Microsoft word - 19.09 _issue 3_.doc

Safe Use of Injectable Medicines – 19.09
SECTION:
MEDICINES
MANAGEMENT
POLICY AND PROCEDURE NO:
NATURE AND SCOPE:
POLICY AND PROCEDURE - TRUST WIDE
SUBJECT:
SAFE USE OF INJECTABLE MEDICINES
This policy and procedure is concerned with the safe use of injectable medicines
within the Trust
DATE OF LATEST RATIFICATION:
JUNE 2013
RATIFIED BY:
TRUST DRUG & THERAPEUTIC COMMITTEE
IMPLEMENTATION
ASSOCIATED TRUST POLICIES
AND PROCEDURES:
Safe and Secure Handling of Medicines – 19.01 Occupational Exposure to Blood Bourne Viruses - 18.6 Unlicensed Use of Medicine – 19.07 Hand Hygiene – 18.04 Use of Intramuscular or Intravenous Medication in Rapid Tranquillisation – 19.05 Sharps Management - 18.11
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
NOTTINGHAMSHIRE HEALTHCARE NHS TRUST
SAFE USE OF INJECTABLE MEDICINES POLICY
CONTENTS
1.0 Introduction
9.0 Consultation
10.0 Relevant Trust Policies
11.0 Monitoring Compliance
12.0 Equality Impact Assessment
13.0 Legislation
14.0 Champion & Expert Writer
15.0 Implementation
16.0 References/Source
Core Procedures All Injections
Mechanically Engineered Safety Systems (Retractable Needle/Syringes)
Procedures for Preparing Injections
Administering an Intramuscular Injection
Additional Guidance on the Preparation and Administration of Depot Antipsychotics including Risperdal Consta Long Acting Injection and Paliperidone Palmitate Long Acting Injection
Administering a Subcutaneous Injection
Appendix 7 Additional Information on the Administration of Specific Drugs (e.g. vaccines,
Pabrinex IMHP, adrenaline 1:1000, insulins, LMWHs)
Appendix 8 Record of Changes
Appendix 9 Record of Employee Having Read the Policy/Procedure
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
NOTTINGHAMSHIRE HEALTHCARE NHS TRUST
SAFE USE OF INJECTABLE MEDICINES POLICY AND PROCEDURE
1.0 INTRODUCTION
1.1
Injectable medicines are being used to a greater extent in the NHS than ever before. Research evidence indicates that the incidence of errors in prescribing, preparing and administering injectable medicines, is higher than for other forms of medicine. The National Patient Safety Agency (NPSA) issued a patient safety alert in March 2007 "Promoting Safer Use of Injectable Medicines" and recommending that NHS organisations take a number of steps to improve patient safety in this area.
This policy and any associated Divisional operational procedures, detail how the Trust and its staff, will ensure that injectable medicines are used safely and in line with best practice, to reduce risks of potential harm to patients and to increase staff safety, through reduction in needle stick injuries.
This policy has a number of core operational procedures attached in the appendices which apply across the Trust. Divisions may produce additional, more specific, procedural guidance for staff working in their clinical areas.
This policy and procedure should be read in conjunction with the appropriate Divisional Medicines Code.
2.0 DEFINITIONS
2.1
"Injectable medicines" refer to those sterile medicines intended for administration by bolus injection, perfusion or infusion by any of the following routes: subcutaneous, intramuscular, intravenous, intrathecal, intra-arterial, intradermal, intraventricular, epidural, intravesicular, intravitreal, intrapleural and intraocular.
"High-risk products/procedures" are those medicinal products/procedures, whose preparation and/or administration, have been identified by risk assessment as most likely to pose a significant risk to patients.
"Purchasing for safety" is the process whereby medicinal products are reviewed by pharmacy, drug and therapeutic committee groups, so that only those products - presentations and formulations - designed in such a way to promote safer practice, are selected for purchase.
PRINCIPLES
The Trust's operational divisions will undertake regular risk assessments of injectable medicinal products and procedures, in all clinical areas, to identify high risks and develop action plans to minimize them.
All clinical areas will have access to up-to-date protocols and procedures, for prescribing, preparing and administering injectable medicines.
Essential technical information on injectable medicines, will be available and accessible to healthcare staff in clinical areas at the point of use.
Pharmacy services will ensure that healthcare staff have access to full technical information, for all injectable medicinal products used in clinical areas. This will include provision of the manufacturer's information leaflet and access to the product's Summary of Product Characteristics (via http://www.medicines.org.uk/emc/ ). Further information and advice can be obtained from the Pharmacy intranet (e.g. link to the Medusa Injectable Medicines Guide) or from the Pharmacy Medicines Information Service.
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
The Trust will implement a "purchasing for safety" policy, to promote procurement of injectable medicines with inherent safety features.
It is recognised that the actions of a healthcare professional, can influence the patient's physical and emotional experience and intended outcome. Poor injection technique can cause adverse outcomes for the patient e.g. pain, local bruising.
Injections should be prepared and administered only by healthcare staff (or patients/carers) who: • understand the risks involved
• have been trained to use safe procedures
• have demonstrated their competence for the task in line with professional codes of
The Trust will provide training for, and supervision of, all healthcare staff involved in prescribing, preparing, administering and monitoring injectable medicines, to enable staff to have the necessary work competencies to undertake their duties safely.
As part of the annual medicines management audit programme, the Trust will include an audit of medication practice with injectable medicines.
3.10 The associated procedures aim to introduce clear and consistent procedures, for the safe
preparation and administration of injectable medicines.
3.11 The Trust has developed a specific policy and procedure for the use of injectable medicines
in rapid tranquillisation. This policy (19.05) and associated Divisional procedures can be found on the Trust intranet site and should be used in conjunction with the guidance found in this policy.
3.12 The administration of intravenous medication is very rare in the Trusts mental health
settings. In the Trusts Health Partnerships Division this form of administration can take place. The administration of intravenous medication will always be undertaken by staff members trained to do so, usually medical staff. All intravenous (IV) administration procedures are outlined in the local Acute Hospital (Nottingham University Hospital) Trust's Guide to Intravenous (IV) Therapy (available via the Trust Pharmacy Intranet).
3.13 Aseptic (non-touch) technique should be used during preparation and administration of
injections. Avoid touching areas where bacterial contamination may be introduced (e.g. syringe tips, needles, vial tops). Never put down a syringe attached to an unsheathed needle.
3.14 Injectable medicines prepared in clinical areas, should always be administered immediately
after preparation. They should not be stored for a period of time before use. Preparation and administration should be one un-interrupted process.
3.15 If in exceptional circumstances, an injection is prepared but is not administered, it must be
labelled immediately after preparation by the person who prepared it (see Appendix 3). Prepared injectable medicines are sterile and at risk of microbiological contamination. Manufacturer's recommendations and advice from pharmacy should be followed, regarding the length of time a prepared product can be kept before it is administered. It is recommended that any prepared injectable medication is stored for the shortest length of time possible.
