Exp21-couv-anglais


Exp21-pages anglais 4-15 24/05/04 16:14 Page 6
SLIT vs SCIT - The first placebo
controlled trial
The eighty-nine patients enrolled had at least 2years of seasonal birch pollen rhino-conjunctivitis
Sublingual immunotherapy (SLIT) has been
uncontrolled by conventional pharmacotherapy (2).
investigated for at least fifteen years. Many
Allergy to birch pollen was verified using a stan-
controlled studies have been conducted and
dard extract in skin prick and conjunctival provo-cation tests. A radioallergosorbent test (RAST)
most prove that SLIT is effective, has a good
was used to confirm the presence of specific IgE
safety profile and is convenient. However,
there has been no placebo-controlled study
Although, the study did not set out to determine
in which the efficacy and safety of SLIT and
efficacy in asthma, patients with mild seasonalbirch pollen induced asthma were included
subcutaneous immunotherapy (SCIT) have
been compared – until now.
Patients were randomized by minimization, whichis a method that distributes patients evenlyaccording to severity disease, gender and age(3).
The findings of the first such study have The study was double-blinded throughout the
been published by respected team of
two-year treatment phase.
Danish investigators (1). It represents alandmark in the development of effecti-
To take SLIT, the patient administered drops that
ve and practical specific immunotherapy (SIT).
were allowed to remain under the tongue for two
The main aim of the study was to compare effi-
minutes and then swallowed. SCIT was administe-
cacy and frequency and severity of side-effects
red in a clinic and patients were kept under obser-
when SLIT or SCIT was administered to adults
vation for thirty minutes after each injection.
suffering from rhino-conjunctivitis as a result ofallergy to birch pollen. Birch pollen allergy has a
For SLIT there was a 30-day sublingual induction
well-defined season and provides a good clinical
phase followed by a maintenance phase of bet-
model for other allergen systems.
ween 21 and 23 months. The starting dose was
Furthermore, there have been few controlled stu-
0.0164 µg Bet v 1 and the highest dose adminis-
dies of birch pollen allergen extract in rhino-
tered was 49.2 µg Bet v 1 administered every two
• Perennial allergy (chronic,
conjunctivitis, so additional data is welcome. The
days. For SCIT, the starting dose in the induction
non-allergic rhinitis or sinusitis)
secondary aim of the study was to determine the
phase was also 0.0164 µg Bet v 1 and the month-
magnitude of clinical efficacy of SLIT and SCIT
ly maintenance dose was 3.28 µg Bet v 1. Dosage
• Prior immunotherapy with
compared with that of placebo.
was adjusted according to each patient's tolerance
birch pollen within the last
to treatment and during the pollen season, doses
The investigators devised a randomized, double-
were reduced by between 20 and 40% in patients
• Current immunotherapy
blind, placebo-controlled, double-dummy study,
who exhibited allergic symptoms (Table 2).
with other allergens
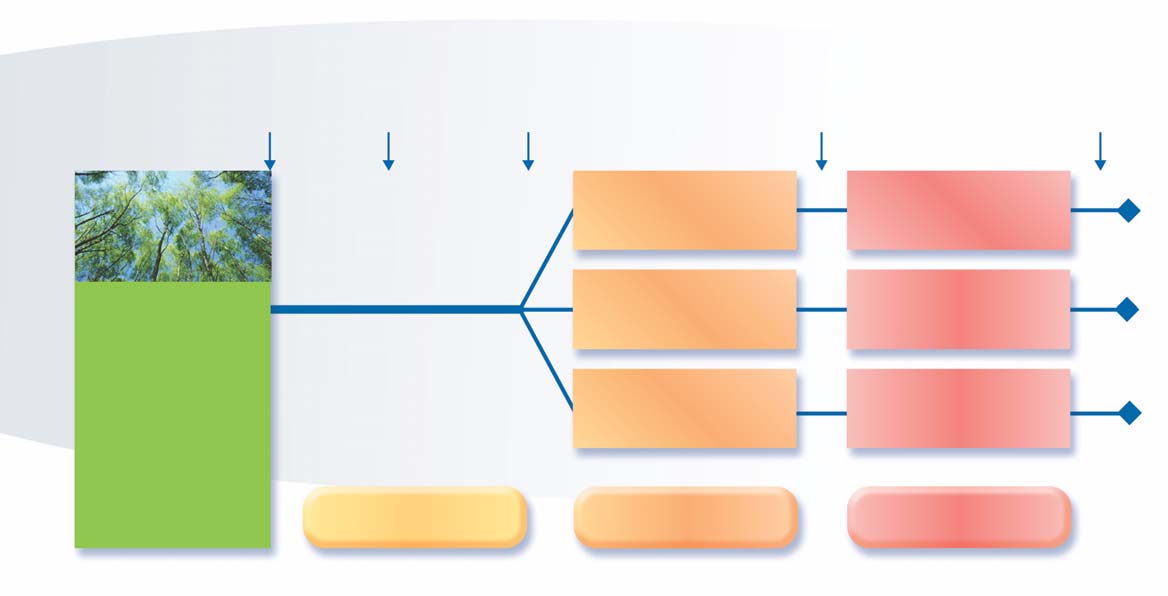
including a pre-treatment evaluation period ofone year, followed by two treatment periods over
Patients completed diary cards during each birch
• Treatment with ß-blockers,
the next two years (Figure 1).
