Ongoing monitoring of viral isolates Should the virus spread, perform testing to track the virus' introduction into local areas, and monitor changes in the virus, including development of antiviral resistance (BSL3). Ensure sufficient resources Activate contingency plans for full mobilization of facilities and workers at national and sub-national levels. Enhance biosafety practices in laboratory settings Influenza laboratory test and recording logic Proceed as described in phase 1-3 for laboratory practice. When the number of cases increases and becomes unmanageable: Stop testing all suspected cases, test
all/ a lot of cases in new regions/population groups that become affected all severe cases in the country in areas already affected, continue to test a portion of cases. If this becomes too much, then select severe cases with atypical symptoms (eg.
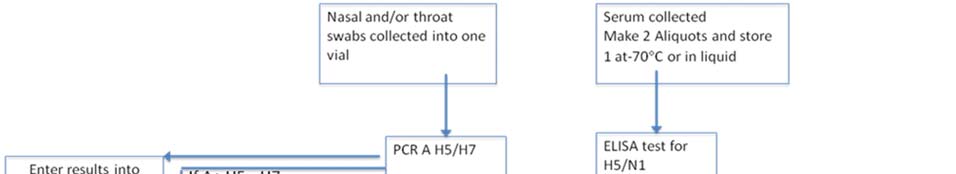
Figure 4: Testing algorithm in veterinary laboratories for specimen collected from animals
Annex 6. Interim Planning guideline for the use of non-pharmaceutical interventions to mitigate influenza pandemic
The guideline is presented in a separate document.
Annex 7. Recommendations on Vaccination against Seasonal Influenza
THE JUSTIFICATION FOR VACCINE USE Influenza vaccination is the primary and single most cost-effective method of preventing influenza and its severe complications. Antiviral agents used for chemoprophylaxis or treatment of influenza are adjuncts to vaccine, but are not substitutes for annual vaccination. Most of the widely licensed influenza vaccines are manufactured according to the quality requirements defined by the WHO. New influenza vaccines must be designed annually to match the circulating viruses which are expected to cause the next epidemic. Current influenza vaccines contain antigens from two influenza A virus strains (an H3N2 and an H1N1 strain) and one B strain, according to the annual recommendation of the WHO. This recommendation is based on intensive surveillance of new influenza strains around the globe to ensure optimal antigenic match between the virus strains in the vaccine and the viruses circulating in the subsequent influenza season. The effectiveness of influenza vaccine depends primarily on the age and immunocompetence of the vaccine recipient and the degree of similarity between the viruses in the vaccine and those in circulation. Vaccines containing strains which match the predominant circulating strains have been reported to be 70-90% efficacious for preventing (laboratory-confirmed) illness in healthy adults. Retrospective studies of people with predisposing medical conditions have found reductions of up to 50% in the rates of severe respiratory illness and death. TYPES OF INFLUENZA VACCINE Inactivated and live attenuated influenza vaccines are available and can be used to reduce the risk for influenza virus infection and its complications. Although both types vaccines are effective, they differ in several aspects. Inactivated influenza vaccine contains killed viruses, and thus can not produce signs or symptoms of influenza virus infection. In contrast, live attenuated influenza vaccine contains live, attenuated viruses, and therefore, has a potential to produce mild signs or symptoms related to influenza virus infection. Only inactivated vaccine is currently registered and available in Georgia. There are three types of inactivated influenza vaccine that show comparable efficacy but differ in terms of reactogenicity.
The whole virus vaccines often cause local reactions in children lasting for 1-2 days. Transient
systemic reactions such as fever, malaise and myalgias may occur in a minority of vaccine recipients within 6-12 hours of vaccination.
Split vaccines [vaccine formulations consisting of disrupted viral particles] and Subunit vaccines [the ones containing the HA and NA surface glycoproteins purified from other
viral components] show reduced systemic reactogenicity both in children and adults as compared to whole virus preparations. Consequently, they are more attractive, particularly for use in children.