3.16 The Trust has fully supported the introduction of syringe/needle devices, with engineered
safety mechanisms to reduce incidents of needlestick injuries. The use of retractable needles and other syringe devices with in-built safety mechanisms are now established in the Trust as the device of choice for the administration of most injectable medicines.
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
3.17 Trust staff are expected to use retractable needles and or other devices with engineered
safety mechanisms, to administer injectable medicines. Conventional needles should only be used in exceptional circumstances and their use must be authorised by the Associate Directors of Nursing, Local, Health Partnerships and Forensic Divisions. For further information on retractable/other syringe devices with built-in safety mechanisms, see Appendix 2.
4.0 PROCEDURES
Core procedures for the safe administration of injectable medication are detailed in the appendices to this policy. The core procedures apply across the Trust. Should a need arise within a Division for additional procedures to be developed this can be done however these must be approved by the Divisional Drug and Therapeutics Committee or Medicines Management Committee and be supported by the Divisions Associate Director of Nursing.
5.0 DUTIES
The Trust has a responsibility to provide adequate and appropriate clinical areas, for the preparation of injectable medications in all in-patient and out-patient settings and appropriate equipment for the safe administration of injectable medicines in the community.
It is the responsibility of each employee of the Trust, to maintain the highest standard of practice, in preparing and administering injectable medicines. Staff members should understand what is expected of them and be confident and competent in discharging their responsibilities.
All of the professional regulatory bodies include, as part of their core standards, a requirement for individual practitioners to be aware that there is a potential risk to patients and to take all necessary steps to ensure that their practice is such that it minimises or removes these.
Executive Directors, Clinical Directors, General Managers, Associate Directors of Nursing and Modern Matrons, will be responsible for ensuring that this policy and associated procedures are implemented within each Care Group they manage.
6.0 TRAINING
It is recognised that basic training in the preparation and administration of injectable medication, forms part of the pre-registration training programme for nursing and other healthcare professionals.
Healthcare professionals are required to maintain their competencies as part of their continued professional registration.
The Trust will provide support, supervision and update training, for individual healthcare staff involved in prescribing, preparing, administering and monitoring injectable medicines, where required. Staff should assess and review their competencies as part of annual performance review and seek guidance or update training as identified.
The NPSA have produced four injectable medicine competency statements, to assist healthcare professionals maintain their competencies:
• Competence 1 – Prescribing injectable medicines
• Competence 2 – Preparation of injectable medicines
• Competence 3 – Administration of injectable medicines
• Competence 4 – Monitoring the administration of injectable medicines
These can be downloaded from: http://www.npsa.nhs.uk/nrls/alerts-and-directives/alerts/injectable-medicines/
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
The BMJ have produced an interactive e-learning module (free registration) on injectable medicines: prescribing, preparing and administering. This can be accessed at http://n3.learning.bmj.com/learning/module-intro/injectable-medicines-prescribing-preparing-administering.html?locale=en_GB&moduleId=10009161
The United Kingdom Psychiatric Pharmacy Group (UKPPG) have written guidance on the administration to adults of oil-based depot and other long-acting intramuscular antipsychotic injections. This is available via the Pharmacy intranet or can be downloaded from the following website: http://www2.hull.ac.uk/fhsc/newsandevents/news/injectionguide.aspx
7.0 TARGET
AUDIENCE
The target audience for this policy and procedure are qualified nursing, medical and pharmacy staff involved in the purchasing, supply, prescribing, preparation and administration of all injectable medicinal products.
Healthcare professionals will be required to signify that they have received and read the policy and associated operational procedures.
8.0 REVIEW
This procedure will be reviewed 3 years after implementation, or in the light of changing legislation, or in circumstances (such as changes to best clinical practice) that require a review.
9.0 CONSULTATION
9.1
The following groups have been included in the consultation: Trust Drug and Therapeutics Committee, Divisional Drug and Therapeutics Committees, Modern Matrons and the Executive Leadership Council (ELC).
RELEVANT TRUST POLICIES
• Safe and Secure Handling of Medicines – 19.01
• Occupational Exposure to Blood Bourne Viruses - 18.06
• Unlicensed Use of Medicine – 19.07
• Hand Hygiene – 18.04 • Use of Intramuscular or Intravenous Medication in Rapid Tranquillisation – 19.05
• Safe Management and Disposal of Sharps - 18.11
11.0 MONITORING
COMPLIANCE
11.1 An annual multidisciplinary injectable medicines audit should be conducted along side other
Trust-wide medicines management audits.
11.2 The audit should include a review of risk assessments of injectable medicine procedures
and products, a review of incident reports, and an assessment of compliance with NPSA recommendations.
11.3 The report should be forwarded to the Trust Risk Management Group and to the Drug and
Therapeutics Committees.
12.0 EQUALITY
ASSESSMENT
12.1 This policy has been screened to identify its relevance to Equality and Diversity. Actions
identified have been incorporated.
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
13.0 LEGISLATION COMPLIANCE
13.1 The procedure has been considered in the context of relevant legislation, as outlined in the
Trust Safe and Secure Handling of Medicines Policy (19.01).
CHAMPION AND EXPERT WRITER
14.1 The Champion of this policy is Dean Howells, Executive Director of Nursing, Quality and
Patient Experience. The Expert Writers are Steve Williamson (Senior Nurse), John Lawton (Clinical Pharmacy Services Manager, Nottingham Services).
15.0 IMPLEMENTATION
15.1 The policy will be implemented immediately after ratification.
REFERENCES /SOURCE DOCUMENTS
16.1 NPSA Patient Safety Alert 20 (March 2007). Promoting Safer Use of Injectable
Medicines (NPSA/2007/20). Available at: http://www.npsa.nhs.uk/nrls/alerts-and-directives/alerts/injectable-medicines/
16.2 Resuscitation Council (UK). Emergency treatment of anaphylactic reactions:
guidelines for healthcare providers (January 2008). Available at: www.resus.org.uk
16.3 Department of Health Immunisation against Infectious Diseases, "The Green Book".
Available at: http://immunisation.dh.gov.uk/category/the-green-book/
16.4 BNF. Available at: http://www.bnf.org/bnf/index.htm 16.5 Palliative Care Formulary (PCF). Available at:
http://www.palliativedrugs.com/palliative-care-formulary.html Note: Requires a simple registration for free access to on-line PCF.
16.6 NUH Guide to Intravenous Therapy. Available via the Pharmacy Local Services Intranet
under "Injectable Medicines".
16.7 Electronic Medicines Compendium (eMC). Available at: http://www.medicines.org.uk/emc/ 16.8 Medusa Injectable Medicines Guide. Available at: http://medusa.wales.nhs.uk/ (registration
required – see Pharmacy Local Services Intranet under "Injectable Medicines"). Alternatively, contact your Pharmacy Medicines Information Service.
