Sbri.fr
The Journal of Neuroscience, December 16, 2009 • 29(50):15675–15683 •
15675
Frontal Feedback-Related Potentials in Nonhuman Primates:
Modulation during Learning and under Haloperidol
Julien Vezoli1,2
and Emmanuel Procyk1,2
1Inserm, U846, Stem Cell and Brain Research Institute, 69500 Bron, France, and 2Universite´ de Lyon, Lyon 1, UMR-S 846, 69003 Lyon, France
Feedback monitoring and adaptation of performance involve a medial reward system including medial frontal cortical areas, the medial
striatum, and the dopaminergic system. A considerable amount of data has been obtained on frontal surface feedback-related potentials
(FRPs) in humans and on the correlate of outcome monitoring with single unit activity in monkeys. However, work is needed to bridge
knowledge obtained in the two species. The present work describes FRPs in monkeys, using chronic recordings, during a trial and error
task. We show that frontal FRPs are differentially sensitive to successes and failures and can be observed over long-term periods. In
addition, using the dopamine antagonist haloperidol we observe a selective effect on FRP amplitude that is absent for pure sensory-
related potentials. These results describe frontal dopaminergic-dependent FRPs in monkeys and corroborate a human-monkey homol-
ogy for performance monitoring signals.
using inexpensive noninvasive functional evaluations of adap-
Performance monitoring, i.e., the continuous checking of goal
tive cognitive processes.
achievement (Ullsperger, 2006), lies at the heart of adaptation by
The ERN and fERN might be used to indicate the integrity of
inducing the regulation of cognitive control, emotional re-
a whole system rather than to directly measure local processing of
sponses, and motivational adjustments. Experiments in humans
errors (Ullsperger, 2006). The neural origin of ERN and fERN
have revealed a neural signature of performance monitoring: a
indeed does encompass several brain structures devoted to rein-
frontal medial evoked potential [error-related negativity (ERN)]
forcement learning and cognitive control. The anterior cingulate
observed during incorrect motor performance (Falkenstein et al.,
cortex (ACC), the striatum, the orbitofrontal cortex, the lateral
1991; Gehring et al., 1993). A frontal negative signal has also been
prefrontal cortex, the supplementary eye field, and the aminergic
observed in relation to external feedback of performance [feed-
systems are directly or indirectly involved. The ACC is of partic-
back error-related negativity (fERN)]. The two signals, ERN and
ular interest since its activation increases in concert with the pro-
fERN, might reflect the same underlying mechanism (for re-
duction of the ERN and because source reconstructions often
view see Holroyd and Coles, 2002). These signals have since
point to the ACC (Dehaene et al., 1994; Debener et al., 2005). An
been correlated with subsequent behavioral adaptation sug-
influential hypothesis posits that the error-related negativity is
gesting a role in adjusting performance and in reinforcement
generated from ACC activity changes when the consequences of
learning (Gehring et al., 1993; Debener et al., 2005; Frank et al.,
an action are worse than expected (Holroyd and Coles, 2002).
2005; Cohen et al., 2007; Taylor et al., 2007). The brain potential
Holroyd and Coles referred to Schultz's (2000) work in monkeys,
is sensitive to pharmacological challenges in particular when
showing that dopaminergic neurons increase and decrease their
aminergic transmission is concerned. Haloperidol (a dopaminer-
activity for respectively positive and negative reward prediction
gic antagonist) reduces the amplitude of ERN although it induces
errors. They proposed that through the direct mesocortical do-
mixed or even no behavioral effects (Zirnheld et al., 2004; de
paminergic pathway, a dopamine-mediated negative reward pre-
Bruijn et al., 2006). This supports the idea of functional rela-
diction error signal disinhibits ACC neurons, which thereby
tionships between dopamine, prediction error signals, and the
produce the cortical error signal. A recent extension posits that
ERN and fERN (Holroyd and Coles, 2002). Importantly, the
conversely a positive prediction error should inhibit ACC feedback-
negativity appears abnormal in a wide range of neurological
related activity and thus reduce surface feedback-related potentials
and psychiatric disorders. This, in itself, is of considerable
(Holroyd, 2004; Holroyd et al., 2008).
interest and opens the door to preclinical or clinical studies
Before the discovery of a human ERN, Brooks (1986) had
observed a local field potential evoked by incorrect motor perfor-mance recorded in the vicinity of monkey's ACC (Brooks, 1986).
Later, local recordings in the banks of anterior cingulate sulcus
Received Oct. 5, 2009; accepted Oct. 25, 2009.
revealed increased activity after errors, reduced rewards, and the
de la Recherche Grant ANR JCJC-0048 (E.P.), and Fondation pour la Recherche Me´dicale (J.V.). We are very grateful to
absence of expected rewards (Ito et al., 2003; Amiez et al., 2005;
H. Kennedy and K. Knoblauch for help with this manuscript and with data analyses.
Emeric et al., 2008; Quilodran et al., 2008). Although error-
Correspondence should be addressed to Emmanuel Procyk, Inserm U846, Stem Cell and Brain Research Institute,
related activity has been reported in other areas (e.g., supplemen-
18 avenue du Doyen Jean Le´pine, 69500 Bron, France. E-mail:
[email protected].
tary eye field, lateral prefrontal cortex, and orbitofrontal cortex),
Copyright 2009 Society for Neuroscience 0270-6474/09/2915675-09$15.00/0
the incidence of outcome-related activity is particularly high in
15676 • J. Neurosci., December 16, 2009 • 29(50):15675–15683
Vezoli and Procyk • Chronic Recordings of Feedback-Related Potentials
the ACC. Yet, the homology between hu-man and monkey ACC regarding perfor-mance monitoring has been questionedbecause of recurring contradictions be-tween data obtained in monkeys and hu-mans (Botvinick et al., 2004).
In this context, we used chronic frontal
transcranial recordings in behaving mon-keys to establish three issues: First, we showa frontal medial surface potential related toperformance feedback (FRP) and modu-lated during cognitive tasks; Second, themonkey feedback-related potential is sensi-tive to dopaminergic transmission; Third,we confirm with this model that long-termFRP follow-up can be performed for longi-tudinal investigations.
Materials and Methods
Housing, surgical, electrophysiological, and his-
tological procedures were performed according
to the European Community Council Directive
(1986) (Ministe re de l'Agriculture et de la Foreˆt,
Commission nationale de l'expe´rimentation ani-
male) and Direction De´partementale des Services
Ve´te´rinaires (Lyon, France).
Subjects. Two 14-year-old rhesus monkeys
(Macaca mulatta; monkey S and R) served assubjects in this study, one male and one femaleweighting 8 kg and 7 kg, respectively. Duringsessions, the animal was seated in a primatechair (Crist Instrument) within arm's reach ofa tangent touch-screen coupled to a TV moni-tor (Microtouch System). In the front panelof the chair, an arm-projection window wasopened, allowing the monkey to touch thescreen with one hand. A computer recordedthe position and accuracy of each touch. It alsocontrolled the presentation via the monitor ofvisual stimuli (color shapes), which served aslight-targets (CORTEX software, National In-
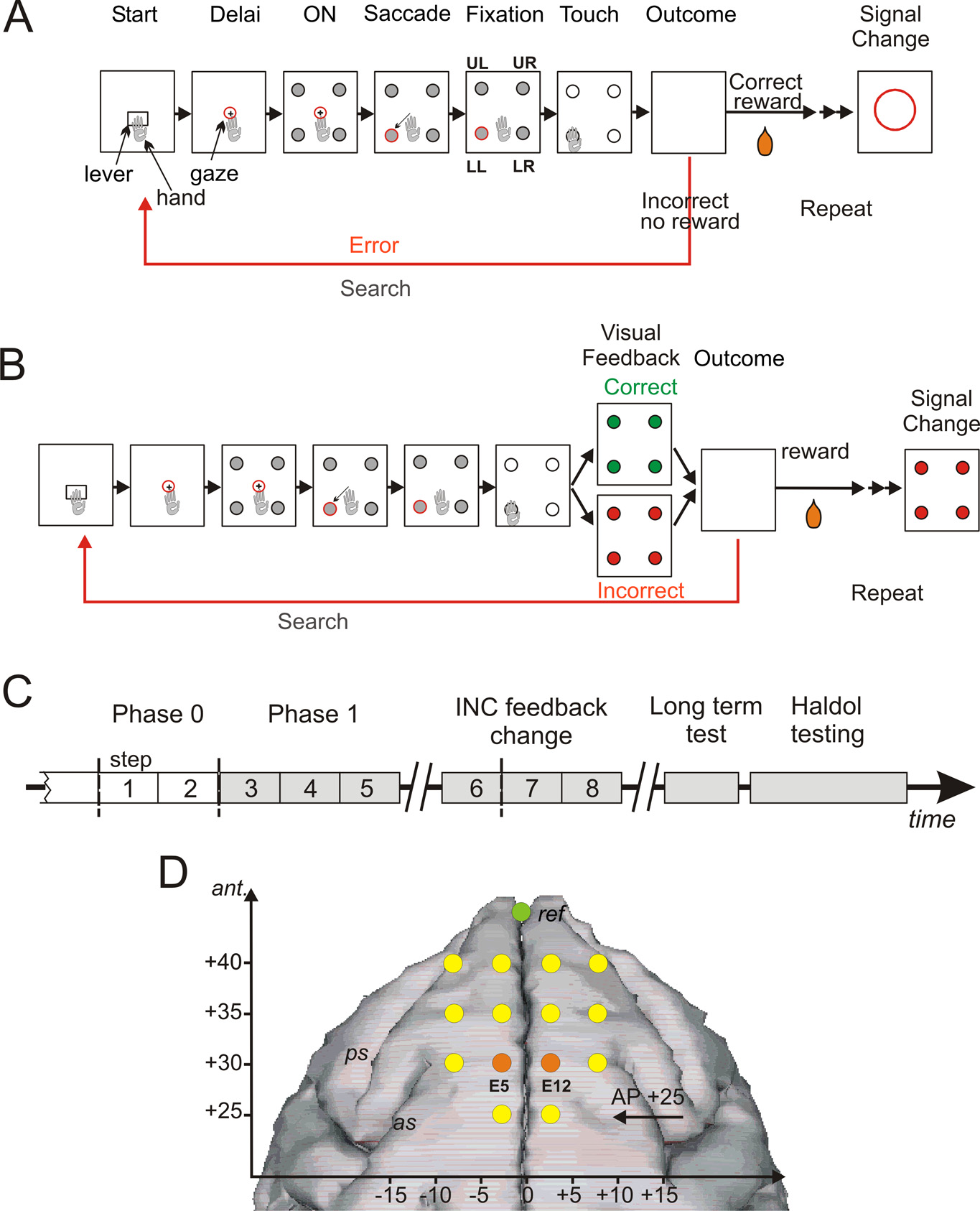
Figure 1. PST, phases of the protocol, and electrodes position. A, PST during phase 0. The task consisted in searching by trial and
stitute of Mental Health Laboratory of Neuro-
error the correct target and then in repeating the discovered correct response. Rectangles symbolize the successive steps in a trial
psychology, Bethesda, MD). Eye movements
and in a problem. Eye fixation and touches were controlled. See Materials and Methods for timing details. During phase zero at the
were monitored using an Iscan infrared system
touch on one target, all target items switched off and the animal either received a reward when correct or no reward when
(Iscan). Four target items (disks of 5 mm in
incorrect. After the outcome, another trial was initiated to proceed the search period or to enter the repetition period after the first
diameter) were used: upper left (UL), upper
correct choice. At the end of repetition, a visual signal (red circle) flashed on the screen to indicate the initiation of a new problem.