Whole virus vaccines are being replaced by less reactogenic split virus and subunit vaccines. RECOMMENDATIONS FOR USING INACTIVATED INFLUENZA VACCINES Objective: The primary objective for the prevention of influenza is to reduce the incidence of severe illness and premature death in groups at increased risk of severe disease, and, as a consequence, to reduce the need for specialized health care services and pharmacological supplies, in particular antibiotics. The inactivated vaccine is approved for persons aged >6 months, including those with high-risk conditions. Annual influenza vaccination is recommended for the following groups:
Residents of institutions for the elderly, disabled and orphanages. All individuals >6 months of age with one or more of the following chronic conditions – chronic cardiovascular6, pulmonary, metabolic (such as diabetes mellitus or renal dysfunction), or immunodeficiency (caused by
medications or HIV)
Persons aged >50 years
Children aged 6-59 months
Women who will be pregnant during the influenza season Individuals who are receiving long-term aspirin therapy, and therefore, might be at risk of experiencing Reye syndrome after influenza virus infection
Persons who Health care workers live with or Healthy household contacts and caregivers of children aged 0-59 months care for
and persons at high risk for severe complications from influenza
persons at high-risk for influenza-related complications
Notes: General population: In addition to the groups for which annual influenza vaccination is recommended, vaccination providers should administer influenza vaccine to any person who wishes to reduce the likelihood of becoming ill with influenza or retransmitting influenza to others should they become infected Persons who provide essential community services should be considered for vaccination to minimize disruption of essential activities during influenza outbreaks. Students and other persons in institutional settings (e.g., those who reside in dormitories) should be encouraged to receive vaccine to minimize the disruption of routine activities during epidemics.
6 Hypertension is not considered a high-risk condition
Breastfeeding mothers: Inactivated influenza vaccine is safe for mothers who are breastfeeding and their infants. USE OF SEASONAL INFLUENZA VACCINES IN HUMANS AT RISK OF H5N1 INFECTION Targeted vaccination with the current seasonal influenza vaccine is now recommended as one of several measures for reducing opportunities for the simultaneous infection of humans with avian and human influenza viruses. Minimizing the opportunities for dual infections reduces the chance for viral re-assortment and for the emergence of a novel influenza virus with pandemic potential7. In addition to the above target groups, the following populations should be considered for current seasonal influenza vaccination:
1. All persons who are expected to be in contact with poultry or poultry farms potentially being
affected with highly pathogenic avian influenza (HPAI); especially cullers involved in destruction of poultry; people living and working on poultry farms where highly pathogenic avian influenza has been reported or is suspected or where culling takes place;
2. Hunters, zoo workers, vendors in live animal markets, etc. 3. Health care workers involved in the daily care of human cases of HPAI. 4. Health care workers in emergency care facilities in areas where there is confirmed occurrence of
5. Close contacts of HPAI human cases.
OTHER ASPECTS OF INACTIVATED INFLUENZA VACCINE USE:
In a refrigerator at +2-80C Usually 1 dose (consult the manufacturer's package insert). Two doses
administered at least 1 month apart are recommended for children aged 6 months – 9 years who are receiving influenza vaccine for the first time. Intramuscular. Adults and older children should be vaccinated in the deltoid
muscle; infants and young children should be vaccinated in the anterolateral aspect of the thigh. 1. Persons known to have anaphylactic hypersensitivity to eggs or to other
components of the influenza vaccine.
indications 2. Persons with moderate-to-severe acute febrile illness.
The optimal time for vaccination efforts is usually during November-December, but can often be extended into January. Providers should routinely
offer influenza vaccine throughout the influenza season, even after influenza activity has been documented in the community. People have peak antibody protection against influenza virus infection 2 weeks after vaccination.
7 Note: This vaccination does not protect against infection with bird flu. This fact must be understood by those exposed so that they are still aware of the need for general protective measures.
