Comparison of chemically and pharmaceutically modified titanium and zirconia implant surfaces in dentistry: a study in sheep
YIJOM-1445; No of Pages 8
Int. J. Oral Maxillofac. Surg. 2008; xxx: xxx–xxxdoi:available online at http://www.sciencedirect.com
Comparison of chemically and J. D. K. Voelter,
D. M. Schnabelrauch, F.
T. Hefti, K. K. B. von Rechenberg1Musculoskeletal Research Unit, Equine
titanium and zirconia implant
Hospital, Vetsuisse Faculty ZH, University ofZurich, Winterthurerstr. 260, CH-8057 Zurich,Switzerland; 2Max Bergmann Center forBiomaterials, Institute of Materials Science,
surfaces in dentistry: a study in DresdenUniversityofTechnology,
Budapester Str. 27, 01069 Dresden,Germany; 3Biomaterials Department,
INNOVENT e. V., Pruessingstrasse 27B, D-07745 Jena, Germany; 4Thommen Medical,Hauptstrasse 26d, CH 4437 Waldenburg,Switzerland; 5Veterinary Anesthesiology,Equine Hospital, Vetsuisse Faculty ZH,University of Zurich, Winterthurerstr. 260, CH-
J. D. Langhoff, K. Voelter, D. Scharnweber, M. Schnabelrauch, F. Schlottig, T. Hefti,
8057 Zurich, Switzerland
K. Kalchofner, K. Nuss, B. von Rechenberg: Comparison of chemically andpharmaceutically modified titanium and zirconia implant surfaces in dentistry: a studyin sheep. Int. J. Oral Maxillofac. Surg. 2008; xxx: xxx–xxx. # 2008 InternationalAssociation of Oral and Maxillofacial Surgeons. Published by Elsevier Ltd. All rightsreserved.
Abstract. Advanced surface modifications and materials were tested on the sameimplant geometry. Six types of dental implants were tested for osseointegrationafter 2, 4 and 8 weeks in a sheep pelvis model. Four titanium implant types weretreated with newly developed surface modifications, of which two were chemicallyand two were pharmacologically modified. One implant was made of zirconia. Asandblasted and acid-etched titanium surface was used as reference. The chemicallymodified implants were plasma-anodized or coated with calcium phosphate. Thepharmacological coatings contained either bisphosphonate or collagen type I with
con superficie "Zerafil" de ZERAMEX-T
chondroitin sulphate. The implants were evaluated using macroscopic, radiographicand histomorphometric methods.
All implants were well osseointegrated at the time of death. All titanium implants
had similar bone implant contact (BIC) at 2 weeks (57–61%); only zirconia wasbetter (77%). The main BIC increase was between 2 and 4 weeks. The
Keywords: dental implants; zirconia; titanium;
pharmacologically coated implants (78–79%) and the calcium phosphate coating
surface modification; histomorphometry;
(83%) showed similar results compared with the reference implant (80%) at 8
weeks. There were no significant differences in BIC. Compared with previousstudies the results of all implants were comparatively good.
Accepted for publication 3 September 2008
Over the last decades, titanium or its alloys
patibilityThese properties ensure good
gery, while allowing efficient osseointe-
has become a gold standard as a base for
anchorage within the mandible or maxil-
gration. Apart from good implant design
tooth reconstruction in dental implantol-
lary bone. The aim is to achieve shorter
ogy, because of its mechanical strength,
healing periods for implants, in order to
* The first two authors contributed equally
chemical stability and excellent biocom-
load them as soon as possible after sur-
to this work.
# 2008 International Association of Oral and Maxillofacial Surgeons. Published by Elsevier Ltd. All rights reserved.
Please cite this article in press as: Langhoff JD, et al., Comparison of chemically and pharmaceutically modified titanium and zirconiaimplant surfaces in dentistry: a study in sheep, Int J Oral Maxillofac Surg (2008),
YIJOM-1445; No of Pages 8
Langhoff et al.
related to mechanical anchorage, these
degeneration associated with implants
requirements may be met by modifying
often uncover parts of the metal implant
The calcium phosphate surface was coated
the implant surface, bearing in mind that
showing a bluish discoloration of the over-
using electrochemical assistance in an
the most important surface properties for
lying gingiva. The use of zirconia implants
aqueous solution containing calcium and
(metallic) implants are topography, chem-
avoids this complication and accedes to
phosphate ions. The coating consists of the
istry, surface charge and
the request of many patients for metal-free
two calcium phosphate phases, hydroxya-
To improve surface properties two
implants. The material also provides high
patite and brushite, and is commercially
main approaches were used either opti-
strength, fracture toughness and biocom-
available. The anodic plasma chemical
mizing the micro-roughness (e.g. sand-
patibility. Osseointegration is approxi-
blasting and acid-etching) or applying
mately the same as with
advanced anodization method, which
bioactive coatings (e.g. calcium phos-
The authors hypothesize that chemical
allows anodic oxide layer formation and
phate, bisphosphonate, collagen). Opti-
and pharmacological surface modifica-
mizing the micro-roughness results in
tions to titanium initiate a stronger bone
phases in a single process step. The
enlarged surfaces providing improved
response than an advanced sandblasted
method exploits the dielectric breakdown
conditions for osteogenic cell attachment
and acid-etched surface alone. They tested
of anodic oxide films to produce a porous
and proliferation. In recent studies, histo-
whether a surface-treated zirconia can
oxide layer that contains significant
mophometric and biomechanical compar-
compete with sophisticated titanium sur-
amounts of electrolyte components. The
isons of such optimized implant surfaces
faces. The bone response to the implant
electrolyte contained calcium and phos-
to machined implants showed better
modifications was tested on the identical
phate ions, leading to a porous surface
values for short time osseointegration.
established implant geometry using histo-
containing calcium phosphate.