16.9 The Royal Marsden Hospital Manual of Clinical Nursing Procedures. Wiley-Blackwell. 16.10 Feetam C. and White J. Eds. Guidance on the administration to adults of oil-based depot
and other long-acting intramuscular antipsychotic injections. 3rd Edition. September 2011. This can be downloaded from the Pharmacy intranet or the following website: http://www2.hull.ac.uk/fhsc/newsandevents/news/injectionguide.aspx
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
APPENDIX 1
CORE PROCEDURES FOR ALL INJECTIONS
1. Before Preparing the Injection
- Check the patient's care plan. All patients will have a physical assessment which details any known risks that may need to be incorporated into their care plans for medications administered via injectable routes. These may include:
• Any known adverse drug reactions, allergies or hypersensitivities
• Previous complications following the administration of an injectable medication
• Any known physical health condition, such as high blood pressure, diabetes
mellitus, raised cholesterol, liver or renal impairment, or the confirmed presence of a blood borne virus
• General condition of the injection site
• Other relevant factors
- Read all prescription details and confirm that the prescription is for the identified patient to be treated and that the patient has no known allergy to the medicine.
- When a patient is being treated under the Mental Health Act (1983), consideration must be given to patients rights under the Act. Where a patient is being treated, refuses treatment, or is unable to consent to treatment, a copy of the relevant Mental Health Act documentation (Form T2/T3/T4) should be attached to the medication card. This documentation must be checked prior to preparing the injection, to ensure it is covered by the treatment plan. The Mental Health Act Office must be notified of any medication given under Section 62 (urgent cases).
2. Preparing the Injection
- Prepare the injection in a designated clean area, free from interruption and distractions, preferably in a clinic room. Select and assemble the equipment required and check that all materials are intact and in date. The following items may be included:
• Prescription
• Medicine ampoule(s)/vial(s), diluent (flushing solution if required)
• Appropriate size syringe with retractable/safety needle
• Ampoule snapper device (if required)
• Disposable Injection tray
• Gauze or cotton wool
• Non-sterile latex-free gloves
• Plasters ( check allergy)
• Sharps disposal container, as close as possible to area of administration.
- When selecting the prescribed medication, check the expiry date, check for damage to containers including, contamination and that the medication has been stored in line with manufacturer's guidance (e.g. in the refrigerator). - Read medication labels carefully and be aware of similar looking medicine packs, names and strengths. If unsure contact your Pharmacy Department.
- Check that the formulation, dose, diluent, infusion fluid and rate of administration
correspond to the prescription and product information. If you do not understand the
method of preparation, STOP NOW, and take advice from another healthcare professional.
- Calculate the volume of medicine solution needed to give the prescribed dose. Write the calculation down. For more complex calculations it is always good practice for a second registered healthcare professional to check the calculation independently in order to minimize the risk of error.
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
- There are a number of "higher-risk" medicines that must be prepared and checked by TWO qualified nurses (e.g. all IV injections, Controlled Drugs (CDs), insulin and heparin injections). Refer to your Divisional Medicines Code. For all other injections it is good practice to have another registered healthcare professional "double-check" the prescription to confirm the dose is due and that it hasn't already been given. The checker should also confirm that any dose calculation is accurate and that the preparation of the injection for administration is correct and that it is in date. In the absence of a second professional it is good practice to consider asking the patient to check the expiry date of the injection and that the dose is correct according to the prescription.
- Cleans hands in line with local procedures by washing hands and/or perform hand decontamination using an alcohol gel/hand wipe and put on a pair of disposable protective gloves. Please refer to Trust Hand Hygiene Policy - 18.04 for full details.
- Prepare the injection by following the approved procedures as detailed in Appendix 2 or the manufacturer's product information.
3. Pre-filled Syringes, Pens and Cartridges
- Some medicines are packaged in pre-filled syringes, disposable pens or pen cartridges. For example, enoxaparin sodium pre-filled syringes, range of min-i-jet pre-filled syringes, Epipen Auto Injector, insulin pre-filled pens (disposable or refillable types), Revaxis injection, GluCagen HypoKit. - The manufacturer's instruction sheet should be referred to when preparing these formulations for injection.
4. Before Administering Any Injection
- Check all the following against the prescription:
• patient's name, hospital number, date of birth
• prescriber's
• approved medicine name
• dose and frequency of administration
• end and start date of prescription and date of administration
• route of administration
• allergy status of the patient - check allergy box is signed/dated
- Also check, where relevant:
• brand name and formulation of the medicine • concentration or total quantity of medicine, in the final syringe or infusion
• name and volume of diluent and/or infusion fluid
• rate and duration of administration
• age and weight of any patient under 16 years of age
• date on which treatment should be reviewed
- Check that the medicine is due for administration at that time and has not already been given. - Assemble all the equipment you need in a disposable injection card tray, along with the patient's prescription chart, and sharps container.
5. Preparing the Patient Prior to Administering the Injection
- Confirm the identity of the patient.
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
- Explain the procedure and check the patients understanding of it. A patient advice leaflet on injectable medicines is available via the Trust Intranet.
- Seek the consent of the patient and offer choices in line with patient preferences, if possible, taking into account diversity and cultural issues.
- Agree a suitable environment with the patient for them to receive the injection.
- Allow the patient the opportunity to talk openly about their treatment. If the patient receives regular injections, discuss any previous effects of injections, such as side-effects or current problems with injection sites, and report them back to the patient's, MDT or care co-coordinator. Use the opportunity to reinforce positive health messages on diet, physical activity, smoking and alcohol if appropriate.
- Privacy and dignity issues, such as gender sensitivities, are addressed wherever possible.
- Agree with the patient which position they prefer to be in to receive the injection. Some patients may have a preference to lie down or stand, when receiving deep intramuscular depot antipsychotic injections into the gluteal muscle.
- Inspect the injection site and avoid areas where there is evidence of inflammation, scarring or abrasions/lesions. Rotate the use of injection sites to avoid irritation, scarring, hardening of the tissues and pain. Record the site used (e.g. left gluteal or right gluteal) in the patient's clinical record and on the drug chart.
6. Administering an Intramuscular Injection - Refer to Appendix 4.
7. Administering a Subcutaneous Injection - Refer to Appendix 6.
8. After Administration of an Injectable Medicine
- After completion of an injection, ask the patient to report promptly any soreness, leakage or inflammation at the injection site and discomfort of any sort.
- Discard any syringes, needles, empty ampoules/vials from which the injection was prepared and any unused medicine in a sharps disposal container according to Trust policy. - Ampoules or vials should never be used to prepare more than one injection, unless specifically labelled by the manufacturer for ‘multi-dose' use.
- Make a detailed record of administration, on the prescription chart and in the patient's healthcare running records.
- In the case of infusions, re-check the administration site for signs of leakage, infection, or inflammation, at regular intervals. Continue to monitor the patient, the contents of the infusion container and the rate of infusion, according to the Local Acute Trust Guide to Intravenous Therapy.
