Buuconference.buu.ac.th

Available online at www.buuconference.buu.ac.th
The 5th Burapha University International Conference 2016
"Harmonization of Knowledge towards the Betterment of Society"
Effects of ethanol and surfactants on physical properties of
elastic liposomes
Chungrida Kao-ian, Waraporn Suwakul, Nontima Vardhanabhuti*
Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmaceutical Sciences, Chulalongkorn University,
Abstract
Elastic liposomes are highly flexible and able to deliver many substances into the deeper skin layers. Two main compositions are phosphatidylcholine and an edge activator. In this study, elastic liposomes composed of various concentrations of ethanol (5-25% v/v) and Tween® 80 or Span® 80 (15-25% w/w and 5-10% w/w of total lipid, respectively) as the edge activator were designed to investigate the effects of type and amount of surfactants, and concentration of ethanol on physical properties of these liposomes. The physical properties monitored included liposome formation, size and size distribution and elasticity. The complete vesicle formation was observed at low ethanol concentrations (5-15% v/v). The size and size distribution were dependent on type and amount of surfactant, and concentration of ethanol. The vesicle size of liposomes composed of Span® 80 and Tween® 80 was in the range of 5.84-8.08 µm and 3.73-5.27 µm, respectively. The elasticity of liposomes formulations containing Span®80 and Tween®80 was in the range of 27.07-79.86 % and 8.44-15.74 %, respectively. The elasticity of the vesicles tended to decrease when the concentration of ethanol was increased. Formulation containing Span® 80 gave higher elasticity than those containing Tween® 80. The highest elastic property was shown in the formulation that contained 7.5% w/w of Span® 80 and 5% v/v of ethanol. The combination of surfactant and ethanol showed significant effects on physical properties of elastic liposomes.
2016 Published by Burapha University.
Keyword: Elastic liposomes; surfactant; ethanol; elasticity
* Corresponding author. Tel.: +0-662-218-8397; fax: +0-662-218-8401. E-mail address: [email protected].
Proceedings of the Burapha University International Conference 2016, 28-29 July 2016, Bangsaen, Chonburi, Thailand
1. Introduction
Elastic liposomes are liposomes with different features that set them apart from conventional
liposomes. They are highly flexible and are able to deliver many substances into the deeper skin layers (Cevc and Blume, 1992; El Zaafarany et al., 2010; Jain et al. 2008). These liposomes are composed mainly of the structural lipid and an edge activator. The combination renders the vesicles elasticity or deformability. For example, the first reported deformable vesicles Transfersomes® consisted of two main components: phosphatidylcholine as the primary lipid structure and sodium cholate as an edge activator. The surfactants used as an edge activator in the preparations of elastic liposomes can be divided into two groups: (1) anionic surfactants such as sodium cholate (Cevc et al., 1998; Guo et al., 2000; Verma et al., 2003; Lee et al., 2005; Hiruta et al., 2006; Ita et al., 2007; Palino et al., 2012), sodium deoxycholate (Ita et al., 2007), deoxycholic acid (Fang et al., 2006) and (2) nonionic surfactants such as Tween® 80 (El Zaafarany et al., 2010; Lee et al., 2005), Tween® 20 (Oh et al., 2006), Span® 60 (Ita et al., 2007), Span® 80 (Mishra et al., 2007) and Span® 85 (El Zaafarany et al., 2010). Anionic surfactants could be toxic to cells (Paolino et al., 2012) and usually have higher prices than nonionic surfactants (Available from: http://www.sigmaaldrich.com). Ethosomes is another type of modified liposomes using ethanol at higher concentrations (20-50% v/v), which could deliver substances into the skin better than conventional liposomes (Dayan and Toutiou, 2000). Ethosomes are able to deliver hydrophilic and hydrophobic substances both into and through the skin. The higher amounts of ethanol may cause adverse reactions when applied to the skin (Ophaswongse and Maibach, 1994). Thus, both surfactant and ethanol can enhance skin delivery as well as induce skin adverse reactions due to their irritation potentials. The combination of these substances in liposome structure at lower concentrations may be useful in enhancement of skin delivery with reduced adverse effects to skin.