These surfaces were also optimized for
The collagen coating was based on an
their wettability for potentially enhanced
extracellular matrix containing chondroi-
implant–tissue interaction and better
tin sulphate, prepared by fibrillogenesis of
osseointegration, achieved by rinsing
Material and methods
the collagen in the presence of CS, and
under an N2 atmosphere and submersion
performed as dip coating in a collagen/
in an isotonic NaCl solution following
chondroitin sulphate solution. The bispho-
acid-etching. The new generation of thin
Overall, 6 types of implants with identical
sphonate coated implants were immobi-
calcium phosphate based coatings pro-
implant geometry were tested
lized with an alendronate solution, to a
vide high wettability and were described
All titanium and zirconia implants were
final concentration of 10 mg/cm2.
as highly potential
sandblasted and partially etched prior to
The zirconia implants were manufac-
Another approach is to add bioactive
the surface treatments, similar to the refer-
tured from yttrium partially stabilized zir-
components to titanium surfaces. One
ence. The surfaces of the chemically mod-
method uses extracellular matrix ligands,
ified implants were either plasma anodized
implants were sandblasted and etched in
the RGD-peptide sequence, for better
or coated with calcium phosphate. The
an alkaline bath.
osteoblast attachment and enhanced bone
remodellingSCHULER et al.used a
were either coated with bisphosphonate
functionalized coating (poly(L-lysine)-
or collagen type I. An acid-etched and
graft-poly(ethylene glycol)) to present
Animal model and study design
sandblasted implant made of titanium
bioligands for interaction with osteoblasts
(grade 4, SPI1ELEMENT, Thommen
A total of 15 sheep underwent surgery. All
in vitro. Faster colonization of the implant
Medical AG, Waldenburg, Switzerland)
sheep were full-grown, aged 2–3 years,
surface by osteoblasts also inhibits bacter-
served as the reference and control for
not gestating females and 49–87 kg (aver-
the surface modifications.
age 68 kg). General guidelines for care
A new method uses nucleic acid, single
strands, fixed electrochemically via theirtermini by anodically growing an oxide
Table 1. Implant groups, their abbreviations and sample size.
layer on Ti6Al7Nb as anchor structures to
load surfaces with bioactive molecules
Sandblasted and acid etched
linked to complementary
The bioactivity of surfaces can be
enhanced using drug eluting coatings,
Calcium Phosphate
which are supposed to influence bone
healing, for example by activating osteo-
blasts, suppressing osteoclasts or stimulat-
ing the production and distribution of
growth factors such as BMP-2.
Collagen I + Chondroitin Sulfate
An increase in the mechanical fixation
of implants has been achieved with local
delivery of Other stu-
dies showed the high potential of growth
Zirconia has gained attention as an
implant material because of its white col-
our, which makes it aesthetically attrac-
Apical bone loss and gingival
* SPI ELEMENT, Thommen Medical.
Please cite this article in press as: Langhoff JD, et al., Comparison of chemically and pharmaceutically modified titanium and zirconiaimplant surfaces in dentistry: a study in sheep, Int J Oral Maxillofac Surg (2008), doi:
YIJOM-1445; No of Pages 8
Comparison of chemically and pharmaceutically modified titanium and zirconia implant surfaces in dentistry
for the wound before the animal wasturned over to the other side. The contral-ateral pelvis was operated on in an iden-tical manner.
Postoperative treatment consisted of an
antiphlogistic and analgesic, as well asantibiotic medication for 4 days (bupre-norphin 0.01 mg/kg i.m. t.i.d. during thefirst 24 h, benzylpenicillin (30000 I.U./kgi.v. b.i.d.), gentamycin (4 mg/kg i.v. s.i.d.)and carprofen (4 mg/kg i.v. s.i.d.).
Fluorochrome labelling
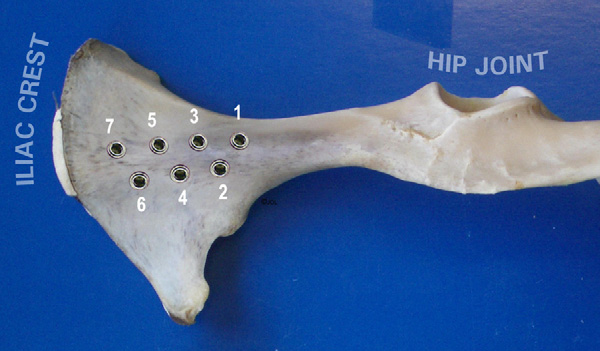
Fig. 1. Implant locations in the iliac bone of a sheep in a dorso-ventral view.
