Pharmaceutical wastewater ozonation at industrial scale
Pharmaceutical Wastewater Ozonation at Industrial Scale.
L. A. Fernández1, E. Veliz1, I. Hernández2, C. Pérez1, L. García Vivó1, S. Padrón2, R. Pérez Rey1,
1Ozone Research Center. National Center of Scientific Research. Havana. Cuba. P.O.Box 6412. e mail:
2Center of Pharmaceutical Research and Development. Havana. Cuba
Abstract
The pharmaceutical production industries generate wastewaters that should be properly treated because in some cases they are highly toxic. For this reason the safe inactivation before disposing them into the environment must be guarantee. This paper deals with an ozone treatment system for the wastewaters generated in the cytostatic production. The cytostatics present in the wastewaters treatment were 5-fluorouracil, methotrexate, doxorubicin and cytarabine. The treated wastewaters fulfilled the parameters established in the Cuban guidelines for disposal. This treatment system was able to inactivate cytostatic wastewaters in an efficient way. It is viable economically and it does not produce harmful wastes to the environment.
The enhancement and diversification of pharmaceutical productions have facilitated the development of this industry in Cuba. Due to this increment it is necessary to find an effective treatment of wastewaters that exit these processes. The wastewater treatment must be effective and economic. In the 90's it was decided to build a Production Plant for cytostatics, which are employed in the treatment of neoplastic diseases. These products are highly toxic, and have carcinogenic and mutagenic properties. For that reason to achieve an appropriate treatment of the wastewaters generated during the productive process, before disposing them into the environment is necessary. It is one of the main objectives of the technicians and heads of this cytostatic production plant. Among their fundamental objectives is preserving human live and also the environment, contributing to the environmental health of the society. An initial research 1 on cytostatic ozonation in water medium at lab scale, showing the possibility of degrading several cytostatics and obtaining suitable elimination times for cytostatic concentrations of about 10-3 mole/L. Taking into account these results a scale up of this procedure was performed. Finally industrial an ozone wastewater system treatment was designed and installed for the elimination of cytostatic traces before wastewater disposal. This plant produces only one kind of cytostatic drug each time, to avoid cross contamination. Therefore the designed wastewater treatment technology must guarantee the total inactivation of one type of cytostatic during its production period. By other hand it is known that efficiency of ozonation processes depends on the mass transfer between phases and the kinetic behavior 2,3. During the scale up of gas-liquid system the relative rate of the transport phenomena in relation with the kinetic and thermodynamic diminish. It causes that the control of the operation is exerted fundamentally by the diffusion phenomenon 4. Taking into account this concept the scaled up was made trying to obtain the same mass transfer performance. This papers deals with the use of ozone in the elimination of cytostatics in water medium and with the evaluation of an industrial ozone treatment system for the wastewaters generated in the cytostatic productions. Results show that these compounds can be inactivated by ozone
Materials and Methods
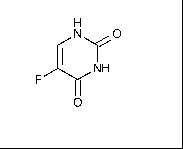
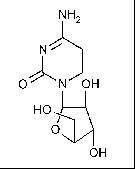
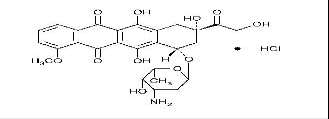
At laboratory, pilot and industrial scale several cytostatics were ozonated. Their molecular structure and molecular weight are shown in Table I.
Table I. Molecular structure and weight of cytostatics
Cytostatic Name
Molecular Structure
Molecular weight
5-Fluoracil
Methotrexate
Cytarabine
Doxorubicin
The scale up was performed in three bubble columns. At lab scale the capacity of column was 100 mL, at bench scale it has 5 L and at pilot scale the effective volume was 70 L. All these bubble columns were provided at the bottom with a porous diffuser with a pore diameter of 250 m. The experiences were conducted at neutral pH and at room temperature. Gas ozone concentration was measured by spectrophotometric method at 256 nm. The concentrations of aqueous solutions of cytostatics during inactivation were measured by HPLC, detection limit was 1.10-6 mol/L. Experimental conditions were previously reported 1.
The Ames test 5 was employed for the detection of mutagenic activity after ozonation. Chemical oxygen demand (COD) and biochemical oxygen demand (BOD) to industrial cytostatic wastewaters were determined according to Standard methods for water and wastewaters 6.
Results and discussion
Results at lab scale
In figure 1 it is shown the results obtained for ozone inactivation of cytostatic at pH 7 are shown. It is clear the decay in concentration of each one of the cytostatic tested during ozonation.
Figure 1. Degradation of aqueous solution of cytostatic.
Gas flow: 3,6 L/h, Gas ozone concentration: 20 mg/L, pH: 7.
It is evident that ozonation can eliminate different cytostatics in an efficient way in 45-60 minutes depending on the specific case.