- Check that arrangements for monitoring fluid balance, or clinical parameters, have been made. Staff must be aware of their responsibilities for monitoring and recording and understand when to seek guidance. - Ensure that relevant documentation is made available for subsequent regular monitoring to take place.
ISSUE 3 – JUNE 2013




Safe Use of Injectable Medicines – 19.09
APPENDIX 2
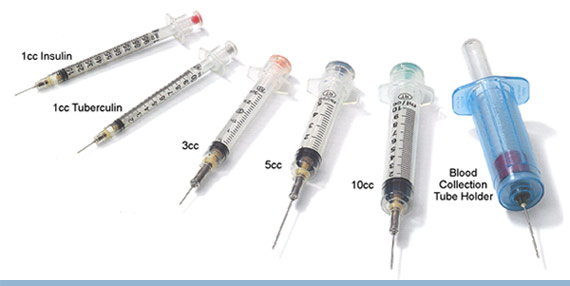
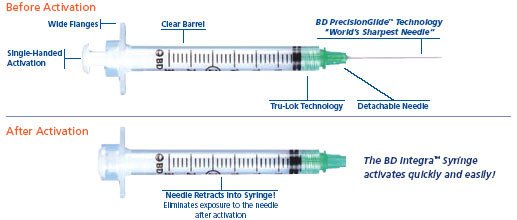
MECHANICALLY ENGINEERED SAFETY DEVICES (RETRACTABLE SYRINGES/NEEDLES):
VanishPoint, BD Integra, BD Eclipse
This information sheet summarises the different products and provides guidance on their place in
clinical practice.
VanishPoint
S.C insulin Syringe_Brochure.
BD Integra
BD Blunt Integra
(non-retractable,
filter needle)
BD Eclipse
(non-retractable)
to fit onto (0.6mmx25mm)
Info Summy Sht 05
(non-retractable)
1 – To open up PDF files click on PDF icon on on-line version, available on Trust Intranet – Pharmacy (Local Services) – Injectable Medicines – Retractable and Safety Needles (JDL Retractable September 2009 (3))
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
MECHANICALLY ENGINEERED SAFETY DEVICES (RETRACTABLE SYRINGES/NEEDLES):
VanishPoint
Syringes with non-detachable retractable needle
BD Integra
Syringe with detachable green retractable needle
Detachable retractable
needles for use with
BD Eclipse
Safety needle (non-retractable)
Fits onto standard BD Plastipak Luer Slip and Luer Lock syringes
BD Blunt Fill red needle (non-retractable),
Safety needles available
compatible with standard
in green, blue and
Slip/Lock syringe
(JDL Retractable June 2009 (2))
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
APPENDIX 3
PROCEDURES FOR PREPARING INJECTIONS
Procedure for Withdrawing Solution or Suspension from an Ampoule (glass or
plastic) into a Syringe
Tap the ampoule gently to dislodge any medicine in the neck.
Snap open the neck of glass ampoules by holding the ampoule at the base and placing thumb over the score line, then apply gentle pressure away from the body to snap the top of the ampoule off. Use an ampoule snapper if required (available from Pharmacy).
Using a needle, draw the required volume of solution into the syringe, taking care not to apply excessive pressure should the needle come into contact with the sides or base of the ampoule. Tilt the ampoule if necessary. For highly viscous preparations (e.g. concentrated oil-based depot antipsychotic injections) it is often easier to draw up using a wide-bore blunt fill needle (e.g. red BD Blunt Fill, 18g (1.2mm)) and then carefully swap this for an administration needle.
If the ampoule contains a suspension rather than solution, it should be gently swirled to mix the contents immediately before they are drawn into the syringe.
Invert the syringe and tap lightly to aggregate the air bubbles at the needle end. Expel the air carefully.
If the injection is not used immediately discard
When labelling a prepared syringe take care not to not obscure the volume graduation markings on the syringe
Keep the ampoule and any unused medicine in the injection tray, until administration is complete to enable further checking procedures to be undertaken.
Withdrawing a Solution or Suspension from a Vial into a Syringe
Remove the tamper-evident seal from the vial and wipe the rubber septum with an alcohol wipe. Allow to dry for at least 30 seconds.
With the needle sheathed, draw into the syringe a volume of air, equivalent to the required volume of solution to be drawn up.
Remove the needle cover and insert the needle into the vial through the rubber septum.
Invert the vial. Keep the needle in the solution and slowly depress the plunger to push the air into the vial.
Release the plunger, so that solution flows back into the syringe.
If a large volume of solution is to be withdrawn, use a push-pull technique. Repeatedly inject small volumes of air and draw up an equal volume of solution until the required total is reached. This ‘equilibrium method' helps to minimise the build-up of pressure in the vial.
Alternatively, the rubber septum may be pierced with a second needle, to let air into the vial as solution is withdrawn. The tip of the vent needle must always be kept above the solution to prevent leakage.
If the vial contains a suspension rather than solution, it should be gently swirled to mix the contents immediately, before they are drawn into the syringe.
With the vial still attached, invert the syringe. With the needle and vial uppermost, tap the syringe lightly to aggregate the air bubbles at the needle end. Push the air back into the vial.
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
Fill the syringe with the required volume of solution, then draw in a small volume air. Withdraw the needle from the vial.
Carefully expel excess air from the syringe.
Keep the vial, and any unused medicine, in the injection tray, until administration is complete to enable further checking procedures to be undertaken.
3 C. Reconstituting Powder in a Vial or Ampoule and Drawing the Resulting Solution or
Suspension into a Syringe
Follow procedure 3A1/3A2 or 3B1 above.
Use procedure 3A3, to withdraw the required volume of diluent (e.g. water for injections or sodium chloride 0.9%) from the ampoule into the syringe.
Inject the diluent into the vial/ampoule. Keeping the tip of the needle above the level of the solution in the vial, release the plunger. The syringe will fill with the air which has been displaced by the solution (if the contents of the vial were packed under a vacuum, solution will be drawn into the vial and no air will be displaced). If a large volume of diluent is to be added, use a push-pull technique (see 3B6 above).
With the syringe and needle still in place, gently swirl the vial to dissolve all the powder, unless otherwise indicated by the product information. This may take several minutes.
Follow the relevant steps above, to withdraw the required volume of solution from the vial into the syringe and label if necessary.
If a purpose-designed reconstitution device is used (e.g. Risperdal Consta long-acting injection), the manufacturer's instructions should be read carefully and followed closely.
3 D. Adding a Medicine to an Infusion Solution and Labeling the Infusion Container
All intravenous (IV) administration procedures, are outlined in the local Area Acute Hospital Trust's Guide to Intravenous (IV) Therapy, available via the Trust Pharmacy Intranet. The general procedure for preparing a medicine for infusion is outlined below.
Prepare the medicine in a syringe, using one of the methods described in 3A/3B/3C above.