Bone healing and remodelling was fol-
and use of animals in research have been
nously thre times a day. In addition, they
lowed by labelling new bone apposition
received 500 Units of equine tetanus
with fluorochrome at defined points
approved by the local veterinary authori-
serum as a single subcutaneous applica-
of time. The first labelling with calcein
ties (approval no.159/2005). The sheep
tion (Tetanus Serum Veterinaria AG, Zur-
green (10 mg/kg s.c.) was performed 2
were kept in groups containing a maxi-
ich, Switzerland).
weeks after implantation. In the 8 week-
mum of 4 animals. Their general condition
The animals were placed in lateral
group, a second label was injected at 6
was checked three times a day to accom-
recumbency and access to the pelvis
weeks using xylenol orange (90 mg/kg
plish pain monitoring, to detect variations
was achieved using a standard operation
in wellbeing and injuries of the musculos-
procedure. A 20 cm long cut was made in
the skin in the longitudinal direction of the
Preparation and evaluation of bone
n = 110) were placed in the iliac bones
iliac bone at the mid-pelvis line. The
of the pelvis. Bone structure was predo-
fascia was cut and the middle gluteal
minantly of cancellous quality in the cra-
muscle and tensor fasciae latae were sepa-
The bones were harvested after killing the
nial part with increasing cortical thickness
rated by blunt dissection. In the distal half
animals. They were freed of all soft tissue,
(up to 3 mm) toward the caudal part. An
of the iliac bone the tendinous insertion of
revealing the implants in the iliac bone.
implantation scheme was worked out to
the deep and middle gluteal muscles was
The firm seat of the implants within the
distribute all implant types homogenously
severed from the iliac crest with a scalpel
bone was tested qualitatively by manual
to 7 implantation sites per iliac bone
and the muscles were bluntly removed
pressure and the caps were removed.
(The study design aimed to
from the iliac bone shaft. A Finocchietto
Thereafter, the intact pelvis bone was
achieve the statistical minimum of 6 sam-
retractor was used to expose the entire
radiographed using a faxitron machine
ples per implant group (one implant type
iliac wing. Holes were drilled using the
(Cabinet X-ray-faxitron series, model
was not evaluated for the present study)
43855A, Hewlett Packard1, USA) for
for each healing period of 2, 4 and 8
Medical AG, Waldenburg, Switzerland)
documentation of implant placement and
weeks, with 5 animals per time point.
with a 2.0 mm pilot drill, widened with
verification of proper seat. Then the bone
Additional implants were placed for a
a 2.8 mm and finally with a 3.5 mm drill.
was cut into 1.5 � 1.5 cm cubes with a
concurrent biomechanical analysis (the
A drill sleeve was used to ensure the
band saw (K 410, Kolbe GmbH, Elchin-
topic of a separate study). Animals were
designated drill depth according to the
gen, Germany), containing one implant.
killed in the University's slaughterhouse
implant design and the depth was con-
Samples were fixed in 40% alcoholic solu-
according to ethical standards.
firmed with a depth gauge (Thommen
tion for 14 days and were routinely pro-
Medical AG, Waldenburg, Switzerland).
The self-tapping implants (SPI1ELE-
histology. They were submitted to a
MENT, Thommen Medical AG, Walden-
dehydration process in an ascending series
After sedation with medetomidine (5 mg/
burg, Switzerland) were placed according
of ethanol solutions (50, 70, 96, 100%),
kg, DomitorTM, Orion Pharma Animal
to the implantation scheme and using the
Health, Finland) anaesthesia was induced
specific instruments supplied with the
vacuum. Samples were infiltrated in
using ketamine (2 mg/kg, Narketan1 10,
implant system. Healing caps were placed
pMMA solution (poly methacrylic acid-
Chassot GmbH, Germany) in combination
to prevent tissue ingrowth in the abutment
methylester; dibuthylphtalate and perka-
with diazepam (0.01 mg/kg, Valium1,
connection area of the implant head.
dox in a proportion 89.5: 10: 0.5) for 7
Roche, Switzerland). After intubation
Implant setting was documented with digi-
days, embedded and polymerized in
anaesthesia was maintained with 0.8
tal photographs. The muscles were reposi-
Teflon containers. Samples were posi-
Vol% isoflurane (Forene1, Abbot AG,
tioned to assure that implants were cut
Switzerland) in O2 and an infusion of
resutured to its origin using a cross pattern
parallel to the longitudinal axis. Two
Ringer's solution with 60 mg/l ketamine
of single (at the edges) and continuous
ground sections were cut at the maximum
(NarketanTM 10, Chassot GmbH, Ger-
sutures. Fascia and subcutis were closed
diameter of the implant using a low speed
many) at a rate of 10 ml/kg/h. As a pro-
with the same synthetic resorbable suture
diamond saw (Leica1 SP 1600, Leica1
phylaxis against infection all animals
(Polyglactin; Vicryl1 2-0, Johnson&-
Instruments GmbH, Nussloch, Germany).
received 30,000 IU/kg penicillin (Hoechst
Johnson Intl.) while the skin was closed
One section of 200 mm was used for nor-
AG, Germany) and 6 mg/kg gentamicin
mal bone histology, applying a surface
(Streuli & Co AG, Switzerland) intrave-
ULC1). Gauze was applied as protection
staining with toluidine blue. The thinner,
Please cite this article in press as: Langhoff JD, et al., Comparison of chemically and pharmaceutically modified titanium and zirconiaimplant surfaces in dentistry: a study in sheep, Int J Oral Maxillofac Surg (2008),
YIJOM-1445; No of Pages 8
Langhoff et al.
ing implantation it was noticed, that thezirconia implants required slightly moreforce for insertion compared with the tita-nium implants.