Aspect of bubble column design
It is well known that the increase in the gas holdup ( ) enhance de mass transfer process. The gas
holdup depends on the gas superficial velocity (UG) and increases linearly with this parameter 7. The dependence of gas hold up on superficial velocity is expressed as (1) 8
U (1)
Taking into account the previous concept it was decided to keep constant the superficial gas velocity in order to maintain a similar mass transfer level in the different scales of cytostatic wastewater treatment. Therefore cytostatic wastewaters are very diluted aqueous solutions, it allowed the use of some empirical correlations develop for gas-aqueous systems. At lab scale the superficial gas velocity was 6 m/h, this value correspond to a bubble flow regime because the superficial velocity is less than 0,021 m/s 8. The gas holdup was evaluated by empirical
correlation (2) [9]. Then, the value was 6.24 X 10-3. Taking into account the results previously
reported [7] and this value, the mass transfer coefficient (k
La) value resulted about 3 X 10-4 s-1.
In the case of bench and pilot scale, mass transfer coefficient (kLa) was estimated from a well known correlation of Akita y Yoshida (3) [10]. The selection was based on that this equation considers the geometry of the two scales.
g d g d
DO
DO3 (cm2/s): ozone diffusivity dc (cm): column diameter L (g/cm3): liquid density
L(poise): liquid viscosity (dina/cm): liquid superficial tension
The diffusivity (DO3) was calculated by Johnson and Davies equation 11. The liquid density, liquid viscosity and superficial tension are reported by Rakness 12. Gas holdup in these cases was determined experimentally by the measure of the liquid height inside the bubble column with (hA) and without gas (h0) employing the following equation (4):
h h
hA
Table II illustrates the kla values obtained for bench and pilot scale. It can be observed that the kla values are in the same order, about 10-4 s-1.
Table II Mass transfer coefficients for different scales
kLa (s-1)
According to scale up results, the criterion of keeping constant the gas superficial velocity to obtain similar mass transfer behavior was a good decision. These results allow predicting that at industrial scale the mass transfer processes do not limit the cytostatic degradation overall process. It means that in scale up the transport time remains more or less constant.
Industrial treatment system
In agreement with the daily volume of wastewaters at industrial scale, the ozone applied doses obtained in the scale up, an industrial ozonation system was designed and built.
Figure 2 illustrates the diagram of the treatment process. It consists of two initials collector tanks, of 1200 L each one, connected to the exit of the production plant where the wastewaters drains by gravity. The wastewaters are pumped to the bubble column which works at semibatch mode. It has an effective volume of 350 L, and a gas ozone flow of 500 L/h. After ozonation the wastewater is pumped to two final collector tanks where the control analyses were performed. If the treated wastewaters do not fulfill the Cuban guidelines for disposals (NC-2799) 13 or cytostatic traces are detected, there is the possibility of leading wastewater to initial collector tanks to be treated again. Although in the wastewater treatment evaluation this situation never happened. The level in the tanks, in the columns and the performances of the pumps are electronically monitored and controlled from a panel for the whole system management with a great level of safe for the operator of the system. This aspect is very important because the cytostatic wastewater have a high risk.
Figure 2. Cytostatic wastewaters treatment plant.
The methodology of cytostatic production in this plant is the production of one type of antineoplastic drug each time. In this way a total cleaning of all the installation is ensure before the start with the new production. This facilitates that wastewater treatment system only treat a solution of water, cytostatic traces and a small quantity of cleaning agents. Table III, shows the results obtained in the ozonation of four cytostatic wastewaters. It is important to notice that the cytostatic initial concentrations are different because they depend on the production level for each drug.
Table III. Kinetics of cytostatic inactivation with ozone at industrial scale.
Cytostatics Concentration (mol/L)
Methotrexate Cytarabine
n.d: not detected
These results demonstrated that ozonation of wastewater can effectively eliminate all the cytostatics treated. The high reductions obtained in their concentration show that ozonation is a reliable method to treat these polluted waters. In table IV the ozone applied doses (DA) are shown. The highest applied doses correspond to 5-fluoracil which have a molecular structure less complex that methotrexate. But in this case the fundamental reason of the high applied doses is the initial concentration of to 5-fluoracil is nine times superior that in methotrexate case.
Table IV. Ozone Applied Doses.
Reaction time (h)
Concentration (mol/L)
A summary of the test performed analysis to treat wastewater according to Cuban guidelines for water disposal is presented in table V. The mutagenicity test to ozonated wastewaters was carried out (Ames test) to ensure the non toxic character of the waste water exiting the plant.
