Rcsb-class.rutgers.edu
Vol 443 7 September 2006 doi:10.1038/nature05114
The structure of H5N1 avian influenzaneuraminidase suggests newopportunities for drug designRupert J. Russell1†, Lesley F. Haire1, David J. Stevens1, Patrick J. Collins1, Yi Pu Lin1, G. Michael Blackburn2,Alan J. Hay1, Steven J. Gamblin1 & John J. Skehel1
The worldwide spread of H5N1 avian influenza has raised concerns that this virus might acquire the ability to passreadily among humans and cause a pandemic. Two anti-influenza drugs currently being used to treat infected patientsare oseltamivir (Tamiflu) and zanamivir (Relenza), both of which target the neuraminidase enzyme of the virus. Reportsof the emergence of drug resistance make the development of new anti-influenza molecules a priority. Neuraminidasesfrom influenza type A viruses form two genetically distinct groups: group-1 contains the N1 neuraminidase of the H5N1avian virus and group-2 contains the N2 and N9 enzymes used for the structure-based design of current drugs. Here weshow by X-ray crystallography that these two groups are structurally distinct. Group-1 neuraminidases contain a cavityadjacent to their active sites that closes on ligand binding. Our analysis suggests that it may be possible to exploit thesize and location of the group-1 cavity to develop new anti-influenza drugs.
Influenza virus membranes contain two glycoproteins: haemagglu-
N9 (refs 8, 9), but the idea that the active sites of the group-1 enzymes
tinin and neuraminidase. Haemagglutinin mediates cell-surface
would be similar was supported by observations that the structures of
sialic acid receptor binding to initiate virus infection. After virus
more distantly related influenza type B neuraminidases10 are similar
replication, neuraminidase removes sialic acid from virus and
to those of the group-2 enzymes (Supplementary Fig. 1). Never-
cellular glycoproteins to facilitate virus release and the spread of
theless, different drug-resistant neuraminidase mutant viruses have
infection to new cells1. The distinct antigenic properties of different
arisen after Tamiflu treatment of humans infected with viruses
haemagglutinin and neuraminidase molecules are used to classify
containing different neuraminidase subtypes11–14. There are also
influenza type A viruses into subtypes: 16 for haemagglutinin (H1–
indications that inhibitor structure/activity relationships do not
H16) and 9 for neuraminidase (N1–N9)2. Numerous combinations
apply across subtypes15. Given these anomalies, and the current
of haemagglutinin and neuraminidase subtypes are found in avian
concerns about the spread of avian H5N1 viruses16, we have deter-
species; in humans, the three pandemics of the twentieth century
mined the crystal structures of three group-1 neuraminidases from
were caused by viruses containing H1N1 in 1918, H2N2 in 1957 and
the N1, N4 and N8 subtypes and of their complexes with the
H3N2 in 1968. The avian influenza virus that currently threatens a
inhibitors oseltamivir, zanamivir, DANA and peramivir, to compare
new pandemic is H5N1 (refs 3, 4). The N1 and N2 neuraminidases of
their active sites with those of the group-2 enzymes against which the
viruses currently circulating in humans belong to two phylogeneti-
current drugs were designed8,9.
cally distinct groups5. Group-1 contains N1, N4, N5 and N8 subtypes
The crystal structures of N1, N4 and N8 were solved by molecular
whereas group-2 contains N2, N3, N6, N7 and N9 (Fig. 1a, b).
replacement (see Methods and Supplementary Table 1).
Neuraminidase has been targeted in structure-based enzyme
inhibitor design programmes that have resulted in the production
Active site comparison
of two drugs, zanamivir (Relenza)6 and oseltamivir (Tamiflu)7, that
Superposition of the structures of N1, N4 and N8 group-1 neurami-
to some extent mimic the transition state of the normal reaction. The
nidases reveals that their active sites are virtually identical (Fig. 1c, d).