Macroscopic and radiological evaluation
After preparation of the muscle above theimplants, the tissue layer directly at thebone–implant surface was gel- and fat-likeafter 2 weeks. A soft tissue layer hadformed after 4 weeks, which developedinto a periosteum-like layer with callusformation later. At 2 and 4 weeks, hae-matoma were rarely visible around theimplants. Overall, no signs of inflamma-tion or infection could be found, indicated
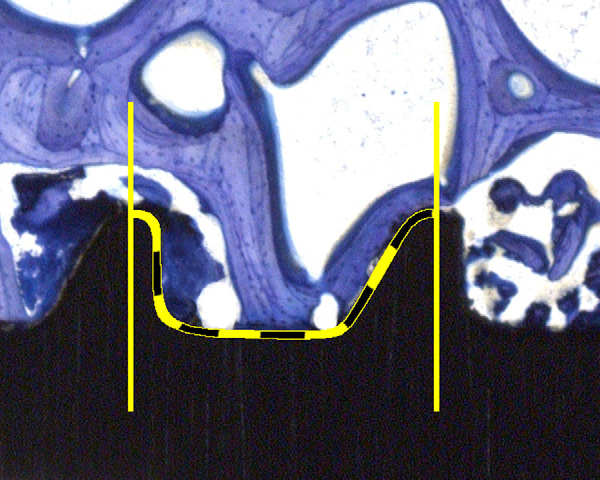
Fig. 2. Illustration of the thread wise evaluation of the bone implant contact (BIC). Estimation
through swelling, reddening or other
of the percentage was supported with a 10% step grid.
degradation of surrounding tissue. Allimplants were firmly seated.
Radiographs demonstrated all implants
native section (150 mm) was used for
tify significant differences, mean values
to be still in place. No fractures or zones of
fluorescence microscopy (Leica, DMR,
and standard deviations using a specific
bone resorption could be found.
UV light source and Filter I3 for calcein
soft ware (SPSS 13.0 for Macintosh). Sig-
green and xylenol orange, Glattbrugg,
nificance level was set at p < 0.05.
Switzerland). Before the 200 mm sectionswere glued to the opal, acrylic Plexiglas
Radiographs of the thick sections con-
slides (Wachendorf, Perspex GS, Acrylic-
firmed the macroradiographic results of
glas Opal 1013) microradiographs, using a
absence of bone resorption. Radiodense
Surgery and postoperative period
high-resolution analogue film (Kodak
structures were visible in detail and could
Oncology Film, Eastman Kodak Com-
All surgery was uneventful and the ani-
be clearly identified as bone (
pany, Rochester, NY), were taken to
mals recovered from anaesthesia quickly.
Radiodense structures and bone tissue
visualize the stage of calcification of the
The sheep were able to walk immediately
stained with toluidine blue matched
bone samples adjacent to the metallic
after recovery, but showed signs of mild
exactly. Except for a small seam of osteoid
muscle soreness for 1–2 days after sur-
all of the new bone formation was calci-
gery. Thereafter, no signs of lameness or
fied at all time points. The microradio-
other discomfort were seen. Insertion of
graphs were not evaluated additionally
all implants proceeded smoothly, but dur-
besides the stained histologies.
Using the toluidine-stained thick sections,a semi-quantitative evaluation of the boneimplant contact (BIC) was made. For this,the percentage of direct contact betweenmineralized bone and the titanium surfacewas determined by intersection countingwithin the thread area. Six thread pitcheswere counted per sample. The evaluationwas performed at calibrated digital pic-tures at 10x magnification (Leica macro-scope M420, Leica DFC320, 3088x2550pixels, Leica Microsystems, Germany).
Two pictures covered the full threadedpart in high resolution. The percentageof BIC was estimated in steps of 10%Means of thread counts perimplant were calculated.
Statistical analysis
In a first step, factors as individual differ-ence and position of the implant could beexcluded as not significant. In a second
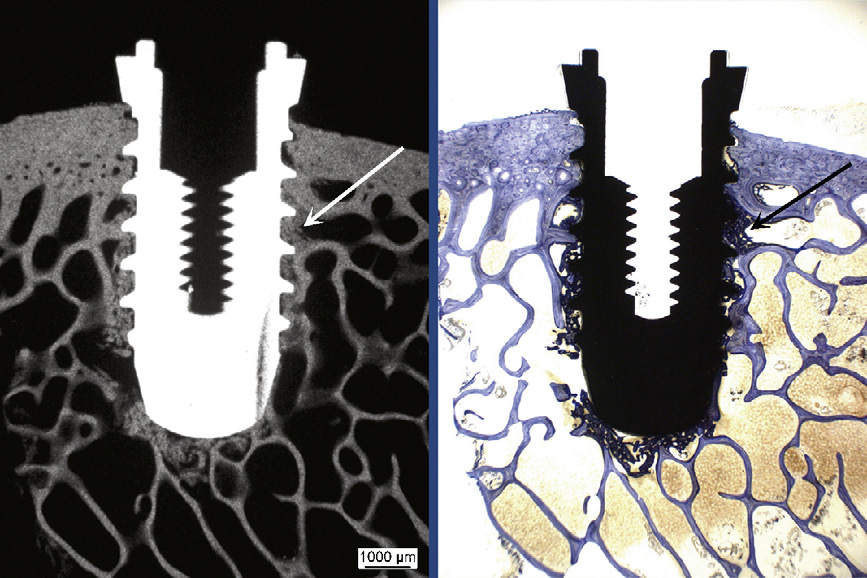
Fig. 3. Calcification of new bone formation (arrows) at the implant was proved by matching
step, comparison of implant types at each
areas of radiodense (microradiograph, on the left) and stained structures (toluidine blue dye, on
point of time was performed. The analysis
the right) in histological thick sections. Overview picture (5.8�) of a bisphosphonate-coated
of variance (ANOVA) was used to iden-
titanium implant at 4 weeks.