In previous studies, ethanol and surfactant were combined in elastic liposomes. Fang et al. (2006)
used 15% v/v of ethanol combined with 0.25% w/v of deoxycholic acid to deliver catechin. Song et al. (2012) used ethanol (30% v/v) and Tween® 80 (0.53% w/v) for enhanced skin delivery of voriconazole. Ethanol was added into these preparations to enhance solubilities of the tested hydrophobic substances. The type and amount of surfactant and the amount of ethanol are known to affect the physical properties of resultant liposomes when present separately in liposomes (Liu et al., 2013; Mishra et al., 2007; Jain et al., 2008). However, the combined effects of surfactant and ethanol on physical properties of liposomes have not been studied systematically. In this present study, we looked at the effect of combinations of commonly used non-ionic surfactants and ethanol on physical properties of liposomes. The physical properties studied included the ease of vesicle formation, particle size and elasticity of the resultant liposomes. The compositions of lipid mixture and hydrating solution included in this study are displayed in Table 1.
2. Materials and methods
2.1. Materials
Soybean phosphatidycholine (SPC) (Epikuron™ 200) was purchased from Cargill, Inc. (Minneapolis, MN,
USA). Tween® 80 was purchased from BL Hua (Bangkok, Thailand). Span® 80 was purchased from Croda (East Yorkshire, UK).The Isopore® polycarbonate membranes (pore size: 2 and 5µm) were from Millipore (Germany). All other reagents were of at least analytical grade. Ultrapure® water was used throughout the experiments.
Proceedings of the Burapha University International Conference 2016, 28-29 July 2016, Bangsaen, Chonburi, Thailand
2.2. Preparation of liposomes
Liposomes were prepared by film hydration method (New, 1990). The total concentration of the lipid (SPC
+ surfactant) was 57.14 mg/ml. Briefly, a mixture of SPC and surfactant was dissolved in chloroform and placed in a round bottom flask. Chloroform was removed by rotary evaporation at 45 ºC under vacuum to make a thin lipid film. The dry lipid film was hydrated with solution of ethanol in Ultrapure™ water. Resultant liposomes were subsequently extruded through polycarbonate membranes with pore diameter of 5 µm to obtain preparations devoid of unreasonably large liposomes. All liposomal formulations were protected from light and kept at 4º C for further study.
Table 1. Compositions of elastic liposomes
Composition of lipid mixture (% w/w)
Composition of hydrating solution
Ethanol in water (% v/v)
Proceedings of the Burapha University International Conference 2016, 28-29 July 2016, Bangsaen, Chonburi, Thailand
2.3. Characterization of liposomes
The complete formation of elastic liposomes with each composition of liposomes was monitored under an
optical microscope and recorded. The physical appearance of the preparation was observed first with naked eyes. The formation of liposomes was confirmed under optical microscopes with both normal light and cross-polarized light. Size and size distribution were determined by laser diffraction particle size analysis with Mastersizer 2000 (Malvern, UK). The elasticity of liposomes was determined by extruding the formulation through 2 µm-pore diameter polycarbonate membranes. The size and size distribution before and after extrusion was compared. Percentage of elasticity of liposomes (E) was calculated using the following formula:
Where, A= overlapping area of the size distribution profiles obtained before and after extrusion; and B=
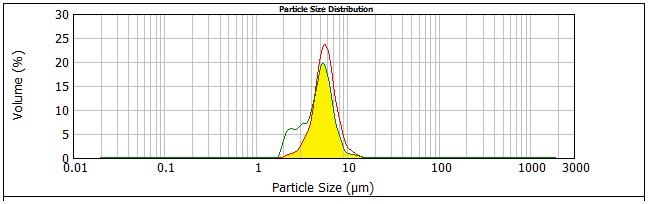
area under the curve of the size distribution profile before extrusion. (Fig. 1)
Figure 1. Particle size distribution profiles of S7.5_ 5 before and after extrusion through 2µm-pore diameter polycarbonate membranes. The overlapping area (A) is shown in bright yellow shaded area.