success of these developments has been attributed, in part, to
However, there are substantial conformational differences between
proposals that the catalytic sites of the enzymes are an invariant
group-1 and group-2 neuraminidases8,9,17 centred on the ‘150-loop'
feature that might be exploited for subtype-independent therapy and
(residues 147–152) and the ‘150-cavity' adjacent to the active site
to observations that they are comparatively rigid, with only minor
(Fig. 1e). The conformation of the 150-loop is such that the Ca
conformational changes in the sites on inhibitor binding. Thus, the
position of group-1-specific Val 149 is about 7 A˚ distant from the
active sites of all influenza neuraminidases contain three arginine
equivalent isoleucine residue in group-2. Moreover, the hydrophobic
residues, Arg 118, Arg 292 and Arg 371, that bind the carboxylate of
side chain at position 149 is pointed away from the active site in group-
the substrate sialic acid; Arg 152 that interacts with the acetamido
1 but towards it in group-2. At the point of closest approach of the 150-
substituent of the substrate; and Glu 276 that forms hydrogen bonds
loop to the active site, there is a difference of 1.5 A˚ in the side-chain
with the 8- and 9-hydroxyl groups of the substrate. The X-ray
position of the conserved aspartic acid residue at position 151 between
crystallographic structural information that supports these con-
group-1 and group-2 neuraminidases. A second nearby acidic residue,
clusions is only available for the group-2 neuraminidases N2 and
Glu 119, is also conserved and adopts a different conformation between
1MRC National Institute for Medical Research, The Ridgeway, Mill Hill, London NW7 1AA, UK. 2Department of Chemistry, University of Sheffield, Sheffield S3 7HF, UK.
†Present address: Centre for Biomolecular Sciences, University of St Andrews, St Andrews KY16 9ST, UK.
2006
Nature Publishing Group

NATURE Vol 443 7 September 2006
the two groups. In group-2 structures this residue forms a hydrogen
drug binding to neuraminidases from influenza B and group-2. For
bond with Arg 156 but in group-1 it adopts a conformation such that
example, in unliganded group-2 (N9) the carboxylate of Glu 276
its carboxylate points in approximately the opposite direction (Fig. 1e).
faces into the active site, but on oseltamivir binding it adopts a
However, comparison of the amino acid residues in the 150-loops
conformation pointing away from the active site so that the carbox-
offers no obvious explanation for the strong conservation of loop
ylate now makes a bidentate interaction with the guanidinium group
structure within, but not between, group-1 and group-2.
of Arg 224 (ref. 7). In the unliganded N1 (group-1) structure the
A major consequence of these differences in structure is that there
conformation of Glu 276 (Fig. 1e) is very similar to that seen in
is a large cavity adjacent to the active site in group-1 but not in group-
unliganded N9 and, as we shall describe later, it undergoes a very
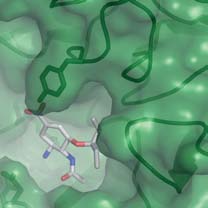
2 neuraminidases (Fig. 2). This cavity is accessible from the active site
similar rearrangement to that in N9 on oseltamivir binding.
because of the differences in position of Asp 151 and Glu 119described above. The combined effect of the difference in position
Group-1 neuraminidase binding to inhibitors
of these two acidic residues is to increase the width of the active site
We have determined the crystal structures of known anti-neurami-
cavity by about 5 A˚. The conserved Arg 156, the side chain of which is
nidase inhibitors in complex with N1, N4 and N8 (see Supplemen-
located approximately mid-way between the two acidic residues,
tary Table 1). Notably, we find that group-1 neuraminidases can bind
adopts approximately the same position in the group-1 and group-2
oseltamivir in either the ‘open' or ‘closed' conformation of the 150-
structures and defines the entrance from the active site cavity into the
loop, depending on the soaking conditions. Thus, the structure of N8
150-cavity. The extent of the 150-cavity is then determined by the
neuraminidase in complex with oseltamivir, resulting from a 30-min
difference in conformation of the 150-loop and by the position of
soak of inhibitor into preformed crystals, reveals that no large-scale
Gln 136 (Fig. 2). In group-2 proteins this residue forms a hydrogen
conformational changes have occurred (Fig. 3a) and that the 150-
bond with the main-chain carbonyl of residue 150 of the loop. In
loop retains the same conformation as in the unliganded structure.