right (UR), lower right (LR), and lower left
B, PST in phase I. A visual feedback is given after a choice and during 500 ms before the actual outcome (reward or no reward). The
(LL) (Fig. 1A). A central white square served as
signal to change was visually equivalent to the negative feedback. C, Experimental schedule indicating the succession of the
fixation point (FP). The lever was disposed just
different phases and periods of testing in time. D, Locations of transcranial electrodes reported on a stereotaxic space and drawn
below the FP. Reward (fruit juice) was deliv-
over a standard reconstructed brain surface fitted to a three-dimensional image (BrainMaps B3D) from http://brainmaps.org.
ered via a reward-delivery-system (Crist In-
The reference is shown in green. The two electrodes used for il ustration and analyses are shown in orange.
strument). All neurophysiological recordingswere performed with an Alpha-Omega mul-
choice was incorrect (no reward, negative feedback), the monkey
tichannel system (AlphaLab, AlphaOmega). Analyses of neurophysi-
could select another target in the following trial and so on until the
ological signals were made using NeuroExplorer, Elan-Pack (Inserm
solution was discovered (end of search period). The animal was then
U821, Lyon, France), and MatLab homemade scripts.
allowed to repeat for at least 3 trials the correct choice (3 trials in 90%
Behavioral task. Monkeys were trained in a problem solving task (PST)
of cases; 7 or 11 trials in 10% of cases). Each block of trials (or
(Procyk and Goldman-Rakic, 2006); they had to find by trial and error
problem) thus contained a search period and a repetition period. A
which target, presented in a set of four, was rewarded (Fig. 1 A, B). Each
visual signal [signal to change (SC)] at the end of the repetition period
trial started by the onset of a starting target named "lever." The animal
indicated the beginning of a new problem. The new correct target was
had to initiate trials by touching the lever and maintaining his touch. A
selected such that it was different from the previous one in !90% of
FP appeared and the animal had to fixate it. A delay period (2 s) followed,
cases. Any break in fixation requirements resulted in trial cessation
and ended by the simultaneous onset of the four gray targets and offset of
(break fixation error).
FP. At the FP offset, the animal is required to make a saccade toward one
Experimental schedule. One important aspect of the experiment was to
target, fixated it (0.5 s), and then touched it following a GO signal (all
verify the existence of FRP and their sensitivity to various behavioral
targets turned white). The time delay from target fixation to GO signal
parameters. In the present paper we focus on data from two phases (0 and
was fixed thus leading to anticipatory patterns of reaction times. If the
I) for behavior and for phase I for event-related potentials. The time line
Vezoli and Procyk • Chronic Recordings of Feedback-Related Potentials
J. Neurosci., December 16, 2009 • 29(50):15675–15683 • 15677
of the protocol is presented in Figure 1C. In the initial phase (phase 0) of
lates D1 receptors inducing cognitive dysfunctions (Lidow and Goldman-
testing all targets turned gray at the touch, and after a 0.4 s delay all targets
Rakic, 1994; Castner et al., 2000; Silvestri et al., 2000). In our protocol, a
switched off and the outcome was given. A reward (fruit juice) was de-
minimum washout period of 10 d was observed between two consecutive
livered for choosing the correct target (positive feedback). If the choice
drug challenges. Behavioral performances and EEG activities were com-
was incorrect no reward was given (negative feedback). In the subsequent
pared to sessions preceding drug treatment. To evaluate the effect of
phase of testing, visual feedbacks were used to dissociate in time visual
time, data acquired 30 min or 3 h after injection were analyzed separately.
performance feedbacks from liquid rewards, and to test the effect of
Testing was performed 11 months after the beginning of Phase I for both
presenting new feedbacks on feedback-related potentials. In phase I, 400
ms after the touch, targets turned red (negative feedback) or green (pos-
Behavioral data analysis. Reaction times (RT) of arm movements from
itive feedback) for incorrect and correct responses, respectively. This
lever to target were computed on each trial. Latencies of saccades from FP
visual feedback was displayed for 500 ms and followed (when correct) by
to peripheral targets were measured using automatic detection of devia-
a reward delivery. The SC was changed to be identical to the negative
tion of the horizontal component of saccades using a Matlab homemade
feedback (Fig. 1 B). In the days following phase I, we tested (1) the effect
script. Other parameters were controlled daily to evaluate performance
of changing visual feedbacks: yellow stars replaced red disks for negative
as follows: n1 and n2, mean number of trials to find the correct target and
feedback and the SC was changed accordingly (only data for negative
to complete the repetition period, respectively; M (Motivation), number
feedback change are reported), (2) the reliable presence of feedback dis-
of trials initiated by the animal (that is during which the monkey at least
crimination after 7 months (long-term test), and (3) the effect of acute
touched the lever) over the total number of trials presented, considered
systemic Haldol injections (Fig. 1C).
as reflecting the motivational state of the animal; P (Perseverance), num-
Phases are composed of sessions (one session corresponding to one
ber of times an incorrect choice is immediately repeated in the search
day of recording). However, for the purpose of event-related potential
period, which we named perseverance (expressed in average number per
(ERP) analyses phases were subdivided in steps, each step being com-
problem solved); Shift, the average number of shifts away from the cor-
posed of several sessions averaged to obtain an equivalent total number
rect response per repetition period.
of trials and with at least 50 events by trial type (see below). Phase 0 was
Parameters n1 and n2 were used during initial training sessions and
composed of the 11 sessions preceding the insertion of visual feedback of
compared to optimal values to increase task difficulty (number of tar-
performance and that met the requirements for number of trials. These
gets). Optimal performances were calculated for an ideal situation where
11 sessions were grouped in 2 successive steps (steps 1 and 2). Phase I was
errors in search are not repeated and the correct response is repeated
subdivided in 3 steps (steps 3 to 5). Data from one step before (step 6) and
without errors (Procyk and Goldman-Rakic, 2006).
two steps after (steps 7 to 8) negative feedback change were also studied.
Trial types were identified according to their position in the search and
Phase I covered 3 months of recordings for both monkeys. One week
repetition periods. Only incorrect trials (INC) in search periods are con-
separated the last session of phase I from the first session of step 6 for both
sidered. Correct trials were grouped as correct trials from the search
monkeys. Tests over steps 6 – 8 covered 3 months of recordings for both
(CO1) and from the repetition (COR, i.e., second, third and fourth cor-
monkeys. The first session of phase I was separated from the first session
rect trials).
of long-term test by 7 months in both monkeys.