Table V Treated wastewater parameters and its comparison with Cuban disposal guidelines
The results show that the treated wastewaters fulfilled the parameters established in the Cuban guidelines. After ozonation none of the wastewaters gave positive results for the Ames test, showing that the reaction products obtained by ozonation are non-mutagenic in all cases. These results allow to establish that the technology was able to inactivate cytostatics wastewaters in an efficient way independently of its molecular structure.
In table VI there is an economic comparison between different investment costs of technologies for this kind of wastewaters. As observed the ozonation is one of the cheapest. Moreover in this case the treatment does not generate secondary residuals, that must be treated as in the case of activated charcoal technology.
Table VI Investment costs for different treatment alternatives for wastewaters of cytostatic production
Treatment systems
Investment cost (USD)
Vacuum evaporation
Activated charcoal
The ozone technology employed allows obtaining good results in elimination of cytostatic and guarantees the protection of the environment.
Conclusions
Ozonation is a suitable method for treated cytostatic wastewaters
The developed technology fulfills the designed requirements and enables the safe disposals
of the treated wastewaters.
The technology developed is simple, with an automation level that avoids the direct contact of
the residual with the operators during performance.
It is economically viable and it does not produce harmful wastes to the environment. For this
reason it is consider as a clean and sustainable technology.
References
1- Pérez Rey R., Padrón Yaquis A. S., García León L., Martínez Pozo M. and Baluja Ch. Ozonation of Cytostatics in Water Medium. Nitrogen Bases. Ozone Science & Engineering, Vol. 21, No. 1, pp. 69-77, 1999. 2- Charpentier J.C. "Advances in Chemical Engineering", Academy Press, New York, Vol. 12, 1981. 3- Beltrán F. J. Ozone Reactions kinetics for water and wastewaters system. Lewis Publisher, 2004. 4- Rehm H.J. y Reed G. Scaling-up of bioreactors. Biotechnology, 2, 581, 1985. 5- Dutka B. J., Jova A., Brechin J. Evaluation of four concentration/extraction procedures on waters and effluent collected for use with Salmonella typhimurium screening procedure for mutagens. Bull Contam. Toxicol. 27, 758, 1981. 6- APHA, AWWA, WPCE, Standard Methods for the Examination of Water and Wastewater, 17th Ed. USA, 1993 7- Sotelo J.L., Benítez F.J., Beltrán-Herédia J. y Rodríguez C. Retención de gas y coeficientes de transferencia de materia en columnas de burbujeo. I. Difusores de placas de vidrio poroso. Anales de Química, 86, 188, 1990 8- Hyndman C.L., Larachi F. y Guy C. Understanding gas-phase hydrodynamics in bubble column: a convective model based on kinetic theory. Chem. Eng. Science, 52, 1, 63, 1997. 9- Roustan M, Wang R. Y., Wolbert D. Modeling hydrodinamics and mass transfer parameters. In a continous ozone bubble column. Ozone Science & Engineering, 18, 99, 1996. 10- Akita K. y Yoshida F. Gas holdup and volumetric mass transfer coefficient in bubble columns. Ind. Eng. Chem. Process Des. Develop, 12, 1, 76, 1973. 11- Johnson P.N. y Davis R.A. Diffusivity of ozone in water. J. Chem. Eng. Data, 41, 1485, 1996. 12- Rakness K.L., Renner R.C., Hegg B.A. y Hill A.G. Practical design model for calculating bubble diffuser contactor ozone transfer efficiency. Ozone Science & Engineering, 10, 173, 1987. 13- Norma Cubana NC 27:1999. Vertimientos de aguas residuales a las aguas terrestres y al alcantarillado. Especificaciones. 1999.
Source: http://www.technozone.in/img/pdf/Cuba-research-paper-VI.pdf
ISA_A1+3mmBleed_Aprill.pdf 1 25.03.2010 9:55:17 „Pabbinn" var frumsýndur í Iðnó þann 25. janúar 2007. Sýningin fékk strax gríðarlega góðar viðtökur hjá gagnrýnendum sem og áhorf-endum. Eftir rúmlega 50 troðfullar sýningar í Iðnó fluttist sýningin í Íslensku óperuna vegna vinsælda. Einnig hefur Pabbinn verið sýndur rúmlega 30 sinnum á landsbyggðinni. Yfir 30 þúsund Íslendingar hafa séð Bjarna Hauk Þórsson túlka föðurhlutverkið og hlegið sig máttlausa kvöld eftir kvöld. Nú gefst íslensku þjóðinni einstakt tækifæri að eignast Pabbann á DVD.
Patient Information Guide to Cardiac Angiogramand Angioplasty Croí works to improve the quality of life for all through the prevention and control of heart disease, stroke, diabetes and obesity. Our specialist health team equip people with lifesaving skills; provide rapid access cardiac diagnostics; and develop and deliver innovative cardiovascular health care in the areas of prevention and