group-1 structures, presumably as a consequence of the different
Presumably as a consequence of the conformation of the 150-loop
loop structure, Gln 136, unable to make this hydrogen bond, adopts a
the acidic residues Asp 151 and Glu 119 are located further from the
conformation that results in its side chain sitting about 3.5 A˚ lower at
nitrogen attached to C4 of the inhibitor than they are in the complex
the base of the cavity. The 150-cavity is therefore about 10 A˚ long and
with N9. Other interactions between oseltamivir and the N8 neur-
5 A˚ wide and deep (Fig. 2).
aminidase are similar to those observed in N9, with the further
The conformation of the active site residue Glu 276 has been of
exception that Tyr 347 makes a hydrogen bond interaction with the
particular interest because it undergoes a marked rearrangement on
C1 carboxylate of oseltamivir (Fig. 2) in addition to the usual
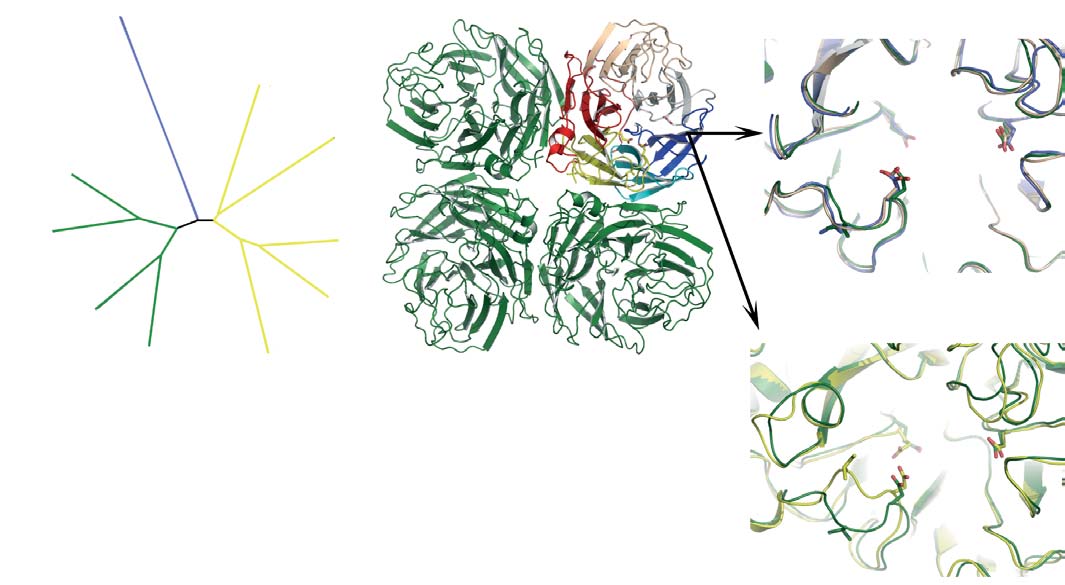
Figure 1 Genetic and structural relationships between neuraminidases from
There is a strong correlation between the extent of sequence identity and the
different influenza viruses. a, Phylogenetic tree containing representative
similarity of the crystal structures. c, Ribbons representation of the group-1
neuraminidases of influenza B and the nine subtypes of neuraminidase from
(N1) neuraminidase tetramer. One monomer is coloured to emphasize the
influenza A. Influenza A neuraminidases fall into two distinct groups, which
molecules' canonical six-bladed b-propeller structure. The active site region at
we have called group-1 and group-2 and coloured in green and yellow,
the centre of the six-bladed b-propeller structure is highlighted and then
respectively; the neuraminidase from influenza B is shown in blue.
shown on a larger scale in d and e. d, Superposition of the active sites of three
(Where appropriate, this colouring scheme is used in subsequent figures.)
neuraminidases from group-1, showing how similar they are: N1, green; N4,
b, Table showing statistics for the similarities of sequence and structures
gold; and N8, blue. e, Superposition of the active site of N1 (green) and N9
between two members of group-1 (N1 and N8) and two from group-2 (N2 and
(yellow) neuraminidases, demonstrating that N9 is markedly different to N1 in
N9) of influenza A with the neuraminidase from influenza B. The percentage
the 150-loop region. Conserved residues such as Glu 119, Asp 151 and Glu 276
sequence identities are shown on the left-hand side and root mean square
and the hydrophobic residue at position 149 are shown in stick representation.
deviations of Ca positions between monomers is given on the right-hand side.
2006 Nature Publishing Group


NATURE Vol 443 7 September 2006
bidentate interaction of that carboxylate with Arg 371. In group-2
and absence of inhibitors. There are two main consequences of this
neuraminidases, residue 347 is a glutamine, rather than a tyrosine,
change in conformation. First, Glu 119 and Asp 151 are now both
which is unable to make such a hydrogen bond.
oriented towards the bound oseltamivir, and second, the size of the
It seems likely that the binding of oseltamivir to N8, at least in the
active site cavity in drug-bound group-1 is now much the same as it is
crystalline state, is a two-step process. First, inhibitor binds to the
for group-2 neuraminidases.