Electrophysiological recordings. Two weeks after surgery, electrophysi-
Surgical procedures. Surgical procedures were performed under aseptic
ological recordings were initiated. All electrodes were referenced to the
conditions. Animals were implanted with a head-holder and intracranial
most frontal electrode (Fig. 1 D). The signal from each electrode was
electrodes. Following premedication with atropine (1.25 mg, i.m.) and
amplified and filtered (1–250 Hz), and digitized at 0.8 kHz. ERPs were
dexamethasone (4 mg, i.m.), chlorpromazine (Largactil 1 mg/kg, i.m),
analyzed off-line (NeuroExplorer software, and Matlab home-made
anesthesia was induced with ketamine hydrochloride (20 mg/kg, i.m.).
scripts). We analyzed ERP peak latencies and amplitudes for target onset
Anesthesia was maintained with halothane in N2O/O2 (70/30). Heart
and FRPs. ERPs were averaged for each session and the mean amplitude
rate was monitored and artificial respiration adjusted to maintain the
of the 200 ms period before the onset of a particular event was subtracted
end-tidal CO2 at 4.5–6%. A bar was attached to the skull with small
from the averaged ERPs for baseline correction. Sessions with #10 prob-
stainless steel screws and then embedded in an acrylic assembly to permit
lems solved were excluded from analysis, and averaged ERPs embodied at
subsequent head fixation. Using stereotaxic guidance, 15 stainless steel
least 260 events by trial type and by step. For the description of FRPs'
surgical screws (Synthes) were fixed in the skull and connected to a
components we based our measures on grand average waveforms cover-
standard female D25-pins connector. The ensemble was then anchored
ing the entire phase I during which no change of feedback occurred. Peak
with dental acrylic to the head-holder. The screws served as transcranial
amplitudes and latencies were measured by detecting maximum or min-
electrodes that were expected to touch the dura. The 14 electrodes im-
imum average amplitude within selected time windows. Windows were
planted 5 mm apart from each other covered a surface area of 175 mm 2
defined based on the observation of overall ERP shapes (see Results).
over the anterior midline (Fig. 1 D). One electrode serving as reference
To detect the latency of the difference in average ERPs between nega-
was screwed on the midline anterior to the set of the 14 active electrodes.
tive and positive feedbacks, we performed an ANOVA [time bins $
The most posterior electrodes were placed at anterior level "25. To keep the
feedback (INC, COR)] using a helmert contrast on time bins. ERPs
connector free of debris a male connector was placed and fixed into the
measures were computed on successive 20 ms time bins. The helmert
implanted connector at return of the animal to the home cage. After surgery,
contrast contrasts the second level with the first, the third with the aver-
monkeys were kept under observation; to prevent pain, morphine was ad-
age of the first two, and so on, and thus enables a detection of the first
ministered after the anesthesia began to wear off; antibiotics were given
time bin showing a significant difference between two conditions.
before surgery and lasted for 6 d.
Peaks of difference-waves were also analyzed; this measure, commonly
Drug testing. We used an antagonist of essentially D2–D4 dopamine
used in human studies to isolate components of interest was applied to
receptors: haloperidol (Haldol, Janssen-Cilag, 5 mg/ml for injection).
contrast ERP for negative and positive feedbacks. In addition this mea-
Drug doses used in testing sessions were defined based on tests for side-
sure allowed us to address the important issues of varying degrees of
effect. Doses were reduced until no global behavioral effects [drowsiness
difference between negative and positive feedback-related potentials
and extra-pyramidal effects: akathisia, dystonia, akinesia, tremor, tardive
(Holroyd et al., 2008). As described in the result section our analyses
dyskinesia, due to the action of the drug on the extra-pyramidal system
focused on the 0.15– 0.25 s window. The average of two electrodes pre-
(Coffin et al., 1989)] could be observed in the home cage. We tested doses
senting the greatest amplitude at first positive peak on INC, CO1, COR,
of 0.02, 0.01, and 0.005 mg/kg (i.m.) and selected 0.01 mg/kg for the final
and on INC-COR difference maps were used for FRPs analyses (Elec-
recording sessions.
trodes E5 and E12) (Fig. 1 D). For haloperidol testing, we compared ERP
The effect of haloperidol was tested in 2 sessions for monkey S and 4
and difference waves obtained during haloperidol sessions to those re-
sessions in monkey R. We tested haloperidol in a minimal number of
corded during earlier control sessions. Differences were tested by calcu-
sessions to avoid effects of repeated challenges. Indeed, chronic exposure
lating prediction intervals from control sessions, and by performing
to haloperidol upregulates D2-dopaminergic receptors and downregu-
permutation tests on control and test sessions ( p values were estimated
15678 • J. Neurosci., December 16, 2009 • 29(50):15675–15683
Vezoli and Procyk • Chronic Recordings of Feedback-Related Potentials
from 10,000 permutations. See supplementalnotes for details, available at www.jneurosci.
org as supplemental material). MatLab, Rv2.5.0 (R Foundation for Statistical Comput-ing), Statistica (StatSoft), and R (R Foundationfor Statistical computing v2.5.0) were used foranalyses and graphics. Alpha level rates were setat 0.05 for all analyses.
Reconstructions of surface maps of poten-
tials were performed with the software packagefor electrophysiological analysis (ELAN-Pack)developed at the Inserm U821 laboratory (pre-viously U280; Lyon, France; http://u821.lyon.
inserm.fr/). Each electrode was given sphericalcoordinates on a unit sphere. To visualize po-tentials distribution, values were interpolatedwith spherical spline functions (Perrin et al.,1987, 1989). To visually compare maps be-tween subjects, data were normalized with theaverage reference as for human high-densityrecordings (Handy, 2005).
Results
During phase 0 of the protocol trial out-
comes were only indicated by the presence
or absence of reward delivery. In phase I
we introduced visual cues—i.e., perfor-
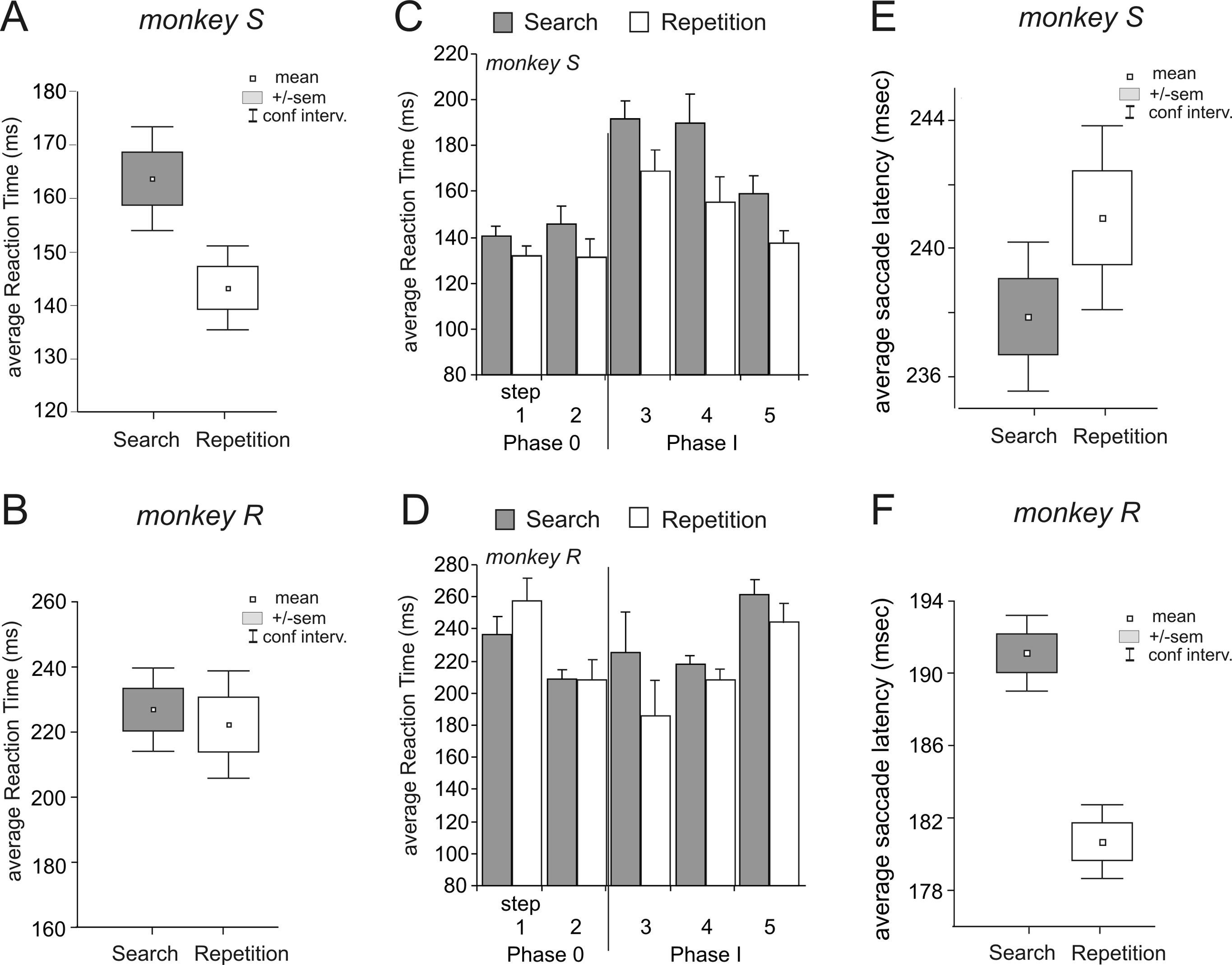
Figure 2. RTs and saccade latencies in search and repetition periods. A, B, Average RTs in search (INC and CO1 trials) and
mance feedbacks—signaling an impend-
repetition (COR trials) for monkeys S (A) and R (B). C, D, RTs in search and repetition during the successive steps and phases
ing reward or no reward.
for the respective monkeys. E, F, Average saccade latencies in search (INC and CO1 trials) and repetition (COR trials) for
monkeys S (E) and R (F ).
difference remained along sessions for monkey S while it was
Performances in the problem-solving task were near optimal and
stably expressed only in phase I sessions for monkey R, suggesting
stable for both monkeys during phase 0 (supplemental Fig.
changes in skill or strategy.