Check the outer wrapper of the infusion container is undamaged.
Remove the wrapper and check the infusion container itself in good light. It should be intact and free of cracks, punctures/leaks.
Check the infusion solution, which should be free of haziness, particles and discolouration.
Where necessary, remove the tamper-evident seal on the additive port, according to the manufacturer's instructions. Wipe the rubber septum of the infusion container with an alcohol wipe and allow to dry for at least 30 seconds.
If the volume of medicine solution to be added is more than 10% of the initial contents of the infusion container (i.e. more than 50ml to a 500ml infusion bag or 100ml to a 1 litre infusion bag), an equivalent volume must first be removed with a syringe and a needle.
Inject the medicine into the infusion container through the centre of the injection port, taking care to keep the tip of the needle away from the side of the infusion container. Withdraw the needle and invert the container at least five times, to ensure thorough mixing before starting the infusion.
Do not add anything to any infusion container when it is hanging on the infusion stand, since this makes adequate mixing impossible.
Check the appearance of the final infusion for absence of particles, cloudiness or discolouration.
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
Label the infusion bag with an IV Additive label and place it on top of the patient's medicine chart. The label must contain:
- name of the medicine, strength - name of diluent - name of practitioner who prepared the medicine - date and time it was prepared - expiry date and time
Diluting a Medicine in a Syringe for Continuous Subcutaneous Infusion (CSCI) using
a Syringe Driver
In end-of-life (palliative) care, some medicines may be administered by continuous subcutaneous infusion (CSCI) using a portable battery-powered syringe driver. For most drugs, this method of administration is unlicensed.
Any healthcare professional setting up a CSCI syringe driver must be competent to do so. Full instructions can be found in the manufacturer's instruction manual. However, clinical areas using syringe drivers are required to follow detailed local procedures. Health Partnerships Division have a written local procedure for the McKinley T34 syringe driver that covers purchasing policy, initial set-up, checks in-use and staff training. Pharmacists are available to provide advice and should be consulted where necessary.
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
APPENDIX 4
ADMINISTERING AN INTRAMUSCULAR INJECTION
Please refer to Appendix 1 – Core Procedures for All Injections.
1.
The intramuscular (IM) route is suitable for a number of drugs, provided they are soluble and non-irritant to soft tissues. Suspensions must be re-suspended immediately prior to administration. Injections stored in the refrigerator should be allowed to reach room temperature naturally, before they are administered.
The sites for intramuscular administration are the upper outer aspect of the arm (deltoid), the outer/middle aspect of the thigh (vastus lateralis and rectus femoris) and the upper outer quadrant of the buttock (gluteal muscle). See Diagrams 1, 2 and 3 respectively.
Clinicians should consider carefully the site chosen for injection, in-line with manufacturer's recommendations and best practice guidance. Consideration should be given to patients where muscle may be wasted, injured or otherwise impaired.
Intramuscular injections should be given with the needle, at a 900 angle to the skin (see Diagram 4). The skin should be stretched, not bunched.
Mid-Deltoid
Upper outer aspect of the arm. Used for small volume injections of less than 2ml e.g. vaccines (see Diagram 1).
The Rectus Femoris and Vastus Lateralis Outer/middle aspect of the thigh. Used for intramuscular injections and self-administered IM injections (see Diagram 2).
To ensure that the drug is injected into the mid-deltoid or outer/middle aspect of the thigh,
the needle needs to be long enough to reach the muscle layer. A 25mm (1 inch) needle is
suitable for all ages. In some larger adults, a longer 38mm length (1½ inch) needle may be
needed. Note: Antipsychotic depot injections should always be given by deep
intramuscular injection and a 38mm (1½inch) or longer needle should used (see 8 and 9
below).
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
The Gluteal Muscles Upper outer quadrant of the buttock (known as the dorsogluteal site). Used for deep intramuscular injections (e.g. depot antipsychotics, Pabrinex IMHP). Care is required to avoid damaging the sciatic nerve and the superior gluteal arteries (see Diagram 3).
Diagram 3. The dotted line represents the upper outer quadrant of the gluteous muscle
Diagrams adapted from Taylor & Lillis, 1997
Depot Antipsychotic Injections
For long acting antipsychotic depot injections, a 21g, 1½ inch (38mm), long green retractable needle, is recommended to give a deep intramuscular administration into the gluteal muscle. Consideration of the patients build and stature may also influence choice of needle length. In some larger adults (e.g. BMI over 30), a longer 2 inch (50mm) length needle may be needed to ensure deep IM administration.
the injection is prepared and the patient is ready to receive the injection.
The recommended method of administering a deep intramuscular injection is using the Z-Tracking technique. The Z-tracking technique prevents leakage of medication into the subcutaneous tissue. Lateral displacement of the skin during the injection, helps seal the medication in the muscle (see Diagram 4).
Place the patient in a comfortable position, ideally lying down, exposing the gluteal muscle to be used as the injection site. Displace the skin laterally by pulling it away from the selected injection site. Hold the syringe with the needle at an angle of 900 to the skin. Keeping the skin taught, quickly push the needle into the skin leaving approximately 3mm of the needle above the surface of the skin (so the needle can be easily removed if it breaks). The speed of entry of the needle into the patient reduces discomfort.
For dorsogluteal injections remember to aspirate; with the needle in position, pull back on the plunger slightly, to check for the presence of blood. If blood is present, remove the needle from the skin and dispose of the syringe into a sharps container and repeat the procedure. If no blood is present, administer the injection.
Slowly (about 1ml per 10 seconds) depress the plunger to allow the muscle fibres time to expand to accommodate the volume of solution. Once the needle has been removed, only then, release the traction from the skin.
For further guidance on the preparation and administration of depot antipsychotic injections, see Appendix 5.
For specific advice on the administration of vaccines, Pabrinex IMHP injection, adrenaline 1:1000 for anaphylaxis, insulins and LMWHs see Appendix 7.