‘open' form of N8 and then a slow conformational change occurs that
We have also determined the structures of three other neurami-
results in the ‘closed' form of the enzyme that is able to make a tighter
nidase inhibitors, DANA6,18,19, zanamivir20 and peramivir21, bound to
interaction with ligand. At this stage we have no information about
group-1 neuraminidases (Supplementary Fig. 3). These structures
how the slow conformational change that occurs in the crystalline
show that the drug-bound complexes of group-1 are very similar to
state relates to the enzyme in solution, but our structural obser-
those seen for group-2 neuraminidases.
vations show that this type of inhibitor is capable of binding to the
Overall, the observation of the open conformation for the 150-
open conformation of group-1 neuraminidases.
loop in the group-1 structures suggests that, for these enzymes, this
Incubating N1 crystals in 20 mM oseltamivir for 150 min also
conformation of the loop is intrinsically lower in energy than the
showed binding of inhibitor with the 150-loop in the open cavity
closed conformation. Group-1 neuraminidases (N1 and N8) initially
conformation (Supplementary Fig. 2), but when N8 crystals were
bind to oseltamivir in this open conformation but eventually adopt
incubated in oseltamivir for 3 days (Fig. 3a, b), or N1 crystals were
the closed conformation. It thus seems that oseltamivir binding to
incubated in a higher concentration of inhibitor (Fig. 3b), the 150-
group-1 neuraminidases favours the higher energy or closed confor-
loop changes its conformation so that it closely resembles the
mation of the 150-loop that it probably accesses via a relatively slow
conformation observed in group-2 neuraminidases in the presence
conformational change. It should therefore be possible to design newinhibitors for group-1 neuraminidases that are selective for the open150-loop conformation and would thereby have the potential to bindmore strongly than oseltamivir or zanamivir.
Examination of the structures suggests, for example, that it may be
possible for a new substituent to be developed from the 4-aminogroup of oseltamivir into the 150-cavity and thereby enhance thebinding of potential inhibitors. The prominent guanidinium sidechain of conserved Arg 156, at the base of the 150-cavity (Fig. 2), is
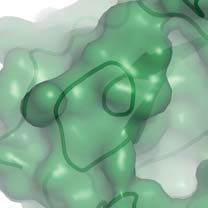
Figure 2 Molecular surfaces of group-1 and group-2 neuraminidases with
Figure 3 Oseltamivir binding to the active sites of group-1
bound oseltamivir showing the 150-cavity in the group-1 structure that
neuraminidases. a, Superposition of the active sites of N8 after a 30-min
arises because of the distinct conformation of the 150-loop. a, b, N1
soak (dark blue) and a 3-day soak (cyan) with 20 mM oseltamivir. There are
(a; green) and N9 (b; yellow) shown in surface representation with the
small changes in the position of Glu 119 and the inhibitor when the 150-loop
protein main chain shown in ‘worm' representation. c, Superposition of the
closes after the longer soaking time. b, Superposition of the active sites of N8
active sites of apo-N1 (green) and N1 complexed with oseltamivir (blue).
with bound oseltamivir after the 3-day soak with 20 mM inhibitor (cyan)
Part of the electron density map from a low-resolution (5.5 A
with N1 soaked for 30 min in 0.5 mM inhibitor (green). In this case, the
Fourier calculated between apo-N1 and oseltamivir-bound N1 data sets is
structures of the two different subtypes of neuraminidase from group-1 are
shown in blue to indicate the position of the 150-cavity.
remarkably similar.
2006 Nature Publishing Group
NATURE Vol 443 7 September 2006
clearly a prospective partner for a salt-bridge or hydrogen bond with
the effects of the mutant Tyr 274 on the orientation of Glu 276. The
a new inhibitor.
importance of the conformation of Glu 276 for oseltamivir bindingby group-2 neuraminidases has been firmly established25. There
Differential oseltamivir resistance of mutant neuraminidases
seems to be at least two factors contributing to the inability of
Three principal oseltamivir-resistant mutant neuraminidases have
group-1 neuraminidases to accommodate the His274Tyr substi-
been characterized from influenza A viruses isolated after Tamiflu
tution (Fig. 4). First, the 270-loop in group-1 neuraminidases
treatment of influenza-infected humans14,22. One, derived from
approaching residue 273 makes a tighter turn than the equivalent
H1N1 and H5N1 infections, contained the amino acid substitution
loop in group-2. Second, in group-1 neuraminidases, but not in
His274Tyr. The other two were from patients infected with H3N2
group-2, there is a conserved tyrosine residue at position 252 that
viruses and contained either Arg292Lys or, less frequently, Glu119Val
makes hydrogen bonds to the main-chain carbonyl at position 273,
substitutions. Although oriented differently in unliganded group-1
to the peptide amide at 250 and to the histidine side chain at 274.