S1A–D, supplemental notes, available at www.jneurosci.org as
As for RTs, saccade latencies were different between search
supplemental material). We checked for stability with a linear fit
and repetition for both monkeys although not with the same
on measures for each parameter over the 11 sessions preceding
pattern (paired t test over all sessions; monkey R, t % 11.39,
phase I. Only parameter n2 (number of trials in repetition)
df % 23, p # 0.0001; monkey S, t % &3.41, df % 29, p # 0.005)
showed a significant reduction for monkey R. In fact, the history
(Fig. 2 E, F ).
of training for animal R was different in that phase 0 was 1 monthafter the first practice of the final task with control on eye move-
Inserting positive and negative visual feedbacks
ments (monkeys were first trained without eye control). For
The insertion of visual feedbacks induced slight changes in per-
monkey S, the data for phase 0 were taken 5 months after the first
formance (supplemental Fig. S1, supplemental notes, available at
practice with control on eye movements.
www.jneurosci.org as supplemental material). It is interesting to
Before addressing the specific effect of inserting visual feed-
note that at this stage monkeys could still rely on the delivery of
backs we tested the overall effect of steps on performance. Among
reward at the extinction of visual feedbacks. The visual feedbacks
behavioral parameters only Shift in repetition for both monkeys
were just giving anticipatory information on the impending oc-
[one-way ANOVA, factor "step" (steps 1–5), at p # 0.05; Shift:
currence of reward delivery.
F(4,25) % 2.95, p % 0.041; F(4,19) % 4.56, p % 0.0095 for monkey S
At the insertion of visual feedbacks RTs for search trials were
and R, respectively] and Motivation for monkey S changed with
increased in the two monkeys (Student's t test between steps 2
steps (M: F(4,25) % 4.66, p % 0.006).
and 3 with individual trials: t % &12.74, df % 2004, p # 0.0001 for
Data for reaction times (RTs) and saccade latencies were ex-
monkey S and t % &2.11, df % 1892, p % 0.035 for monkey R).
tracted for each session (day of recording). RTs were longer in
Both monkeys also showed significant changes in RTs in repeti-
search than in repetition periods on average over all sessions for
tion after inserting feedback (Student's t test between steps 2 and
monkey S (paired t test over phases 0 –I sessions; t % 8.18, df %
3: t % &12.34, df % 2651, p # 0.0001 for monkey S and t % 4.07,
29, p # 0.0001) (Fig. 2A) and only over phase I sessions for
df % 2748, p # 0.0001 for monkey R) (Fig. 2C,D). In addition,
monkey R (paired t test over phase I sessions; t % 3.61, df % 23,
data for monkey S revealed a global reduction of RTs across phase
p % 0.0041) (Fig. 2B,D). These interperiod differences are in ac-
I steps of the protocol that might correspond to a general learning
cordance with previous observations on changes (increase or de-
process initiated by feedback insertion (One-way ANOVA, factor
crease depending on individuals) between search and repetition
"step" (steps 3 to 5), at p # 0.05, search F(2,16) % 5.05, p % 0.020
periods (Procyk and Goldman-Rakic, 2006; Quilodran et al.,
and repetition F(2,16) % 4.52, p % 0.028).
2008). RTs in repetition were strongly affected by steps (one-way
Saccade latencies revealed slight and inconsistent changes af-
ANOVA, factor "step," all tests at p # 0.05, for the two animals),
ter feedback insertion, with a decrease of latencies in repetition
with changes possibly triggered by feedback insertion (see below)
for monkey R (t test between steps 2 and 3 with individual trials,
for monkey S and R (Fig. 2C,D). The search versus repetition
t % 2.94, df % 2612, p % 0.003) and an increase of latencies in
Vezoli and Procyk • Chronic Recordings of Feedback-Related Potentials
J. Neurosci., December 16, 2009 • 29(50):15675–15683 • 15679
The main potentials described in this
study will be labeled FRPs. Note that theconfiguration of our electrodes and refer-ence has no correspondence with theusual ones used in humans. Thus, themost important aspect of potentials willbe the presence of significant discrimina-tions between different feedback typesand in particular between negative andpositive feedbacks.
Shape, latencies, and valence
Signals recorded during phase I averagedfrom two electrodes were selected for il-lustration (see Materials and Methods)(Fig. 1D). These same electrodes wereused for all subsequent analyses. We iden-tified several peaks with similar latenciesfor the two monkeys (supplemental Fig.
S2A, available at www.jneurosci.org assupplemental material). Latencies at peakand peak amplitudes for the two animalsare described in supplemental Table S1,available at www.jneurosci.org as supple-mental material. Two major events are an-alyzed here: the most positive value within0.15 to 0.25 s [early feedback-related po-tential (eFRP)], and the most negativevalue within 0.25– 0.35 s [late feedback-related potential (lFRP)]. Negative feed-backs elicited a large positive deflectionpeaking !170 –220 ms (eFRP) followedby a negative deflection !300 ms (lFRP)(supplemental Fig. S2A, available at www.
jneurosci.org as supplemental material).
Note that the overall shape of potentialsis not strictly identical between the twoanimals for positive feedbacks, althoughmajor effects were reproducible. Forclarity, the grand average waveform ispresented in Figure 3A. All subsequentanalyzes were performed individuallyfor the two monkeys.
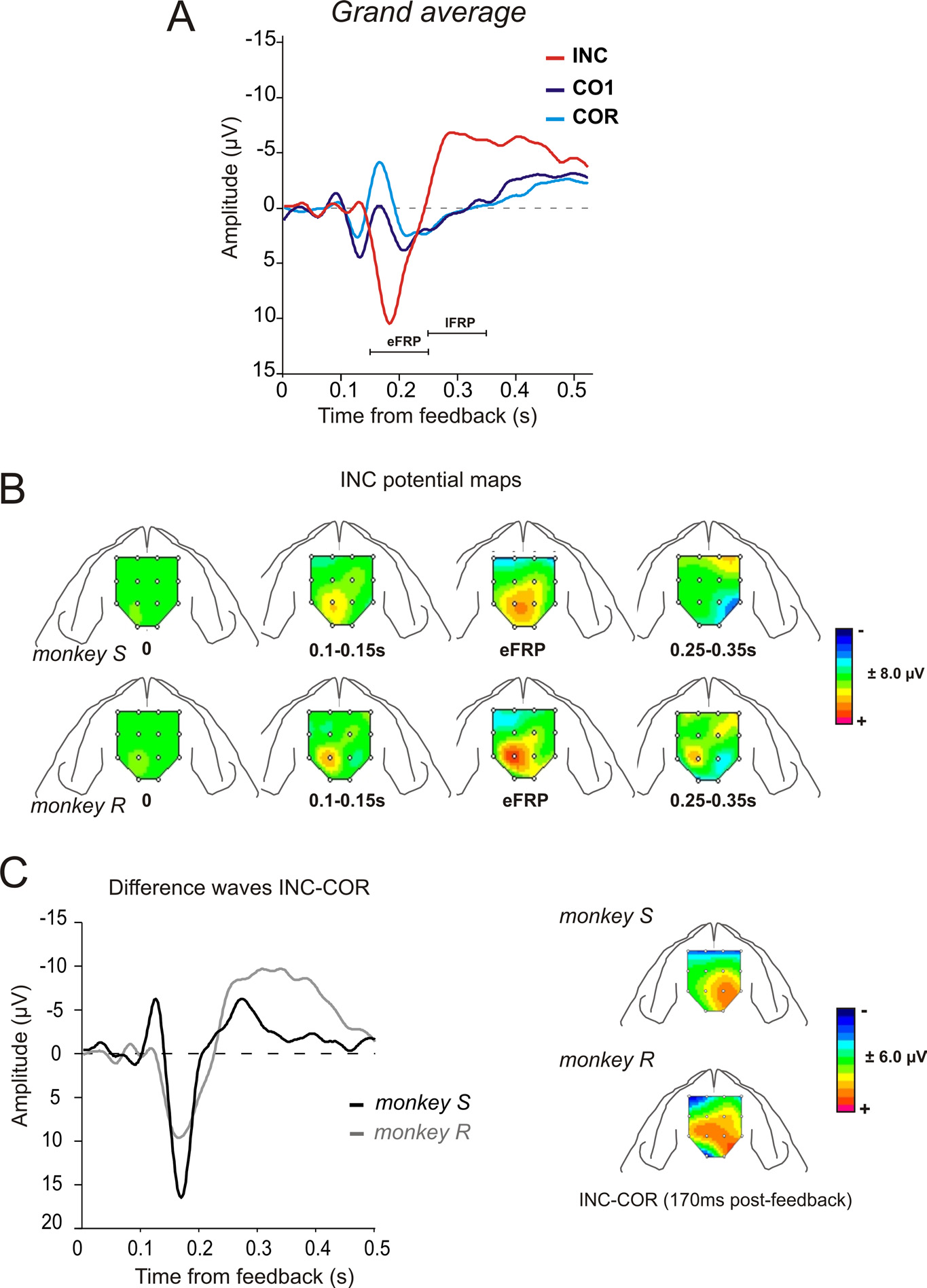
Figure 3. FRPs: surface maps and difference waves. A, Grand average FRPs for the two monkeys for INC, CO1, and COR trials over
We analyzed feedback-related poten-
Phase I. The actual outcome is given at 0.5 s after feedback onset. Peak of interest is indicated. Waveforms correspond to averages
tials by separating three types of trials—
between monkeys and electrodes E5/E12. B, Surface maps reconstructed for INC FRPs during Phase I for the two monkeys and at
INC and CO1 for search periods, and
successive time points (see Materials and Methods). Each map represents the mean values for the time window used to measure
COR. We first tested the dynamic of the
ERP components. The amplitude is represented by a color code. C, Difference waves INC-COR for the two animals for the entire
difference in average amplitudes between
Phase I. On the right are represented the corresponding surface maps reconstructed at the positive peak (170 ms). Difference waves
INC and COR waves reconstructed on
correspond to average between electrodes E5 and E12.