3. Results and discussion
Liposomes were prepared from lipid compositions shown in Table 1 and were characterized for their
physical appearances, size and size distribution and elasticity. The formulations contained Span® 80 and Tween® 80 in the same molar ratio range of 0.1-0.2. The acceptance criteria for completeness of liposome formation included being milky homogeneous dispersion, spherical vesicles, absence of lipid crystals, Brownian movement of vesicles and being birefringent of the lamellar structure under the cross-polarized light. All formulations studied were homogeneous and turbid with white to pale yellow color. All formulations containing 5 and 15% ethanol formed complete vesicles, regardless of type and concentration of surfactant (Table 2). At higher ethanol concentration, complete liposome formation was seen only with the formulation with 25% Tween® 80 (T25_20). The phospholipid might be solubilized at high ethanol and surfactant concentrations (Touitou et al., 2000). In addition, surfactant solubility might be increased at high alcohol concentration (Wu et al., 2007; Yeh et al., 2005). Moreover, mixed micelles could form when surfactant concentration was increased (Lopez et.al, 1998). Thus, the formation of vesicles was disturbed.
Proceedings of the Burapha University International Conference 2016, 28-29 July 2016, Bangsaen, Chonburi, Thailand
Table 2. Vesicle formation of the formulations studied
Average sizes (µm).
Average sizes (µm).
Y = complete formation N = In-complete formation N/A = not available
The vesicle size of liposomes composed of Span® 80 and Tween® 80 was in the range of 5.84-8.08
µm and 3.73-5.27 µm, respectively (Table 2). The vesicle size of liposomes tended to decrease with increasing ethanol contents, except the S7.5 and the S10. This finding agreed well with previous studies (Chourasia, Kang and Chan, 2011; Elsayed et al., 2007; Touitou et al., 2000). The packing of phospholipid bilayer could be tighter with increasing ethanol contents, resulting in a smaller size of vesicles. Mishra et al. (2007) and Jain et al. (2008) found that increased concentration or decreased HLB of surfactant decrease the vesicle size. However, the effect of surfactant concentration was inconclusive in this study (Table 2). However, the size of liposomes composed of Tween ® 80 (HLB = 15) was smaller than that of liposomes composed of Span® 80 (HLB = 4.3). The reason for this finding might be attributed to the result from a previous study that Tween® 80 enhances hydrophilicity of liposome surface produced by polyoxyethylene chain (Liu et. al, 2013).
The elasticity of liposome formulations containing Span®80 and Tween®80 was in the range of
27.07-79.86% and 8.44 - 15.74%, respectively. The results indicated that elasticity of vesicles depended on surfactant type, surfactant concentration and ethanol content. With increasing surfactant concentration, the elasticity was increased and then decreased. Thus, only at a suitable surfactant concentration would result in the highest elasticity. Similar findings have been published (El Zaafarany et al., 2010; Mishra et al., 2007; Jain et al., 2008). The elasticity of liposomes tended to decrease when the concentration of ethanol was increased as shown in Table 3.The incorporation of ethanol in the phospholipid bilayer increases the fluidity of the bilayer that is responsible for increase in elasticity of vesicle membrane (Touitou et al., 2000). At high ethanol contents, the strength of phospholipid bilayer is decreased because the phospholipid solubility is increased. In this study, Span® 80 had higher ability to incorporate into phospholipid bilayer than Tween®80 because it is more hydrophobic. Thus higher elasticity was shown in liposomes composed of Span® 80.
Proceedings of the Burapha University International Conference 2016, 28-29 July 2016, Bangsaen, Chonburi, Thailand
Table 3. Effect of ethanol and surfactant concentrations on % elasticity of formulations studied. The data are shown as mean ± SD, n = 3.
Significant differences were seen among all formulations (p < 0.05)
Ethanol concentration (% v/v)
Ethanol concentration (% v/v)
N/A = not available This present study indicated elastic liposomes could be developed using the combinations of
surfactant and ethanol if the right combinations of the two components were selected. Span®80 could form elastic liposomes with less restriction than Tween®80. The most suitable ethanol concentration was in the range of 5-15% v/v. The type and concentration of surfactant, as well as ethanol concentration, had different effects on the formation and physical properties of elastic liposomes.