compared with group-2 neuraminidases, Glu 119 of both groups
Histidine 274 also forms a hydrogen bond through its other side-
appears to interact similarly with oseltamivir. Comparison of the
chain nitrogen with Glu 276. It appears that introduction of the
structures of group-1 and group-2 neuraminidases reveals group-
bulkier tyrosine residue at position 274 in group-1 (N1) enzymes can
specific differences in the active sites that might explain how the
only be accommodated by the new side chain moving towards, and
mutations at positions 274 and 292 lead to inhibitor resistance.
partially displacing, Glu 276. By contrast, in group-2 enzymes, there
The mutation His274Tyr leads to high resistance of group-1
is a smaller residue at position 252, leaving space for Tyr 274 to
neuraminidases against oseltamivir but has little effect on group-2
occupy without perturbing Glu 276. This interpretation of the
neuraminidases23,24. Inspection of the structures of the group-1
group-specific effect of this mutation is consistent with observations
neuraminidases in complex with oseltamivir, and comparison with
from mutagenic studies that examined the effects of side-chain size at
equivalent group-2 complexes, suggests a reason for this group-
residue 274 on sensitivity to oseltamivir23.
specific difference and indicates how resistance may be mediated by
The mutation Arg292Lys is the commonest substitution in group-
2 (N2) neuraminidases resistant to oseltamivir22. It has already beenthe subject of a detailed crystallographic analysis to show that in thegroup-2 neuraminidase N9, resistance results, in part, from the lossof a hydrogen bond from Arg 292 to the carboxylate group ofoseltamivir25. The substituted Lys 292 also interacts with Glu 276,impeding its movement to accommodate the hydrophobic substi-tuent attached to C6 of oseltamivir. The structures of group-1neuraminidases, and their complexes with oseltamivir, now reveala likely reason for the smaller effect of the mutation on group-1enzymes. As Fig. 4 shows, the conserved tyrosine residue at position347 in the group-1 neuraminidase N1 makes an additional hydrogenbond to the carboxylate group of the inhibitor that cannot be madeby the equivalent residues in group-2 neuraminidases. In this way itseems that the additional hydrogen bond interaction between Tyr 347and the carboxylate of the inhibitor compensates for a weaker, water-mediated interaction between the carboxylate and the substitutedlysine residue at position 292 (ref. 25).
ConclusionAs a proven anti-influenza drug target, neuraminidase continues tobe attractive for the development of new virus inhibitors, not leastbecause of the emergence of viruses resistant to the currentlyavailable drugs14,26–28. The crystal structures of group-1 neuramini-dases described here will add to this attraction. They show that the150-loop, which forms one corner of the enzyme active site, is able toexist in at least two stable conformations. The fact that group-1neuraminidases bind drugs like oseltamivir with similar affinity togroup-2 enzymes22 suggests that the difference in energy between thetwo conformations is not very large. The notion of a degree ofplasticity in the structure of the active site of neuraminidase, or atleast of the group-1 enzymes, is unexpected, but considering theirsimilarities in sequence it would not now be surprising if the 150-loop of group-2 neuraminidases was found to also possess a degree offlexibility. Evidently the closed conformation is energetically pre-ferred in group-2 neuraminidases, both in the absence and presenceof current inhibitors, but a higher energy open conformation may
Figure 4 Locations of the oseltamivir resistance mutations found in
well be accessible to an inhibitor that could make an energetically
group-1 and group-2 neuraminidases. Middle panel: superposition of the
advantageous interaction with it.
active sites of group-1 (green) and group-2 (yellow) neuraminidases with
On the basis of our structural observations, new drugs against
bound oseltamivir; two regions of the active site are highlighted and detailed
group-1 neuraminidases could be obtained by adding extra substi-
in the top and bottom panels. Top panel: residues involved in the group-2-
tuent moieties to existing inhibitor skeletons. Although it may be
specific resistance mutant Arg292Lys also showing that residue 347 is a
considered ideal to focus on compounds that work against all virus
tyrosine residue in the group-1 protein but an asparagine in group-2.
subtypes, an effective group-specific inhibitor could be of consider-
Bottom panel: residues involved in the group-1-specific resistance mutantHis274Tyr showing that the tyrosine at position 252 is involved in a network
able value against the currently circulating human H1N1 viruses, the
of hydrogen bonds in group-1.