Please cite this article in press as: Langhoff JD, et al., Comparison of chemically and pharmaceutically modified titanium and zirconiaimplant surfaces in dentistry: a study in sheep, Int J Oral Maxillofac Surg (2008), doi:
YIJOM-1445; No of Pages 8
Comparison of chemically and pharmaceutically modified titanium and zirconia implant surfaces in dentistry
Fig. 4. A matrix of representative histological pictures of all implant types and time points (2, 4 and 8 weeks) at 10� magnification. Surface wereeither sandblasted and acid etched (Ref), anodic plasma treated (APC), calcium phosphate (CaP), bisphosphonate (BisP), collagen withchondroitin sulfate (Coll+) coated or of zirconia (Zr).
Evaluation of histology samples
Remodeling in the cortical bone started
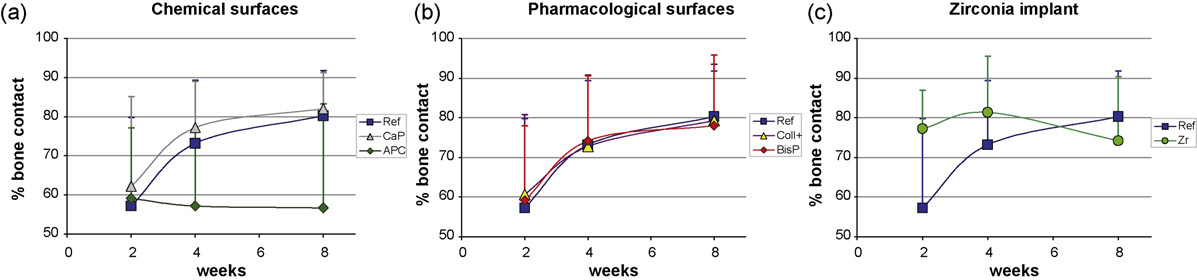
types and time points. All titanium types
by 4 weeks in some samples and was
were nearly similar at 2 weeks (59–62%
All sections were cut precisely in the
prominent at all implant sites at 8 weeks.
BIC) and increased with time (78–83%),
middle axis, capturing the entire implant,
There were no signs of pathological bone
except the plasma anodized surface (58%).
enabling standardized evaluation.
resorption indicative of excessive mechan-
The two chemical surface modifications
ical instability or issues of bioincompat-
performed very differently. The calcium
revealed that implants were generally
ibility (accumulation of inflammatory
phosphate surface showed similar values,
well seated within the bone. New bone
cells) in any implant. Striking differences
with the main increase at 2–4 weeks, like
formation, visible as dark-bluish stain,
between the implant types were not
the reference, and a slight increase
was present around all implants in the
observed in the qualitative evaluation.
towards week 8. In contrast, the plasma
cancellous bone by 2 weeks and built
anodized surface lost 2% bone contact
up steadily until 8 weeks (Bony
initially and did not improve after 4 weeks.
debris was found in the remaining cavity
Evaluation of BIC
Pharmacologically modified surfaces
of the implant tip, where new bone was
Results of the BIC measurements
performed close to the reference. The
found by 2 weeks.
demonstrated clear trends between surface
Please cite this article in press as: Langhoff JD, et al., Comparison of chemically and pharmaceutically modified titanium and zirconiaimplant surfaces in dentistry: a study in sheep, Int J Oral Maxillofac Surg (2008),
YIJOM-1445; No of Pages 8
Langhoff et al.
Fig. 5. Results of the bone-implant contact (BIC) measurements are given according to the three groups of implant types: Chemical (a) andpharmacological (b) titanium surface modifications and a zirconia implant (c) were evaluated for bone response and referenced by a sandblastedand acid etched implant (SPI1ELEMENT). Significant differences were not found between the groups of 6 samples per implant and time point.
collagen with chondroitin sulphate surface
to show better BIC values at 8 weeks
be established as a standard with a zero
showed slightly higher values than the
compared to the anodic plasma treated
failure rate of operation and implantation.
reference implant at 2 weeks and contin-
surface or zirconia implants.
Standard dental equipment (SPI1 -Sys-
ued nearly equally, whereas the bispho-
Finding an appropriate animal model
tem, Thommen Medical) could be used
sphonate coated surface was higher at 2
for testing dental implants is difficult,
without limitations for predrilling and
and 4 weeks.
mainly because the morphology of teeth
implant placement. In this manner, clin-
The zirconia implant presented 20%
in animals is different from that of
ical standard procedures and precision
more bone contact than the titanium
humans. Pigs and dogs are commonly
could be applied. Also sample preparation
implants at 2 weeks, improved toward 4
used as experimental animals, if dental
for histology did not involve complica-
weeks, then reduced at 8 weeks to below
implants are applied . Apart
tions or loss of samples. The sample pre-
the level of the reference surface.
from different root systems and the form
paration proved to be a very effective and
The overall performance of the new
of the incisor and molar teeth, mouth
reliable method for longitudinal sections.
surfaces, except the plasma anodized,
hygiene is a problem in those animals
All sections were cut in the centre of the
was better than the reference. Statistically
after setting dental implants and, thus,
implants with very little variation, so his-
significant differences for BIC were not
healing without infection may pose a pro-
tological evaluation of osseointegration
could be well standardized. The present
Osseointegration is often tested in other
study was mainly focused on the morpho-
locations, such as the femoral condyle.