The authors are grateful to several persons at the Faculty of Pharmaceutical Sciences, Chulalongkorn
University. Professor Garnpimol Ritthidej, Ph.D., of the Department of Pharmaceutics and Industrial Pharmacy for providing the Mastersizer 2000, Assistant Professor Vichien Jongbunprasert of the Department of Pharmacognosy and Pharmaceutical Botany for the access to the optical microscopes. Miss Keaw Kajornchaiyakul of the Scientific and Technological Research Equipment Centre Chulalongkorn University is also highly appreciated for the use of small volume dispersion unit for size analysis. Our appreciation is also directed to Mr. Wichpong Kao-ian of the Department of computer engineering, Faculty of Engineering, Kasetsart University for his computer programming support.
References
Cevc, G., Blume, G., 1992. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force,
Biochimica et Biophysica Acta 1104, pp. 226–232.
Cevc, G., Gebauer, D., Stieber, J., Schatzlein, A., Blume, G.1998. Ultraflexible vesicles, Transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin, Biochimica et Biophysica Acta 1368, pp 201–215. Chourasia, M. K., Kang, L., Chan, S. Y., 2011. Nanosized ethosomes bearing ketoprofen for improved transdermal delivery, Results in
Pharma Sciences 1, pp. 60–67.
Dayan, N., Touitou, E., 2000. Carriers for skin delivery of trihexyphenidyl HCl: ethosomes vs. liposomes, Biomaterials 21, pp. 1879–
El Maghraby, G. M. M, Williams, A. C., Barry, B. W., 2000. Oestradiol skin delivery from ultradeformable liposomes: refinement of
surfactant concentration, International Journal of Pharmaceutics 196, pp. 63-74.
El Zaafarany, G. M., Awad, G. A., Holayel, S. M., Mortada, N. D., 2010. Role of edge activators and surface charge in developing
ultradeformable vesicles with enhanced skin delivery, International Journal of Pharmaceutics 397, pp. 164–172.
Elsayed, M. M. A., Abdallah, O. Y., Naggar, V. F., Khalafallah, N. M., 2007. Deformable liposomes and ethosomes as carriers for skin
delivery of ketotifen, Pharmazie 62, pp. 133-137.
Fang, J. Y., Hwang, T. L., Huang, Y. L., Fang, C. L., 2006. Enhancement of the transdermal delivery of catechins by liposomes
incorporating anionic surfactants and ethanol, International Journal of Pharmaceutics 310, pp. 131–138.
Guo, J., Ping, Q., Zhang, L., 2000. Transdermal Delivery of Insulin in Mice by Using Lecithin Vesicles as a Carrier, Drug Delivery 7, pp.
Proceedings of the Burapha University International Conference 2016, 28-29 July 2016, Bangsaen, Chonburi, Thailand
Hiruta, Y., Hattori, Y., Kawano, K., Obata, Y., Maitani, Y., 2006. Novel ultra-deformable vesicles entrapped with bleomycin and
enhanced to penetrate rat skin, Journal of Controlled Release 113, pp. 146–154.
Ita, K. B., Du Preez, J., Lane, M. E., Hadgraft, J., du Plessis, J., 2007. Dermal delivery of selected hydrophilic drugs from elastic
liposomes: effect of phospholipid formulation and surfactants, The Journal of Pharmacy and Pharmacology 59, pp. 1215–1222.
Jain, S. K., Gupta, Y., Jain, A., Rai, K., 2008. Enhanced transdermal delivery of acyclovir sodium via elastic liposomes, Drug Delivery
15, pp. 141-147.
Lee, E. H., Kim, A., Oh, Y. K., Kim, C. K., 2005. Effect of edge activators on the formation and transfection efficiency of
ultradeformable liposomes, Biomaterials 26, pp. 205–210.
Liu, Y. S., Wen, C. F., Yang, Y. M., 2013. Quercetin deformable liposome: Preparation and efficacy against ultraviolet B induced skin
damages in vitro and in vivo, Journal of Photochemistry and Photobiology B: Biology 127, pp. 8-17.
López, O., de la Maza, A., Coderch, L., López-Iglesias, C., Wehrli, E., Parra, J. L., 1998. Direct formation of mixed micelles in the
solubilization of phospholipid liposomes by Triton X-100, FEBS Letters 426, pp. 314-318.