H3N8 viruses that repeatedly cause influenza in equines and are now
2006 Nature Publishing Group
NATURE Vol 443 7 September 2006
causing widespread disease in canines29, and the avian H5N1 viruses
12. Gubareva, L. V., Kaiser, L., Matrosovich, M. N., Soo-Hoo, Y. & Hayden, F. G.
that currently threaten the human population. In any case, it may
Selection of influenza virus mutants in experimentally infected volunteerstreated with oseltamivir. J. Infect. Dis. 183, 523–-531 (2001).
well be possible that new inhibitors designed to exploit additional
13. Carr, J. et al. Influenza virus carrying neuraminidase with reduced sensitivity to
interactions with the open form of the 150-loop of group-1 neur-
oseltamivir carboxylate has altered properties in vitro and is compromised for
aminidases could select a similar conformation of this loop in group-
infectivity and replicative ability in vivo. Antiviral Res. 54, 79–-88 (2002).
2 neuraminidases.
14. Ward, P., Small, I., Smith, J., Suter, P. & Dutkowski, R. Oseltamivir (Tamiflu)
and its potential for use in the event of an influenza pandemic. J. Antimicrob.
Chemother. 55 (suppl. 1), i5–-i21 (2005).
15. Brouillette, W. J. et al. Pyrrolidinobenzoic acid inhibitors of influenza virus
N1 neuraminidase (NA) was prepared from a WSN-NA (H5N1) recombinant
neuraminidase: modifications of essential pyrrolidinone ring substituents.
virus containing seven genes from WSN and the neuraminidase gene from A/
Bioorg. Med. Chem. 11, 2739–-2749 (2003).
Vietnam/1203/04. N4 and N8 neuraminidases from A/mink/Sweden/E12665/84
16. Webster, R. G., Peiris, M., Chen, H. & Guan, Y. H5N1 outbreaks and enzootic
(H10N4) and A/duck/Ukraine/1/63 (H3N8) were prepared from viruses grown
influenza. Emerg. Infect. Dis. 12, 3–-8 (2006).
in hens' eggs. Neuraminidase was released from the viruses by bromelain
17. Colman, P. M., Varghese, J. N. & Laver, W. G. Structure of the catalytic and
digestion, and further purified, as previously described30. N1 crystals were
antigenic sites in influenza virus neuraminidase. Nature 303, 41–-44 (1983).
18. Bossart-Whitaker, P. et al. Three-dimensional structure of influenza A N9
grown by vapour diffusion in sitting drops dispensed by an Oryx-6 crystal-
neuraminidase and its complex with the inhibitor 2-deoxy 2,3-dehydro-N-
lization robot from Douglas Instruments. A total of 0.1 ml of protein solution, at
acetyl neuraminic acid. J. Mol. Biol. 232, 1069–-1083 (1993).
9 mg ml21 in 10 mM Tris-HCl, pH 8.0, was mixed with an equal volume of
19. Burmeister, W. P., Henrissat, B., Bosso, C., Cusack, S. & Ruigrok, R. W.
reservoir solution (0.1 M MES, pH 6.0, 0.2 M ammonium acetate, 20% w/v PEG
Influenza B virus neuraminidase can synthesize its own inhibitor. Structure 1,
3350). N4 crystals were grown by vapour diffusion in hanging drops consisting
19–-26 (1993).
of 2 ml of reservoir solution (0.1 M HEPES, pH 7.5, 5 mM cobalt chloride, 5 mM
20. Meindl, P., Bodo, G., Palese, P., Schulman, J. & Tuppy, H. Inhibition of
nickel chloride, 5 mM cadmium chloride, 5 mM magnesium chloride and 12%
neuraminidase activity by derivatives of 2-deoxy-2,3-dehydro-N-
w/v PEG 3350) and 2 ml of concentrated protein solution (10 mg ml21 in 10 mM
acetylneuraminic acid. Virology 58, 457–-463 (1974).