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
APPENDIX 5
Additional Guidance on Preparation and Administration of Depot Antipsychotic Injections,
including Risperdal Consta Long-Acting Injection and Paliperidone Palmitate Injection
Manufacturer's recommended site of
Zuclopenthixol Decanoate
Deep intramuscular injection into upper outer
buttock (gluteal region) or lateral thigh
Zuclopenthixol Decanoate
Deep intramuscular injection into upper outer
buttock (gluteal region) or lateral thigh
Deep intramuscular injection into upper outer
Flupentixol Decanoate 20mg/ml Psytixol
buttock (gluteal region) or lateral thigh
Flupentixol Decanoate
Deep intramuscular injection into upper outer
buttock (gluteal region) or lateral thigh
Flupentixol Decanoate
Deep intramuscular injection into upper outer
buttock (gluteal region) or lateral thigh
Fluphenazine Decanoate
Modecate, generic Deep intramuscular injection into upper outer
buttock (gluteal region)
Fluphenazine Decanoate
Deep intramuscular injection into upper outer
buttock (gluteal region)
Haloperidol Decanoate
Deep intramuscular injection into upper outer
50mg/ml, 100mg/ml
buttock (gluteal region)
Pipotiazine Palmitate
Deep intramuscular injection into upper outer
buttock (gluteal region)
Zuclopenthixol Acetate
Deep intramuscular injection into upper outer
Clopixol Acuphase
buttock (gluteal region) or lateral thigh
Deep intramuscular injection into upper outer
Risperidone Long Acting
buttock (gluteal region) or deltoid muscle
Risperdal Consta
Injection 25mg, 37.5mg, 50mg
using the appropriate manufacturer's safety needle (see section 7 below) Deep intramuscular injection into upper outer
Paliperidone Palmitate
buttock (gluteal region) or deltoid muscle
50mg, 75mg, 100mg, 150mg
using the appropriate manufacturer's safety needle(see section 8 below)
For further prescribing information about each product refer to the BNF or Summary of Product Characteristics (available at: http://www.medicines.org.uk/emc/). 1. For depot antipsychotic injections the maximum volume recommended for a single deep
intramuscular injection into the gluteal region, is 2-3ml. A small test dose of the injection must be given before therapy is initiated to confirm tolerability to both the active ingredient as well as the oily vehicle (e.g. sesame oil, fractionated coconut oil) and other excipients (e.g. benzyl alcohol), as any adverse effect will be prolonged (see BNF for details). Therapy may normally be initiated 4-7 days after a successful test dose. Injections should ideally be alternated between the left and right sided muscles.
2. Select the most appropriate strength of product to keep the volume to a minimum, to reduce
patient discomfort. Although different strengths of the same branded drug can be mixed, it is best practice to use a single strength preparation, to minimize risks of error.
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
3. In exceptional cases, it may be necessary to administer a depot antipsychotic injection into the
upper outer aspect of the arm (deltoid muscle). It should be recognized that this (with the exception of Risperdal Consta and paliperidone palmitate (Xeplion) long-acting injections) falls outside of the manufacturer's prescribing recommendations and is considered an "off-label" use of the medicine (see below).
4. Published data supporting such administration is very limited and it is possible that the
pharmacokinetics (slow-release characteristics) of the drug may differ, resulting in changes to efficacy and side-effect profiles. Under such circumstances, the healthcare professional should follow the Trust Unlicensed Use of Medicines Policy (19.07).
5. This means completing a risk assessment, considering all options and providing a clear
rationale for the proposed treatment option. This should be documented in the healthcare record, along with a record that the patient has been informed and has given consent.
6. All antipsychotic depot injections (with the exception of Risperidone Consta and paliperidone
palmitate (Xeplion)) should be given using a 21g, 1½ inch (38mm), green needle. Larger patients with a BMI>30, with thicker adipose tissue, will usually require a slightly longer, 21g, 2 inch (50mm), green needle, to ensure that a deep intramuscular injection is given.
7. Risperidone Long Acting Injection (Risperdal Consta) 7.1 Risperidone (Risperdal Consta) long acting injection, is licensed for gluteal and deltoid
administration. It is formulated differently to other depot antipsychotic injections. Each box contains a vial of risperidone powder, a syringe filled with diluent, a SmartSite vial access device used during the reconstitution process, two Needle-Pro safety needles for administration and a manufacturer's instruction leaflet.
7.2 Do not use anything other than the contents of the pack, to prepare and administer a Risperdal
Consta injection. The needles provided have been specifically designed for the purpose of administering a suspension of risperidone microspheres to prevent blocking. The needles have a slightly larger inner bore diameter and the inner surfaces of the needle are silicone coated, to ensure smooth administration. The 2 inch (50mm) yellow needle is for gluteal administration. The 1 inch green needle is for deltoid administration. Care should be taken to ensure the correct needle is selected depending on the site of the injection.
7.3 Risperidone Consta must be stored under refrigeration (between +20C and +80C) as the
microsphere formulation is unstable at higher temperatures. Pharmacy will deliver Risperidone Consta to clinical areas with ice packs. Upon receipt, boxes of Risperidone Consta must be transferred to the clinic drug fridge as soon as possible, to maintain the cold chain and its original expiry date.
7.4 If a box of Risperidone Consta is left at room temperature (i.e. not greater than +250C) it must
be labelled with a 7 day expiry date and used within the next 7 days. If not, it should be discarded. If in doubt, ring pharmacy for advice.
7.5 Risperdal Consta injection also differs from other antipsychotic depot injections in that there is
a longer lag period following the first injection, before therapeutic blood levels are reached. As a consequence, there is no "test-dose" for Risperidone Consta. It is recommended that risperidone-naïve patients are pre-treated with oral risperidone (2mg daily) for several doses, to assess tolerability/allergy status, before the first injection is given.
7.6 Preparation of Risperdal Consta Injection
7.6.1 Allow the pack contents to naturally come to room temperature before reconstitution.
This will reduce patient discomfort on administration. Do not use any other method to speed up the warming process.
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
7.6.2 Do not touch the spike tip of the SmartSite vial access device at anytime. 7.6.3 Flip off the cap from the vial of Risperidone Consta powder. 7.6.4 Peel back the blister pack and remove the SmartSite device by holding the white luer
7.6.5 Insert the spike tip of the SmartSite device directly into the vial's rubber stopper, by
pressing until the device clicks into place. Do not attempt to slide the SmartSite device on at an angle.
7.6.6 Swab the syringe connection point (the blue circle) with an alcohol wipe and leave to
dry for at least 30 seconds.
7.6.7 Twist off the white cap from the syringe of diluent and remove with the rubber tip inside. 7.6.8 Press the syringe tip into the blue circle of the SmartSite device. Holding the skirt of the
SmartSite device, and keeping it aligned to the syringe to prevent spinning, twist the syringe clockwise to ensure secure attachment.
7.6.9 Inject the diluent into the vial. All 2ml of the diluent must be used for suspension of the
7.6.10 With the vial and syringe still connected, hold the plunger rod down with the thumb;
shake vigorously up and down for at least 10 seconds.
7.6.11 When properly mixed, the suspension should be uniform, thick and milky in
appearance. There should be no dry particles on the vial wall.
7.6.12 Do not store the vial after reconstitution or the suspension will settle. 7.6.13 Invert the vial and slowly withdraw the suspension, using only the syringe provided.
Make sure the entire content is drawn up in to the syringe. The syringe has no graduation marks on it, so it is not possible to accurately administer any dose less than the full dose.
7.6.14 Unscrew the syringe from the SmartSite device by twisting the syringe anti-clockwise.
Discard the vial and SmartSite device in a sharps container.
7.6.15 Select the correct administration needle depending on the site of the intramuscular
injection (2 inch yellow needle for gluteal; 1 inch green needle for deltoid). Safely discard the unwanted needle. Peel the blister pouch of the Needle-Pro device open half way. Grasp the sheath using the plastic peel pouch (non-touch).