logical aspects of osseointegration, as the
Evaluation of fluorochrome labeling
Even though the risk of infection may be
histological picture did not show any
At 4 weeks, calcein green fluorescent dye
excluded, the cancellous bone of the
abnormalities on the cellular level. As a
was exclusively visible in the new bone
femoral condyle is more compact and
common tool in dental research, BIC was
directly at the implant, while in the 8 week
stronger compared to the mandible. The
regarded as an appropriate method to mea-
sections xylenol orange was found directly
authors' group has developed an animal
sure the performance of an implant. The
at the implant surface. In those specimens
model in the iliac shaft of sheep, where the
estimation of bone contact in 10% steps
calcein green was found at a greater dis-
structure of the bone is similar to that of
per screw thread was regarded as ade-
tance from the implant surface. Differ-
the human mandible, as described by the
quate. Calculation of means for each
Lekholm and Zarb . Sheep is a
implant was close to the accuracy of a
fluorochrome dyes could not be found
well-established animal for orthopedic
between the implant types and, therefore,
research, because of the similar remodel-
threaded part of the implant was evalu-
further histomorphometrical evaluations
ing rate, bone structure and bone propor-
were not performed.
tions as humans. The pelvis model allows
Although good standardization of sur-
the implantation of a relatively high num-
gery and sample preparation procedures
ber of implants in one sheep; by operating
could be achieved, differences between
on both sides intra- and inter-individual
groups did not reach statistical signifi-
In this study the osseointegration of mar-
comparisons can be made. The animal
cance. The two main reasons for this were
ket standard dental implants (titanium
model serves well from an ethical stand-
the relatively small sample size and the
grade 4, sandblasted, acid etched) was
point considering animal welfare and pro-
good material properties of all the tested
compared with surface-treated implants
tection, because surgery does not interfere
implants. The minimal sample size for
that were either chemically (plasma ano-
significantly with normal ambulation of
statistical evaluation was chosen consider-
dized, calcium phosphate coated) or phar-
the sheep, housing can be easily provided
ing animal welfare issues and ethical con-
macologically modified (bisphosphonate,
appropriate to the species and handling
cerns related to the use of animals in
collagen type 1 containing chondroitin
does not cause excessive stress.
experimental research. Since the sand-
sulphate) or to zirconia implants. An
The study design achieved the statistical
blasted and acid-etched implants (Thom-
experimental sheep pelvis model was
minimum within the limitations of a jus-
men Medical SPI1-System) used as a
used, where all implants showed good
tifiable use of animals. The implantation
reference show good performance owing
scheme reduced the influences of the indi-
to their original titanium dif-
Although statistically not significant, there
vidual and the implantation site using a
ferences to the modified implants were
was a clear tendency for the chemically
rotation system of sample distribution.
expected to be relatively small. Tenden-
and pharmacologically modified implants
The iliac bone as implantation site could
cies for improved osseointegration follow-
Please cite this article in press as: Langhoff JD, et al., Comparison of chemically and pharmaceutically modified titanium and zirconiaimplant surfaces in dentistry: a study in sheep, Int J Oral Maxillofac Surg (2008), doi:
YIJOM-1445; No of Pages 8
Comparison of chemically and pharmaceutically modified titanium and zirconia implant surfaces in dentistry
ing implant modification were clearly
or pharmacologically treated implants and
for appropriate biomedical use. BMC
shown and allow further research and
the reference titanium implants. Whether
Musculoskelet Disord 2007: 8: 72.
testing to be focused on the implants
this is due to the surface of the implant is
4. Bierbaum S, Douglas T, Hanke T,
showing superior performance.
unknown and will be investigated.
Scharnweber D, Tippelt S, MonseesTK, Funk RH, Worch H. Collagenous
The improved values of the pharmaco-
Calcium phosphate coated implants
matrix coatings on titanium implants
logically modified surfaces may be attrib-
showed similar BIC rates as the pharma-
modified with decorin and chondroitin
cologically treated surfaces, with a BIC
sulfate: characterization and influence
osteoblasts and the suppression of osteo-
rate of approximately 80% after 8 weeks,
on osteoblastic cells. J Biomed Mater
clasts, or a combination of both. The early
comparable with those of other advanced
Res A 2006: 77: 551–562.
attachment of the old bone to the implant,
5. Buser D, Broggini N, Wieland M,
including the inhibition of function of
RK, Denzer AJ, Cochran
osteoclasts (bisphosphonates) can hamper
improved surfaces could not be accepted
DL, Hoffmann B, Lussi A, Steine-
resorption and lead to a better anchorage at
even though there were trends for better
mann SG. Enhanced bone apposition toa chemically modified SLA titanium sur-
the very early stage of bone . The
performance for some surface modifica-
face. J Dent Res 2004: 83: 529–533.
reduction of micromotion at a very early
tions. All tested implant types demon-
6. Buser D, Nydegger T, Hirt HP,
stage after implantation is therefore con-
Cochran DL, Nolte LP. Removal tor-
sidered responsible for good osseointegra-
osseointegration, with only small differ-
que values of titanium implants in the
tion of bisphosphonate-coated implants,
maxilla of miniature pigs. Int J Oral Max-
since initially mainly bone formation
implant surface.
illofac Implants 1998: 13: 611–619.
occurs. Resorption of the old bone matrix
Further studies will refine the concen-
7. Ferguson SJ, Broggini N, Wieland M,
may take place later when the bispho-
tration of bioactive substances used in this
de Wild M, Rupp F, Geis-Gerstorfer J,
sphonates are resorbed and the implant
study and explain the reactions on a cel-
Cochran DL, Buser D. Biomechanical
has gained a certain stability. This dif-
lular level as well as prove those concepts
evaluation of the interfacial strength of achemically modified sandblasted and
ference was not confirmed for BIC in this
in clinical conditions.
acid-etched titanium surface. J Biomed
Mater Res A 2006: 78: 291–297.