Tris-HCl, pH 8.0). N8 neuraminidase crystals were grown by vapour diffusion in
21. Babu, Y. S. et al. BCX-1812 (RWJ-270201): discovery of a novel, highly potent,
hanging drops consisting of 2 ml of reservoir solution (0.1 M imidazole, pH 8.0
orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J. Med. Chem. 43, 3482–-3486 (2000).
and 35% MPD) and 2 ml of concentrated protein solution (10 mg ml21 in 10 mM
22. Mishin, V. P., Hayden, F. G. & Gubareva, L. V. Susceptibilities of antiviral-
Tris-HCl, pH 8.0). Crystals of N4 and N8 were soaked for 30 min in inhibitor
resistant influenza viruses to novel neuraminidase inhibitors. Antimicrob. Agents
made up in crystallization buffer (augmented with 20% glycerol, in the case of
Chemother. 49, 4515–-4520 (2005).
N4, for cryo-protection) at concentrations of 20 mM for oseltamivir, zanamivir
23. Wang, M. Z., Tai, C. Y. & Mendel, D. B. Mechanism by which mutations at
and peramivir and at 20 mM for DANA. Additionally, a crystal of N8 neur-
His 274 alter sensitivity of influenza A virus N1 neuraminidase to oseltamivir
aminidase was soaked in 20 mM oseltamivir for 3 days. N1 was cryo-protected by
carboxylate and zanamivir. Antimicrob. Agents Chemother. 46, 3809–-3816
the addition of 15% v/v ethylene glycol and the oseltamivir soaks were carried
out using 20 mM or 0.5 mM drug made up in this cryo-protectant.
24. Yen, H. L. et al. Neuraminidase inhibitor-resistant influenza viruses may differ
N4 and N8 data were collected at 100 K on an in-house Rigaku-MSC RU200
substantially in fitness and transmissibility. Antimicrob. Agents Chemother. 49,4075–-4084 (2005).
rotating anode coupled to a RaxisIIc detector. N1 data sets were recorded on a
25. Varghese, J. N. et al. Drug design against a shifting target: a structural basis for
Raxis4 detector (100-mm scan) mounted on a Rigaku MicroMax 007 HF
resistance to inhibitors in a variant of influenza virus neuraminidase. Structure
generator. Diffraction data were integrated using Denzo and scaled with
6, 735–-746 (1998).
Scalepack31. N1, N4 and N8 neuraminidase structures were solved by molecular
26. Le, Q. M. et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature 437,
replacement using variously AmoRe (N1) and Phaser32 (N4, N8) with N9
1108 (2005).
neuraminidase as the initial search model. Standard refinement was carried out
27. Kiso, M. et al. Resistant influenza A viruses in children treated with oseltamivir:
with a combination of refmac532 and CNS33 together with manual model building
descriptive study. Lancet 364, 759–-765 (2004).
with O34. Figures were created with Pymol (http://pymol.sourceforge.net/).
28. de Jong, M. D. et al. Oseltamivir resistance during treatment of influenza A
(H5N1) infection. N. Engl. J. Med. 353, 2667–-2672 (2005).
Received 6 June; accepted 25 July 2006.
29. Crawford, P. C. et al. Transmission of equine influenza virus to dogs. Science
Published online 16 August 2006.
310, 482–-485 (2005).
30. Ha, Y., Stevens, D. J., Skehel, J. J. & Wiley, D. C. X-ray structures of H5 avian
Murphy, B. R. & Webster, R. G. in Fields Virology 3rd edn (eds Fields, D. B. N.,
and H9 swine influenza virus hemagglutinins bound to avian and human
Knipe, M. & Howley, P. M.) 1397–-1445 (Lippincott-Raven, Philadelphia, 1996).
receptor analogs. Proc. Natl Acad. Sci. USA 98, 11181–-11186 (2001).
World Health Organization. A revision of the system of nomenclature for
31. Otwinowski, Z. & Minor, W. in Data Collection and Processing (eds Sawyer, L.,
influenza viruses: a WHO memorandum. Bull. World Health Organ. 58, 585–-591
Isaacs, N. & Bailey, S.) 556–-562 (SERC Daresbury Laboratory, Warrington,
Bender, C. et al. Characterization of the surface proteins of influenza A (H5N1)
32. CCP4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr D
viruses isolated from humans in 1997–-1998. Virology 254, 115–-123 (1999).
50, 760–-763 (1994).
World Health Organization Global Influenza Program Surveillance Network.