ROLE OF PHYSICIANS IN INCREASING VACCINATION COVERAGE Health care professionals are in a key position to spread the information regarding influenza vaccine effectiveness, cost-effectiveness and safety. The most important factor influencing the use of influenza vaccine is whether it is recommended by the doctor. Current inactivated influenza vaccines have an excellent safety record. About 300 million vaccine doses are being administered annually around the globe, and the overall rate of adverse reactions is extremely low. The most frequently occurring side effects are local reactions at the site of infection, which usually do not last more than 1-2 days. Generally, the reactions are mild and of a transient nature. When educating patients regarding potential side effects, clinicians should emphasize that inactivated influenza vaccine contains non-infectious killed viruses and can not cause influenza. Increased use of influenza vaccines is expected to significantly reduce epidemics and to improve our preparedness for potential new pandemic outbreaks. SOME ASPECTS OF DEVELOPING A HUMAN VACCINE AGAINST PANDEMIC INFLUENZA Data from initial clinical trials of a vaccine being developed to protect humans against infection with H5N1 avian influenza indicate that the experimental vaccine evoked an immune response in a small group of healthy adults. Although more trials are needed, the findings reconfirm the feasibility of developing an H5N1-specific vaccine.Pandemic vaccine production faces two major challenges: first, two doses would almost certainly be required to compensate for the lack of existing immunity within the world population and second, at least based on current trials of pandemic vaccines, much higher concentrations of antigen8 might be needed to achieve an immune response, further limiting the number of people who can be vaccinated.
Strategies for stretching limited antigen supplies – by adding an adjuvant to the vaccine formulation or injecting the vaccine into the skin rather than into muscle – have been proposed. Adjuvants are chemicals that can be added to the vaccine formulation to boost the immune response, theoretically allowing the use of smaller doses of antigen to achieve an immune response. Such antigen-sparing strategies using adjuvants are currently being tested by several manufacturers.
At present, 90% of production capacity for all influenza vaccines is concentrated in Europe and North America in countries that account for only 10% of the world's population. Current global manufacturing capacity (estimated at 300 million doses of regular trivalent influenza vaccine per year) is inadequate to meet the expected global needs during a pandemic and cannot be rapidly augmented.
Because the present total global manufacturing capacity for influenza vaccine is limited, any decision to manufacture a pandemic vaccine in large quantities prior to the start of a pandemic would, if necessity, compromise the capacity to produce vaccines for seasonal influenza. Seasonal epidemics of influenza predictably cause an estimated 250,000 to 500,000 deaths each year. In the current situation, the capacity to respond to seasonal influenza must be balanced against preparations for pandemic influenza. However, once a pandemic has been declared, all manufacturers would stop production of seasonal vaccines and produce only the pandemic vaccine.
8 Antigen is the component of the vaccine that elicits an immune response.
Even with the use of an adjuvant, however, it is important to remember that current production technologies can take up to six months to produce the seasonal vaccine supply. Therefore, it is doubtful at this time that enough H5N1 vaccine can be produced to meet global needs during the first wave of a pandemic.
Annex 8. Clinical management of Pandemic (H1N1) 2009 Virus Infection (Guideline)
The guideline is presented in a separate document
Annex 9. Novel Influenza A (H1N1, H5N1) Infection Control Guidelines for Health Care Facilities
The guidelines are presented in a separate document
Annex 10. Hospital Pandemic Preparedness Plan
The standard format and guidelines for Hospital Pandemic Preparedness are presented in a separate document. The document has an attachment of an excel tool that will help hospital managers in estimations.
Annex 11. Business Pandemic Influenza Planning Checklist
1. Plan for the impact of a pandemic on your business
Identify a pandemic coordinator and/or team with defined roles and
responsibilities for preparedness and response planning. Identify essential employees and other critical inputs (e.g. raw materials,
suppliers, sub-contractor services/products, and logistics) required to
maintain business operations by location and function during a pandemic. Train and prepare ancillary workforce (e.g. contractors, employees in other
job titles/descriptions, retirees). Develop and plan for scenarios likely to result in an increase or decrease in
demand for your products and/or services during a pandemic (e.g. effect of
restriction on mass gatherings, need for hygiene supplies). Determine potential impact of a pandemic on company business financial
using multiple possible scenarios that affect different product lines and/or
production sites. Determine potential impact of a pandemic on business-related domestic and
international travel (e.g. quarantines, border closures). Find up-to-date, reliable pandemic information from the NCDC and other
sources and establish sustainable information links. Establish an emergency communications plan and revise periodically. This
plan includes identification of key contacts (with back-ups), chain of
communications (including suppliers and customers), and processes for tracking and communicating business and employee status.
Implement an exercise/drill to test your plan, and revise periodically.