20 ms bins. An ANOVA design with a
search for monkey S (t test between steps 2 and 3, t % &1.9983,
built-in helmert contrast (at p # 0.05; see
df % 1896, p % 0.046).
Materials and Methods) revealed initial and stable significant dif-ference between INC and COR potentials at 100 –120 ms and
120 –140 ms for monkey S and R respectively (supplemental Fig.
Analyses of event-related signals during phase 0 revealed brain
S2B, available at www.jneurosci.org as supplemental material).
potentials that differed between positive and negative feedbacks.
The eFRP was larger for INC than COR trials. The later compo-
However, reward delivery in case of correct trials prevented a
nent was more negative in amplitude for negative compared to
pure comparison between the two trial types. We thus inserted
positive feedbacks (Fig. 3A; supplemental Table S1, available at
visual feedbacks that preceded actual outcomes by 500 ms, with-
www.jneurosci.org as supplemental material). Note that a late
out any other change in the task. When aligned on visual feedback
positive component for positive feedback was not observed on
onset, the recordings made in the subsequent phases revealed
the average waveform in one subject (supplemental Fig. S2A,
feedback-related potentials sensitive to successes and failures. We
available at www.jneurosci.org as supplemental material). How-
describe here the main characteristics of these potentials.
ever, a session per session analysis showed that this component
15680 • J. Neurosci., December 16, 2009 • 29(50):15675–15683
Vezoli and Procyk • Chronic Recordings of Feedback-Related Potentials
emerged progressively through phase I sessions. The surface
Table 1. Amplitudes and latencies at the first peak of difference waves (mean (SD))
maps reconstructed for FRPs following negative feedbacks re-
for the two monkeys
vealed comparable variations between monkeys, in time, ampli-
tude, and topography, especially for the early positive component
(eFRP) (Fig. 3B). Before subtracting the average of all electrodesfrom each channels (Fig. 3 B,C), we verified that maps computed
Peak amplitude (!V)
with the original reference presented similar spatial features.
Each map in Figure 3B represents themean values for a particular time window.
To clearly evaluate the contrast be-
tween negative and positive feedbacks wecomputed the difference waves betweenfeedback types as previously applied in theliterature (Yeung et al., 2005). The differ-ence wave INC-COR for phase I con-firmed, for the two monkeys, a maximumeffect of valence !170 ms (167 ' 13 msand 168 ' 5 ms for monkey S and R, re-spectively) (Fig. 3C, Table 1). Note thehigh similarity in peak latencies and over-
all time course between the two animals,
as well as in the topographic distribution
of the early positive peak. Maps ob-
at each step 'SEM. Statistics correspond to pairedt test (*p # 0.05) between INC-CO1 and INC-COR.
tained from the difference (at 170 ms)between negative feedbacks in search
visual attributes of the negative feedback on the potential evoked
and positive feedbacks in repetition trials (INC-COR) pre-
by the SC signaling each end of repetition periods and that had
sented similar topography for the two animals and evidenced a
the same visual properties (see Materials and Methods). Paired t
positive difference-potential more lateralized over the right
tests ( p # 0.05) applied on session measures in phase I returned
hemisphere for both subjects (Fig. 3C, right).
a significant difference between eFRPs for INC and SC; the dif-
Analyses at the peak of difference revealed for both monkeys,
ference remained significant ( p # 0.05) after negative feedback
differences between INC and CO1, but also between CO1 and
change (supplemental Fig. S3A, available at www.jneurosci.org as
COR although only marginally for monkey S (average signal on
supplemental material). If the visual attributes were the sole cause
time-windows 150 –180 ms; Student's t test, INC vs CO1: t %
of ERP changes then changing the negative feedback should have
9.07, df % 36, p # 10 – 4 for monkey S and t % 5.01, df % 26, p #
had the same effect on INC- and SC-related potentials. The over-
10 – 4 for monkey R; CO1 vs COR: t % 1.94, df % 36, p % 0.06 for
all evolution of the SC potential was opposite to the evolution for
monkey S and t % 3.40, df % 26, p % 0.0022 for monkey R). Thus,
INC: eFRP for INC was marginally reduced after change of neg-
FRPs were sensitive to negative and to positive feedbacks, but
ative feedback whereas eFRP increased for SC (supplemental Fig.
were also sensitive to the period (search vs repetition for positive
S3A, available at www.jneurosci.org as supplemental material).
This supports a selective action of feedback change on INC FRP.
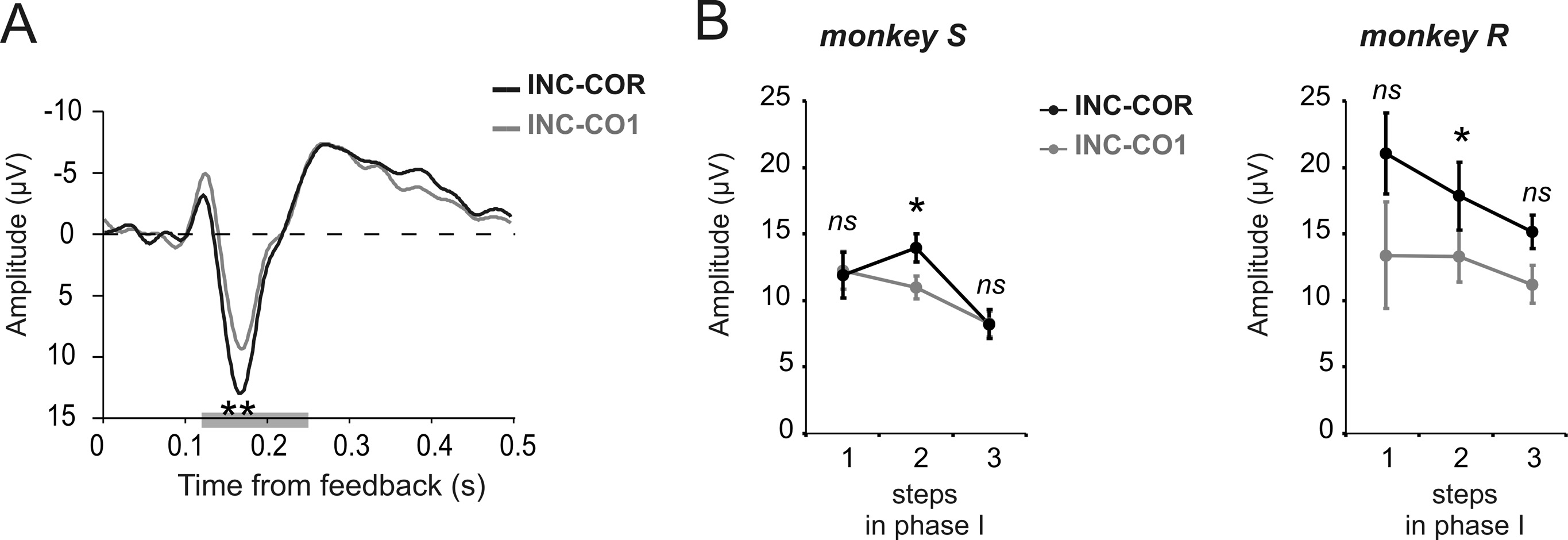
Measures over 10 sessions taken 7 months after the beginning
Effects of trial and error learning and expectations on
of phase I revealed a stable presence of the peak of difference
INC-COR (supplemental Fig. S3B, available at www.jneurosci.
The difference between INC and COR reveals one aspect of the
org as supplemental material) (peak latency: 150 ' 8 ms, ampli-
effect of valence induced by negative and positive feedbacks.
tude: 7.36 ' 2.2 !V for monkey R; peak latency: 212 ' 33 ms,
However, the reinforcement learning theory of the ERN (RL-
amplitude: 9.13 ' 2.5 !V for monkey S. Expressed as mean '
ERN) also predicts that FRPs should vary according to reward
SD). During these sessions eFRPs continued to discriminate INC
prediction error i.e., according to the level of reward expectation.
from COR trials (bin 140 –160 ms, t % 7.5538, df % 18, p # 10&6
In our case, expectation varies between CO1 and COR trials. The
for monkey R; bin 200 –220 ms, t % 6.1975, df % 18, p # 10&5 for
RL-ERN theory precisely predicts that unexpected positive feed-
backs should have a larger effect on frontal-medial potential thanexpected positive feedback when compared to negative feedback
Modulations under haloperidol
(Holroyd, 2004; Holroyd et al., 2008). Contrary to predictions,
In humans the response-locked ERN is sensitive to dopaminergic
the difference curve for INC-CO1 was marginally smaller at the 170
pharmacology although the underlying mechanisms are disputed
ms peak than INC-COR (Fig. 4A). Although the effect was not
(de Bruijn et al., 2006; Jocham and Ullsperger, 2009). We thus
significant over the 3 steps for each monkey individually, the
tested whether FRPs were changed after systemic haloperidol ad-
effect was consistent on step 2 for both monkeys (paired t test
ministration. Haloperidol was given at a fixed dose of 0.01
over sessions, monkey S: t % &4.46, p # 0.05; monkey R: t %
mg ! kg&1. Data acquired 30 min or 3 h after injections were
&2.9, p # 0.05) (Fig. 4B).
analyzed separately. We observed a time-dependent effect bothon reaction times and INC-COR difference waves, with stronger
Long-term follow-up of feedback-related potentials
Chronic recordings give the opportunity to observe long-term
alterations after 3 h.