Collagen containing chondroitin sul-
8. Jones AA, Buser D, Schenk R, Woz-
phate surfaces increased cell proliferation
Acknowledgements. Implants were pro-
J, Cochran DL. The effect of
and activated osteoblasts in cell cultures as
vided by Thommen Medical AG, Walden-
rhBMP-2 around endosseous implants
demonstrated through higher values of
burg, Switzerland. The excellent work by
with and without membranes in the
bone markers (osteopontin, alkaline phos-
S. Bierbaum (Biomaterials Department,
canine model. J Periodontol 2006: 77:
phatase) and larger cell sizeAdhesion
INNOVENT e. V) for providing the col-
molecules such as vinculin, actin and
lagen containing chondroitin sulfate coat-
9. Kohal RJ, Weng D, Bachle M, Strub
JR. Loaded custom-made zirconia and
integrins were up-regulated in vitro. Inhi-
titanium implants show similar osseoin-
bition of osteoclasts does not occur in
Mathys Foundation, Bischmattstrasse 12,
tegration: an animal experiment. J Period-
parallel. Recruitment and activation of
2544 Bettlach, Switzerland) for providing
ontol 2004: 75: 1262–1268.
osteoclasts and subsequent bone resorp-
the plasma anodized coating, by A.Kautz
10. Lekholm U, Zarb G. Patient selection
tion at the surface of the bone lesion is not
(Biomaterials Department, INNOVENT e.
and preparation. Chicago Quintessence
inhibited and takes its normal course. As
V) for providing the bisphosphonate coat-
Publishing Co 1985: pp. 199–209.
bone resorption normally precedes new
ing and P.Zeggel (DOT GmbH, Charles-
11. Leutenegger CM, von Rechenberg B,
bone formation and deposition, it may
Darwin-Ring 1a, 18059 Rostock, Ger-
Huder JB, Zlinsky K, Mislin C, Akens
result in temporary microinstability at
many) for providing the calcium phos-
MK, Auer J, Lutz H. Quantitative real-
the bone–implant interface and thus, less
phate coating is highly appreciated.
time PCR for equine cytokine mRNA innondecalcified bone tissue embedded in
stability of implants in the immediate and
methyl methacrylate. Calcif Tissue Int
early postoperative
1999: 65: 378–383.
Cell proliferation, cell size and regula-
12. Michael J, Beutner R, Hempel U,
tion of adhesion molecules were not inves-
1. Abrahamsson I, Zitzmann NU, Ber-
Scharnweber D, Worch H, Schwen-
tigated in the current study, where
glundh T, Wennerberg A, Lindhe J.
zer B. Surface modification of titanium-
osseointegration was assessed using the
Bone and soft tissue integration to tita-
based alloys with bioactive molecules
histology of non-decalcified bone samples
nium implants with different surface
using electrochemically fixed nucleic
topography: an experimental study in
containing the implants alone. Although it
acids. J Biomed Mater Res B Appl Bio-
the dog. Int J Oral Maxillofac Implants
would be interesting to understand the
mater 2007: 80: 146–155.
2001: 16: 323–332.
13. Nuss KM, Auer JA, Boos A, von
exact mechanism of osseointegration on
2. Albrektsson T, Wennerberg A. Oral
B. An animal model in
a molecular level, it would not change the
implant surfaces: Part 1–review focusing
sheep for biocompatibility testing of bio-
practical and clinical results, where histol-
on topographic and chemical properties
materials in cancellous bones. BMC Mus-
ogy demonstrated a sound performance
of different surfaces and in vivo responses
culoskelet Disord 2006: 7: 67.
for bisphosphonate-coated implants.
to them. Int J Prosthodont 2004: 17: 536–
14. Oliva J, Oliva X, Oliva JD. One-year
follow-up of first consecutive 100 zirco-
osseointegration in histology. The addi-
3. Auer JA, Goodship A, Arnoczky S,
nia dental implants in humans: a compar-
S, Price J, Claes L, von
tional etching process and the roughness
ison of two different rough surfaces. Int J
achieved was good for cell attachment and
Oral Maxillofac Implants 2007: 22: 430–
brinck M, Schneider E, Muller-Ter-
bone apposition and seemed to make a
pitz R, Thiele F, Rippe KP, Grainger
15. Peter B, Gauthier O, Laib S, Bujoli
difference in the early postoperative phase
DW. Refining animal models in fracture
B, Guicheux J, Janvier P, van Lenthe
at 2 and 4 weeks. Later the BIC values
research: seeking consensus in optimising
GH, Muller R, Zambelli PY, Bouler
were lower compared with the chemically
both animal welfare and scientific validity
Nota: Dentalpoint ha comprado los patentes de Thommen Medical AG, Suiza
Please cite this article in press as: Langhoff JD, et al., Comparison of chemically and pharmaceutically modified titanium and zirconiaimplant surfaces in dentistry: a study in sheep, Int J Oral Maxillofac Surg (2008),
YIJOM-1445; No of Pages 8
Langhoff et al.