2. Plan for the impact of a pandemic on your employers and customers
Forecast and allow for employee absences during a pandemic due to factors
such as personal illness, family member illness, community containment
measures and quarantines, school and/or business closures, and public transportation closures. Implement guidelines to modify the frequency and type of face-to-face
contact (e.g. hand-shaking, seating in meetings, office layout, shared
workstations) among employees and between employees and customers according to the SES recommendations.
Encourage and track annual influenza vaccination for employees.
Evaluate employee access to and availability of healthcare and social services
during a pandemic, and improve services as needed.
Identify employees and key customers with special needs, and incorporate
the requirements of such persons into your preparedness plan. 3. Establish policies to be implemented during a pandemic
Establish policies for employee compensation and sick-leave absences unique
to a pandemic (e.g. non-punitive liberal leave), including policies on when a
previously ill person is no longer infectious and can return to work after illness. Establish policies for flexible worksite (e.g. telecommuting) and flexible
work hours (e.g. staggered shifts). Establish policies for preventing influenza spread at the worksite (e.g.
promoting respiratory hygiene/cough etiquette, and prompt exclusion of
people with influenza symptoms). Establish policies for employees who have been exposed to pandemic
influenza, are suspected to be ill, or become ill at the worksite (e.g. infection
control response, immediate mandatory sick leave). Establish policies for restricting travel to affected geographic areas (consider
both domestic and international sites), evacuating employees working in or
near an affected area when an outbreak begins, and guidance for employees returning from affected areas. Set up authorities, triggers, and procedures for activating and terminating
the company's response plan, altering business operations (e.g. shutting
down operations in affected areas), and transferring business knowledge to key employees. 4. Allocate resources to protect your employees and customers during a pandemic
Provide sufficient and accessible infection control supplies (e.g. hand-
products, tissues and receptacles for their disposal) in all business locations. Enhance communications and information technology infrastructures as
needed to support employee telecommuting and remote customer access. Ensure availability of medical consultation and advice for emergency
response. 5. Communicate and educate your employees
Develop and disseminate programs and materials covering pandemic
fundamentals (e.g. signs and symptoms of influenza, modes of transmission),
personal and family protection and response strategies (e.g. hand hygiene, coughing/sneezing etiquette, contingency plans). Anticipate employee fear and anxiety, rumors and misinformation and plan
communications accordingly. Disseminate information to employees about your pandemic preparedness an
response plan. Provide information for the at-home care of ill employees and family
members. Develop platforms (e.g. hotlines, dedicated websites) for communicating
pandemic status and actions to employees, vendors, suppliers, and customers
inside and outside the worksite in a consistent and timely way, including the emergency contact system. 6. Coordinate with other organizations and help your community
Collaborate the local health administration, major local healthcare facilities
to share your pandemic plans and understand their capabilities and plans. Collaborate with the national, regional and local public health services and
other emergency responders to participate in their planning processes, share
your pandemic plans, and understand their capabilities and plans. Communicate with local public health services about the assets and/or
services your business could contribute to the community. Share best practices with other businesses in your communities and
associations to improve community response efforts.
Annex 12. Contingency Plan for Avian Influenza _Ministry of Agriculture of Georgia
The plan is presented in a separate document
Source: http://ncdc.ge/AttachedFiles/Pandemic%20Preparedness%20Plan_(eng)-final_1_86789a39-7874-4089-aea3-255baf442797.pdf
LIVING WELL WITH DEMENTIA IN LEEDS Our local strategy First published draft, June 25th 2012 Open for comment to 30th September 2012 Our vision, values and approach The Dementia Journey - diagram The views of people with dementia, families and carers Dementia-friendly Leeds Prevention and research Early detection and diagnosis
GAIPOS-2318; No of Pages 8 Gait & Posture xxx (2006) xxx–xxx Long-term monitoring of gait in Parkinson's disease Steven T. Moore ,, Hamish G. MacDougall , Jean-Michel Gracies Helen S. Cohen William G. Ondo a Department of Neurology, Mount Sinai School of Medicine, New York, NY 10029, USA b Bobby R. Alford Department of Otolaryngology—Head and Neck Surgery, Baylor College of Medicine, Houston, TX, USA