changes in brain signals and modifications reflecting learning
mechanisms. To test the contribution of the visual properties of
Haloperidol injections had only weak effects on the trial and error
feedback stimuli per se, we evaluated the effect of changing the
strategy, at least as evaluated by our analyses. Both monkeys per-
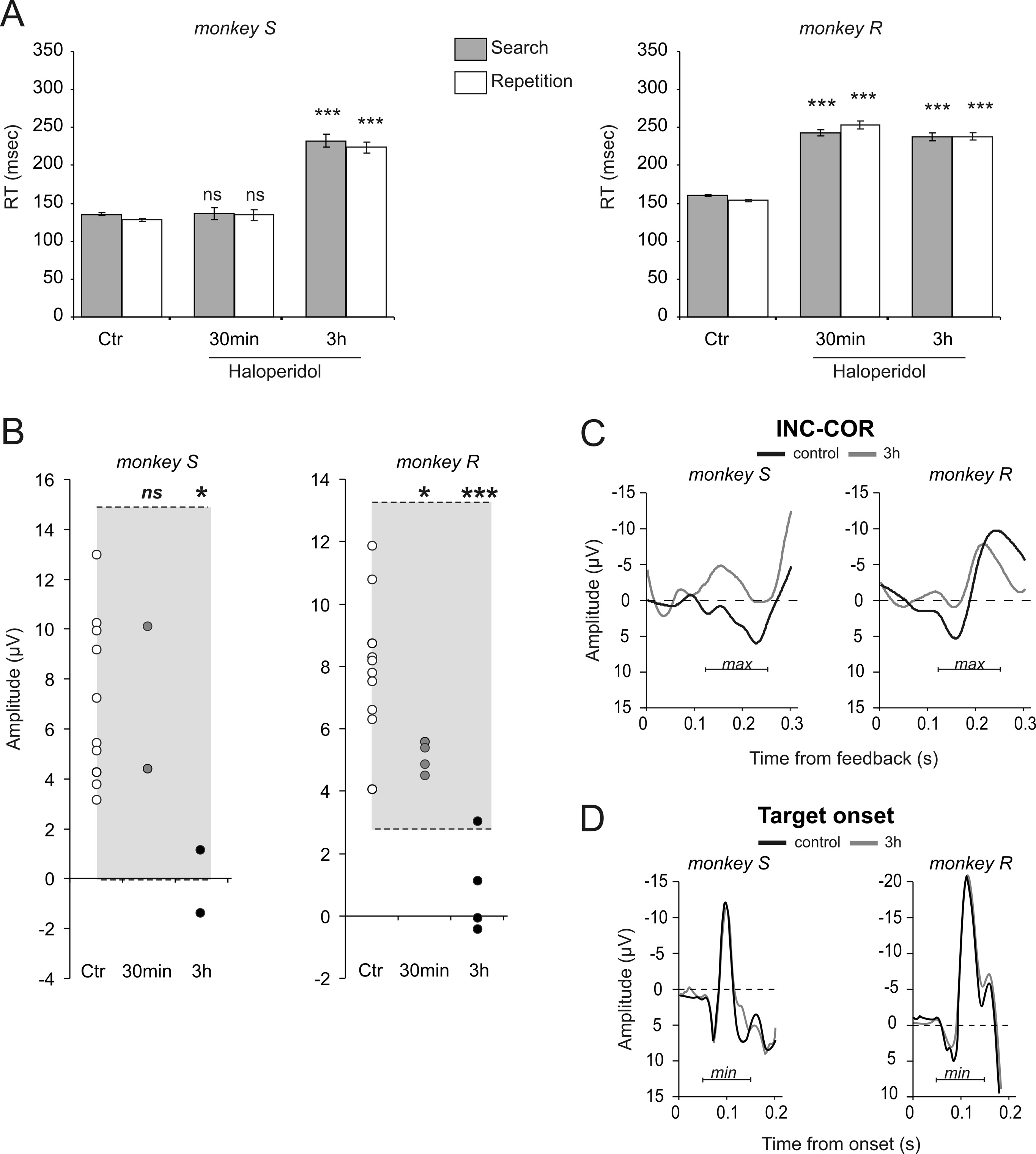
Vezoli and Procyk • Chronic Recordings of Feedback-Related Potentials
J. Neurosci., December 16, 2009 • 29(50):15675–15683 • 15681
evaluate the significance of decreases andconfirmed the effect at 3 h for both mon-keys (see statistics in Fig. 5B). A controlanalysis revealed that the time effect (i.e., areduction of difference wave between thebeginning and end of a session) was ab-sent during control conditions (t test, be-tween the first and last 45 min in controlconditions; monkey S: t % &1.76, df % 16,p % 0.1; monkey R: t % &1.25, df % 18,p % 0.23).
We also tested whether the effect of
haloperidol was selective of ERP related toperformance feedbacks, or if haloperidolaffected any evoked potentials. The lit-erature shows that treatment with halo-peridol does not alter stimulus-locked N1potentials (Zirnheld et al., 2004; de Bruijnet al., 2006). Accordingly, we looked foreffects on evoked potentials triggered bystimuli onset. There was clearly no effecton the measured negativity peaking 100ms after target onset 3 h after haloperidolinjection (permutation tests, all tests non-significant. See supplemental Fig. S4, avail-able at www.jneurosci.org as supplementalmaterial).
Discussion
We reported the first evidences of surface
frontal feedback-related potentials recorded
chronically in monkeys during a cognitive
task. The properties of these potentials dur-
ing trial and error and their sensitivity to the
administration of haloperidol support their
Figure 5. Selective effects of haloperidol. A, Systemic haloperidol injections induced increased RT for both monkeys especially
homology with FRPs observed in humans.
3 h after injections. B, Values of the peak of INC-COR difference curves, for each control session (Ctr: white disks) and haloperidol
sessions (30 min: measured 30 min after injection, gray disks; 3 h: measured 3 h after injection, black disks) for the two monkeys.
Each disk corresponds to the average measure from a single session. The gray areas represent prediction intervals calculated from
Medial frontal feedback-related
control sessions. Permutation tests showed significant differences between control and Haldol sessions at 3 h (see Results for
potentials in primates
details). Statistical significance from permutation tests: *p # 0.05; **p # 0.001; ns, nonsignificant. C, Average difference curves
The major finding of the present work is
INC-COR for control and haloperidol (3 h) sessions for the two monkeys. D, Haloperidol had no effect on N100 potentials evoked by
the description of a medial frontal poten-
visual target onset. See supplemental Figure S4, available at www.jneurosci.org as supplemental material, for session per session
tial related to performance feedback in the
monkey. Our initial motivation to searchfor this potential was to assess the some-
formed the task as well as in control conditions (parameters n1,
times criticized homology between data obtained in monkeys—
n2, and P were not significantly affected by haloperidol). A drop
especially using single unit recordings- and in humans— using
in motivation was observed for both monkeys (Student's t test,
EEG or functional magnetic resonance imaging (fMRI) (Botvinick
control vs haloperidol, M: monkey S t % 4.13, df % 13, p % 0.012,
et al., 2004). In the present context the functional homologies are
and monkey R t % 5.15, df % 13, p % 0.0002). A significant effect
discussed especially regarding the ACC. To address this problem,
was observed on RTs that increased following Haldol injections
one important approach is to compare data acquired in the two
especially for the 3 h conditions (Fig. 5A), for which all RTs were
species with the same technology. For instance, unit recordings in
significantly increased for both monkeys (Control vs 3 h, Stu-
human ACC confirmed its role in processing reinforcement-related
dent's t test, search RT: monkey S t % &9.57, df % 1511, p # 10&4
information as shown in monkey experiments (Ito et al., 2003;
and monkey R t % &16.3, df % 6139, p # 10&4; repetition RT:
Matsumoto et al., 2003; Williams et al., 2004; Quilodran et al., 2008).
monkey S t % &13.06, df % 2003, p # 10&4 and monkey R t %
fMRI in behaving monkeys will be a further important step to com-
&20.4, df % 8415, p # 10&4).
pare data between the two species. Here, we chose to address the
problem using event-related potentials, a technique widely used to
The effect of haloperidol was weak and inconsistent when mea-
study performance monitoring. Note, similarities between monkey
sured on FRP. However, a consistent significant effect of DA
and human ERPs have been documented for other stimulus-locked
blockade was observed for the first peak of INC-COR difference-
potentials (Pineda et al., 1997; Woodman et al., 2007).
waves. For both monkeys, the peak amplitude in haloperidol ses-
The FRPs that we describe in monkeys are in many ways com-
sions was slightly reduced 30 min after injections, and was clearly
parable to the medial frontal event-related potentials described in
lower 3 h after injections (Fig. 5B). Permutation tests were used to
humans (Donkers et al., 2005; Cohen et al., 2007). Like human
15682 • J. Neurosci., December 16, 2009 • 29(50):15675–15683
Vezoli and Procyk • Chronic Recordings of Feedback-Related Potentials
ERP, monkey FRP is sensitive to alteration of dopamine trans-
2008). However, as for unit and local field potential recordings in
mission (Zirnheld et al., 2004; de Bruijn et al., 2006). While this in
ACC, signals for positive feedback in COR trials are weaker.
itself is interesting, the impact of dopaminergic alteration onFRPs and its prevalence compared to other neurotransmitters
Perspectives for chronic observations of
remains debated (Jocham and Ullsperger, 2009). Future experi-
ments could directly address these issues.