7.6.16 Attach the luer connection of the Needle-Pro device to the syringe in a clockwise
7.6.17 Do NOT use the orange safety guard until after the product has been used. It is not
7.6.18 Prepare the patient for injection as per core procedures (see Appendix 1). 7.6.19 If 2 minutes pass before injection, resuspend by shaking vigorously. 7.6.20 Ideally, Risperidone Consta should be used immediately after it has been reconstituted.
It will, however, remain stable at or below room temperature (+250C), for up to 6 hours.
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
7.6.21 Pull the sheath away from the needle, but avoid twisting the sheath, or the needle may
7.6.22 Tap the syringe gently, to remove any air bubbles, before injecting the entire contents
intramuscularly deep into the gluteal or deltoid muscle as per Trust procedure (see Appendix 4).
7.6.23 If necessary, label the syringe as per Trust procedure (see Appendix 1). There is a
tear-off section from the vial that can be used to clearly identify the reconstituted dose.
7.6.24 After injection, using one hand and a hard surface, gently press the needle into the
orange safety guard, ensuring the needle is fully engaged in the protection sheath.
7.6.25 Discard the needle in an appropriate sharps container immediately. 7.6.26 Reconstitution and administration of Risperidone Consta doses greater than 50mg.
Guidance for nurses, summarising the key points for consideration, has been written by the Wells Road Centre Pharmacy. It can be accessed via the Pharmacy – Local Services Intranet or via Pharmacy direct.
8. Paliperidone Palmitate Long Acting Injection (Xeplion) 8.1 Paliperidone palmitate (Xeplion) depot injection is licensed for gluteal and deltoid
administration. It is formulated differently to all other depot antipsychotic injections. Each box contains a pre-filled syringe of paliperidone palmitate, two safety needles for administration and a manufacturers instruction leaflet.
8.2 The 1 inch 23g (blue hub) needle is for deltoid administration. If the patient is over 90kg the
1½ inch 22g (grey hub) needle is recommended for deltoid administration. The 1½ inch 22g (grey hub) needle is recommended for gluteal administration. Care should be taken to ensure the correct needle is selected depending on the site of the injection.
8.3 There is no "test-dose" for paliperidone palmitate. It is recommended that paliperidone- or
risperidone-naïve patients are pre-treated with oral risperidone 2mg/day for 2 days to assess hypersensitivity/allergy status before the first injection is given.
8.4 Paliperidone palmitate injection also differs from other antipsychotic depot injections in that two
loading doses are required in most cases. The two loading doses of 150mg on day 1 and 100mg on day 7 need to be given into the deltoid muscle. Subsequent injections are given at monthly (±7 days) intervals in either the gluteal or deltoid muscle. When switching from risperidone long-acting injection (Risperdal Consta) or any other antipsychotic depot injection the two loading dose injections are not required.
8.5 Preparation of Paliperidone Palmitate Depot Injection
8.5.1 Please read the manufacturer's instruction leaflet before proceeding. 8.5.2 Each pack contains a pre-filled syringe containing a suspension of paliperidone
palmitate and two safety needles for intramuscular injection.
- 1½ inch 22 gauge grey hub needle for gluteal injections and deltoid injections if patient ≥90kg - 1 inch 23 gauge blue hub needle for deltoid injections if patient weighs <90kg
8.5.3 It is important to shake the syringe vigorously for a minimum of 10 seconds to ensure
thorough mixing of the suspension.
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
8.5.4 Select the appropriate needle from the pack and carefully twist it onto the syringe. 8.5.5 Pull the sheath away from the needle with a straight pull. Do not twist the sheath as
this may loosen the needle from the syringe.
8.5.6 Ensure all air has been expelled from the syringe/needle, then inject the entire contents
intramuscularly into the selected muscle as per approved procedures.
9. Further Information about depot antipsychotics 9.1 The UKPPG have written guidance on the administration of depot antipsychotic injections
9.2 The Nottinghamshire Area Prescribing Committee (NAPC) have ratified AMBER 2 depot
antipsychotic prescribing and monitoring guidelines for both primary care and secondary care healthcare professionals. These can be accessed via the Pharmacy – Local Services intranet under "Prescribing Policies and Guidelines".
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
APPENDIX 6
ADMINISTERING A SUBCUTANEOUS INJECTION
Please refer to Appendix 1 – Core Procedures for All Injections.
1.
Injection via the subcutaneous (SC) route is chosen when slow, continuous absorption of the drug is required, for example, insulin or low molecular weight heparin (LMWH). The medication is injected beneath the epidermis, into the fat and connective tissue underlying the dermis, where there is less blood flow and therefore a slower absorption rate. In some situations, fluids can also be infused in this way; it is a less invasive route for the hydration of some patients.
The sites suitable for administering subcutaneous injections, are the lateral aspect of the upper arm, thighs and umbilical region of the abdomen (see Diagram 5). The abdomen is probably the most common site of choice for LMWH and insulin, as the skin here has thicker subcutaneous tissue.
A 25g, 5/8 inch (16mm) orange safety needle, is recommended for SC injections.
Lateral aspect of upper arms and thighs
Umbilical region of abdomen
Diagram 5: Sites commonly used for subcutaneous injections
With one hand, pinch the skin using the thumb and forefinger, to lift the adipose tissue from the underlying muscle and prevent the solution from being injected into the muscle (see Diagram 6).
Diagram 6: Correct lifted skin fold
Incorrect lifted skin fold
Insert the needle smoothly into the subcutaneous skin, at an angle of 900. The angle may depend on the amount of subcutaneous tissue available. It is recommended that vaccines given by deep subcutaneous injection, should be given with the needle at a 450 angle to the skin (see Appendix 7D).
Inject the solution by pushing carefully and slowly on the plunger. Wait briefly before retracting or withdrawing the needle, to help prevent backtracking, and then release the lifted skin fold. Use a tissue to wipe away any fine capillary blood that might be leaking away. Do not massage the area.
For additional information on the administration of subcutaneous insulin injections, see Appendix 7D. For a specific procedure on the administration of subcutaneous infusion of fluids (hypodermoclysis) refer to The Royal Marsden Hospital Manual of Clinical Nursing Procedures. Health Partnerships Division have a written local procedure for the subcutaneous administration of medicines using the McKinley T34 syringe driver.
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
APPENDIX 7
ADDITIONAL INFORMATION ON THE ADMINISTRATION OF SPECIFIC DRUGS
Vaccines
Most vaccinations should be given by intramuscular injection into the deltoid muscle. Injections given intramuscularly rather than by deep subcutaneous injection, are less likely to cause local reactions. There are, however, exceptions.
Healthcare professionals preparing and administering vaccines, should always refer to the latest edition of the Department of Health Immunization Against Infectious Diseases "The Green Book". Available at: http://immunisation.dh.gov.uk/category/the-green-book/
For individuals with a bleeding disorder, vaccines normally given by an intramuscular route, should be given by deep subcutaneous injection, to reduce the risk of bleeding.