Mishra, D., Garg, M., Dubey, V., Jain, S., Jain, N. K., 2007. Elastic liposomes mediated transdermal delivery of an anti-hypertensive
agent: propranolol hydrochloride, Journal of Pharmaceutical Sciences 96, pp. 145–155.
New, R. R. C., 1990. Liposomes A Practical Approach. Oxford University Press, New York, pp. 37-40; 105-109. Ophaswongse, S., Maibach, H.I., 1994. Alcohol dermatitis: allergic contact dermatitis and contact urticarial syndrome, Contact
Dermatitis 30, pp. 1-6.
Oh, Y. K., Kim, M. Y., Shin, J. Y., Kim, T. W., Yun, M. O., Yang, S. J., Choi, S. S., Jung, W. W., Kim, J. A., Choi, H. G., 2006. Skin
permeation of retinol in Tween 20-based deformable liposomes: in-vitro evaluation in human skin and keratinocyte models, The Journal of Pharmacy and Pharmacology 58, pp. 161–166.
Paolino, D., Cosco, D., Cilurzo, F., Trapasso, E., Morittu, V. M., Celia, C., Fresta, M., 2012. Improved in vitro and in vivo collagen
biosynthesis by asiaticoside-loaded ultradeformable vesicles, Journal of Controlled Release 162, pp. 143-151.
Sigma-Aldrich Co. LLC. Available fro2015, January, 4]. Song, C. K., Balakrishnan, P., Shim, C. K., Chung, S. J., Chong, S., Kim, D. D., 2012. A novel vesicular carrier, transethosome, for
enhanced skin delivery of voriconazole: characterization and in vitro/in vivo evaluation, Colloids and Surfaces B: Biointerfaces 92, pp. 299–304.
Touitou, E., Dayan, N., Bergelson, L., Godin, B., Eliaz M., 2000. Ethosomes - novel vesicular carriers for enhanced delivery:
characterization and skin penetration properties, Journal of Controlled Release 65, pp. 403 – 418.
Verma, D. D., Verma, S., Blume, G., Fahr, A., 2003. Liposomes increase skin penetration of entrapped and non-entrapped hydrophilic
substances into human skin: a skin penetration and confocal laser scanning microscopy study, European Journal of Pharmaceutics and Biopharmaceutics 55, pp. 271–277.
Wu, K. C, Huang, Z. L, Yang Y. M, Chang C. H., Chou, T. H., 2007. Enhancement of catansome formation by means of cosolvent effect:
Semi-spontaneous preparation method, Colloids and Surfaces A: Physicochemical and Engineering Aspects 302, pp. 599-607.
Yeh, S. J., Yang, Y. M., Chang, C. H., 2005. Cosolvent Effects on the Stability of Catanionic Vesicles Formed from Ion-Pair
Amphiphiles, Langmuir 21, pp. 6179-6184.
Submission_BUUConf2016 Osteoporosis Dr.Kunavut Vannajak.pdf
S12 kunavut.pdf
Submission_BUUConf2016 Osteoporosis Dr.Kunavut Vannajak.pdf
Vuorinen, J., Tuominen, J. 1994. Fieller's Confidence Intervals for the Ratio of Two Means in the Assessment of Average Bioequivalence from Crossover Data, Statistics in Medicine 13, p. 2531-2545.
7.1 Program.pdf
Source: http://www.buuconference.buu.ac.th/registration/getdownload.php?name=paper108&file=file/BUU2016/paper108.pdf
Guide du futur transplanté du foie Qu'est-ce qu'une transplantation ? La transplantation est une opération par laquelle un organe malade est remplacé par un organe sain prélevé sur une personne décédée. La personne transplantée est appelée un receveur, celle qui est décédée est un donneur. L'organe prélevé est un greffon. Quand transplante-t-on ?
Exp21-pages anglais 4-15 24/05/04 16:14 Page 6 SLIT vs SCIT - The first placebo controlled trial The eighty-nine patients enrolled had at least 2years of seasonal birch pollen rhino-conjunctivitis Sublingual immunotherapy (SLIT) has been uncontrolled by conventional pharmacotherapy (2). investigated for at least fifteen years. Many