It is very likely that monkey FRPs reflect, as in humans, the integ-
Although we observed interindividual variability of the ERP
rity of the performance monitoring system (Ullsperger and von
shapes, the calculation of difference waves demonstrated a strong
Cramon, 2006). The sensitivity to dopaminergic transmission
similarity between subjects. This reflects the consistency of one of
could be explained by the modulatory role of dopamine on the
the main characteristics of information processing within the
cingulo-accumbens loop that is on striatal and/or cortical pro-
performance monitoring system that is the discrimination be-
cessing of performance feedback. It has been shown that ge-
tween negative and positive feedbacks. Importantly, the differ-
notypic characteristics of subjects concerning D2 receptor
ence waves demonstrated a peak at !170 ms that differs from the
regulation (DRD2-TAQ-IA polymorphism) are correlated with
human reports [!290 –300 ms (Nieuwenhuis et al., 2005; Yeung
learning strategies and with stronger activations of ACC and dor-
et al., 2005)] by a 3/5 ratio that approximately fits the proposed
solateral prefrontal cortex for performance feedbacks (Klein et
rule of correspondence between human and nonhuman primates
al., 2007). Furthermore, several studies showed attenuated ERN
(Schroeder et al., 2004; Foxe and Schroeder, 2005).
in Parkinsonian patients, including at early stages of the disease(Falkenstein et al., 2001; Ito, 2004; Stemmer et al., 2007; Willemssenet al., 2008). However, the link between dopamine and FRP re-
Feedback-related potentials and expectations
mains unresolved and its sensitivity to other neuromodulators
In humans, FRPs follow several properties predicted by rein-
needs to be further investigated (Jocham and Ullsperger, 2009).
forcement learning rules and, in particular, a sensitivity to out-
The chronic EEG model in monkeys allows for direct investiga-
come predictability (Frank et al., 2005; Cohen et al., 2007;
tions of the relationships between neuromodulatory systems,
Eppinger et al., 2008; Holroyd et al., 2009). The description of a
ACC activity, and FRPs.
larger FRP for the first, unsure, correct trial compared to certaincorrect trials in repetition also concurs with such sensitivity tooutcome predictability. Investigations on modulations by reward
size and probability during the trial and error period are in
Amiez C, Joseph JP, Procyk E (2005) Anterior cingulate error-related activ-
ity is modulated by predicted reward. Eur J Neurosci 21:3447–3452.
It has been proposed recently that the main variance observed
Botvinick MM, Cohen JD, Carter CS (2004) Conflict monitoring and ante-
rior cingulate cortex: an update. Trends Cogn Sci 8:539 –546.
in the difference waves between positive and negative feedback-
Brooks VB (1986) How does the limbic system assist motor learning? a lim-
related ERP should be attributed to positive feedbacks and not to
bic comparator hypothesis. Brain Behav Evol 29:29 –53.
negative feedbacks (Holroyd et al., 2008). Based on the reinforce-
Castner SA, Williams GV, Goldman-Rakic PS (2000) Reversal of antipsychotic-
ment learning theory of the ERN, Holroyd et al. (2008) proposed
induced working memory deficits by short-term dopamine D1 receptor
that during unexpected positive feedback the dopaminergic af-
stimulation. Science 287:2020–2022.
ferent to the ACC reduces a phenomenon otherwise observed
Coffin VL, Latranyi MB, Chipkin RE (1989) Acute extrapyramidal syn-
drome in Cebus monkeys: development mediated by dopamine D2 but
after negative feedbacks or other task-relevant events, and hence
not D1 receptors. J Pharmacol Exp Ther 249:769 –774.
reduces the observed surface feedback-related potential. Based
Cohen MX, Elger CE, Ranganath C (2007) Reward expectation modulates
on this hypothesis, one can predict that after unexpected positive
feedback-related negativity and EEG spectra. Neuroimage 35:968 –978.
feedback, the FRP should be smaller than after expected positive
Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK
feedback. Several experiments in humans support this hypothesis
(2005) Trial-by-trial coupling of concurrent electroencephalogram and
(Holroyd et al., 2008; Martin et al., 2009). With the present pro-
functional magnetic resonance imaging identifies the dynamics of perfor-mance monitoring. J Neurosci 25:11730 –11737.
tocol, the peak of difference waves INC-CO1 should be larger
de Bruijn ER, Sabbe BG, Hulstijn W, Ruigt GS, Verkes RJ (2006) Effects of
than INC-COR. On the contrary, we described a weak but inverse
antipsychotic and antidepressant drugs on action monitoring in healthy
effect. Further experiments are needed to confirm this effect and
volunteers. Brain Res 1105:122–129.
possible divergence between human and monkey data. Neverthe-
Dehaene S, Posner MI, Tucker DM (1994) Localization of a neural system
less, data from previous neurophysiological studies in monkeys
for error detection and compensation. Psychol Sci 5:303–305.
might help explain this discrepancy. Unit and local field potential
Donkers FC, Nieuwenhuis S, van Boxtel GJ (2005) Mediofrontal negativi-
ties in the absence of responding. Brain Res Cogn Brain Res 25:777–787.
recordings during the PST have revealed that ACC neurons par-
Emeric EE, Brown JW, Leslie M, Pouget P, Stuphorn V, Schall JD (2008)
ticipate in the detection and evaluation of positive and negative
Performance monitoring local field potentials in the medial frontal cortex
feedbacks (INC and CO1) when those are meaningful for adap-
of primates: anterior cingulate cortex. J Neurophysiol 99:759 –772.
tation i.e., during the search period (Quilodran et al., 2008).
Eppinger B, Kray J, Mock B, Mecklinger A (2008) Better or worse than
Emeric et al. (2008) recently described negative feedback-related
expected? Aging, learning, and the ERN. Neuropsychologia 46:521–539.
potentials from recordings of ACC local field potentials. These
Falkenstein M, Hohnsbein J, Hoormann J, Blanke L (1991) Effects of cross-
modal divided attention on late ERP components. II. Error processing in
data support at least a partial source of surface feedback-related
choice reaction tasks. Electroencephalogr Clin Neurophysiol 78:447– 455.
potentials within the ACC. Here we show that FRPs are larger for
Falkenstein M, Hielscher H, Dziobek I, Schwarzenau P, Hoormann J, Sun-
INC and CO1 feedbacks than for COR. One interpretation is then
derman B, Hohnsbein J (2001) Action monitoring, error detection, and
that the different FRPs reflect the processing by different neural
the basal ganglia: an ERP study. Neuroreport 12:157–161.
entities, and the activation of different ACC neuronal popula-
Foxe JJ, Schroeder CE (2005) The case for feedforward multisensory con-
tions, of negative and positive feedbacks in search periods. The
vergence during early cortical processing. Neuroreport 16:419 – 423.
Frank MJ, Woroch BS, Curran T (2005) Error-related negativity predicts
increased signals for CO1 and INC compared to COR might also
reinforcement learning and conflict biases. Neuron 47:495–501.
come for nonselective processing of feedbacks in search as ob-
Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E (1993) A neural
served in ACC recordings (INC/CO1 neurons in Quilodran et al.,
system for error detection and compensation. Psychol Sci 4:385–390.
Vezoli and Procyk • Chronic Recordings of Feedback-Related Potentials
J. Neurosci., December 16, 2009 • 29(50):15675–15683 • 15683
Handy T (2005) Event-related poptentials: a methods handbook. Cam-
text and context-dependent noradrenergic influences. Int J Psychophysiol
bridge, MA: MIT press.
Holroyd C (2004) A note on oddball N200 and the feedback ERN. In: Er-
Procyk E, Goldman-Rakic PS (2006) Modulation of dorsolateral prefrontal
rors, conflicts, and the brain. Current opinions on performance monitor-
delay activity during self-organized behavior. J Neurosci 26:11313–11323.
ing (Ullsperger M, Falkenstein M, eds), pp 211–218. Leipzig, Germany:
Quilodran R, Rothe´ M, Procyk E (2008) Behavioral shifts and action valua-
MPI of Cognitive Neuroscience.
tion in the anterior cingulate cortex. Neuron 57:314 –325.
Holroyd CB, Coles MG (2002) The neural basis of human error processing:
Schroeder CE, Molholm S, Lakatos P, Ritter W, Foxe JJ (2004) Human-
reinforcement learning, dopamine, and the error-related negativity. Psy-
simian correspondence in the early cortical processing of multisensory
chol Rev 109:679 –709.
cues. Cogn Process 5:140 –151.
Holroyd CB, Pakzad-Vaezi KL, Krigolson OE (2008) The feedback correct-
Schultz W (2000) Multiple reward signals in the brain. Nat Rev Neurosci
related positivity: sensitivity of the event-related brain potential to unex-
1:199 –207.
pected positive feedback. Psychophysiology 45:688 – 697.
Silvestri S, Seeman MV, Negrete JC, Houle S, Shammi CM, Remington GJ,
Holroyd CB, Krigolson OE, Baker R, Lee S, Gibson J (2009) When is an
Kapur S, Zipursky RB, Wilson AA, Christensen BK, Seeman P (2000)
error not a prediction error? An electrophysiological investigation. Cogn
Increased dopamine D2 receptor binding after long-term treatment with
Affect Behav Neurosci 9:59 –70.
antipsychotics in humans: a clinical PET study. Psychopharmacology
Ito J (2004) Error processing in patients with Parkinson's disease. Int Congr
152:174 –180.