The preferred site for intramuscular immunization in adults is the deltoid area of the upper arm (see Diagram 1). A 23g (blue), 1 inch, 25mm needle is preferable, although a longer 21g (green), 1 ½ inch, 38mm needle may be required for some larger adults.
7 B. Pabrinex IMHP Injection
Pabrinex Intramuscular High Potency (IMHP) injection contains high doses of vitamins B (thiamine, nicotinamide, pyridoxine and riboflavin) and C (ascorbic acid). It is used to treat and prevent severe deficiency states seen in for example, chronic alcoholism.
There are two formulations of Pabrinex High Potency Injection; one for deep intramuscular use (IMHP) and one for intravenous injection/infusion (IVHP). These formulations are not interchangeable and practitioners must take care to ensure the correct product has been selected. Packaging is similar and both are stored in the refrigerator.
Parenterally administered thiamine, can rarely cause serious allergic adverse reactions such as anaphylaxis. Although the risk is less with the intramuscular route, facilities for treating anaphylaxis (including resuscitation facilities) should be available when parenteral thiamine is administered.
To prepare Pabrinex Intramuscular High Potency (IMHP) injection first allow the unopened pair of ampoules to naturally come to room temperature before mixing. This will reduce patient discomfort on administration. Do not use any other method to speed up the warming process.
Then, draw up the contents of Ampoule Number 1 (5ml) and Ampoule Number 2 (2ml) into a single syringe, to mix them just before use.
The 7ml injection is then injected slowly into the upper outer quadrant of the gluteal (buttock) muscle, 5cm below the iliac crest (see Diagram 3 in Appendix 4).
7 C. Intramuscular Adrenaline 1:1000 for Anaphylaxis
1.
The best site for an intramuscular injection of adrenaline, for the treatment of an anaphylactic reaction, is the anterolateral aspect of the middle third of the thigh (see Diagram 2 in Appendix 4).
The needle needs to be long enough to ensure that the adrenaline is injected into muscle. The current Resuscitation Council (UK) guidance states that a 23g, 1 inch (25mm), blue needle is best and suitable for all ages. In larger patients, a longer 21g, 1½ inch (38mm), green needle may be needed.
7 D. Subcutaneous Insulin injections
1.
The usual places for insulin injections are the abdomen, the upper thighs and the buttocks. Avoid the arms. Vary the site and point of the injection. See Appendix 6.
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
Repeated injections into the same area can result in fatty lumps (lypohypertrophy). Insulin may not be absorbed properly from sites where lumps have developed and this can affect blood sugar control. Further advice and information can be obtained from your local diabetic service e.g. Nottingham University Hospital Diabetes Service (Available at http://www.nottinghamdiabetes.co.uk/insulin.html).
For additional information about the prescribing, administration and storing of insulin refer to your Divisional Medicines Code (e.g. Nottingham Medicines Code PH/LS/02 – Appendix 12: Safer Insulin Therapy).
Insulin from 10ml, single-patient only, multi-dose vials, should be drawn up following procedure in Appendix 3, using a 1ml (100unit) retractable insulin syringe (29g ½inch (12mm) orange needle). A 0.3ml and 0.5ml insulin safety syringe is available for very small doses of insulin.
Some insulin pen devices require product-specific disposable needles (e.g. Novofine, BD Micro-Fine). These come in a range of sizes so it is important to check which type the patient has been recommended to use (e.g. 28g 12mm, 29g 12.7mm, 30g 8mm, 31g 5mm, 6mm and 8mm). Safety needles for insulin pen devices are now available and should be used by staff (e.g. Ypsomed My Life Click Fine Auto Protect 29g 8mm).
Cloudy insulin suspensions must be thoroughly mixed before use. This means gently rocking the vial or pen backwards and forwards about 20 times. Do not shake vigorously. Always refer to the product-specific manufacturer's instruction leaflet/booklet.
Low Molecular Weight Heparins (LMWHs)
Low molecular weight heparins (LMWHs) e.g. enoxaparin sodium (Clexane) are usually given by subcutaneous injection into the abdomen (see Appendix 6).
There are a wide range of strengths of enoxaparin sodium injection available for both prophylaxis and treatment doses. Treatment doses are calculated based on the patients body weight.
For additional information about prescribing LMWHs refer to your Divisional Medicines Code (e.g. Nottingham Medicines Code PH/LS/02 – Appendix 13: Reducing Treatment Dose Errors with LMWHs. A dose calculation aid for enoxaparin sodium can be accessed via the Pharmacy – Local Services intranet under "Injectable Medicines".
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
APPENDIX 8
Procedure for:
Safe Use of Injectable Medicines
APPROVED
Author Name and Title:
John Lawton, Clinical Pharmacy Services Manager (Nottingham
Services)
Approved by:
TRUST DRUG & THERAPEUTIC COMMITTEE
Distribution/Access:
RECORD OF CHANGES
DETAILS OF CHANGE
PROCEDURE
Appendix 3 and Appendix 4 updated to reflect
changes to SPC for risperidone long-acting injection (Risperdal Consta). 3.6 and core procedure 4.2
Changes to sections: 3.4, 6.6, 16.0, Appendix 2 (2A7), Appendix 3 (9.1), Appendix 4 (table, 1,
19.09 3, 6, 8, 9), Appendix 6 (6C2, 6D1, 6D3, 6D4,
Changes to sections 3.12, 3.17, 4.1, and all
appendices as part of general policy review.
ISSUE 3 – JUNE 2013
Safe Use of Injectable Medicines – 19.09
APPENDIX 9
EMPLOYEE RECORD OF HAVING READ THE POLICY/PROCEDURE
Title of Policy/Procedure: Safe Use of Injectable Medicines
I have read and understand the principles contained in the named policy/procedure.
PRINT FULL NAME
SIGNATURE
ISSUE 3 – JUNE 2013
Source: http://www.nottinghamshirehealthcare.nhs.uk/download.cfm?doc=docm93jijm4n960.pdf&ver=744
COMMENTARY ON: Preservation, Management and Identification of Sources of Information that are Not Reasonably Accessible A Project of The Sedona Conference® Working Group on Electronic Document Retention & Production (WG1) The Sedona Conference® Preservation, Management and Identification of Sources of Information that are Not Reasonably Accessible
A Drug Abuse Prevention Guide For Teens Table of Contents Introduction:Substance Abuse Guide For Teens 1 Part One:Today's Drug Problem 2Extent of Problem 2 Drugs of Abuse 3• Cannabis • Heroin • Cocaine 4• Methamphetamine • Prescription Drugs 5• GHB • Ecstasy 6 • LSD • PCP • Ketamine 7• Anabolic Steroids • Inhalants • Over the Counter (OTCs) 8