JM, Pioletti DP. Local delivery of
20. Shibutani T, Inuduka A, Horiki I,
From Microroughness to Resorbable
bisphosphonate from coated orthopedic
Luan Q, Iwayama Y. Bisphosphonate
Bioactive Coatings. In: Ellingsen JE,
implants increases implants mechanical
inhibits alveolar bone resorption in
stability in osteoporotic rats. J Biomed
Interface. CRC Press 2003: 73–100.
Mater Res A 2006: 76: 133–143.
in dogs. Clin Oral Implants Res 2001:
25. Tanzer M, Karabasz D, Krygier JJ,
16. Piconi C, Maccauro G. Zirconia as a
12: 109–114.
Cohen R, Bobyn JD. The Otto Aufranc
ceramic biomaterial. Biomaterials 1999:
21. Stadlinger B, Pilling E, Mai R, Bier-
Award: bone augmentation around and
baum S, Berhardt R, Scharnweber D,
within porous implants by local bispho-
17. Rahn BV, Bacellar FC, Trapp L, Per-
Eckelt U. Effect of biological implant
sphonate elution. Clin Orthop Relat Res
ren SM. A method for morphometry of
surface coatings on bone formation,
2005: 441: 30–39.
bone formation using fluorochromes.
applying collagen, proteoglycans, glyco-
Aktuelle Traumatol 1980: 10: 109–115.
saminoglycans and growth factors. J
18. Rammelt S, Illert T, Bierbaum S,
Mater Sci Mater Med 2008: 19: 1043–
Scharnweber D, Zwipp H, Schneiders
Musculoskeletal Research Unit
W. Coating of titanium implants with col-
22. Steinemann SG. Titanium–the material
lagen. RGD peptide and chondroitin sul-
of choice? Periodontol 2000 1998: 17:
Vetsuisse Faculty ZH
fate. Biomaterials 2006: 27: 5561–5571.
University of Zurich
19. Schuler M, Owen GR, Hamilton DW,
23. Sul YT, Johansson CB, Jeong Y,
Winterthurerstr. 260
de Wild M, Textor M, Brunette DM,
Roser K, Wennerberg A, Albrekts-
Tosatti SG. Biomimetic modification of
son T. Oxidized implants and their influ-
titanium dental implant model surfaces
ence on the bone response. J Mater Sci
Tel: +41 76 432 27 38
using the RGDSP-peptide sequence: a
Mater Med 2001: 12: 1025–1031.
Fax: +41 44 635 8905
cell morphology study. Biomaterials
24. Szmukler-Moncler S, Zeggel P, Per-
2006: 27: 4003–4015.
D, Bernard JP, Neumann HG.
Please cite this article in press as: Langhoff JD, et al., Comparison of chemically and pharmaceutically modified titanium and zirconiaimplant surfaces in dentistry: a study in sheep, Int J Oral Maxillofac Surg (2008), doi:
Source: http://www.s217469054.mialojamiento.es/zeramexPT/index_htm_files/Zerafiloberflaeche%20Studie%20Thommen%20mit%20Kommentar%20.pdf
Cosmetic ingredients database Chemical Type Other information Compound which dissolves in water to make a solution with a pH less than 7 Compound which dissolves in water to make a solution with a pH above 7. Aloe barbadensis Softens skin, soothes burns and injuries. Name not used in cosmetics. Aloe vera (Latin) See Aloe barbadensis.
Stellenwert der extrakorporalen Membranoxygenierung bei schwerst traumatisierten Patienten mit ARDSN. Madershahian, U. Franke, T. Wittwer, S. Sakka, K. Schwarzkopf, M. Kaluza, T. WahlersFriedrich-Schiller Universität Jena, Klinik für Herz-, Thorax- und Gefäßchirurgie, Jena Das ARDS infolge eines schweren Thoraxtraumas ist mit einer sehr hohen Mortalität vergesellschaf-tet. Die extrakorporale Membranoxygenierung (ECMO) könnte als ultima ratio das Überleben dieser schwerst traumatisierten Patienten sichern. Häufig stellen schwere Begleitverletzungen aufgrund der notwendigen Antikoagulation eine absolute Kontraindikation für diese Maximaltherapie dar. Anhand von 3 Kasuistiken soll der Stellenwert der ECMO bei Patienten mit Polytrauma dargestellt werden.Bei Pat. 1 wurde nach schwerem Polytrauma (Schädelbasisfraktur mit Schädelhirntrauma, Thorax-trauma, stumpfes Bauchtrauma mit Milzruptur, Unterarmfraktur) ein Hauptbronchusabriß rechts diag-nostiziert. Bei foudryanter Entwicklung eines ARDS musste zunächst die ECMO implantiert werden. Die Oberlappenmanschettenresektion erfolgte an der ECMO. Pat. 2 und 3 entwickelten bei Polytraumatisie-rung ohne wesentliche Lungenverletzung ein ARDS. Bei einem Oxygenierungsindex < 70 mmHg und schwerer, therapierefraktärer, respiratorischer Azidose wurde die Indikation zur ECMO gestellt.Die ECMO wurde für 116 ± 30 h aufrechterhalten. Es traten keine ECMO-assoziierten, thrombembo-lischen oder Blutungskomplikationen auf. Alle 3 Patienten konnten erfolgreich von der maschinellen Unterstützung entwöhnt und nach 34 ± 26 d in die Rehabilitationsklinik verlegt werden.Der Einsatz der ECMO ist bei Pat. mit posttraumatischem Lungenversagen als ultima ratio Therapie möglich ohne zusätzliche Komplikationen zu verursachen.