Stemmer B, Segalowitz SJ, Dywan J, Panisset M, Melmed C (2007) The error
Ito S, Stuphorn V, Brown JW, Schall JD (2003) Performance monitoring by
negativity in nonmedicated and medicated patients with Parkinson's dis-
the anterior cingulate cortex during saccade countermanding. Science
ease. Clin Neurophysiol 118:1223–1229.
302:120 –122.
Taylor SF, Stern ER, Gehring WJ (2007) Neural systems for error mon-
Jocham G, Ullsperger M (2009) Neuropharmacology of performance mon-
itoring: recent findings and theoretical perspectives. Neuroscientist
itoring. Neurosci Biobehav Rev 33:48 – 60.
13:160 –172.
Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M
Ullsperger M (2006) Performance monitoring in neurological and psychi-
(2007) Neural correlates of error awareness. Neuroimage 34:1774– 1781.
Lidow MS, Goldman-Rakic PS (1994) A common action of clozapine, hal-
atric patients. Int J Psychophysiol 59:59 – 69.
operidol, and remoxipride on D1- and D2-dopaminergic receptors in the
Ullsperger M, von Cramon DY (2006) The role of intact frontostriatal cir-
primate cerebral cortex. Proc Natl Acad Sci U S A 91:4353– 4356.
cuits in error processing. J Cogn Neurosci 18:651– 664.
Martin LE, Potts GF, Burton PC, Montague PR (2009) Electrophysiological
Willemssen R, Mu¨ller T, Schwarz M, Hohnsbein J, Falkenstein M (2008)
and hemodynamic responses to reward prediction violation. Neuroreport
Error processing in patients with Parkinson's disease: the influence of
20:1140 –1143.
medication state. J Neural Transm 115:461– 468.
Matsumoto K, Suzuki W, Tanaka K (2003) Neuronal correlates of goal-
Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN (2004) Hu-
based motor selection in the prefrontal cortex. Science 301:229 –232.
man anterior cingulate neurons and the integration of monetary reward
Nieuwenhuis S, Slagter HA, von Geusau NJ, Heslenfeld DJ, Holroyd CB
with motor responses. Nat Neurosci 7:1370 –1375.
(2005) Knowing good from bad: differential activation of human cortical
Woodman GF, Kang MS, Rossi AF, Schall JD (2007) Nonhuman primate
areas by positive and negative outcomes. Eur J Neurosci 21:3161–3168.
event-related potentials indexing covert shifts of attention. Proc Natl
Perrin F, Bertrand O, Pernier J (1987) Scalp current density mapping: value
Acad Sci U S A 104:15111–15116.
and estimation from potential data. IEEE Trans Biomed Eng 34:283–288.
Yeung N, Holroyd CB, Cohen JD (2005) ERP correlates of feedback and
Perrin F, Pernier J, Bertrand O, Echallier JF (1989) Spherical splines for scalp
reward processing in the presence and absence of response choice. Cereb
potential and current density mapping. Electroencephalogr Clin Neuro-
physiol 72:184 –187.
Zirnheld PJ, Carroll CA, Kieffaber PD, O'Donnell BF, Shekhar A, Hetrick WP
Pineda JA, Westerfield M, Kronenberg BM, Kubrin J (1997) Human and
(2004) Haloperidol impairs learning and error-related negativity in hu-
monkey P3-like responses in a mixed modality paradigm: effects of con-
mans. J Cogn Neurosci 16:1098 –1112.
- Supplementary notes
- Table S1
- Figures S1, S2, S3, and S4
Effect of insertion and change of visual feedbacks on behavioral performance.
Before the insertion of visual feedbacks (phase 0) monkeys presented relatively
stable performances. This was evaluated by applying a linear fit to the successive measures in phase 0. Only parameter n2 significantly reduced for monkey R during this period, which might imply that this monkey was still optimizing its performances regarding the repetition period.
Insertion of visual feedbacks induced slight changes in performance (Fig. S1). This
adaptation of performances consisted, for both subjects, in an increased number of shift
after introduction of visual feedbacks (Fig. S1E). This effect might indicate that
monkeys were perturbed by the presence of visual feedbacks. However, no deleterious effect was found on perseverance in search.
Statistics. Prediction intervals. Permutation tests.
Because of the limited number of haloperidol sessions we addressed the statistical significance of effects in two ways. First, single test session measures were tested for being within the 95% prediction interval calculated from the population of control measures. Upper and lower limits of the intervals (see figures 5 and S4) were computed as follows:
Up=mean(x) + qt(0.025, n - 1) * sqrt((n + 1)/n) * sd(x)Low=mean(x) - qt(0.025, n - 1) * sqrt((n + 1)/n) * sd(x)
where x is the vector containing control data points, and qt(0.025, n-1) is the quantile
function for the t distribution with n-1 degrees of freedom.
Second, to evaluate the significance of effects, permutation tests were realized for
testing the difference in means of control and test measures. Double sided tests were performed with 10000 permutations of combined control (n1 sessions) and test (n2 sessions) sessions (30min and 3h tested separately). The test evaluated the probability of the difference of the control and test means to be within the population of 10000 differences of means of n1 and n2 permuted data points.
monkey S
monkey R
latency (msec)
peak amplitude (µV)
latency (msec)
peak amplitude (µV)
Trial number by step
468 (16)
321 (8)
Table S1: Individual FRPs amplitudes and latencies at peak. Measures obtained from phase I average
waveforms for both monkeys and for each type of trials (INC, CO1, COR) at average electrode E5-E12. The mean numbers of trials ± sd per step are presented below tables.
r = -0.0266, p = 0.9380
r = 0.0103, p = 0.9759
r = 0.1252, p = 0.7137
r = 0.548, p = 0.0809
n1: r = 0.0541, p = 0.8745
n1: r = -0.0607, p = 0.8592
r = 0.1921, p = 0.5715
r = -0.5014, p = 0.1161
n2: r = -0.3840, p = 0.2437
n2: r = -0.7574, p = 0.0069 **
monkey R ns
Figure S1. Changes in behavioral performance. A-D. Performances in Phase 0 i.e. without visual
feedbacks. Measured were tested for stability using a linear fit. A. Mean number of perseverance in the search
period per problem (P); B. Mean number of shift in the repetition period per problem; C. Mean number of trials
to achieve the search (n1) and repetition (n2) periods; D. Percentage of trials engaged per session
(=Motivation : M). All parameters were stable except n2 for monkey R (C, right) which evidenced an
improvement of performances at the end of Phase 0. E-G. Performances were compared between phases 0
and I. The effect of feedback changes was assessed applying a Student t-test between steps at phase
transition. E. P (in grey) and Shift (in green); F. n1 (in blue) and n2 (in orange); and G. motivation (M). Student t-
test performed for steps before vs. after feedback introduction (step2 vs. step3). ns: not significant ; *: p<0.05 ; **: p<0.01; ***: p<0.001.
Time from feedback (s)
Time from feedback (s)
20ms time bins from feedback onset - Corresponding time from feedback onset (s)
Figure S2. Different FRPs for the different feedbacks. A. Individual FRPs waveforms for the
average of electrodes E5 and E12. Measures obtained from phase I average waveforms for both monkeys and for each type of trials (INC, CO1, COR). The main peaks of interest are labeled
eFRP and lFRP. B. ANOVA helmert contrast on the factor time bin, tested for conditions INC and
COR. The graph shows the p-values (on a log axis) at each bin which is contrasted with the average of all previous bins. Note that the beginning of significant difference between INC and COR is observed in the bin 100-120ms and 120-140ms for monkey S and R respectively.
negative feedback
negative feedback
Time from feedback (s)
Time from feedback (s)
Figure S3. Long term tests on FRP and difference curves A. Changes in eFRP amplitude
across time for INC and SC. Average amplitude by step ±sem for monkey S (left) and monkey R
(right). The effect of negative feedback change was tested with t-test between steps 6 and 7. ns: not significant ; *: p<0.05 ; **:
p<0.01. . INC-COR difference waves after 7 months. INC-COR
difference waves for monkey S (left) and monkey R (right). Waveforms represent the average of a group of 10 sessions obtained 7 months after introducing visual performance feedback in phase I.
Figure S4. Haloperidol had no effect on ERP related to target onset. The figure shows
values of the peak of ERP at 100ms after target onset, for each control session (Ctr, white disks) and haloperidol sessions (30min: measured 30 minutes after injection, gray disks; 3h: measured 3 hours after injection, black disks) for the two monkeys. The grey areas represent prediction intervals calculated from control sessions. Permutation tests for 30min and 3h measures showed no difference between control and Haldol sessions (see results).
Source: http://www.sbri.fr/files/publications/vezoli%202009%20j%20neurosci.pdf
B-4000 LIEGE – PLACE X. NEUJEAN 39 TEL.: 04/223.74.10 - FAX : 04/223.75.61 LA NAMIBIE CIRCUIT INDIVIDUEL EN VOITURE DE LOCATION Circuit en lodge de 14 jours / 13 nuits L'Afrique préservée. Un territoire des savanes, des rivages léchés par l'océan, des dunes vertigineuses, une succession de déserts chaotiques de sable et de rocaille, habitat d'une faune incroyablement riche.
Share this: March 2016 Volume 11 Number 1 Now Is the Time… to Register From the Editor for the 2016 BIO Conference The generosity of BIO memberswhen it comes to stepping up andhelping out is one of the mostgratifying things for me here atTBC. When I put out a call forassistance, people respond. Casein point: This month differentorganizations in New York areoffering programs of interest tobiographers that I would like tocover in the April issue. Ourdedicated and intrepid NYCcorrespondent, Dona M unker,would attend all of them if shecould, but logistics make itimpossible. At BIO board member