33. Brunger, A. T. et al. Crystallography & NMR system: a new software suite for
Evolution of H5N1 avian influenza viruses in Asia. Emerg. Infect. Dis. 11,
macromolecular structure determination. Acta Crystallogr. D 54, 905–-921
Thompson, J. D., Higgins, D. G. & Gibson, T. J. Improved sensitivity of profile
34. Jones, T. A., Zhou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for
searches through the use of sequence weights and gap excision. Comput. Appl.
building protein models in electron density maps and the location of errors in
Biosci. 10, 19–-29 (1994).
these models. Acta Crystallogr. A 47, 110–-119 (1991).
von Itzstein, M. et al. Rational design of potent sialidase-based inhibitors ofinfluenza virus replication. Nature 363, 418–-423 (1993).
Supplementary Information is linked to the online version of the paper at
Kim, C. U. et al. Influenza neuraminidase inhibitors possessing a novel
hydrophobic interaction in the enzyme active site: design, synthesis, andstructural analysis of carbocyclic sialic acid analogues with potent anti-
Acknowledgements This work was supported by the MRC (UK) and by an
influenza activity. J. Am. Chem. Soc. 119, 681–-690 (1997).
International Partnership Research Award in Veterinary Epidemiology from the
Varghese, J. N., Laver, W. G. & Colman, P. M. Structure of the influenza virus
Wellcome Trust. R.J.R. acknowledges the Wellcome Trust and the University of
glycoprotein antigen neuraminidase at 2.9 A
˚ resolution. Nature 303, 35–-40
St Andrews for support and Biocryst for supply of peramivir. We thank P. Walker
for assistance with data collection and preparation of the manuscript, and
Baker, A. T., Varghese, J. N., Laver, W. G., Air, G. M. & Colman, P. M. Three-
Rigaku (Europe) and CRUK (Lincoln's Inn) for access to data collection facilities.
dimensional structure of neuraminidase of subtype N9 from an avian influenzavirus. Proteins 2, 111–-117 (1987).
Author Information Coordinates have been deposited with the Protein Data
10. Burmeister, W. P., Ruigrok, R. W. & Cusack, S. The 2.2 A
˚ resolution crystal
Bank and the relevant accession codes (2HTY, 2HU0, 2HU4, 2HT5, 2HTR,
structure of influenza B neuraminidase and its complex with sialic acid. EMBO
2HT7, 2HT8, 2HTQ, 2HTU, 2HTV and 2HTW) are described in Supplementary
J. 11, 49–-56 (1992).
Table 1. Reprints and permissions information is available at www.nature.com/
Ives, J. et al. Anti-viral drug resistance: an oseltamivir treatment-selected
reprints. The authors declare no competing financial interests. Correspondence
influenza A/N2 virus with a R292K mutation in the neuraminidase gene has
and requests for materials should be addressed to J.J.S.
reduced infectivity in vivo. J. Clin. Virol. 18, 251–-269 (2000).
2006 Nature Publishing Group
Source: http://rcsb-class.rutgers.edu/Summer2009/files/H5N1-nature2006.pdf
Cannabis in Multiple Sclerosis: Women's Health Concerns SUMMARY. Women's health has received greater attention with therecognition of significant differences in disease expression and drug ac-tion in men and women. Multiple sclerosis is a neurological disorderwith important gender differences. MS patients have employed cannabisto treat a number of symptoms associated with the disease includingspasticity, pain, tremor, fatigue, and autonomic dysfunction. The scien-tific literature includes supportive case reports, single-patient (N-of-1)trials and randomized clinical trials. Large-scale clinical trials are under-way to answer questions concerning the efficacy and safety of cannabisin patients with MS. While these studies will answer important questionsconcerning the actions of cannabinoids on the nervous system, addi-tional studies in female MS patients will be needed to address issues suchas gender-specific actions on symptoms such as pain and autonomic dys-function along with studies in menopausal and post-menopausal women.Since the drug-drug interactions have been reported with cannabinoids,the effects of cannabis on the actions of other centrally-acting drugsshould be explored. [Article copies available for a fee from The HaworthDocument Delivery Service: 1-800-HAWORTH. E-mail address: <[email protected]> Website: 2002 byThe Haworth Press, Inc. All rights reserved.]
Patient Quality of Life Questionnaire (baseline) PLEASE DO NOT WRITE ON THIS QUESTIONNAIRE. IT IS FOR INFORMATION ONLY. ALL ANSWERS WILL BE RECORDED BY THE RESEARCH PROFESSIONAL The CONSTRUCT study Biobank Suite (rm 244), Grove Building, School of Medicine, Swansea University Singleton Park, Swansea SA2 8PP Phone: +44(0)1792 513426