pollen season, recording allergic symptoms, medi-
• Pregnancy or planned
� Tab.2 TREATMENT FORMULATIONS
• Participation in another
• Standard contraindications
Standardized birch
Standardized birch
SLIT placebo inclu-
for immunotherapy (2)
pollen extract (major
pollen extract (major
contained histamine
allergen Bet v 1)
allergen Bet v 1)
dihydrochloride (0.01,
as glycero-saline
adsorbed on calcium
visual appearance
0.1 and 0.5 mg/ml)
and taste of SLIT.
to mimic induction oflocal reactions.
Exp21-pages anglais 4-15 24/05/04 16:14 Page 7
� Fig.1 STUDY DESIGN
Patients with
pollen rhino-
conjunctivitis
and verified birch
pollen allergy
(n=89, mean age
30 years -
FIRST SEASON: 1997
range 20–58 )
Pre-treatment year
First treatment year
Second treatment year
� Tab.3 PERMITTED RESCUE MEDICATION
Permitted treatments:
Treatments not permitted:
• acrivastine 8 mg
• nasal corticosteroids
• levocabastine 0.5 mg/ml eye drops
• long-acting antihistamines
• levocabastine 50 µg/dose nasal spray
• other anti-allergic drugs
• prednisolone 5 mg tablets
(used as rescue medicine as required)
cation consumption, and adverse events. Each
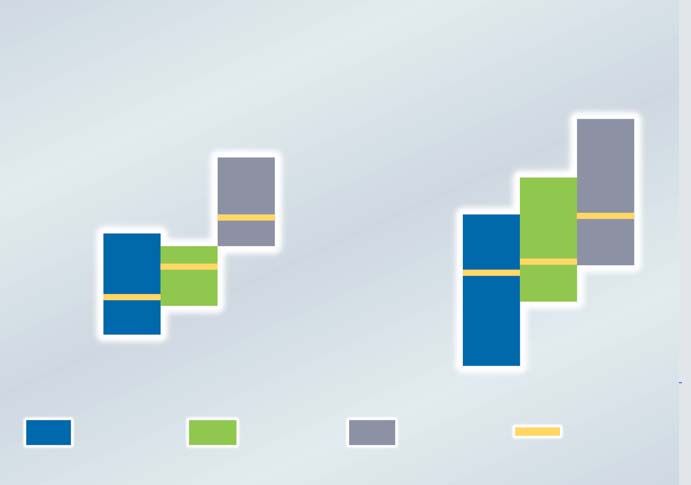
� Fig.2 DIFFERENCES IN DISEASE SEVERITY:
patient's quality of life was assessed by question-
PRE-TREATMENT TO FIRST TREATMENT PERIOD
naire at the end of each pollen season, includingthe pre-treatment season (Table 3 and 4).
Because the level of pollen exposure was so low in
the second treatment season (1999), patients in all
groups experienced few symptoms and used little
rescue medicine. As a result, this season was not
included in the evaluation of efficacy.
After the first treatment season, the cumulative
allergen dose in the SLIT group was 4717 µg Bet v 1. On average they received 175 times more
Change in mean daily score
Change in mean daily score
allergen than the SCIT group ( 27 µg Bet v 1).
The treatment effect was calculated in two different
ways. One involved subtracting the pre-treatment
scores for individual patients from those obtainedin the treatment season (Figure 2).
� Tab.4 SCORING AND ASSESSMENTS
0 = absent, 1 = mild, 2 = moderate, 3 = severe
Two drops levocabastine (eye drops)
Two puffs levocabastine (nasal spray)
One prednisolone tablet
SF-36 Health Status Questionnaire (47–49)
Pollen trap placed 15 m above ground (Danish Aerobiological Group) (50)


Exp21-pages anglais 4-15 24/05/04 16:14 Page 8
The other was to calculate the ratio of first treat-
by a factor 1.03. Disease severity in the SLIT group
ment season scores to those of pre-treatment.
was being only half that of the placebo group. In
Both methods have in-built effects, the subtrac-
the SCIT group symptom and medication also
tion method, weighting results in patients with
improved and disease severity was one-third of the
This is the first placebo-
severe disease, and the ratio method weighting
placebo group. There were no statistically signifi-
results in those with mild disease.
cant differences in efficacy between the SLIT and
of SLIT and SCIT. It
the SCIT groups using either method of measuring
shows that birch pollen
Using the subtraction method both SLIT and SCIT
treatment showed a significantly better outcome
rapy produces a signifi-
compared with placebo treatment. Results obtai-
Local side-effects associated with SLIT (itching
ned by the ratio method indicated that the seve-
or mild œdema in the mouth and/or throat) occur-
cant and clinically
rity of the disease was reduced to a greater extent
red mostly during the induction phase and resolved
relevant reduction of
in the SCIT group, to one-third of the severity in
when the dose was temporarily reduced.
symptoms and rescue
the placebo group. In the SLIT group severity was
In the quality of life assessment, there were no sta-
medication usage in
reduced to half that observed in placebo treated
tistically significant differences between groups.
Similarly, for adverse events, including grade 2
patients allergic to birch
systemic side-effects, there were no differences.
In the first treatment season (1998), pollen
However whereas one grade 4 and five grade 3
pollen. SLIT efficacy is
exposure was 2.29 times higher than in the pre-
systemic reactions occurred in the SCIT group (all
not statistically different
treatment season. Not surprisingly, the placebo-
were successfully treated without withdrawal from
to that of SCIT. SLIT
treated patients had increased symptom scores (by
the study), no grade 3 or 4 reactions occurred in
was well tolerated with
a factor 1.45) and increased medication scores (by
the SLIT group. There were no withdrawals due to
only harmless local
a factor of 2.01). By contrast, in the SLIT group,
grade 3 or 4 reactions.
symptom scores fell by a factor 0.78 and drug use
Reviewed by Professor P. Scheinman.
INTERVIEW… with Pr P. Scheinman, Necker Hospital, Paris (France)
Expressions. What lessons can we
allergens. There is no theoretical reason
ned to use immunotherapy, which in turn
draw from this study?
why not. Furthermore, the results were
means that fewer physicians in general are
obtained in adults but they can be extrapo-
taught (BMJ June 2003).There were also
Professor P. Scheinman. There are two
lated to children. You cannot do the same
some bad experiences with early trials of
important lessons. First, there is no signifi-
trial in children because it would be une-
immunotherapy which may make British
cant difference in efficacy between subcu-
thical to give them placebo injections.
physicians more wary in general. In Fran-
taneous and sublingual therapy. There is a
ce, for example, immunotherapy is a regu-
suggestion that sublingual therapy may be
E. Is compliance a problem with
lar feature of the continuing medical edu-
a little less effective but the tolerance is so
cation programme. Sublingual immuno-
good with no systemic adverse events. It
therapy has made a difference too. I no
makes a strong argument for SLIT.
Pr P. S. As far as we can see, compliance
longer use injectable allergens except for
Secondly, these patients had rhino-
is good. It may be that some sort of tablets
immunisation against venom.
conjunctivitis but almost a third had asth-
would be preferable to drops.
ma and that reflects real life.
E. The study is described as a
E. Why is immunotherapy not used
"cornerstone" in immunotherapy,
E. Can we assume that SLIT would
more widely in, say, the United
what, for you would be the next
be effective with other allergens?
Pr P. S. I think the results obtained with
Pr P. S. There is a shortage of allergolo-
Pr P. S. That's easy. Perennial asthma due to
birch pollen can be extrapolated to other
gists and few specialist physicians are trai-
dust mites with sublingual immunotherapy.
(1) Khinchi MS, Poulsen LK, Carat F, André C, Hansen AB, Malling H-J. Clinical efficacy of sublingual and subcutaneous birch pollen allergen-specific immunotherapy: a ran-domized, placebo-controlled, double-blind, double-dummy study. Allergy 2004; 59: 45–53. Comments 37-38.
(2) Malling H-J, Weeke B (eds). EAACI position paper: immunotherapy. Allergy 1993; 48 (Suppl. 14):7-35.
(3) Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Therap 1974; 15: 443-453.
(4) Cockrane 2004.
Source: http://www.stallergenes.be/uploads/tx_stlgnexpressions/Clinical_Issues_Exp21_en_07.pdf
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Mar. 2008, p. 1394–1401 0099-2240/08/$08.00⫹0 doi:10.1128/AEM.01463-07Copyright © 2008, American Society for Microbiology. All Rights Reserved. Two Different Tetracycline Resistance Mechanisms, Plasmid-Carried tet(L) and Chromosomally Located Transposon-Associated tet(M), Coexist in Lactobacillus sakei Rits 9䌤
RESOLUCIÓN 05-03-ARCOTEL-2016 EL DIRECTORIO DE LA AGENCIA DE REGULACIÓN Y CONTROL DE LAS Que, el artículo 226 de la Constitución de la República del Ecuador dispone que: "Las instituciones del Estado, sus organismos, dependencias, las servidoras o servidores públicos y las personas que actúen en virtud de una potestad estatal ejercerán solamente las competencias y facultades que les sean atribuidas en la Constitución y la Ley. Tendrán el deber de coordinar acciones para el cumplimiento de sus fines y hacer








